Optimization and Characterization of PEG Extraction Process for Tartary Buckwheat-Derived Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Experimental Methods
2.2.1. Tartary Buckwheat Pretreatment
2.2.2. Extraction Process of TBDNs
2.2.3. Optimization of the Extraction Process of TBDNs
- (1)
- One-way experiment
- (2)
- Response surface experimental design
2.2.4. Determination of Biological Activity of TBDNs
- (1)
- Determination of total polyphenol content
- (2)
- Determination of total flavonoid content
- (3)
- Measurement of DPPH Free Radical Scavenging
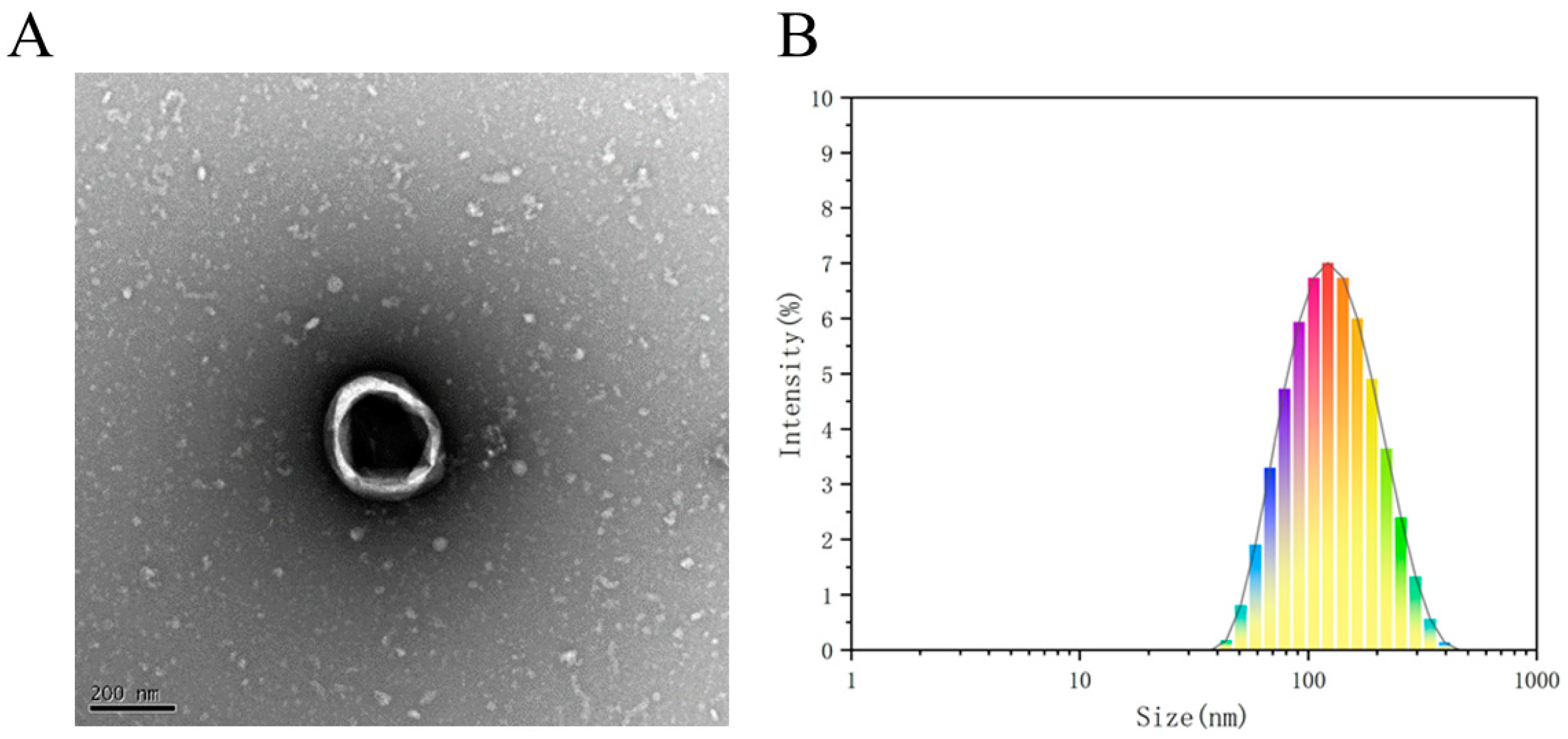
2.2.5. Characterization of TBDNs
- (1)
- Electron microscopic detection of TBDNs
- (2)
- Particle size detection of TBDNs
2.2.6. Statistics and Analysis of Data
3. Result and Discussion
3.1. Results of the One-Way Test
3.1.1. Effect of Different Extraction Processes on the Extraction Yield of TBDNs
3.1.2. Effects of Different Extraction Processes on the Total Phenolic Content of TBDNs
3.1.3. Effects of Different Extraction Processes on the Total Flavonoid Content of TBDNs
3.1.4. Effect of Different Extraction Processes on the Antioxidant Activity of TBDNs
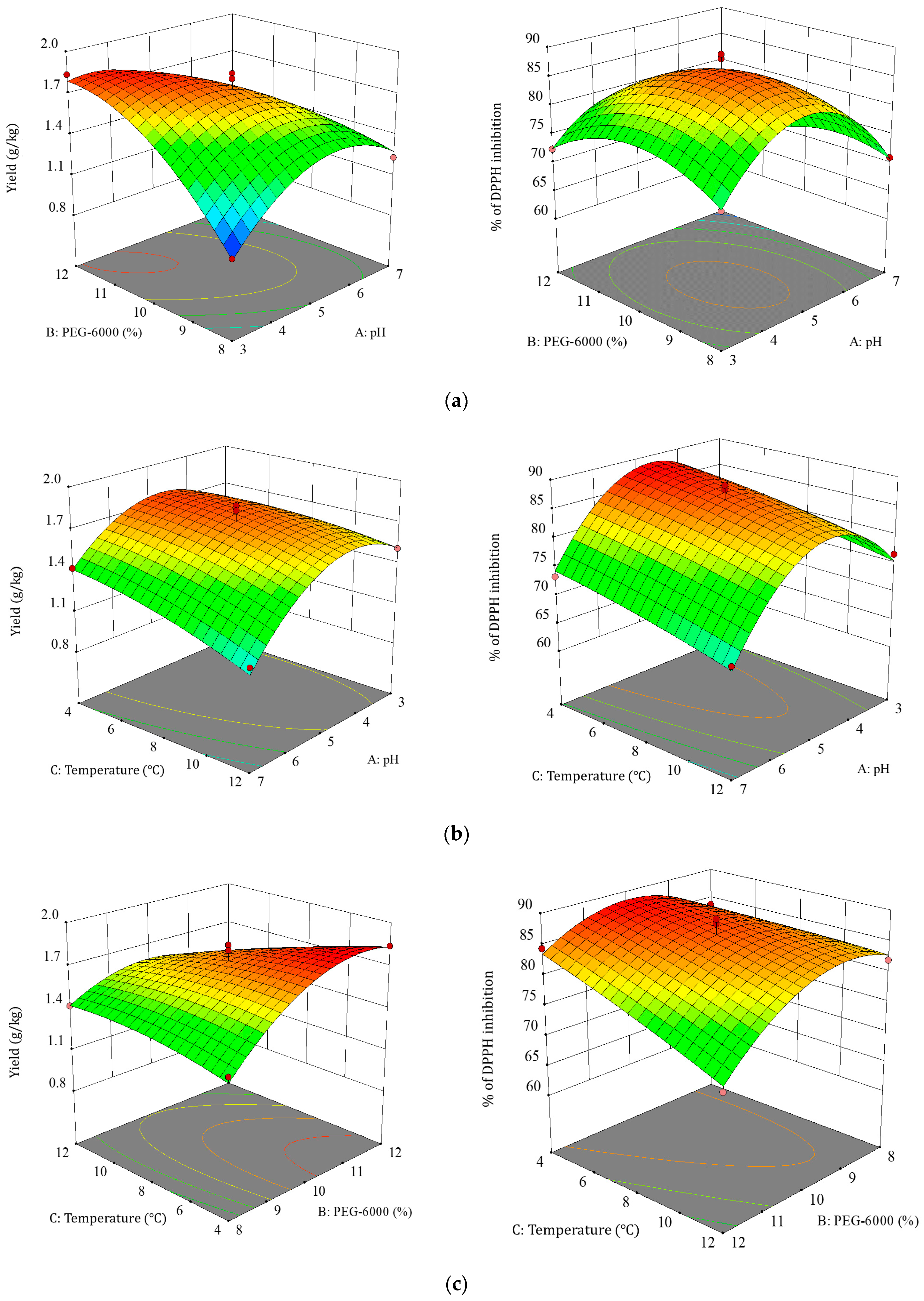
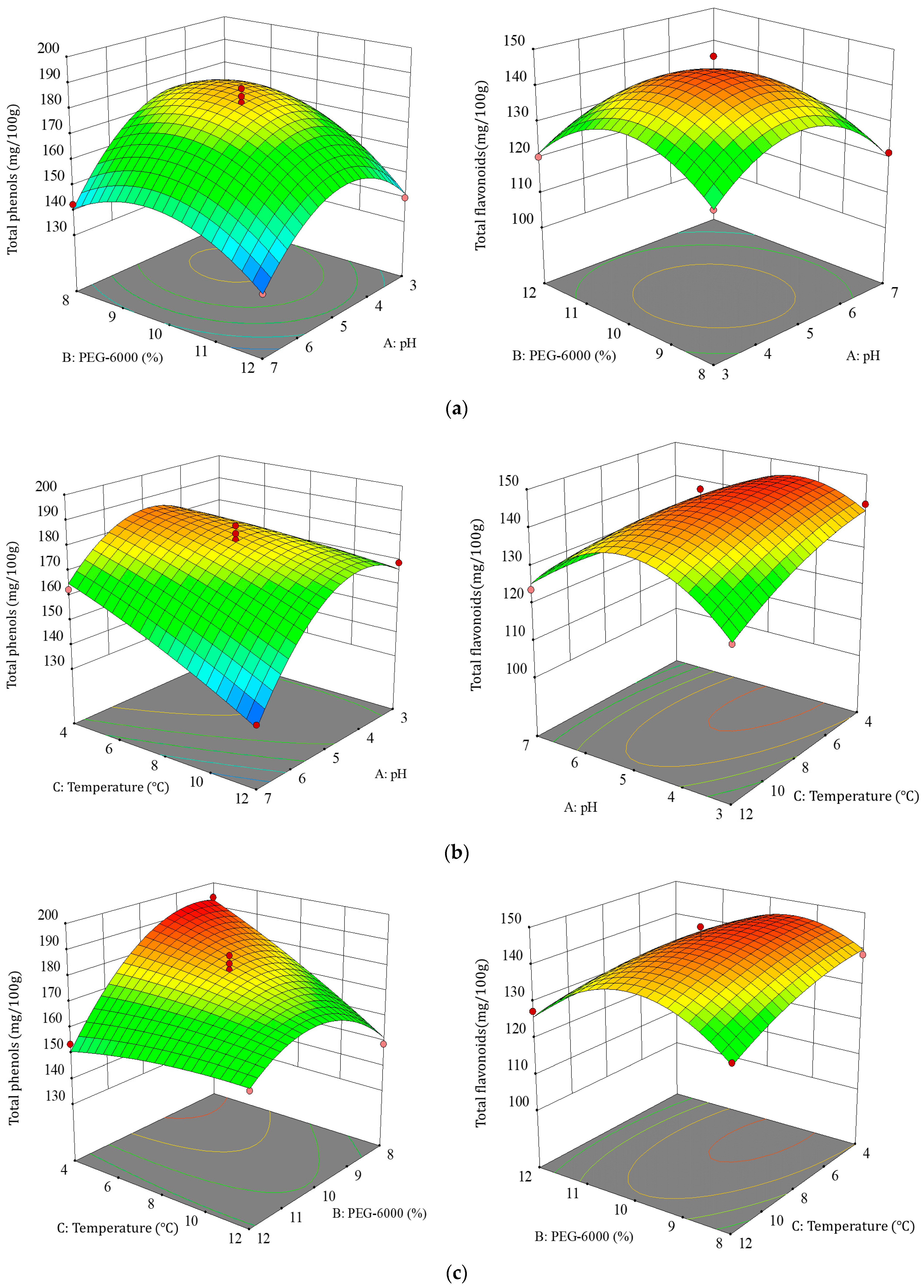
3.2. Response Surface Optimization Tests
3.2.1. Response Surface Optimization and ANOVA Results
3.2.2. Effect of Factor Interactions on TBDNs
3.2.3. Optimal Process Validation
3.3. Characterization of TBDNs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.-Y.; Ren, C.-Q.; Cao, Y.-N.; Ren, Y.; Zou, L.; Zhou, C.; Peng, L.-X. Role of MicroRNAs in Dietary Interventions for Obesity and Obesity-Related Diseases. J. Agric. Food Chem. 2023, 71, 14396–14412. [Google Scholar] [PubMed]
