Insight into the Probiogenomic Potential of Enterococcus faecium BGPAS1-3 and Application of a Potent Thermostable Bacteriocin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
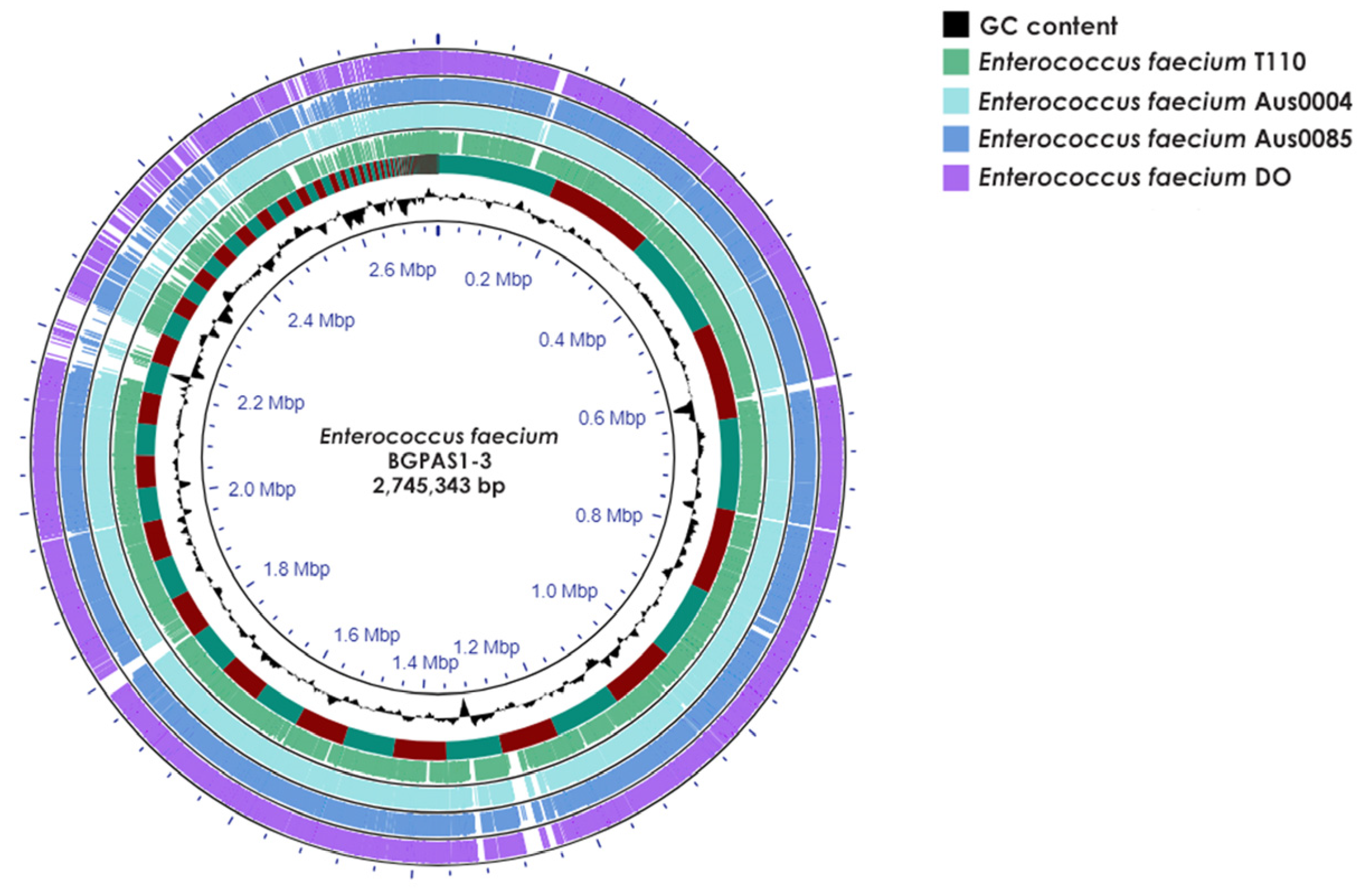
2.2. Whole-Genome Sequencing, Standard Analysis, and Data Deposition
2.3. Genotypic Characterization for Safety and Probiotic-Related Traits
2.4. DNA Manipulations
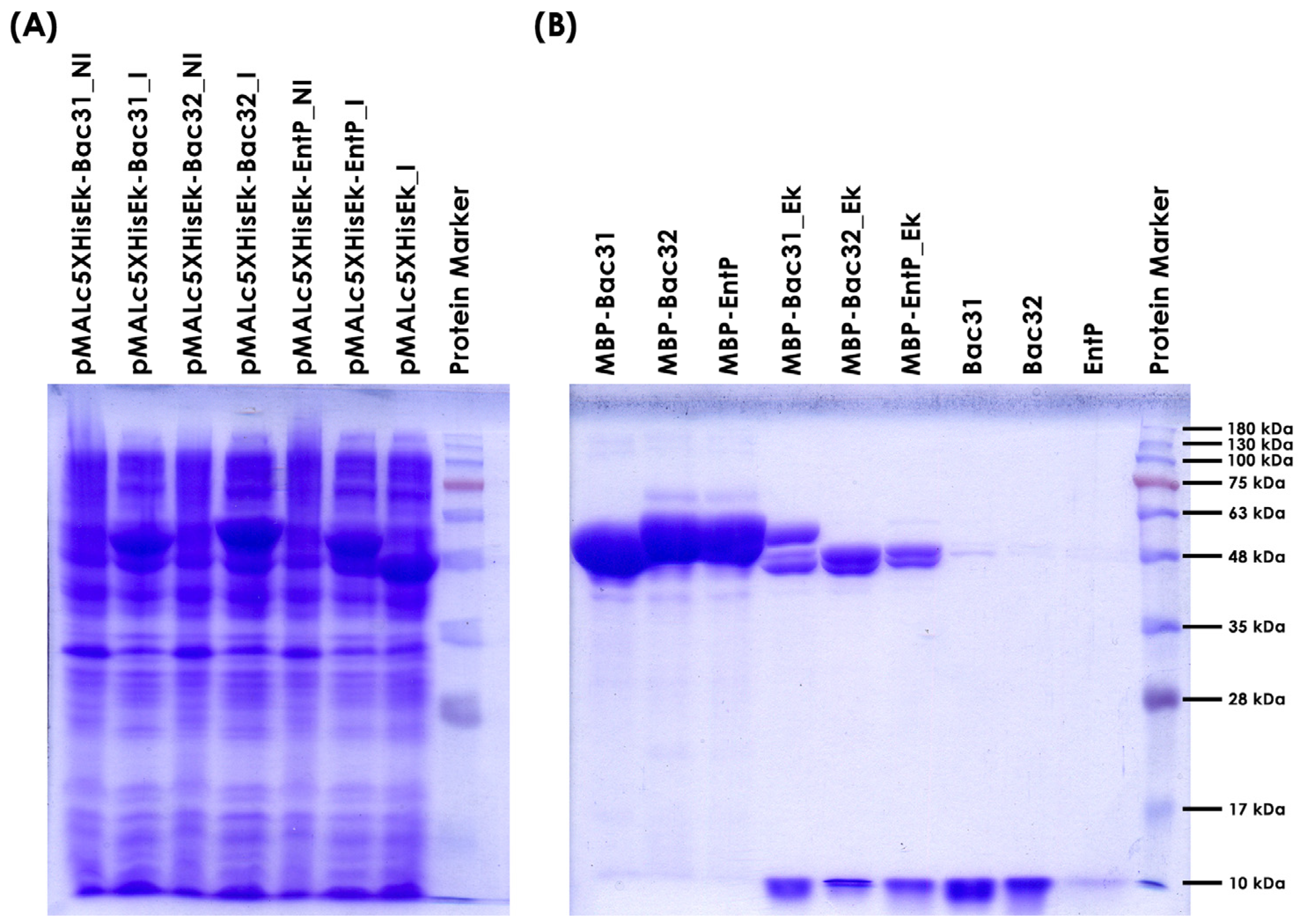
2.5. Overexpression of Bacteriocins from Enterococcus faecium BGPAS1-3 in Escherichia coli ER2523
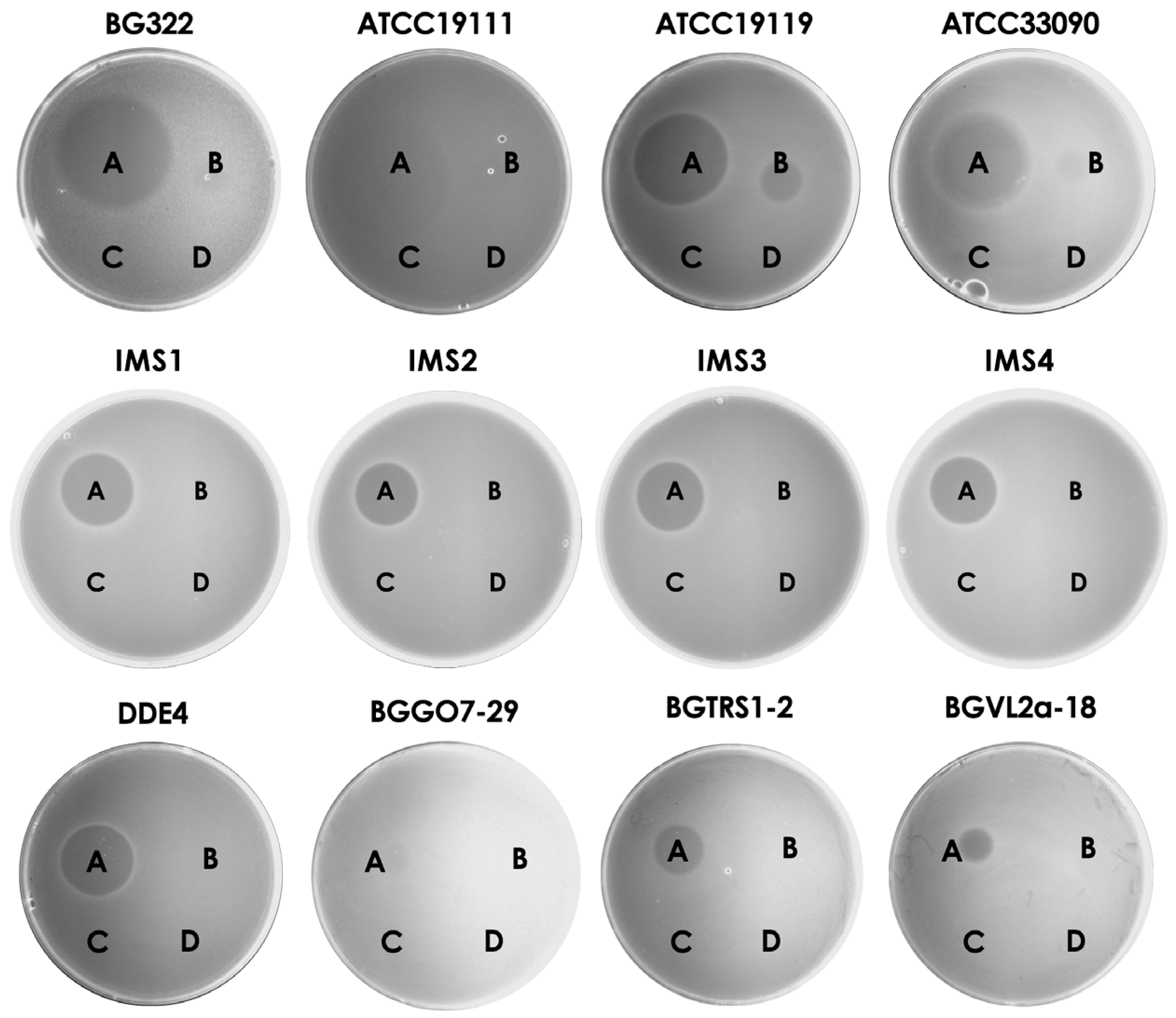
2.6. Bacteriocin Activity Assay
2.7. Amplified Fragments and Constructs Sequencing and Sequence Analysis
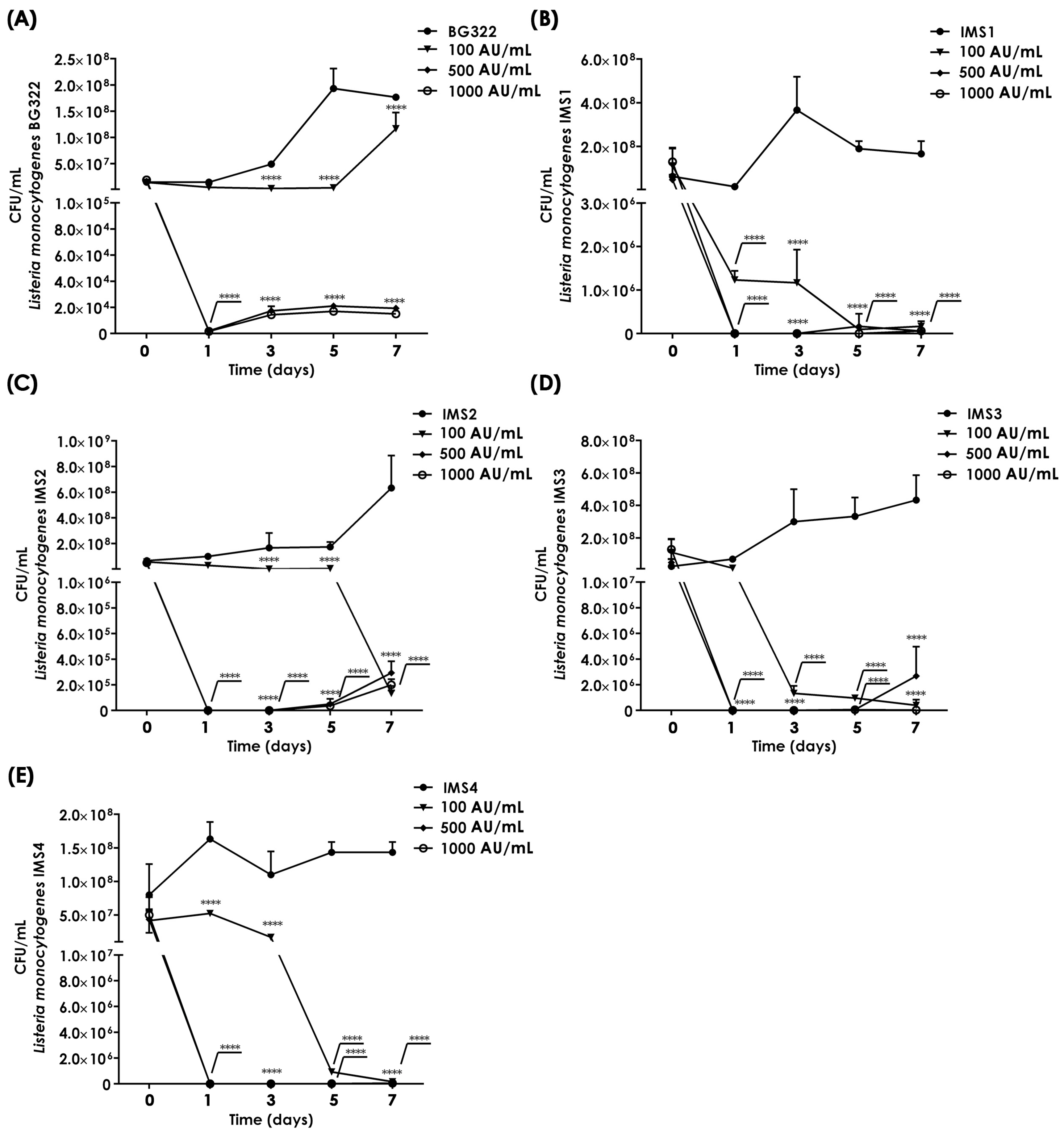
2.8. Effects of Purified Bacteriocin 31 on the Abundance of Listeria monocytogenes in a Milk Model
2.9. Statistical Analysis
3. Results
3.1. Enterococcus faecium BGPAS1-3 Was Predicted as a Non-Human Pathogen
3.2. Enterococcus faecium BGPAS1-3 Contains Genes Predicted to Be Involved in Biosynthetic Pathways of Essential Amino Acids
3.3. Enterococcus faecium BGPAS1-3 Contains Operons/Gene Clusters Predicted to Encode the Production of Four Antimicrobial Peptides
3.4. Overexpression of Bacteriocin Genes from Enterococcus faecium BGPAS1-3 in Escherichia coli ER2523
3.5. Purified Bacteriocins Are Active against Listeria sp. and Lactic Acid Bacteria
