Effects of Microwave Treatment on Physicochemical Attributes, Structural Analysis, and Digestive Characteristics of Pea Starch–Tea Polyphenol Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.2.1. Preparation of Samples Treated with Microwave
2.2.2. Preparation of the PS and TP Physical Mixture
2.3. Determination of TP Content in the Complex
2.4. Scanning Electron Microscopy (SEM)
2.5. Laser Scattering Measurement
2.6. Wide-Angle XRD
2.7. Fourier Transform Infrared Spectral (FTIR) Analysis
2.8. Solubility and Swelling Power
2.9. Thermal Properties
2.10. In Vitro Digestion
2.11. Statistical Analysis
3. Results
3.1. Complexation Index of PS-TPs
3.2. SEM Analysis
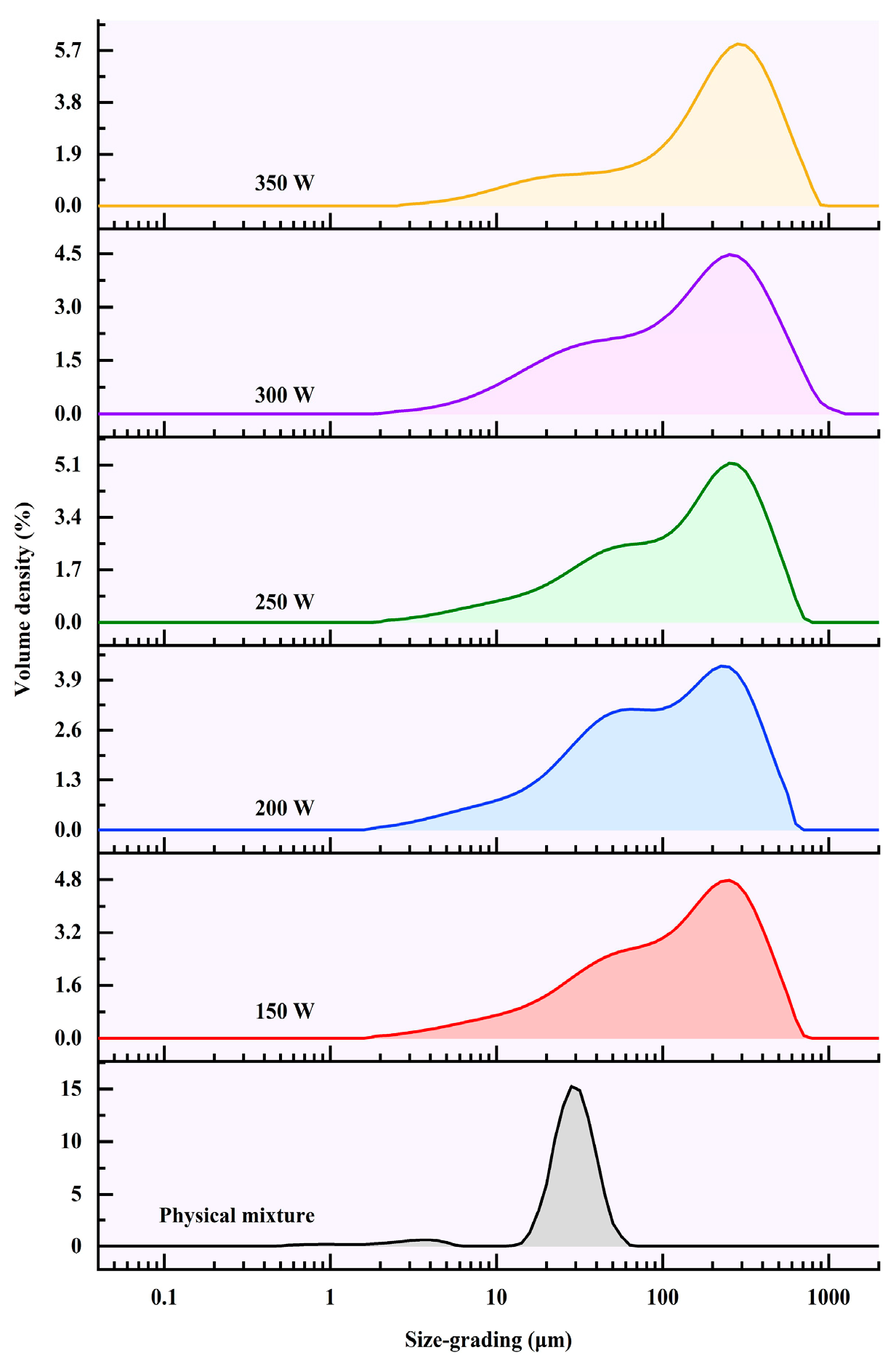
3.3. Particle Size Distributions
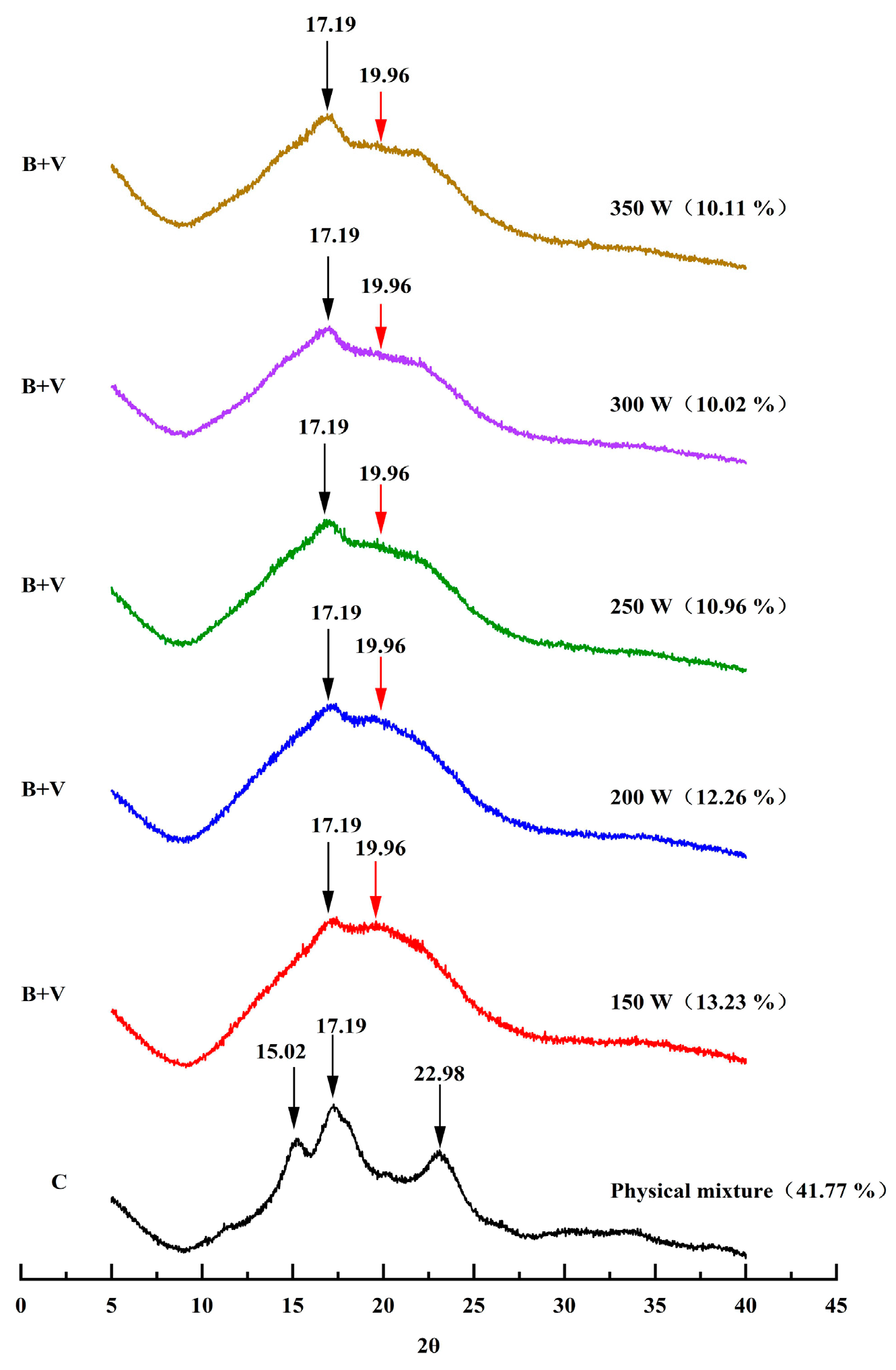
3.4. X-ray Diffraction Spectra Analysis
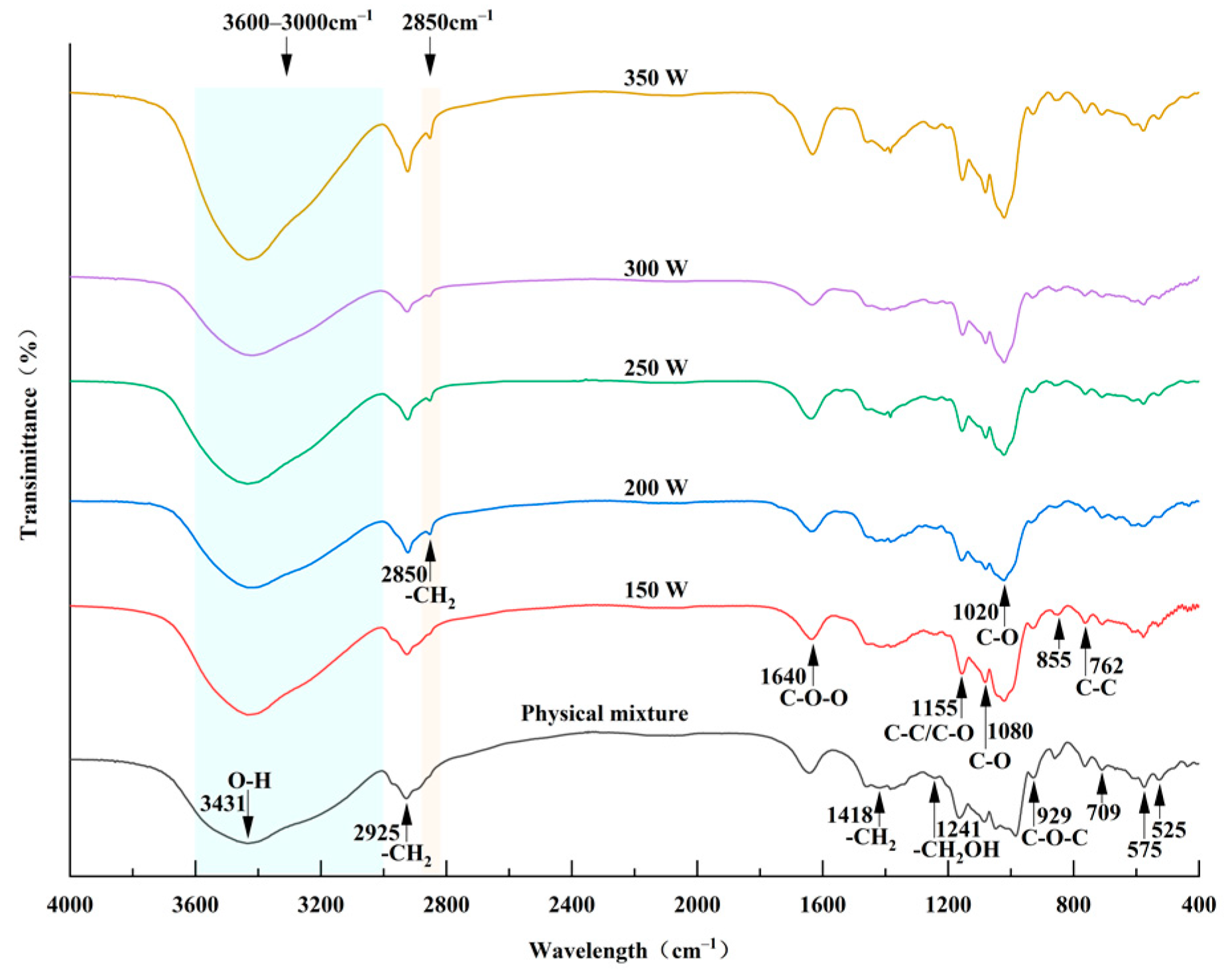
3.5. FT-IR Spectra Analysis
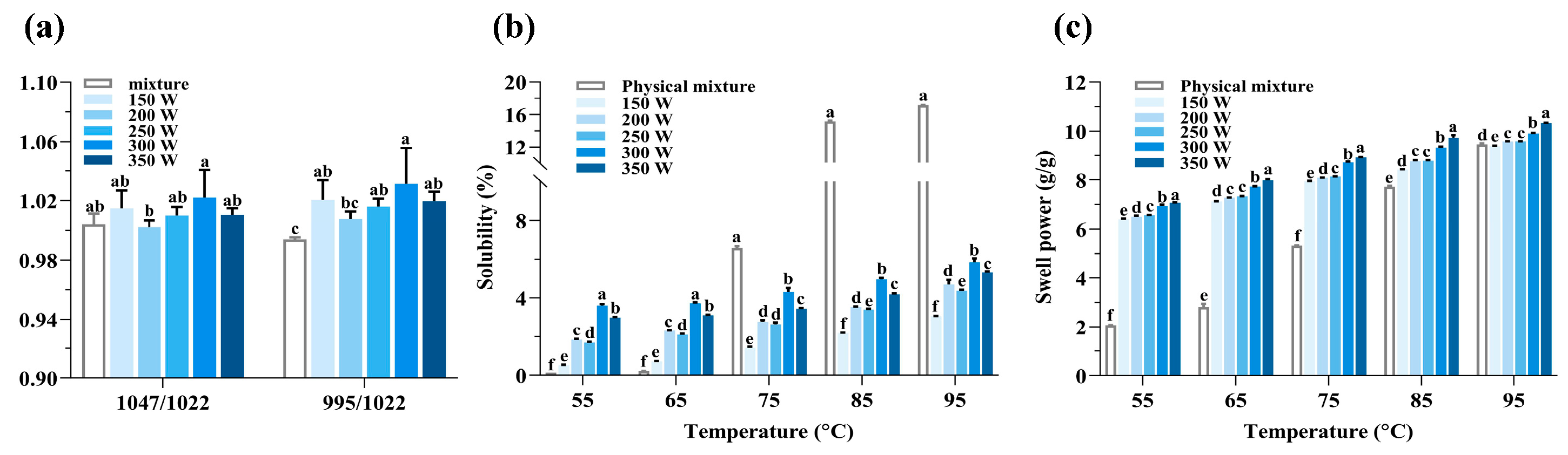
3.6. Solubility and Swelling Power
3.7. Thermal Properties Analysis
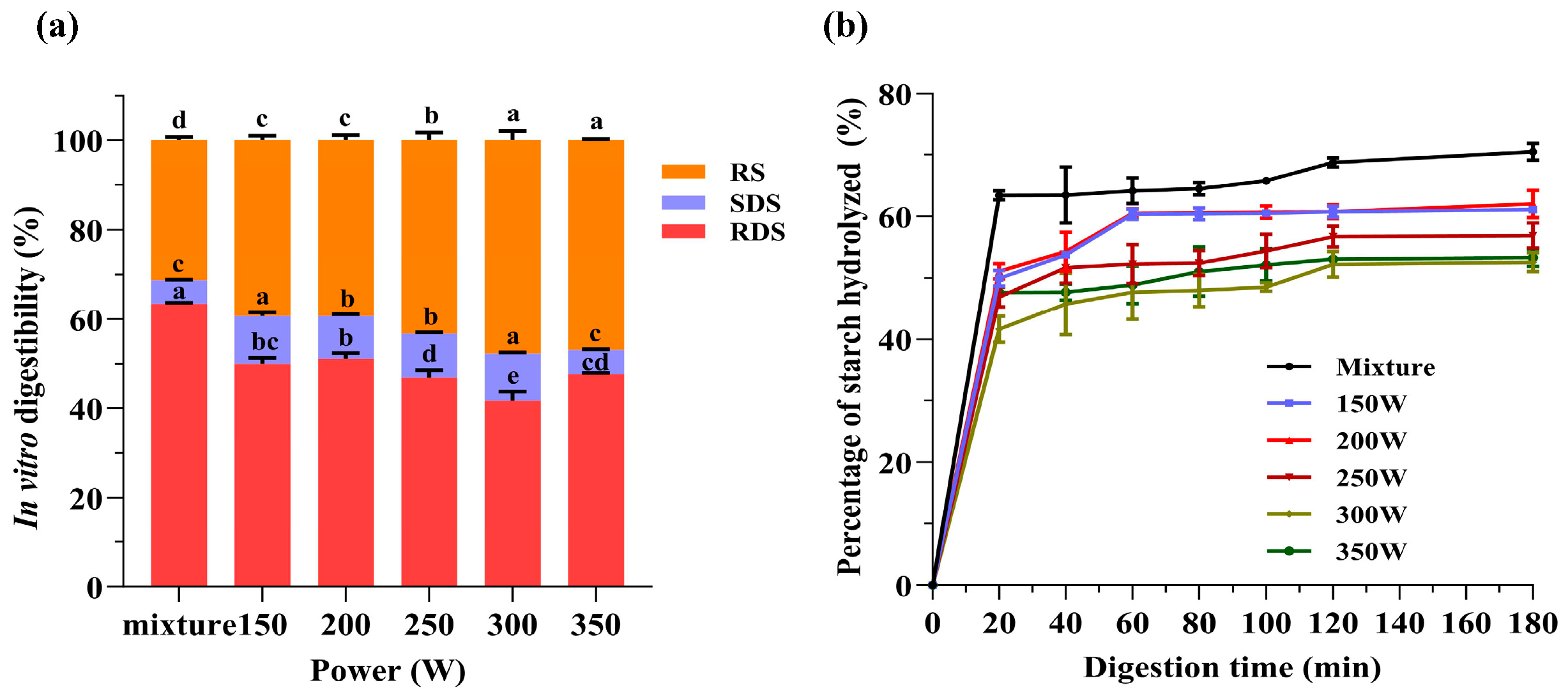
3.8. In Vitro Digestion Characteristics
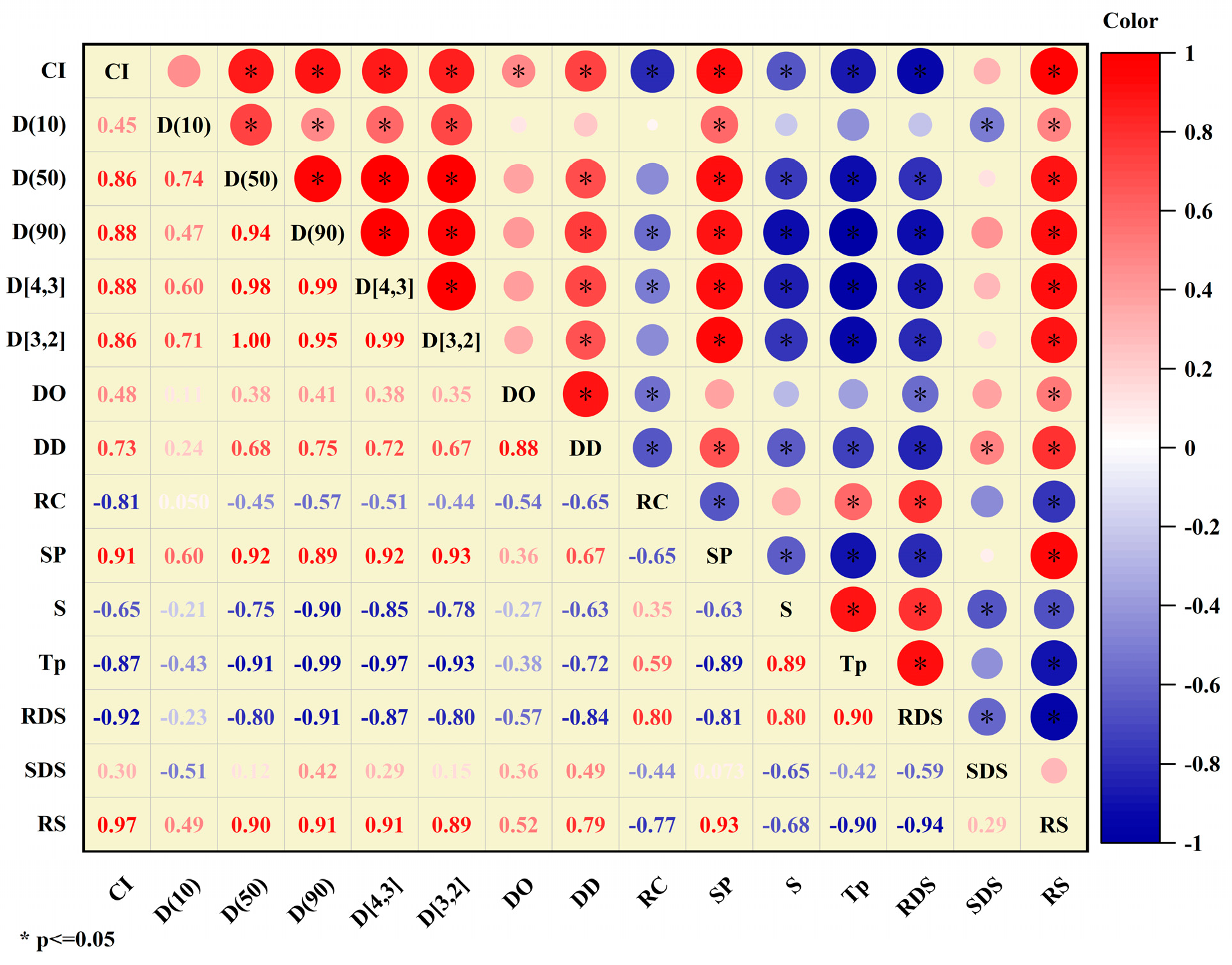
3.9. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Englyst, K.N.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br. J. Nutr. 2003, 89, 329–340. [Google Scholar] [CrossRef]
- Ren, Y.K.; Setia, R.S.; Warkentin, T.D.; Ai, Y.F. Functionality and starch digestibility of wrinkled and round pea flours of two different particle sizes. Food Chem. 2021, 336, 127711. [Google Scholar] [CrossRef]