- Liu, Y. Effect of Tartary Buckwheat—Derived Nanoparticles(TBDNs) on Gut Microbiota and Development of Fermented Beverages. Master’s Thesis, Chengdu University, Chengdu, China, 2022. [Google Scholar]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [PubMed]
- Liu, Y.; Tan, M.-L.; Zhu, W.-J.; Cao, Y.-N.; Peng, L.-X.; Yan, Z.-Y.; Zhao, G. In Vitro Effects of Tartary Buckwheat-Derived Nanovesicles on Gut Microbiota. J. Agric. Food Chem. 2022, 70, 2616–2629. [Google Scholar] [PubMed]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 522–534. [Google Scholar]
- Zu, M.; Xie, D.; Canup, B.S.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials 2021, 279, 121178. [Google Scholar]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.-K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar]
- Guo, M.; Wu, F.; Hu, G.; Chen, L.; Xu, J.; Xu, P.; Wang, X.; Li, Y.; Liu, S.; Zhang, S.; et al. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci. Transl. Med. 2019, 11, eaat5690. [Google Scholar]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar]
- Teng, Y.; Mu, J.; Hu, X.; Samykutty, A.; Zhuang, X.; Deng, Z.; Zhang, L.; Cao, P.; Yan, J.; Miller, D.; et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget 2016, 7, 25683–25697. [Google Scholar]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar]
- Liu, Y.; Ren, C.; Zhan, R.; Cao, Y.; Ren, Y.; Zou, L.; Zhou, C.; Peng, L. Exploring the Potential of Plant-Derived Exosome-like Nanovesicle as Functional Food Components for Human Health: A Review. Foods 2024, 13, 712. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G., Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar]