3.6. Application of Purified Bacteriocin 31 Effectively Reduces Listeria monocytogenes in a Milk Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jibo, G.G.; Raji, Y.E.; Salawudeen, A.; Amin-Nordin, S.; Mansor, R.; Jamaluddin, T.Z.M.T. A Systematic Review and Meta-Analysis of the Prevalence of Listeria monocytogenes in South-East Asia; a One-Health Approach of Human-Animal-Food-Environment. One Health 2022, 15, 100417. [Google Scholar] [CrossRef] [PubMed]
- Hafner, L.; Pichon, M.; Burucoa, C.; Nusser, S.H.A.; Moura, A.; Garcia-Garcera, M.; Lecuit, M. Listeria monocytogenes Faecal Carriage Is Common and Depends on the Gut Microbiota. Nat. Commun. 2021, 12, 6826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Chen, X.; Qu, C. Review Controlling Listeria monocytogenes in Ready-to-Eat Meat and Poultry Products: An Overview of Outbreaks, Current Legislations, Challenges, and Future Prospects. Trends Food Sci. Technol. 2021, 116, 24–35. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes Persistence in Food-Associated Environments: Epidemiology, Strain Characteristics, and Implications for Public Health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Olanya, O.M.; Hoshide, A.K.; Ijabadeniyi, O.A.; Ukuku, D.O.; Mukhopadhyay, S.; Niemira, B.A.; Ayeni, O. Cost Estimation of Listeriosis (Listeria monocytogenes) Occurrence in South Africa in 2017 and Its Food Safety Implications. Food Control 2019, 102, 231–239. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Libera, K.; Konieczny, K.; Grabska, J.; Szopka, W.; Augustyniak, A.; Pomorska-Mól, M. Selected Livestock-Associated Zoonoses as a Growing Challenge for Public Health. Infect. Dis. Rep. 2022, 14, 63–81. [Google Scholar] [CrossRef]