- Wu, D.T.; Li, W.X.; Wan, J.J.; Hu, Y.C.; Gan, R.Y.; Zou, L. A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods 2023, 12, 2527. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.K.; Yuan, T.Z.; Chigwedere, C.M.; Ai, Y.F. A current review of structure, functional properties, and industrial applications of pulse starches for value-added utilization. Compr. Rev. Food Sci. F 2021, 20, 3061–3092. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.Y.; Yu, L.; Zhu, P.T.; Zhou, X.P.; Liu, H.S.; Yang, Y.Y.; Zhou, J.Q.; Gao, C.C.; Bao, X.Y.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Z.X.; Li, X.X.; Li, M. Effect of tea polyphenols on the retrogradation of rice starch. Food Res. Int. 2009, 42, 221–225. [Google Scholar] [CrossRef]
- Zhao, B.B.; Sun, S.W.; Lin, H.; Chen, L.D.; Qin, S.; Wu, W.G.; Zheng, B.D.; Guo, Z.B. Physicochemical properties and digestion of the lotus seed starch-green tea polyphenol complex under ultrasound-microwave synergistic interaction. Ultrason. Sonochem. 2019, 52, 50–61. [Google Scholar] [CrossRef]
- Xie, F.; Huang, Q.; Fang, F.; Chen, S.Q.; Wang, Z.G.; Wang, K.; Fu, X.; Zhang, B. Effects of tea polyphenols and gluten addition on In Vitro wheat starch digestion properties. Int. J. Biol. Macromol. 2019, 126, 525–530. [Google Scholar] [CrossRef]
- Limwachiranona, J.; Jiang, L.; Huang, H.; Sun, J.; Luo, Z.S. Improvement of phenolic compounds extraction from high-starch lotus (Nelumbo nucifera G.) seed kernels using glycerol: New insights to amylose/amylopectin-Phenolic relationships. Food Chem. 2019, 274, 933–941. [Google Scholar] [CrossRef]
- Li, N.N.; Wang, L.L.; Zhao, S.M.; Qiao, D.L.; Jia, C.H.; Niu, M.; Lin, Q.L.; Zhang, B.J. An insight into starch slowly digestible features enhanced by microwave treatment. Food Hydrocoll. 2020, 103, 105690. [Google Scholar] [CrossRef]