- Yu, L.L.; Zhu, J.; Liu, J.-X.; Jiang, F.; Ni, W.-K.; Qu, L.-S.; Ni, R.-Z.; Lu, C.-H.; Xiao, M.-B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. BioMed Res. Int. 2018, 2018, 3634563. [Google Scholar]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar]
- Gao, Y.; Liu, K.; Zhu, X.; Ren, S.; Ye, X.; Ru, W.; Niu, Y.; Liu, Y.; Gao, X.; Huang, Z. Optimization of total phenolics and total flavonoids enrichment process of black Tartarybuckwheat by solid-state fermentation and its antioxidant activity. J. Henan Univ. Technol. China Nat. Sci. Ed. 2024, 1–17. [Google Scholar] [CrossRef]
- Wang, J.; Yi, Q.; Tie, Q.; Peng, L.; Zhao, G. Effect on flavonoids and antioxidant activity of the bud of tartary buckwheat aftertreatment of different LED light sources. Food Sci. Technol. 2021, 44, 213–218. [Google Scholar]
- Lewis, G.D.; Metcalf, T.G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol. 1988, 54, 1983–1988. [Google Scholar]
- Bian, Y.P. Garlic-derived Exosomes-like Nanoparticles Attenuate Inflammation Response in the White Adipose of High-fat Diet-fed C57BL/6 Mice. Master’s Thesis, Chongqing University of Technology, Chongqing, China, 2021. [Google Scholar]
- Zhao, W.J. Blueberry-derived exosomes-like nanoparticles antagonize oxidative stress and its associated molecular mechanisms in the hepatocytes of HFD-fed mice. Master’s Thesis, Chongqing University of Technology, Chongqing, China, 2021. [Google Scholar]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar]