- Thakur, M.; Asrani, R.K.; Patial, V. Listeria monocytogenes: A Food-Borne Pathogen. In Foodborne Diseases; Holban, A.M., Grumezescu, A.M., Eds.; Handbook of Food Bioengineering, Academic Press: Cambridge, MA, USA, 2018; pp. 157–192. ISBN 978-0-12-811444-5. [Google Scholar]
- Drolia, R.; Bhunia, A.K. Crossing the Intestinal Barrier via Listeria Adhesion Protein and Internalin A. Trends Microbiol. 2019, 27, 408–425. [Google Scholar] [CrossRef]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and Virulence of Listeria monocytogenes : A Trip from Environmental to Medical Microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef]
- Mazaheri, T.; Cervantes-Huamán, B.R.H.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes Biofilms in the Food Industry: Is the Current Hygiene Program Sufficient to Combat the Persistence of the Pathogen? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef]
- Skowron, K.; Hulisz, K.; Gryń, G.; Olszewska, H.; Wiktorczyk, N.; Paluszak, Z. Comparison of Selected Disinfectants Efficiency against Listeria monocytogenes Biofilm Formed on Various Surfaces. Int. Microbiol. 2018, 21, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Givskov, M. Quorum-Sensing Inhibitors as Anti-Pathogenic Drugs. Int. J. Med. Microbiol. 2006, 296, 149–161. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of Antibiotic Resistance in Listeria monocytogenes Isolated from Food Products: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- Abril, A.G.; Carrera, M.; Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Sánchez-Pérez, A.; Villa, T.G. Proteomic Characterization of Antibiotic Resistance in Listeria and Production of Antimicrobial and Virulence Factors. Int. J. Mol. Sci. 2021, 22, 8141. [Google Scholar] [CrossRef]
- Farber, J.M.; Zwietering, M.; Wiedmann, M.; Schaffner, D.; Hedberg, C.W.; Harrison, M.A.; Hartnett, E.; Chapman, B.; Donnelly, C.W.; Goodburn, K.E.; et al. Alternative Approaches to the Risk Management of Listeria monocytogenes in Low Risk Foods. Food Control 2021, 123, 107601. [Google Scholar] [CrossRef]
- Ceruso, M.; Liu, Y.; Gunther, N.W.; Pepe, T.; Anastasio, A.; Qi, P.X.; Tomasula, P.M.; Renye, J.A. Anti-Listerial Activity of Thermophilin 110 and Pediocin in Fermented Milk and Whey. Food Control 2021, 125, 107941. [Google Scholar] [CrossRef]
- Liu, Y.; Ceruso, M.; Jiang, Y.; Datta, A.R.; Carter, L.; Strain, E.; Pepe, T.; Anastasi, A.; Fratamico, P. Construction of Listeria monocytogenes Mutants with In-Frame Deletions in the Phosphotransferase Transport System (PTS) and Analysis of Their Growth under Stress Conditions. J. Food Sci. 2013, 78, M1392–M1398. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Kasimin, M.E.; Shamsuddin, S.; Molujin, A.M.; Sabullah, M.K.; Gansau, J.A.; Jawan, R. Enterocin: Promising Biopreservative Produced by Enterococcus sp. Microorganisms 2022, 10, 684. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural Preservatives for Extending the Shelf-Life of Seafood: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, Synthesis, Mechanism of Action and Resistance Development in Food Spoilage Causing Bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Lozo, J.; Topisirovic, L.; Kojic, M. Natural Bacterial Isolates as an Inexhaustible Source of New Bacteriocins. Appl. Microbiol. Biotechnol. 2021, 105, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.A.P.; van Belkum, M.J.; Holzapfel, W.H.; Abriouel, H.; Gálvez, A. Diversity of Enterococcal Bacteriocins and Their Grouping in a New Classification Scheme. FEMS Microbiol. Rev. 2007, 31, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as Probiotics and Their Implications in Food Safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef]
- Favaro, L.; Barretto Penna, A.L.; Todorov, S.D. Bacteriocinogenic LAB from Cheeses—Application in Biopreservation? Trends Food Sci. Technol. 2015, 41, 37–48. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Peng, X.; Ed-Dra, A.; Yue, M. Whole Genome Sequencing for the Risk Assessment of Probiotic Lactic Acid Bacteria. Crit. Rev. Food Sci. Nutr. 2023, 63, 11244–11262. [Google Scholar] [CrossRef]
- EFSA Statement on the Requirements for Whole Genome Sequence Analysis of Microorganisms Intentionally Used in the Food Chain-2021—EFSA Journal—Wiley Online Library. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2021.6506 (accessed on 3 July 2024).