- Guo, Q.S.; Sun, D.-W.; Cheng, J.-H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Akintayo, O.A.; Adebo, O.A.; Kayitesi, E.; Njobeh, P.B. A review on the physicochemical properties of starches modified by microwave alone and in combination with other methods. Int. J. Biol. Macromol. 2021, 176, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.Y.; Tian, Y.; Liu, X.X.; Ding, L.; Kirkensgaard, J.J.K.; Hebelstrup, K.; Putaux, J.L.; Blennow, A. Influence of microwave treatment on the structure and functionality of pure amylose and amylopectin systems. Food Hydrocoll. 2021, 119, 106856. [Google Scholar] [CrossRef]
- Jiang, X.F.; Wang, J.Y.; Ou, Y.J.; Zheng, B.D. Effect of chlorogenic acid on the structural properties and digestibility of lotus seed starch during microwave gelatinization. Int. J. Biol. Macromol. 2021, 191, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.H.; Song, Y.; Hong, Y.; Tao, S.; Sun, J.; Liu, C.; Wu, Z.Y.; Cao, L. The extension of vacuum microwave drying time improved the physicochemical properties, In Vitro digestibility and antioxidant activity of brown rice flour. LWT-Food Sci. Technol. 2023, 184, 115023. [Google Scholar] [CrossRef]
- Du, J.J.; Yang, Z.K.; Xu, X.N.; Wang, X.N.; Du, X.F. Effects of tea polyphenols on the structural and physicochemical properties of high-hydrostatic-pressure-gelatinized rice starch. Food Hydrocoll. 2019, 91, 256–262. [Google Scholar] [CrossRef]
- Chen, B.Y.; Zeng, S.X.; Zeng, H.L.; Guo, Z.B.; Zhang, Y.; Zheng, B.D. Properties of lotus seed starch–glycerin monostearin complexes formed by high pressure homogenization. Food Chem. 2017, 226, 119–127. [Google Scholar] [CrossRef]
- Guo, Z.B.; Zeng, S.X.; Lu, X.; Zhou, M.L.; Zheng, M.J.; Zheng, B.D. Structural and physicochemical properties of lotus seed starch treated with ultra-high pressure. Food Chem. 2015, 186, 223–230. [Google Scholar] [CrossRef]