- Suresh, A.P.; Kalarikkal, S.P.; Pullareddy, B.; Sundaram, G.M. Low pH-Based Method to Increase the Yield of Plant-Derived Nanoparticles from Fresh Ginger Rhizomes. ACS Omega 2021, 6, 17635–17641. [Google Scholar]
- Ban, J.J.; Lee, M.; Im, W.; Kim, M. Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 2015, 461, 76–79. [Google Scholar]
- Zhao, Y.; Chen, K.; Li, H.; Wu, H. Effect of pH on the isolation of urinary exosome. Int. Urol. Nephrol. 2017, 49, 165–169. [Google Scholar]
- Zhuo, W.W.; Li, F.; Hu, J. Optimization of Ultrasonic-assisted Extraction of Total Flavonoids from Nutmeg by Response Surface and Its Antioxidant Activity Analysis. Food Res. Dev. 2021, 42, 126–133. [Google Scholar]
- Zhang, S.-Y.; Xu, N.-X.; Fang, X.-A.; Cui, N.; Wang, P.; Chen, L.; Gao, J.; Li, C.-Q. Optimization in Ultrasonic Assisted Solvent Extraction Process of Total Flavonoids from Wuyuan Chrysanthemum morifolium by Response Surface Methodology. Chem. Bioeng. 2022, 39, 32–37. [Google Scholar]
- Guo, L.F. Study on preparation of polysaccharide from pecan peel and its antioxidant activity in vitro. Feed Res. 2022, 45, 92–97. [Google Scholar]
- Yi, R.L.; Ge, G.T.; Wang, Z.J.; Hu, R.C.; Bai, S. Study on the Extraction Process of Total Flavonoids and Total Phenolic Acids in Leontopodium leontopodioides (Wild.) Beauv. Acta Agrestia Sin. 2024, 32, 935–944. [Google Scholar]
- Lu, J. Study on response surface optimization of extraction process and antioxidant of total flavonoids from walnut peel. Feed Res. 2023, 46, 97–102. [Google Scholar]
- Wang, Z.W. Optimization of extraction technology and antioxidant activity of Fructus mori polysaccharide by response surface method. Feed Res. 2022, 45, 87–92. [Google Scholar]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 1345–1357. [Google Scholar]
