An Analysis of the Intestinal Microbiome Combined with Metabolomics to Explore the Mechanism of How Jasmine Tea Improves Depression in CUMS-Treated Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Depressive Rat Model
2.3. Body Weight Measurement
2.4. Behavioral Tests
2.5. Fecal Sample Collection
2.6. Tissue Collection
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Hematoxylin and Eosin (HE) and Nissl Staining
2.9. 16S rRNA Sequencing
2.9.1. Intestinal Microbial DNA Extraction
2.9.2. 16S rRNA Sequencing and Analysis
2.10. Serum Metabolomics Analysis
2.11. Data Processing and Metabolite Identification
2.12. Data Correlation Analysis
2.13. Statistical Analysis
3. Results
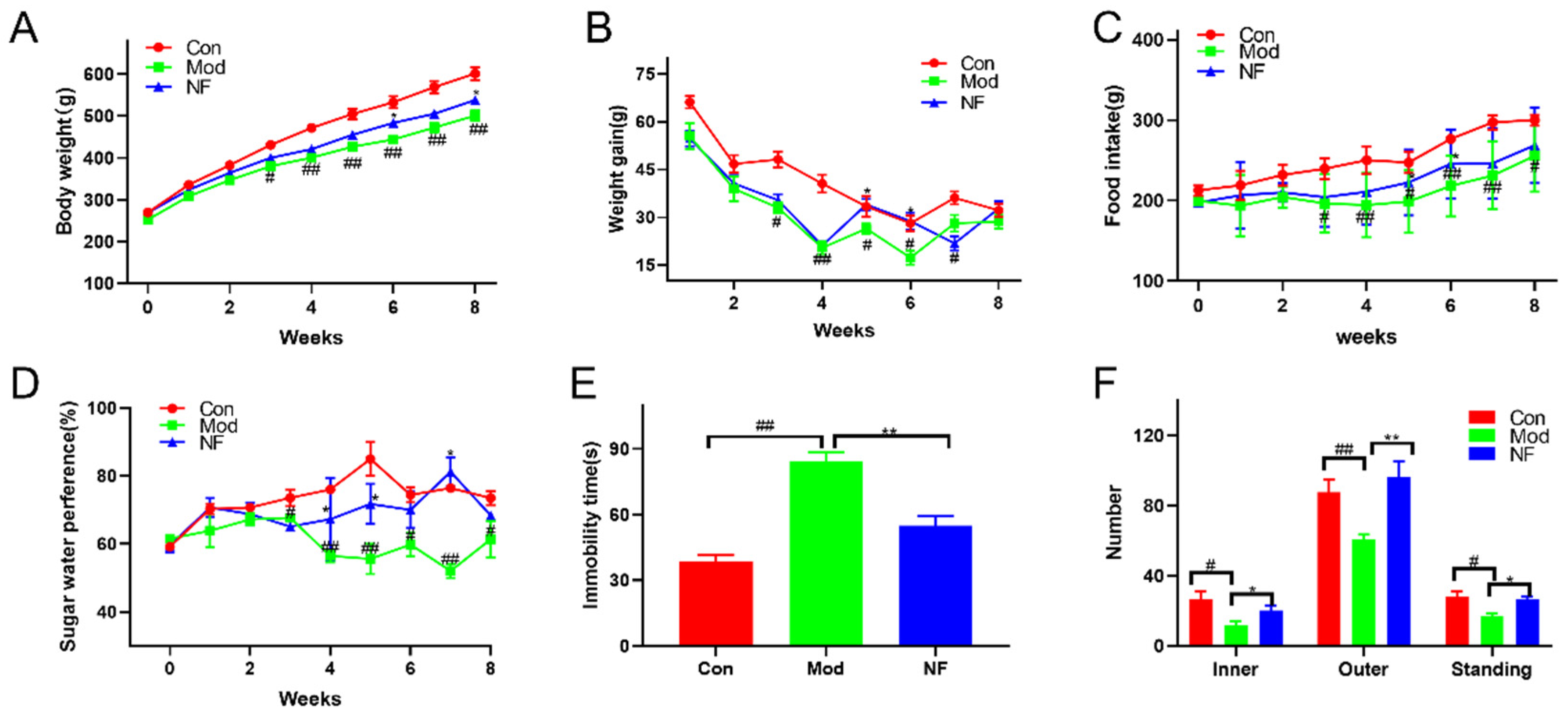
3.1. Jasmine Tea Intake Ameliorates the Behavioral Characteristics of Depressive-like Rats Induced by CUMS
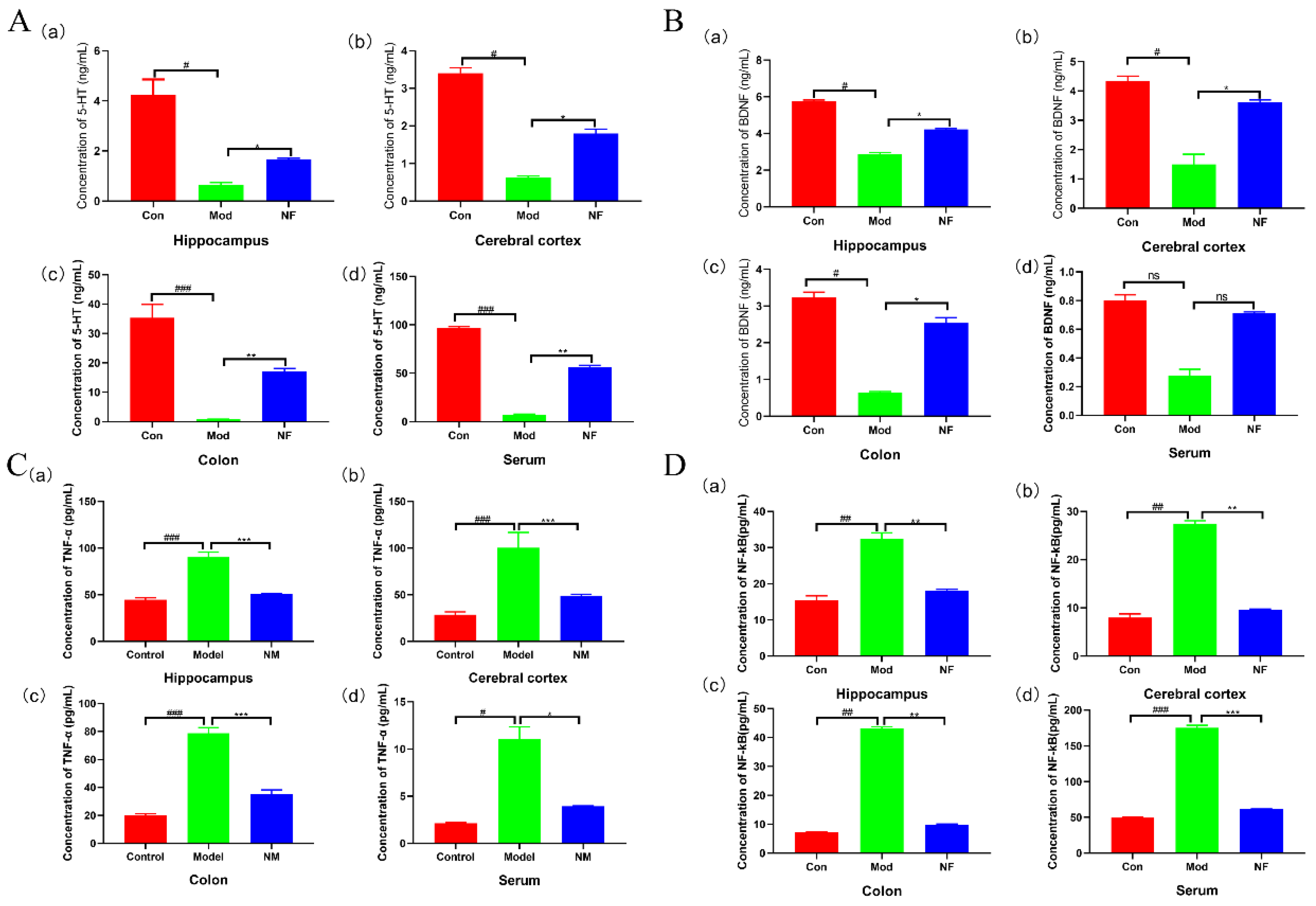
3.2. Jasmine Tea Intake Alleviates Neurotransmitters and Inflammatory Factors of Depressive-like Rats Induced by CUMS
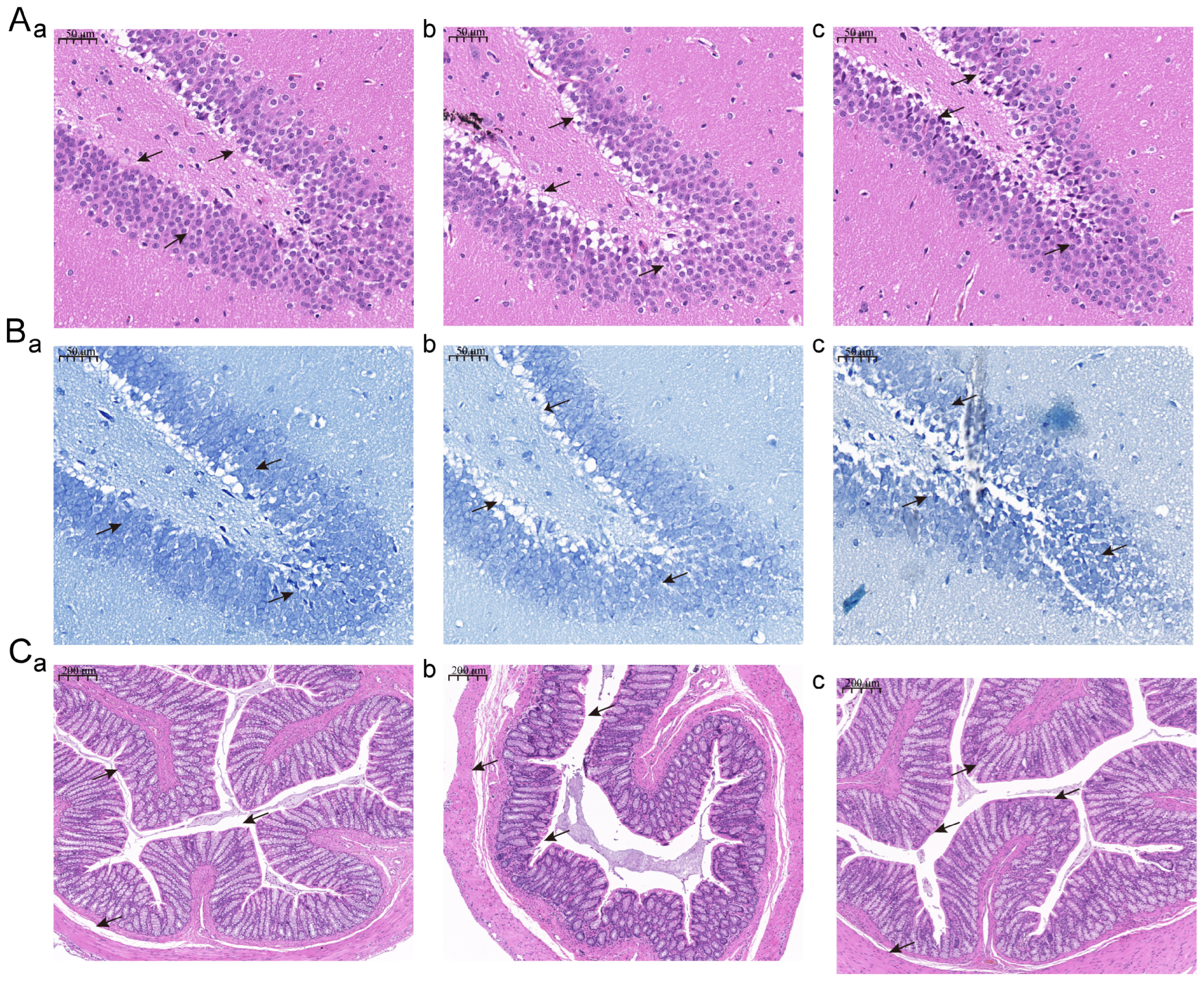
3.3. Jasmine Tea Intake Alleviates the Phenotype of the Hippocampus and Colon of Depressive-like Rats Induced by CUMS
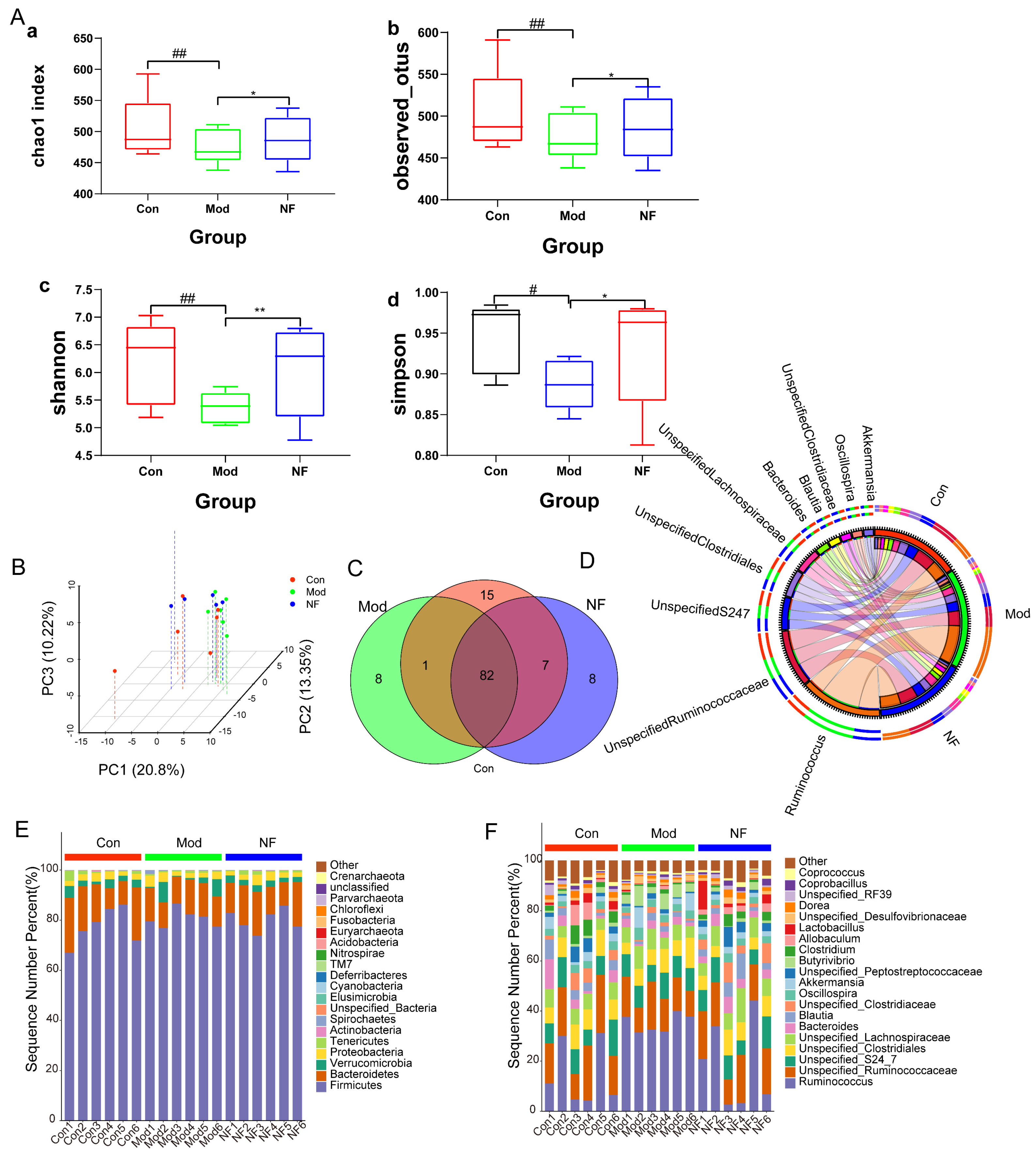
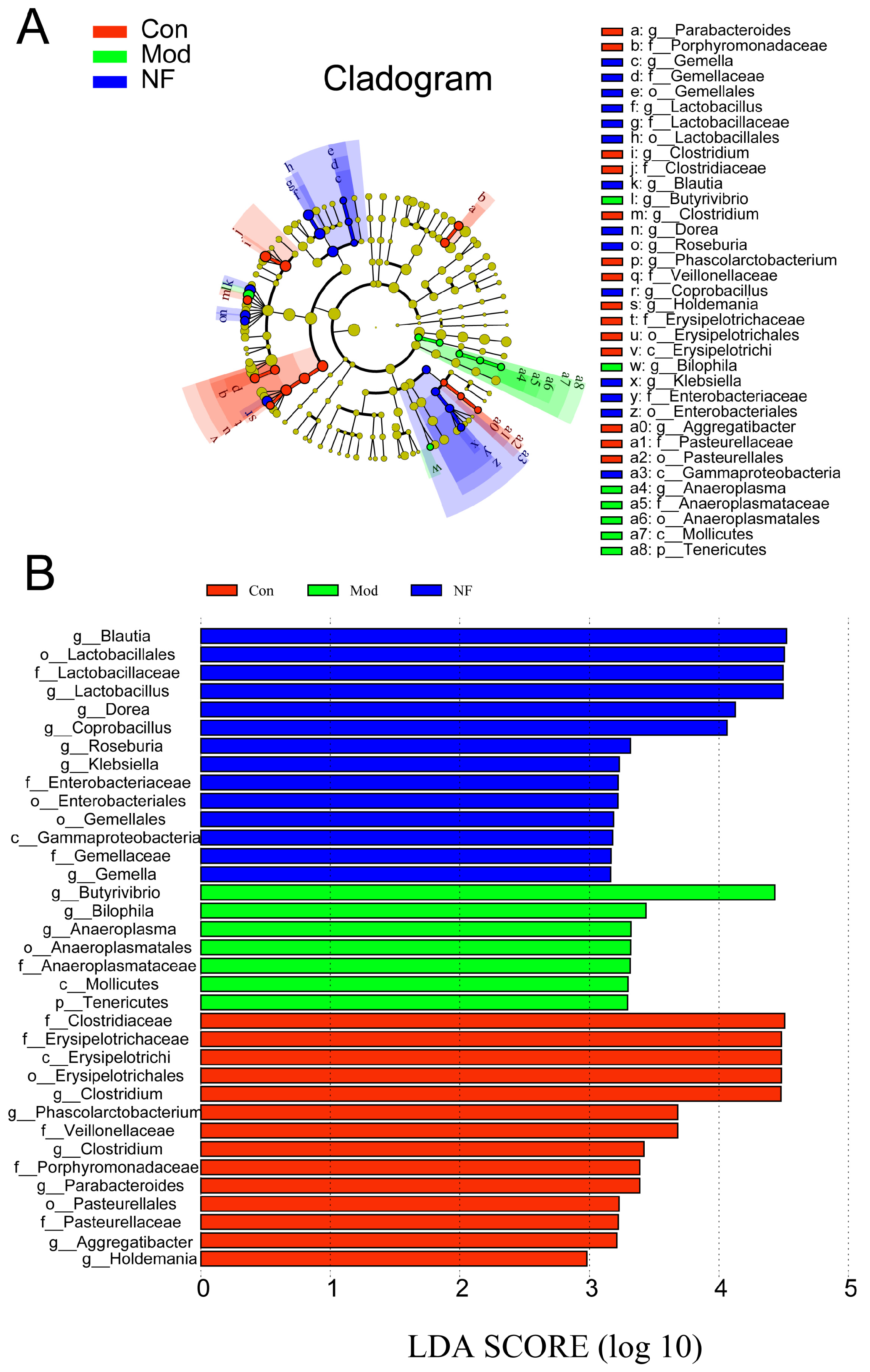
3.4. The Amelioration of Gut Microbiota of Depressive-like Rats Induced by CUMS after Jasmine Tea Administration
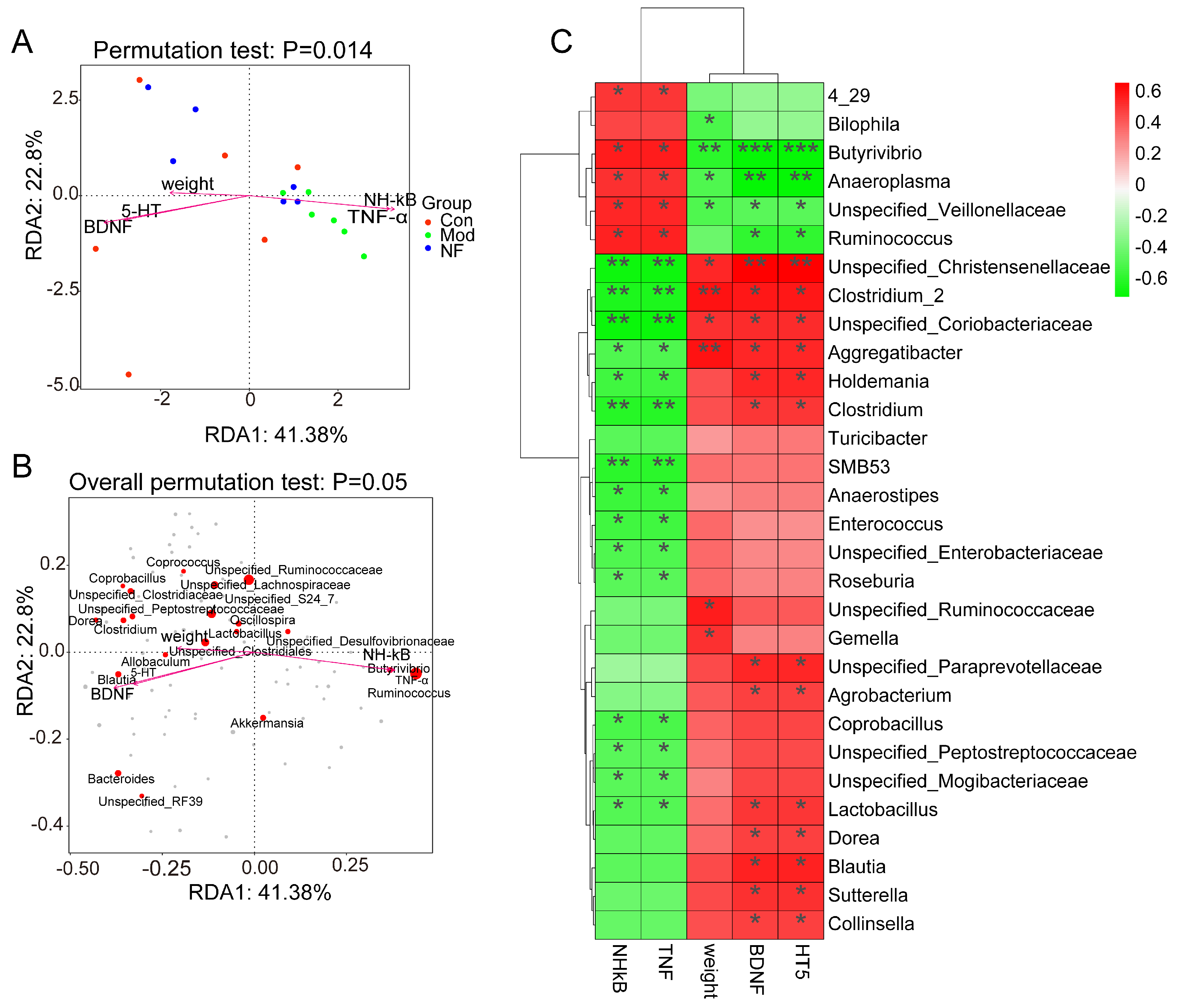
3.5. Correlations of Neurotransmitters and Inflammation with Altered Gut Microbes in Depressive-like Rats Induced by CUMS Treated with Jasmine Tea
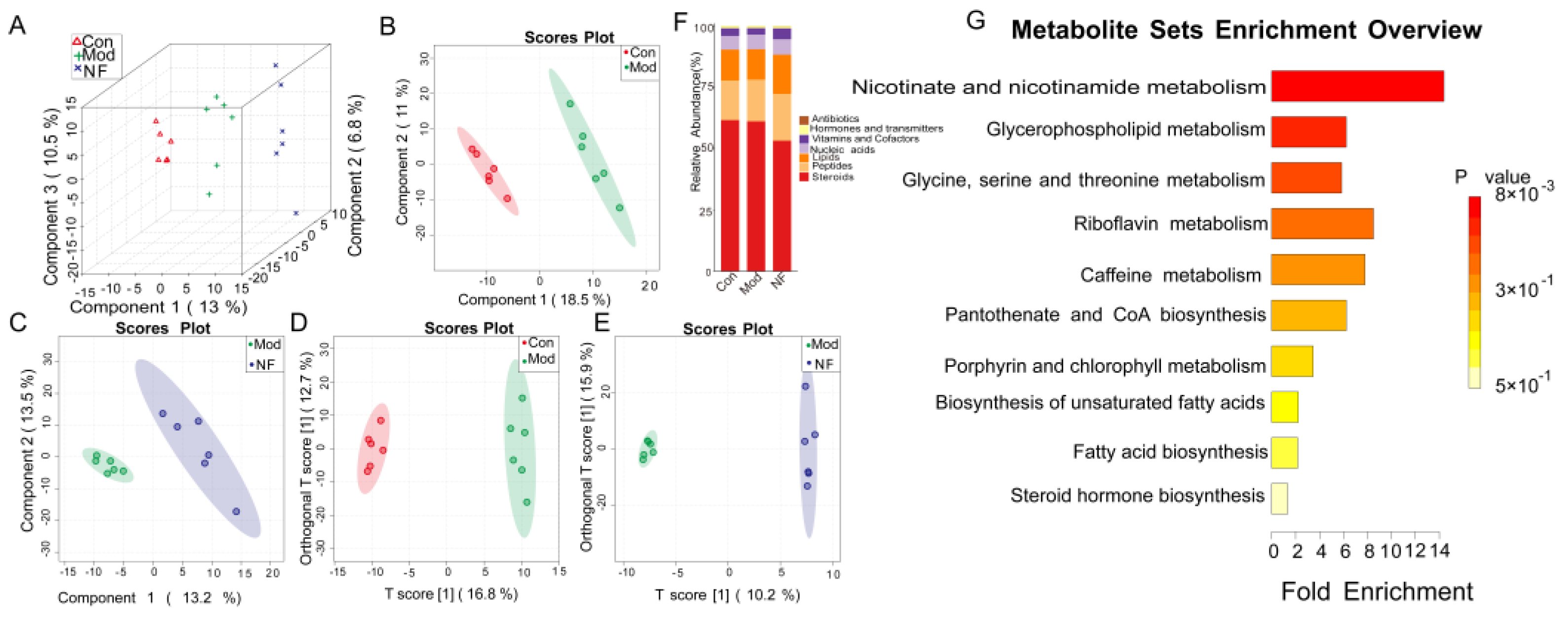
3.6. The Alleviation of Blood Metabolisms in Depressive-like Rats Induced by CUMS after Jasmine Tea Administration
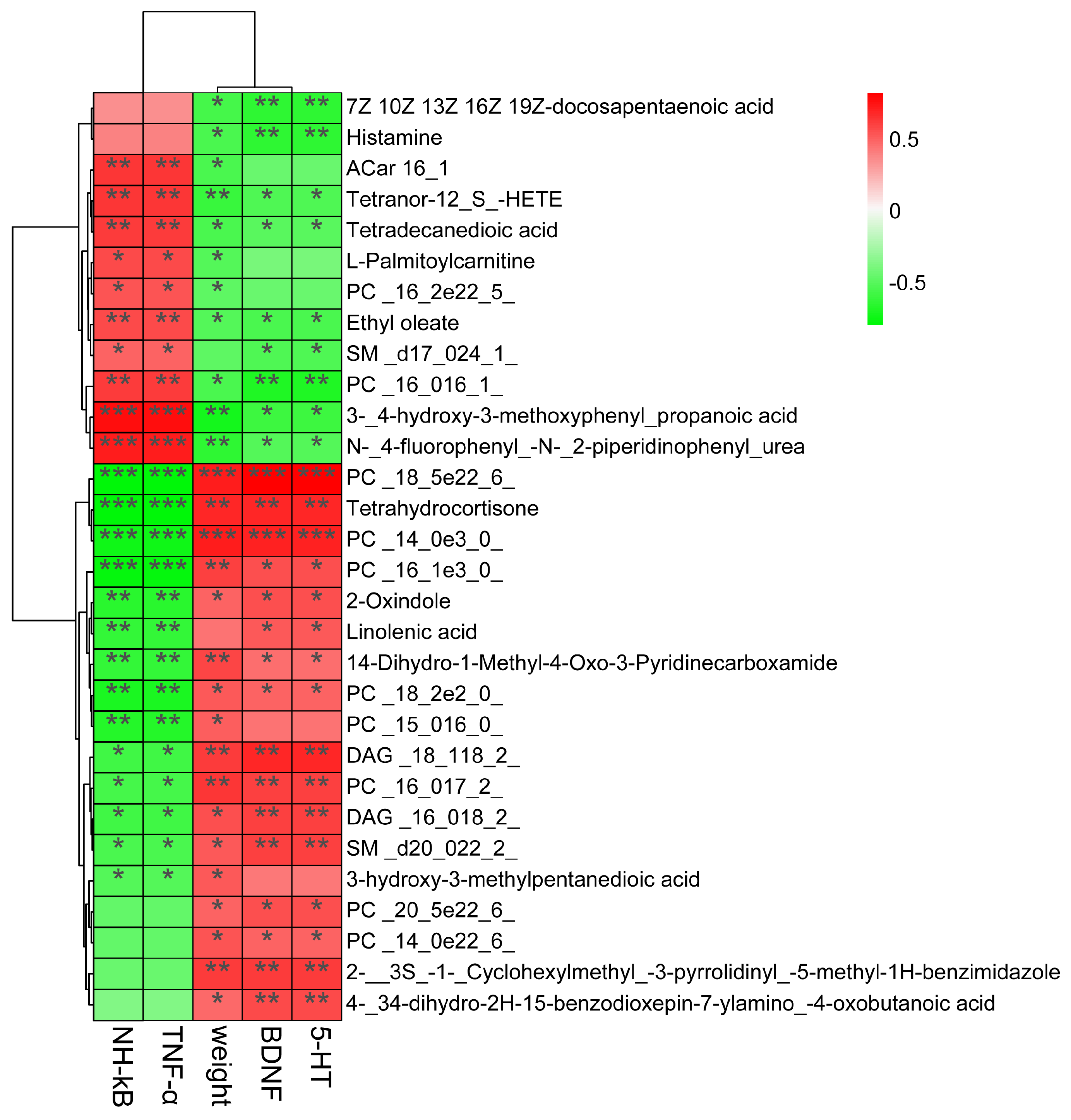
3.7. Correlations Among Neurotransmitters, Inflammation, and Serum Metabolites in Depressive-like Rats Induced by CUMS Treated with Jasmine Tea