- Flores-Silva, P.C.; Roldan-Cruz, C.A.; Chavez-Esquivel, G.; Vernon-Carter, E.J.; Bello-Perez, L.A.; Alvarez-Ramirez, J. In Vitro digestibility of ultrasound-treated corn starch. Starch-Stärke 2017, 69, 1700040. [Google Scholar] [CrossRef]
- Lv, Y.Z.; Zhang, L.M.; Li, M.N.; He, X.H.; Hao, L.M.; Dai, Y.J. Physicochemical properties and digestibility of potato starch treated by ball milling with tea polyphenols. Int. J. Biol. Macromol. 2019, 129, 207–213. [Google Scholar] [CrossRef]
- Raza, H.; Ameer, K.; Ren, X.F.; Liang, Q.F.; Chen, X.X.; Chen, X.X.; Ma, H.L. Physicochemical properties and digestion mechanism of starch-linoleic acid complex induced by multi-frequency power ultrasound. Food Chem. 2021, 364, 130392. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jiang, X.F.; Guo, Z.B.; Zhang, Y. Insights into the multi-scale structural properties and digestibility of lotus seed starch-chlorogenic acid complexes prepared by microwave irradiation. Food Chem. 2021, 361, 130171. [Google Scholar] [CrossRef]
- Luo, D.; Xie, Q.; Chen, C.; Mu, K.Y.; Wang, Z.M.; Gu, S.M.; Xue, W.T. Increasing the pressure during high pressure homogenization regulates the starch digestion of the resulting pea starch-gallic acid complexes. Int. J. Biol. Macromol. 2023, 235, 123820. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Shen, M.Y.; Xiao, W.H.; Li, Y.L.; Pan, W.T.; Xie, J.H. Regulating the physicochemical and structural properties of different starches by complexation with tea polyphenols. Food Hydrocoll. 2023, 142, 108836. [Google Scholar] [CrossRef]
- Matia-Merino, L.; Prieto, M.; Roman, L.; Gomez, M. The impact of basil seed gum on native and pregelatinized corn flour and starch gel properties. Food Hydrocoll. 2019, 89, 122–130. [Google Scholar] [CrossRef]