- Pinedo, M.; de la Canal, L.; de Marcos Lousa, C. A call for Rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles 2021, 10, e12048. [Google Scholar]
- Man, F.; Meng, C.; Liu, Y.; Wang, Y.; Zhou, Y.; Ma, J.; Lu, R. The Study of Ginger-Derived Extracellular Vesicles as a Natural Nanoscale Drug Carrier and Their Intestinal Absorption in Rats. AAPS PharmSciTech 2021, 22, 206. [Google Scholar] [PubMed]
- Liu, W.; Wang, S.; Dong, K.; Cheng, T. Research on optimization of perforation parameters for formation fractures based on response surface optimization method. PLoS ONE 2021, 16, e0255793. [Google Scholar]
- Xu, W.Q.; Hao, X.L.; Dai, S.J. Study on optimization of extraction conditions of flavonoids from angelica dahurica root by responses surface methodology. Cereals Oils 2021, 34, 129–131. [Google Scholar]
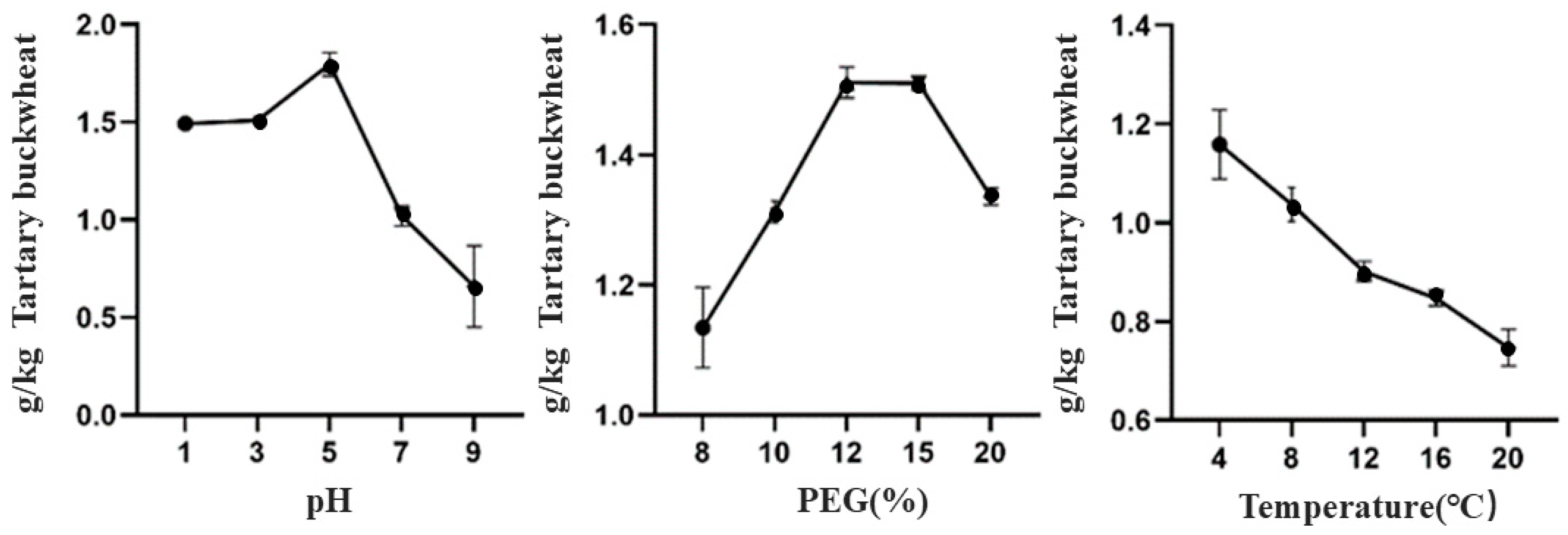
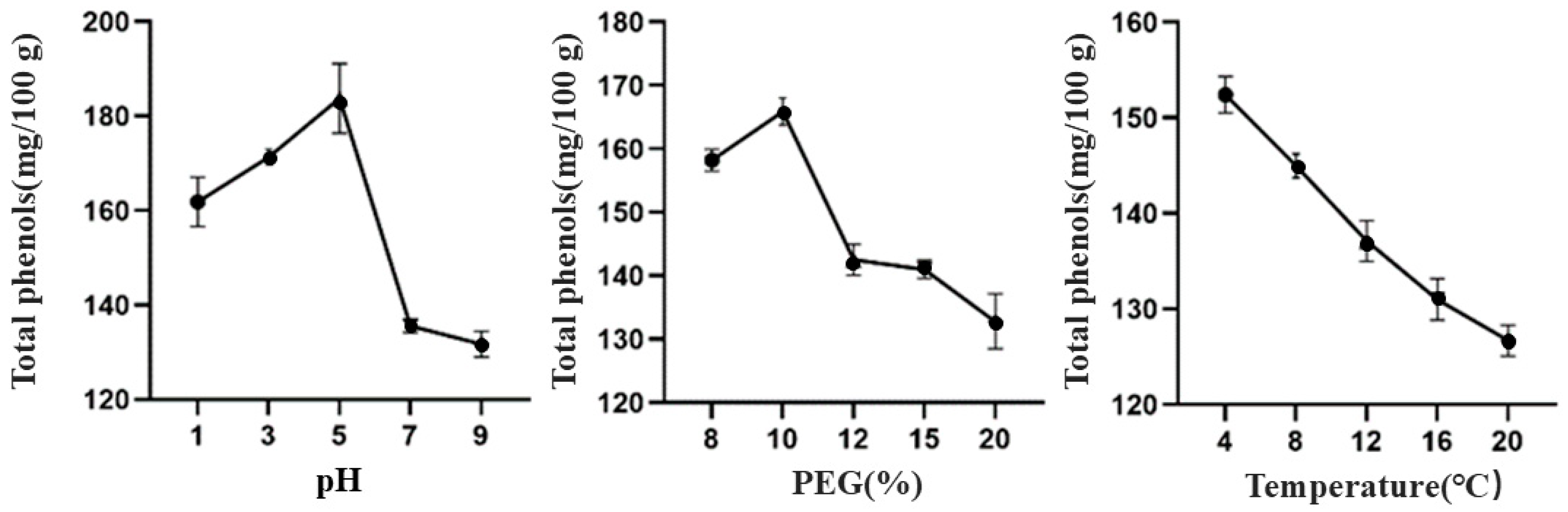
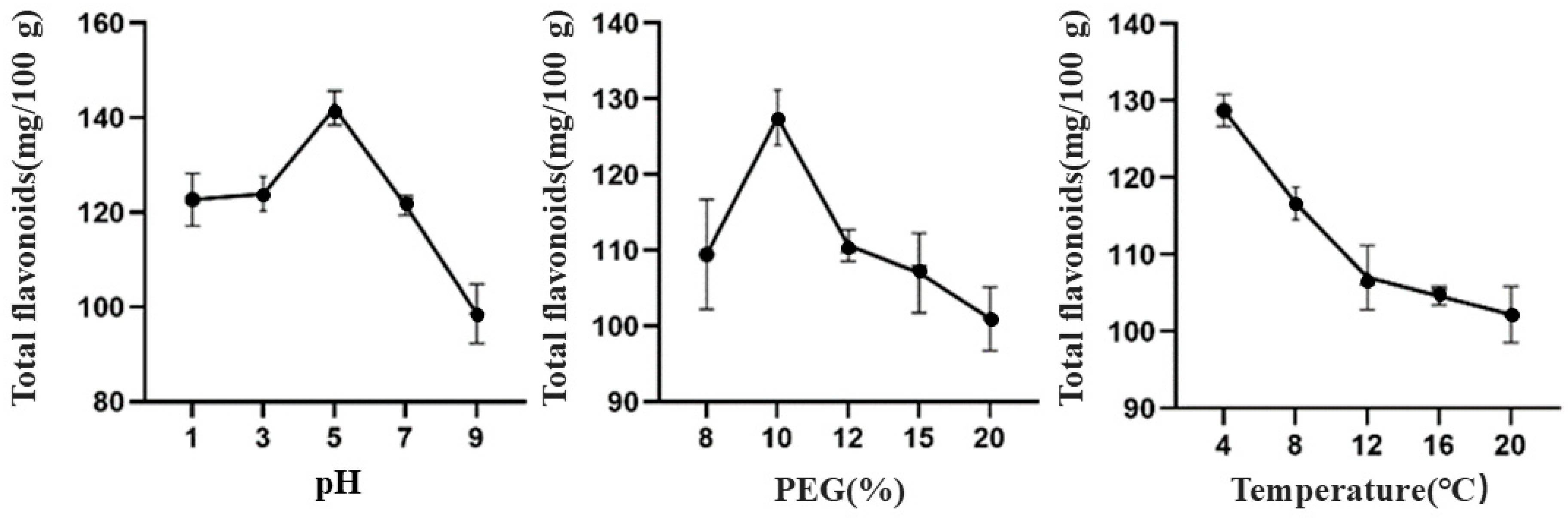
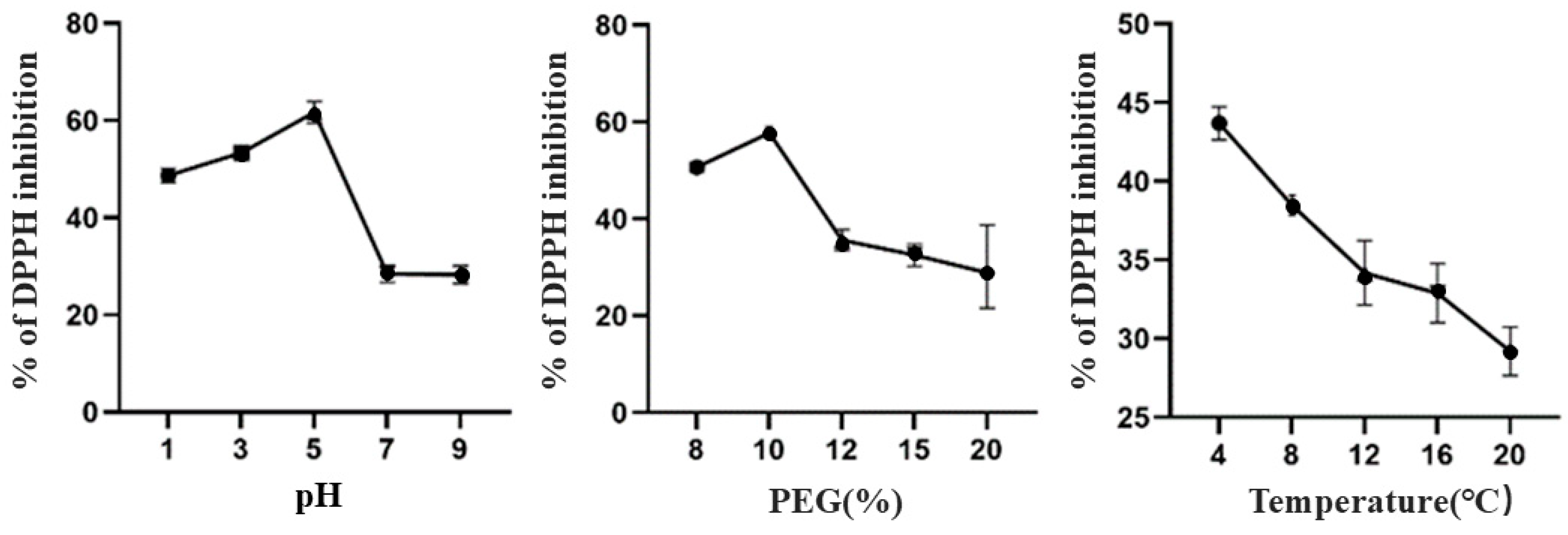
| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: pH | 3 | 5 | 7 |
| B: PEG6000 (%) | 8 | 10 | 12 |
| C: temperature (°C) | 4 | 8 | 12 |
| Number | A | B | C | Y1: Yield (g/kg) | Y2: DPPH Inhibition (%) | Y3: Total Phenol (mg/100 g) | Y4: Total Flavonoid (mg/100 g) |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 8 | 12 | 1.419 | 81.900 | 148.85 | 127.54 |
| 2 | 5 | 12 | 12 | 1.325 | 71.77 | 157.22 | 127.54 |
| 3 | 3 | 8 | 8 | 0.973 | 72.85 | 167.12 | 123.91 |
| 4 | 5 | 10 | 8 | 1.662 | 84.43 | 172.94 | 148.07 |