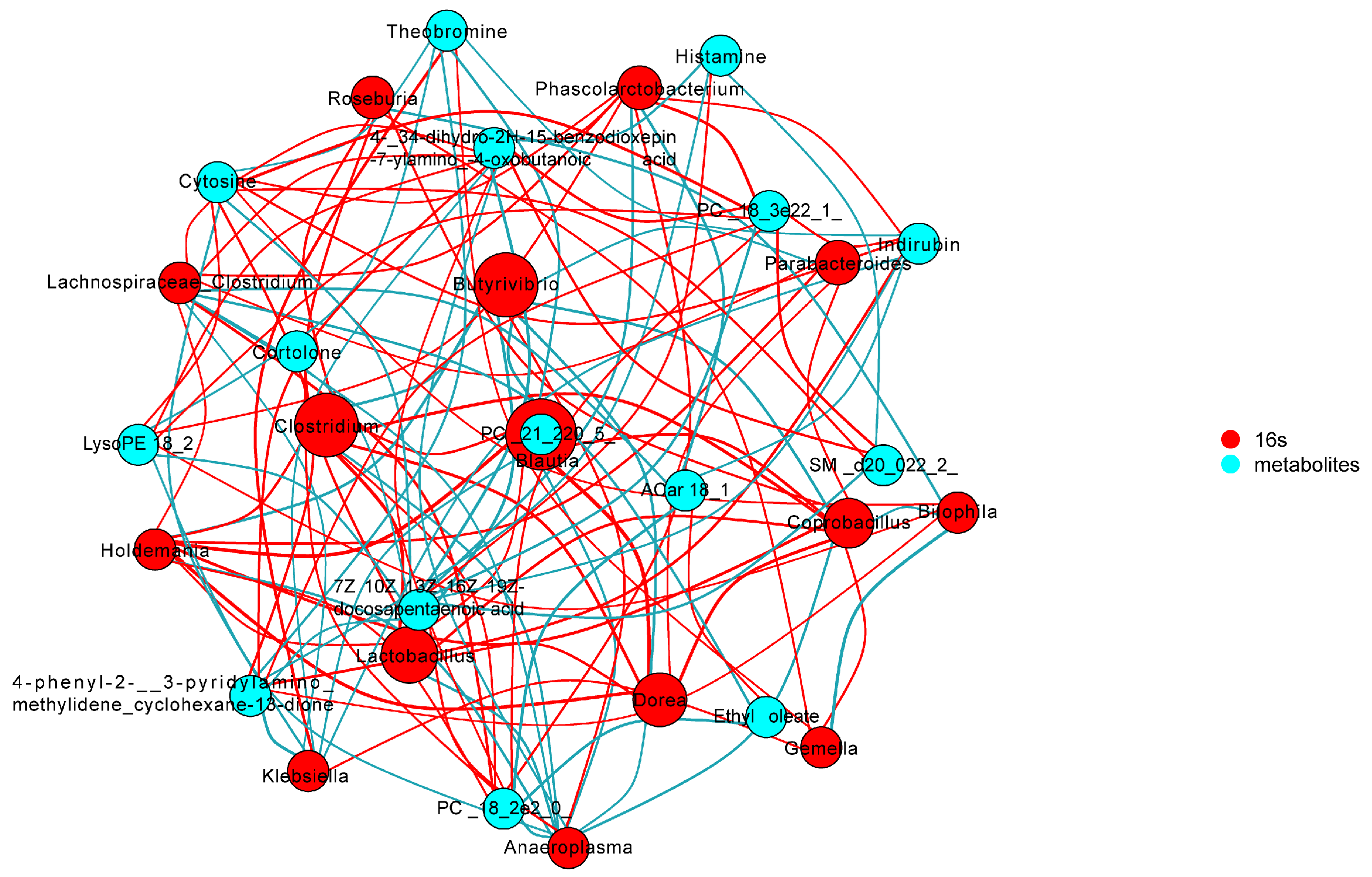
3.8. Correlations between Gut Microbes and Serum Metabolites in Depressive-like Behavior Induced by CUMS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CUMS | Chronic unpredictable mild stress. |
| Con | Control group. |
| Mod | CUMS group. |
| NF | CUMS-treated rats treated with jasmine tea. Scented by new process in middle dose. |
| SPT | Sugar preference test. |
| FST | Force swimming test. |
| OFT | Open field test. |
| 5-HT | 5-hydroxytryptamine. |
| BDNF | Brain-derived neurotrophic factor. |
| TNF-α | Tumor necrosis factor alpha. |
| NF-κB | Nuclear factor kappa B. |
References
- Qiao, Y.; Zhao, J.; Li, C.; Zhang, M.; Wei, L.; Zhang, X.; Kurskaya, O.; Bi, H.; Gao, T. Effect of combined chronic predictable and unpredictable stress on depression-like symptoms in mice. Ann. Transl. Med. 2020, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Nutritional and therapeutic approaches for protecting human gut microbiota from psychotropic treatments. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2021, 108, 110182. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Bear, T.; Dalziel, J.E.; Jane, C.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.Z.; Li, X.J.; Zhang, P.W.; Chen, J.X. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 2019, 284, 112691. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Collins, J.; Blennerhassett, P.A.; Ghia, J.E.; Verdu, E.F.; Bercik, P.; Collins, S.M. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 2013, 25, 733-e575. [Google Scholar] [CrossRef]
- Mendoza, C.; Perez-Urrutia, N.; Alvarez-Ricartes, N.; Barreto, G.E.; Perez-Ordas, R.; Iarkov, A.; Echeverria, V. Cotinine Plus Krill Oil Decreased Depressive Behavior, and Increased Astrocytes Survival in the Hippocampus of Mice Subjected to Restraint Stress. Front. Neurosci. 2018, 12, 952. [Google Scholar] [CrossRef]