- Raza, H.; Li, S.Q.; Zhou, Q.; He, J.Y.; Cheng, K.W.; Dai, S.D.; Wang, M.F. Effects of ultrasound-induced V-type rice starch-tannic acid interactions on starch In Vitro digestion and multiscale structural properties. Int. J. Biol. Macromol. 2023, 246, 125619. [Google Scholar] [CrossRef]
- Liu, Y.F.; Chen, L.; Xu, H.S.; Liang, Y.; Zheng, B. Understanding the digestibility of rice starch-gallic acid complexes formed by high pressure homogenization. Int. J. Biol. Macromol. 2019, 134, 856–863. [Google Scholar] [CrossRef]
- Raza, H.; Zhou, Q.; Cheng, K.-W.; He, J.Y.; Wang, M.F. Synergistic impact of heat-ultrasound treatment on the properties and digestibility of Sagittaria sagittifolia L. starch-phenolic acid complexes. Food Chem. 2024, 445, 138785. [Google Scholar] [CrossRef]
- Xiao, H.X.; Lin, Q.L.; Liu, G.-Q.; Wu, Y.; Wu, W.; Fu, X.J. Inhibitory Effects of Green Tea Polyphenols on the Retrogradation of Starches from Different Botanical Sources. Food Bioprocess. Technol. 2013, 6, 2177–2181. [Google Scholar] [CrossRef]
- Perez, S.; Vergelati, C. Solid state and solution features of amylose and amylosic fragments. Polym. Bull. 1987, 17, 141–148. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Chi, C.D.; Li, X.X.; Zhang, Y.P.; Chen, L.; Li, L.; Wang, Z.J. Digestibility and supramolecular structural changes of maize starch by non-covalent interactions with gallic acid. Food Funct. 2017, 8, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.J.; Zhao, Y.; Li, X.X.; Li, L.; Xie, F.W.; Chen, L. Supramolecular structural changes of waxy and high-amylose cornstarches heated in abundant water. Food Hydrocoll. 2014, 35, 700–709. [Google Scholar] [CrossRef]