| 5 | 7 | 8 | 8 | 1.235 | 71.00 | 142.43 | 121.50 |
| 6 | 3 | 12 | 8 | 1.836 | 72.46 | 139.87 | 120.29 |
| 7 | 5 | 10 | 8 | 1.845 | 88.00 | 182.94 | 143.24 |
| 8 | 7 | 12 | 8 | 0.901 | 62.15 | 131.00 | 107.00 |
| 9 | 5 | 10 | 8 | 1.808 | 88.77 | 180.49 | 148.07 |
| 10 | 5 | 10 | 8 | 1.731 | 85.75 | 186 | 142.03 |
| 11 | 3 | 10 | 4 | 1.495 | 79.40 | 164.88 | 142.03 |
| 12 | 7 | 10 | 12 | 1.129 | 68.88 | 131.51 | 123.91 |
| 13 | 7 | 10 | 4 | 1.42 | 73.34 | 162.63 | 121.50 |
| 14 | 3 | 10 | 12 | 1.51 | 76.59 | 169.06 | 123.91 |
| 15 | 5 | 12 | 4 | 1.838 | 84.35 | 153.75 | 123.91 |
| 16 | 5 | 10 | 8 | 1.631 | 84.74 | 175.69 | 142.03 |
| 17 | 5 | 8 | 4 | 1.355 | 83.71 | 193.75 | 138.41 |
| Source | Sum of Squared Deviations | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 933.18 | 9 | 103.69 | 33.16 | <0.0001 | ** |
| A: pH | 84.05 | 1 | 84.05 | 26.88 | 0.0013 | ** |
| B: PEG6000 | 43.85 | 1 | 43.85 | 14.03 | 0.0072 | ** |
| C: temperature | 58.64 | 1 | 58.64 | 18.76 | 0.0034 | ** |
| AB | 17.89 | 1 | 17.89 | 5.72 | 0.048 | * |
| AC | 0.68 | 1 | 0.68 | 0.22 | 0.655 | |
| BC | 29 | 1 | 29 | 9.28 | 0.0187 | * |
| A2 | 537.78 | 1 | 537.78 | 172.01 | <0.0001 | ** |
| B2 | 123.76 | 1 | 123.76 | 39.58 | 0.0004 | ** |
| C2 | 0.99 | 1 | 0.99 | 0.32 | 0.5919 | |
| Residual | 21.89 | 7 | 3.13 | |||
| Misfit term | 6.67 | 3 | 2.22 | 0.58 | 0.6564 | ns |
| Pure error | 15.22 | 4 | 3.8 | |||
| Total | 955.07 | 16 | ||||
| R2 = 0.9771 Adj R2 = 0.9476 Pre R2 = 0.8634 | ||||||
| Source | Sum of Squared Deviations | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 5631.38 | 9 | 625.71 | 29.22 | <0.0001 | ** |
| A: pH | 672.71 | 1 | 672.71 | 31.42 | 0.0008 | ** |