- Matthews, D.M.; Jenks, S.M. Ingestion of Mycobacterium vaccae decreases anxiety-related behavior and improves learning in mice. Behav. Process. 2013, 96, 27–35. [Google Scholar] [CrossRef]
- Wiedlocha, M.; Marcinowicz, P.; Janoska-Jazdzik, M.; Szulc, A. Gut microbiota, kynurenine pathway and mental disorders—Review. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2021, 106, 110145. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef]
- Cacabelos, R.; Torrellas, C.; Fernandez-Novoa, L.; Aliev, G. Neuroimmune Crosstalk in CNS Disorders: The Histamine Connection. Curr. Pharm. Des. 2016, 22, 819–848. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Xia, X.T.; Sun, N.; Liu, C.C.; Qin, X.M.; Tian, J.S. Discovering potential biomarkers of depression and drug intervention of paroxetine based on (1) H NMR metabolomics. Acta Pharm. Sin. 2016, 51, 595–599. [Google Scholar]
- Li, Z.-Y.; Zheng, X.-Y.; Gao, X.-X.; Zhou, Y.-Z.; Sun, H.-F.; Zhang, L.-Z.; Guo, X.-Q.; Du, G.-H.; Qin, X.-M. Study of plasma metabolic profiling and biomarkers of chronic unpredictable mild stress rats based on gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, G.; Cheng, K.; Yang, D.; Chen, G.; Liu, Z.; Zhang, R.; Zhou, J.; Fang, L.; Fang, Z.; et al. Peripheral blood mononuclear cell-based metabolomic profiling of a chronic unpredictable mild stress rat model of depression. Mol. Biosyst. 2014, 10, 2994–3001. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, C.; Lin, J.; Chen, T.; Zhao, T.; Jia, Z.; Xie, X.; Oju, Y.; Su, M.; Jiang, T.; et al. Metabonomics Approach to Assessing the Modulatory Effects of St John’s Wort, Ginsenosides, and Clomipramine in Experimental Depression. J. Proteome Res. 2012, 11, 6223–6230. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, C.-h.; Li, Z.-m.; Yang, Z.; Xiao, Z.