- Zhang, X.L.; Mi, T.T.; Gao, W.; Wu, Z.Z.; Yuan, C.; Cui, B.; Dai, Y.Y.; Liu, P.F. Ultrasonication effects on physicochemical properties of starch–lipid complex. Food Chem. 2022, 388, 133054. [Google Scholar] [CrossRef]
- Wang, M.T.; Shen, Q.; Hu, L.L.; Hu, Y.Q.; Ye, X.Q.; Liu, D.H.; Chen, J.C. Physicochemical properties, structure and In Vitro digestibility on complex of starch with lotus (Nelumbo nucifera Gaertn.) leaf flavonoids. Food Hydrocoll. 2018, 81, 191–199. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, Y.H.; Shen, M.Y.; Wen, H.L.; Ren, Y.M.; Yang, J.; Han, X.Y.; Xie, J.H. Effect of Mesona chinensis polysaccharide on the retrogradation properties of maize and waxy maize starches during storage. Food Hydrocoll. 2020, 101, 105538. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, M.X.; Zhou, D.T.; Li, X.P.; Hu, X.Z. The retrogradation characteristics of pullulanase debranched field pea starch: Effects of storage time and temperature. Int. J. Biol. Macromol. 2019, 134, 984–992. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Liu, S.C.; Shen, M.Y.; Jiang, L.; Ren, Y.M.; Luo, Y.; Xie, J.H. Effect of different polysaccharides on pasting, gelation, structural properties and digestibility of tapioca starch-polysaccharides gels. Food Hydrocoll. 2020, 99, 105327. [Google Scholar] [CrossRef]
- Carcea, M.; Acquistucci, R. Isolation and Physicochemical Characterization of Fonio (Digitaria exilis Stapf) Starch. Starch-Stärke 1997, 49, 131–135. [Google Scholar] [CrossRef]
- Hedayati, S.; Niakousari, M. Microstructure, pasting and textural properties of wheat starch-corn starch citrate composites. Food Hydrocoll. 2018, 81, 1–5. [Google Scholar] [CrossRef]