| B: PEG6000 | 617.94 | 1 | 617.94 | 28.86 | 0.001 | ** |
| C: temperature | 584.31 | 1 | 584.31 | 27.29 | 0.0012 | * |
| AB | 62.57 | 1 | 62.57 | 2.92 | 0.1311 | ** |
| AC | 311.52 | 1 | 311.52 | 14.55 | 0.0066 | * |
| BC | 584.91 | 1 | 584.91 | 27.32 | 0.0012 | |
| A2 | 1759.09 | 1 | 1759.09 | 82.16 | <0.0001 | ** |
| B2 | 833.21 | 1 | 833.21 | 38.92 | 0.0004 | ** |
| C2 | 19.5 | 1 | 19.5 | 0.91 | 0.3717 | ** |
| Residual | 149.88 | 7 | 21.41 | |||
| Misfit term | 37.33 | 3 | 12.44 | 0.44 | 0.7357 | ns |
| Pure error | 112.55 | 4 | 28.14 | |||
| Total | 5781.26 | 16 | ||||
| R2 = 0.9741 Adj R2 = 0.9407 Pre R2 = 0.8663 | ||||||
| Source | Sum of Squared Deviations | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 5631.38 | 9 | 625.71 | 29.22 | 0.0001 | ** |
| A: pH | 672.71 | 1 | 672.71 | 31.42 | 0.0008 | ** |
| B: PEG6000 | 617.94 | 1 | 617.94 | 28.86 | 0.001 | ** |
| C: temperature | 584.31 | 1 | 584.31 | 27.29 | 0.0012 | * |
| AB | 62.57 | 1 | 62.57 | 2.92 | 0.1311 | ** |
| AC | 311.52 | 1 | 311.52 | 14.55 | 0.0066 | * |
| BC | 584.91 | 1 | 584.91 | 27.32 | 0.0012 | |
| A2 | 1759.09 | 1 | 1759.09 | 82.16 | <0.0001 | ** |
| B2 | 833.21 | 1 | 833.21 | 38.92 | 0.0004 | ** |
| C2 | 19.5 | 1 | 19.5 | 0.91 | 0.3717 | ** |
| Residual | 149.88 | 7 | 21.41 | |||
| Misfit term | 37.33 | 3 | 12.44 | 0.44 | 0.7357 | ns |
| Pure error | 112.55 | 4 | 28.14 | |||
| Total | 5781.26 | 16 | ||||
| R2 = 0.9741 Adj R2 = 0.9407 Pre R2 = 0.8663 | ||||||
| Source | Sum of Squared Deviations | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 2195.81 | 9 | 243.98 | 32.45 | <0.0001 | ** |
| A: pH | 164.08 | 1 | 164.08 | 21.83 | 0.0023 | ** |