-j.; Duan, J.-j.; Zhou, T.; Xu, F. Chronic Stress Disturbs Metabolome of Blood Plasma and Urine in Diabetic Rats. Front. Psychiatry 2018, 9, 525. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurother. J. Am. Soc. Exp. NeuroTher. 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Kunhachan, P.; Banchonglikitkul, C.; Kajsongkram, T.; Khayungarnnawee, A.; Leelamanit, W. Chemical Composition, Toxicity and Vasodilatation Effect of the Flowers Extract of Jasminum sambac (L.) Ait. “G. Duke of Tuscany”. Evid. Based Complement. Alternat. Med. 2012, 2012, 471312. [Google Scholar] [CrossRef]
- Li, D.; Tang, X.; Liu, C.; Li, H.; Li, S.; Sun, S.; Zheng, X.; Wu, P.; Xu, X.; Zhang, K.; et al. Jasmine (Jasminum grandiflorum) Flower Extracts Ameliorate Tetradecanoylphorbol Acetate Induced Ear Edema in Mice. Nat. Prod. Commun. 2020, 15, 1934578X2091749. [Google Scholar] [CrossRef]
- Sengar, N.; Joshi, A.; Prasad, S.K.; Hemalatha, S. Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. J. Ethnopharmacol. 2015, 160, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Latif, F.; Edou, P.; Eba, F.; Mohamed, N.; Dicko, M.H. Antimicrobial and antioxidant activities of essential oil and methanol extract of Jasminum sambac from Djibouti. Afr. J. Plant Sci. 2010, 4, 38–43. [Google Scholar]
- Wikee, S.; Cai, L.; Pairin, N.; McKenzie, E.H.C.; Su, Y.-Y.; Chukeatirote, E.; Thi, H.N.; Bahkali, A.H.; Moslem, M.A.; Abdelsalam, K.; et al. Colletotrichum species from Jasmine (Jasminum sambac). Fungal Divers. 2010, 46, 171–182. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, Y.; Liu, B.; Chen, Z.; Zheng, J.; Guan, M.; Shi, H.; Wang, Y.; Yang, W. Changes in the volatiles, chemical components, and antioxidant activities of Chinese jasmine tea during the scenting processes. Int. J. Food Prop. 2017, 20, 681–693. [Google Scholar] [CrossRef]