- Zavareze, E.d.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Zhao, B.B.; Wang, B.L.; Zheng, B.D.; Chen, L.D.; Guo, Z.B. Effects and mechanism of high pressure homogenization on the characterization and digestion behavior of lotus seed starch-green tea polyphenol complexes. J. Funct. Foods 2019, 57, 173–181. [Google Scholar] [CrossRef]
- Karunaratne, R.; Zhu, F. Physicochemical interactions of maize starch with ferulic acid. Food Chem. 2016, 199, 372–379. [Google Scholar] [CrossRef]
- Zhou, X.; Baik, B.-K.; Wang, R.; Lim, S.T. Retrogradation of waxy and normal corn starch gels by temperature cycling. J. Cereal Sci. 2010, 51, 57–65. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.L.; Liu, G.-Q.; Yu, F.X. Evaluation of Black Tea Polyphenol Extract Against the Retrogradation of Starches from Various Plant Sources. Molecules 2012, 17, 8147–8158. [Google Scholar] [CrossRef] [PubMed]
- Qadir, N.; Wani, I.A. In-Vitro digestibility of rice starch and factors regulating its digestion process: A review. Carbohydr. Polym. 2022, 291, 119600. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, M.; Tang, A.X.; Jane, J.L.; Dhital, S.; Guo, B. RS Content and eGI Value of Cooked Noodles (I): Effect of Cooking Methods. Foods 2020, 9, 328. [Google Scholar] [CrossRef]
- Chai, Y.W.; Wang, M.Z.; Zhang, G.Y. Interaction between Amylose and Tea Polyphenols Modulates the Postprandial Glycemic Response to High-Amylose Maize Starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]
- He, T.; Wang, K.; Zhao, L.; Chen, Y.; Zhou, W.X.; Liu, F.M.; Hu, Z.Y. Interaction with longan seed polyphenols affects the structure and digestion properties of maize starch. Carbohydr. Polym. 2021, 256, 117537. [Google Scholar] [CrossRef]
- Li, M.; Pernell, C.; Ferruzzi, M.G. Complexation with phenolic acids affect rheological properties and digestibility of potato starch and maize amylopectin. Food Hydrocoll. 2018, 77, 843–852. [Google Scholar] [CrossRef]