- Haranahalli Nataraj, B.; Behare, P.V.; Yadav, H.; Srivastava, A.K. Emerging Pre-Clinical Safety Assessments for Potential Probiotic Strains: A Review. Crit. Rev. Food Sci. Nutr. 2023, 11, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Zhao, X.; Zhen, Z.; Wang, X.; Guo, N. Synergy of a Combination of Nisin and Citric Acid against Staphylococcus aureus and Listeria monocytogenes. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 2058–2068. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial Production of Bacteriocins: Latest Research Development and Applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Abriouel, H.; López, R.L.; Ben Omar, N. Bacteriocin-Based Strategies for Food Biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Parish, J.H. Genetic Manipulation of Streptomyces—A Laboratory Manual: By D A Hopwood, M J Bibb, K F Chater; T Kieser CJ Bruton, H M Kieser, D J Lydiate, C P Smith, J M Ward and H Schrempf. Pp 356. The John Innes Foundation, Norwich, UK and Cold Spring Harbour Laboratory. 1985. $25 ISBN 0-7084-0336-0. Biochem. Educ. 1986, 14, 196. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. J. Comput. Mol. Cell Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast Metagenomic Sequence Classification Using Exact Alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Florensa, A.F.; Kaas, R.S.; Clausen, P.T.L.C.; Aytan-Aktug, D.; Aarestrup, F.M. ResFinder—An Open Online Resource for Identification of Antimicrobial Resistance Genes in Next-Generation Sequencing Data and Prediction of Phenotypes from Genotypes. Microb. Genom. 2022, 8, 000748. [Google Scholar] [CrossRef]
- Kleinheinz, K.A.; Joensen, K.G.; Larsen, M.V. Applying the ResFinder and VirulenceFinder Web-Services for Easy Identification of Acquired Antibiotic Resistance and E. coli Virulence Genes in Bacteriophage and Prophage Nucleotide Sequences. Bacteriophage 2014, 4, e27943. [Google Scholar] [CrossRef]
- Enuh, B.M.; Gedikli, S.; Aytar Çelik, P.; Çabuk, A. Genome Sequence and Probiotic Potential of Newly Isolated Enterococcus durans Strain MN187066. Lett. Appl. Microbiol. 2023, 76, ovad035. [Google Scholar] [CrossRef]
- Cosentino, S.; Voldby Larsen, M.; Møller Aarestrup, F.; Lund, O. PathogenFinder—Distinguishing Friend from Foe Using Bacterial Whole Genome Sequence Data. PLoS ONE 2013, 8, e77302. [Google Scholar] [CrossRef]
- Carattoli, A.; Hasman, H. PlasmidFinder and In Silico pMLST: Identification and Typing of Plasmid Replicons in Whole-Genome Sequencing (WGS). Methods Mol. Biol. 2020, 2075, 285–294. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An Automatic Genome Annotation and Pathway Reconstruction Server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef]
- van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A User-Friendly Web Server to Thoroughly Mine RiPPs and Bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.-Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-Depth Characterization and Visualization of Bacterial Genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Hanahan, D. Studies on Transformation of Escherichia coli with Plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef] [PubMed]
- Kojic, M.; Svircevic, J.; Banina, A.; Topisirovic, L. Bacteriocin-Producing Strain of Lactococcus lactis subsp. diacitilactis S50. Appl. Environ. Microbiol. 1991, 57, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Vukotic, G.; Polovic, N.; Mirkovic, N.; Jovcic, B.; Stanisavljevic, N.; Fira, D.; Kojic, M. Lactococcin B Is Inactivated by Intrinsic Proteinase PrtP Digestion in Lactococcus lactis subsp. lactis BGMN1-501. Front. Microbiol. 2019, 10, 874. [Google Scholar] [CrossRef]
- Ansari, A.; Zohra, R.R.; Tarar, O.M.; Qader, S.A.U.; Aman, A. Screening, Purification and Characterization of Thermostable, Protease Resistant Bacteriocin Active against Methicillin Resistant Staphylococcus aureus (MRSA). BMC Microbiol. 2018, 18, 192. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.L.; Gaya, P.; Medina, M.; Nuñez, M. Bactericidal Effect of Enterocin 4 on Listeria monocytogenes in a Model Dairy System. J. Food Prot. 1997, 60, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Đokić, J.; Brdarić, E.; Dinić, M.; Terzić-Vidojević, A.; Golić, N.; Veljović, K. The Influence of Heat-Killed Enterococcus faecium BGPAS1-3 on the Tight Junction Protein Expression and Immune Function in Differentiated Caco-2 Cells Infected With Listeria monocytogenes ATCC 19111. Front. Microbiol. 2019, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Gardijan, L.; Miljkovic, M.; Obradovic, M.; Borovic, B.; Vukotic, G.; Jovanovic, G.; Kojic, M. Redesigned pMAL Expression Vector for Easy and Fast Purification of Active Native Antimicrobial Peptides. J. Appl. Microbiol. 2022, 133, 1001–1013. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, L. Encapsulated Natural Antimicrobials: A Promising Way to Reduce Microbial Growth in Different Food Systems. Food Control 2021, 123, 107678. [Google Scholar] [CrossRef]