- Inoue, N.; Kuroda, K.; Sugimoto, A.; Kakuda, T.; Fushiki, T. Autonomic nervous responses according to preference for the odor of jasmine tea. Biosci. Biotechnol. Biochem. 2003, 67, 1206–1214. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Xiong, Y.; Zhang, X.; Lin, Y.; Liu, Z. Jasmine Tea Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior in Rats via the Gut-Brain Axis. Nutrients 2022, 14, 99. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Hu, X.; Yang, C.; Xu, H.; Yao, Y.; Liu, J. Clostridium butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior in Mice via the Gut-Brain Axis. J. Agric. Food Chem. 2018, 66, 8415–8421. [Google Scholar] [CrossRef]
- Mou, Z.; Huang, Q.; Chu, S.F.; Zhang, M.J.; Hu, J.F.; Chen, N.H.; Zhang, J.T. Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis. Biomed. Pharmacother. 2017, 92, 962–971. [Google Scholar] [CrossRef]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Liu, Z. Fuzhuan Brick Tea Attenuates High-Fat Diet-Induced Obesity and Associated Metabolic Disorders by Shaping Gut Microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, G.; Jiang, S.; Liu, Y.-X. Wekemo Bioincloud: A user-friendly platform for meta-omics data analyses. IMETA 2024, 3, e175. [Google Scholar] [CrossRef]
- Dunn, W.B.; David, B.; Paul, B.; Eva, Z.; Sue, F.M.; Nadine, A.; Marie, B.; Knowles, J.D.; Antony, H.; Haselden, J.N. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2018, 6, 1060–1083. [Google Scholar] [CrossRef]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Barri, T.; Dragsted, L.O. UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: Effect of experimental artefacts and anticoagulant. Anal. Chim. Acta 2013, 768, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yieh, L.; Yang, T.; Drinkenburg, W.; Peeters, P.; Steckler, T.; Narayan, V.A.; Wittenberg, G.; Ye, J. Metabolomic biosignature differentiates melancholic depressive patients from healthy controls. BMC Genom. 2016, 17, 669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Preparation and Mechanism of Anti-Anxious Depression Peptide. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2020. [Google Scholar]