| B: PEG6000 | 133.01 | 1 | 133.01 | 17.69 | 0.004 | ** |
| C: temperature | 65.84 | 1 | 65.84 | 8.76 | 0.0211 | * |
| AB | 29.59 | 1 | 29.59 | 3.94 | 0.0877 | ** |
| AC | 105.37 | 1 | 105.37 | 14.02 | 0.0072 | * |
| BC | 52.56 | 1 | 52.56 | 6.99 | 0.0332 | |
| A2 | 826.77 | 1 | 826.77 | 109.98 | <0.0001 | ** |
| B2 | 657.92 | 1 | 657.92 | 87.52 | <0.0001 | ** |
| C2 | 33.91 | 1 | 33.91 | 4.51 | 0.0713 | ** |
| Residual | 52.62 | 7 | 7.52 | |||
| Misfit term | 13.52 | 3 | 4.51 | 0.46 | 0.7246 | ns |
| Pure error | 39.1 | 4 | 9.78 | |||
| Total | 2248.44 | 16 | ||||
| R2 = 0.9766 Adj R2 = 0.9465 Pre R2 = 0.8766 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhou, C.; Tan, M.; Cao, Y.; Ren, Y.; Peng, L. Optimization and Characterization of PEG Extraction Process for Tartary Buckwheat-Derived Nanoparticles. Foods 2024, 13, 2624. https://doi.org/10.3390/foods13162624
Zhang J, Zhou C, Tan M, Cao Y, Ren Y, Peng L. Optimization and Characterization of PEG Extraction Process for Tartary Buckwheat-Derived Nanoparticles. Foods. 2024; 13(16):2624. https://doi.org/10.3390/foods13162624
Chicago/Turabian StyleZhang, Jiyue, Chuang Zhou, Maoling Tan, Yanan Cao, Yuanhang Ren, and Lianxin Peng. 2024. "Optimization and Characterization of PEG Extraction Process for Tartary Buckwheat-Derived Nanoparticles" Foods 13, no. 16: 2624. https://doi.org/10.3390/foods13162624
APA StyleZhang, J., Zhou, C., Tan, M., Cao, Y., Ren, Y., & Peng, L. (2024). Optimization and Characterization of PEG Extraction Process for Tartary Buckwheat-Derived Nanoparticles. Foods, 13(16), 2624. https://doi.org/10.3390/foods13162624