- Archer, D.L. The Evolution of FDA’s Policy on Listeria monocytogenes in Ready-to-Eat Foods in the United States. Curr. Opin. Food Sci. 2018, 20, 64–68. [Google Scholar] [CrossRef]
- Sebastianski, M.; Bridger, N.A.; Featherstone, R.M.; Robinson, J.L. Disease Outbreaks Linked to Pasteurized and Unpasteurized Dairy Products in Canada and the United States: A Systematic Review. Can. J. Public. Health Rev. Can. Santé Publique 2022, 113, 569–578. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakur, M.; Singh, S.; Tripathy, S.; Gupta, A.K.; Baranwal, D.; Patel, A.R.; Shah, N.; Utama, G.L.; Niamah, A.K.; et al. Bacteriocins as Antimicrobial and Preservative Agents in Food: Biosynthesis, Separation and Application. Food Biosci. 2022, 46, 101594. [Google Scholar] [CrossRef]
- Golić, N.; Veljović, K.; Popović, N.; Đokić, J.; Strahinić, I.; Mrvaljević, I.; Terzić-Vidojević, A. In vitro and in vivo Antagonistic Activity of New Probiotic Culture against Clostridium difficile and Clostridium perfringens. BMC Microbiol. 2017, 17, 108. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef]
- Giraffa, G. Functionality of Enterococci in Dairy Products. Int. J. Food Microbiol. 2003, 88, 215–222. [Google Scholar] [CrossRef]
- Terzić-Vidojević, A.; Veljović, K.; Popović, N.; Tolinački, M.; Golić, N. Enterococci from Raw-Milk Cheeses: Current Knowledge on Safety, Technological, and Probiotic Concerns. Foods 2021, 10, 2753. [Google Scholar] [CrossRef]
- Popović, N.; Dinić, M.; Tolinački, M.; Mihajlović, S.; Terzić-Vidojević, A.; Bojić, S.; Djokić, J.; Golić, N.; Veljović, K. New Insight into Biofilm Formation Ability, the Presence of Virulence Genes and Probiotic Potential of Enterococcus sp. Dairy Isolates. Front. Microbiol. 2018, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Dubin, K.; Pamer, E.G. Enterococci and Their Interactions with the Intestinal Microbiome. Microbiol. Spectr. 2014, 5, 1110–1128. [Google Scholar] [CrossRef]
- Panthee, S.; Paudel, A.; Hamamoto, H.; Ogasawara, A.A.; Iwasa, T.; Blom, J.; Sekimizu, K. Complete Genome Sequence and Comparative Genomic Analysis of Enterococcus faecalis EF-2001, a Probiotic Bacterium. Genomics 2021, 113, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Gorrie, C.; Higgs, C.; Carter, G.; Stinear, T.P.; Howden, B. Genomics of Vancomycin-Resistant Enterococcus faecium. Microb. Genom. 2019, 5, e000283. [Google Scholar] [CrossRef]
- Gao, W.; Howden, B.P.; Stinear, T.P. Evolution of Virulence in Enterococcus faecium, a Hospital-Adapted Opportunistic Pathogen. Curr. Opin. Microbiol. 2018, 41, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ghattargi, V.C.; Nimonkar, Y.S.; Burse, S.A.; Davray, D.; Kumbhare, S.V.; Shetty, S.A.; Gaikwad, M.A.; Suryavanshi, M.V.; Doijad, S.P.; Utage, B.; et al. Genomic and Physiological Analyses of an Indigenous Strain, Enterococcus faecium 17OM39. Funct. Integr. Genom. 2018, 18, 385–399. [Google Scholar] [CrossRef]
- Negash, A.W.; Tsehai, B.A. Current Applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef]
- Popović, N.; Stevanović, D.; Radojević, D.; Veljović, K.; Đokić, J.; Golić, N.; Terzić-Vidojević, A. Insight into the Postbiotic Potential of the Autochthonous Bacteriocin-Producing Enterococcus faecium BGZLM1-5 in the Reduction in the Abundance of Listeria monocytogenes ATCC19111 in a Milk Model. Microorganisms 2023, 11, 2844. [Google Scholar] [CrossRef]
- Malesevic, M.; Stanisavljevic, N.; Miljkovic, M.; Jovcic, B.; Filipic, B.; Studholme, D.J.; Kojic, M. TThe Large Plasmidome of Lactococcus lactis subsp. lactis Bv. diacetylactis S50 Confers Its Biotechnological Properties. Int. J. Food Microbiol. 2021, 337, 108935. [Google Scholar] [CrossRef]