- Polyakova, M.; Beyer, F.; Mueller, K.; Sander, C.; Witte, V.; Lampe, L.; Rodrigues, F.; Riedel-Heller, S.; Kratzsch, J.; Hoffmann, K.T.; et al. Serum BDNF levels correlate with regional cortical thickness in minor depression: A pilot study. Sci. Rep. 2020, 10, 14524. [Google Scholar] [CrossRef] [PubMed]
- Bajor, A.; Gillberg, P.G.; Abrahamsson, H. Bile acids: Short and long term effects in the intestine. Scand. J. Gastroenterol. 2010, 45, 645–664. [Google Scholar] [CrossRef]
- Del Grande da Silva, G.; Wiener, C.D.; Barbosa, L.P.; Araujo, J.M.G.; Molina, M.L.; Martin, P.S.; Oses, J.P.; Jansen, K.; de Mattos Souza, L.D.; da Silva, R.A. Pro-inflammatory cytokines and psychotherapy in depression: Results from a randomized clinical trial. J. Psychiatr. Res. 2016, 75, 57–64. [Google Scholar] [CrossRef]
- Song, Q.; Fan, C.; Wang, P.; Li, Y.; Yang, M.; Yu, S.-Y. Hippocampal CA1 betaCaMKII mediates neuroinflammatory responses via COX-2/PGE2 signaling pathways in depression. J. Neuroinflamm. 2018, 15, 338. [Google Scholar] [CrossRef]
- He, H.; He, H.; Mo, L.; You, Z.; Zhang, J. Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice. Brain Behav. Immun. 2024, 115, 280–294. [Google Scholar] [CrossRef]
- Kelly, J.R.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbiota axis: Challenges for translation in psychiatry. Ann. Epidemiol. 2016, 26, 366–372. [Google Scholar] [CrossRef]
- Lv, M.; Wang, Y.; Qu, P.; Li, S.; Yu, Z.; Qin, X.; Liu, X. A combination of cecum microbiome and metabolome in CUMS depressed rats reveals the antidepressant mechanism of traditional Chinese medicines: A case study of Xiaoyaosan. J. Ethnopharmacol. 2021, 276, 114167. [Google Scholar] [CrossRef]