- Fathizadeh, H.; Saffari, M.; Esmaeili, D.; Moniri, R.; Salimian, M. Evaluation of Antibacterial Activity of Enterocin A-Colicin E1 Fusion Peptide. Iran. J. Basic Med. Sci. 2020, 23, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.; Criado, R.; Citti, R.; Martín, M.; Herranz, C.; Nes, I.F.; Cintas, L.M.; Hernández, P.E. Cloning, Production and Functional Expression of Enterocin P, a Sec-Dependent Bacteriocin Produced by Enterococcus faecium P13, in Escherichia coli. Int. J. Food Microbiol. 2005, 103, 239–250. [Google Scholar] [CrossRef]
- Cintas, L.M.; Casaus, P.; Håvarstein, L.S.; Hernández, P.E.; Nes, I.F. Biochemical and Genetic Characterization of Enterocin P, a Novel Sec-Dependent Bacteriocin from Enterococcus faecium P13 with a Broad Antimicrobial Spectrum. Appl. Environ. Microbiol. 1997, 63, 4321–4330. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, B.; Cocolin, L.; Zeppa, G.; Field, D.; Cotter, P.D.; Hill, C. Technological Characterization of Bacteriocin Producing Lactococcus lactis Strains Employed to Control Listeria monocytogenes in Cottage Cheese. Int. J. Food Microbiol. 2012, 153, 58–65. [Google Scholar] [CrossRef]


| Bacterial Strain | Growth Conditions | Source |
|---|---|---|
| Listeria monocytogenes ATCC19111 | GM17; 37 °C | ATCC a |
| Listeria monocytogenes BG322 | GM17; 37 °C | Laboratory collection |
| Listeria monocytogenes IMS1 | GM17; 37 °C | Laboratory collection |
| Listeria monocytogenes IMS2 | GM17; 37 °C | Laboratory collection |
| Listeria monocytogenes IMS3 | GM17; 37 °C | Laboratory collection |
| Listeria monocytogenes IMS4 | GM17; 37 °C | Laboratory collection |
| Listeria ivanovii ATCC19119 | GM17; 37 °C | ATCC |
| Listeria inocua ATCC33090 | GM17; 37 °C | ATCC |
| Enterococcus faecium DDE4 | GM17; 37 °C | Laboratory collection |
| Strepotococcus thermophiles BGKMJ1-36 | GM17; 37 °C; CO2 | Laboratory collection |
| Lactobacillus delbrueckii subsp. bulgaricus BGVL1-21 | MRS b; 37 °C; CO2 | Laboratory collection |
| Lactococcus lactis subsp. lactis BGMN1-596 | GM17; 30 °C | Laboratory collection |
| Lactobacillusplantarum BGGO7-29 | MRS; 37 °C; CO2 | Laboratory collection |
| Leuconostocmesenteroides subsp. cremoris BGTRS1-2 | GM17; 30 °C | Laboratory collection |
| LactobacillusplantarumBGVL2a-18 | MRS; 37 °C; CO2 | Laboratory collection |
| Lactococcuslactis subsp. lactis BGVL2-8 | GM17; 30 °C | Laboratory collection |
| Lactococcuslactis subsp. lactis BGTRK4-21 | GM17; 30 °C | Laboratory collection |
| Lactococcus lactis subsp. lactis biovar. diacetylactis BGTRK10-2 | GM17; 30 °C | Laboratory collection |
| Lactococcuslactis subsp. cremoris BGTRM1-22 | GM17; 30 °C | Laboratory collection |
| Bacteriocin Name | Amino Acid Sequence | The Node of the Genome Enterococcus faecium BGPAS1-3 | The Best Score for Identity |
|---|---|---|---|
| Bacteriocin 31 | MKKKFVSIFMILGIVLLSVSTLGITVDAATYYGNGVYCNTQKCWVDWNKASKEIGKIIVNGWVQHGPWAPR | 56 | 98% class II bacteriocin [Enterococcus] ID: WP_048340632.1 |
| Bacteriocin 32 | MKKTKLLVASLCLFSSLLAFTPSVSFSQNGGVVEAAAQRGYIYKKYPKGAKVPNKVKMLVNIRGKQTMRT CYLMSWTASSRTAKYYYYI | 44 | 100% MULTISPECIES: hypothetical protein [Bacteria] ID: WP_002313303.1 |
| Enterocin P | MTNFGTKVDAATRSYDNGIYCNNSKCWVNWGEAKENIAGIVISGWASGLAGMGH | 49 | Class II bacteriocin [Enterococcus] ID: WP_002298900.1 |
| Primer Name | Sequence 5′-3′ | Source/ Reference |
|---|---|---|
| EntP_Fw | AGCTACGCGTTCATATGATAATGG | This study |
| EntP_Rev | TAGAAGCTTAATGTCCCATACCTGCCAAGCCAGAAGCCC | This study |
| Bac31_Fw | AGCAACTTATTATGGAAATGGTG | This study |
| Bac31_Rev | AACGGATCCTTTCTATCTAGGAGCCC | This study |
| Bac32_Fw | ATTCACCCCTTCTGTTTCATTTTCTC | This study |
| Bac32_Rev | TTTAAGCTTACTAAATGTAGTAATAATATTTGGC | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović, N.; Veljović, K.; Radojević, D.; Brdarić, E.; Stevanović, D.; Živković, M.; Kojić, M. Insight into the Probiogenomic Potential of Enterococcus faecium BGPAS1-3 and Application of a Potent Thermostable Bacteriocin. Foods 2024, 13, 2637. https://doi.org/10.3390/foods13162637
Popović N, Veljović K, Radojević D, Brdarić E, Stevanović D, Živković M, Kojić M. Insight into the Probiogenomic Potential of Enterococcus faecium BGPAS1-3 and Application of a Potent Thermostable Bacteriocin. Foods. 2024; 13(16):2637. https://doi.org/10.3390/foods13162637
Chicago/Turabian StylePopović, Nikola, Katarina Veljović, Dušan Radojević, Emilija Brdarić, Dušan Stevanović, Milica Živković, and Milan Kojić. 2024. "Insight into the Probiogenomic Potential of Enterococcus faecium BGPAS1-3 and Application of a Potent Thermostable Bacteriocin" Foods 13, no. 16: 2637. https://doi.org/10.3390/foods13162637