- Winter, G.; Hart, R.A.; Charlesworth, R.P.G.; Sharpley, C.F. Gut microbiome and depression: What we know and what we need to know. Rev. Neurosci. 2018, 29, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Cheng, R.; Liu, H.; Zhao, Y.; Liu, Y.; Chen, Y.; Sun, Z.; Zhai, Z.; Wu, M.; et al. Alteration of the gut microbiome and correlated metabolism in a rat model of long-term depression. Front. Cell. Infect. Microbiol. 2023, 13, 1116277. [Google Scholar] [CrossRef]
- Li, J.M.; Yu, R.; Zhang, L.P.; Wen, S.Y.; Kong, L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef]
- Alauzet, C.; Cunat, L.; Wack, M.; Lanfumey, L.; Legrand-Frossi, C.; Lozniewski, A.; Agrinier, N.; Cailliez-Grimal, C.; Frippiat, J.-P. Impact of a Model Used to Simulate Chronic Socio-Environmental Stressors Encountered during Spaceflight on Murine Intestinal Microbiota. Int. J. Mol. Sci. 2020, 21, 7863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef]
- An, Q.; Li, C.; Chen, Y.; Yang, Y.; Song, R.; Zhou, L.; Li, J.; Tong, A.; Luo, Y. Scaffold hopping of agomelatine leads to enhanced antidepressant effects by modulation of gut microbiota and host immune responses. Pharmacol. Biochem. Behav. 2020, 192, 172910. [Google Scholar] [CrossRef]
- Abildgaard, A.; Kern, T.; Pedersen, O.; Hansen, T.; Wegener, G. A diet-induced gut microbiota component and related plasma metabolites are associated with depressive-like behaviour in rats. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2020, 43, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Chang, H.; Ma, S.; Guo, J.; She, G.; Zhang, F.; Li, L.; Li, X.; Lu, Y. Tiansi Liquid Modulates Gut Microbiota Composition and Tryptophan–Kynurenine Metabolism in Rats with Hydrocortisone-Induced Depression. Molecules 2018, 23, 2832. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Yang, Q.; Li, J.; Wang, L.; Liu, X.; Huang, Y.; Liu, L. Beneficial Effect of Alkaloids from Sophora alopecuroides L. on CUMS-Induced Depression Model Mice via Modulating Gut Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 665159. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jia, H.; Zhou, C.; Yang, Y.; Zhao, Y.; Yang, M.; Zou, Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.-z.; Ma, Q.-y.; Tao, G.; Huang, J.-q.; Chen, J.-x. Oral coniferyl ferulate attenuated depression symptoms in mice via reshaping gut microbiota and microbial metabolism. Food Funct. 2021, 12, 12550–12564. [Google Scholar] [CrossRef]
- Xiong, Z.; Yang, J.; Huang, Y.; Zhang, K.; Bo, Y.; Lu, X.; Su, G.; Ma, J.; Yang, J.; Zhao, L.; et al. Serum metabonomics study of anti-depressive effect of Xiao-Chai-Hu-Tang on rat model of chronic unpredictable mild stress. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1029, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lin, Y.; Xiong, Y.; Huang, J.; Liu, Z. An Analysis of the Intestinal Microbiome Combined with Metabolomics to Explore the Mechanism of How Jasmine Tea Improves Depression in CUMS-Treated Rats. Foods 2024, 13, 2636. https://doi.org/10.3390/foods13162636
Zhang Y, Lin Y, Xiong Y, Huang J, Liu Z. An Analysis of the Intestinal Microbiome Combined with Metabolomics to Explore the Mechanism of How Jasmine Tea Improves Depression in CUMS-Treated Rats. Foods. 2024; 13(16):2636. https://doi.org/10.3390/foods13162636
Chicago/Turabian StyleZhang, Yangbo, Yong Lin, Yifan Xiong, Jianan Huang, and Zhonghua Liu. 2024. "An Analysis of the Intestinal Microbiome Combined with Metabolomics to Explore the Mechanism of How Jasmine Tea Improves Depression in CUMS-Treated Rats" Foods 13, no. 16: 2636. https://doi.org/10.3390/foods13162636