| Power (W) | TP Content (mg GAE/g PS) |
|---|---|
| mixture | 0.04 ± 0.01 f |
| 150 W | 8.40 ± 0.30 e |
| 200 W | 9.30 ± 0.17 d |
| 250 W | 16.76 ± 0.08 c |
| 300 W | 21.06 ± 0.51 a |
| 350 W | 18.53 ± 0.75 b |
| Power (W) | D [4,3]/μm | D [3,2]/μm | D (10)/μm | D (50)/μm | D (90)/μm |
|---|---|---|---|---|---|
| mixture | 27.14 ± 0.20 f | 14.59 ± 0.13 e | 17.33 ± 0.25 d | 27.07 ± 0.08 e | 38.89 ± 0.77 f |
| 150 W | 157.85 ± 0.72 d | 40.59 ± 0.26 c | 18.04 ± 0.18 c | 122.38 ± 0.85 c | 355.31 ± 2.27 d |
| 200 W | 138.14 ± 1.71 e | 36.15 ± 0.33 d | 16.20 ± 0.17 e | 96.49 ± 2.01 d | 323.61 ± 3.24 e |
| 250 W | 169.22 ± 0.33 c | 44.32 ± 0.04 b | 19.19 ± 0.01 b | 137.70 ± 0.67 b | 372.94 ± 0.36 c |
| 300 W | 176.68 ± 0.34 b | 44.35 ± 1.30 b | 17.29 ± 0.33 d | 137.44 ± 5.50 b | 406.79 ± 0.61 b |
| 350 W | 222.99 ± 4.41 a | 60.36 ± 0.71 a | 22.98 ± 0.34 a | 197.34 ± 2.14 a | 455.10 ± 5.03 a |
| Power (W) | TO (°C) | TO (°C) | TP (°C) | TC (°C) |
|---|---|---|---|---|
| mixture | 55.53 ± 0.93 a | 65.70 ± 0.82 a | 73.93 ± 4.81 a | 11.84 ± 0.86 a |
| 150 W | 43.45 ± 0.35 c | 56.80 ± 1.13 b | 71.75 ± 0.49 ab | 3.04 ± 0.46 c |
| 200 W | 41.90 ± 1.13 d | 56.95 ± 0.35 b | 68.27 ± 0.35 bc | 2.30 ± 0.25 d |
| 250 W | 45.15 ± 1.06 b | 56.35 ± 1.06 b | 66.10 ± 0.99 c | 3.51 ± 0.24 c |
| 300 W | 44.67 ± 0.60 bc | 54.83 ± 0.45 c | 64.57 ± 1.27 c | 3.23 ± 0.23 c |
| 350 W | 43.70 ± 1.39 bc | 53.83 ± 0.55 c | 64.45 ± 2.98 c | 4.50 ± 0.28 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Qin, P.; Wu, D.; Huang, J.; Zhang, J.; Gong, Y.; Zou, L.; Hu, Y. Effects of Microwave Treatment on Physicochemical Attributes, Structural Analysis, and Digestive Characteristics of Pea Starch–Tea Polyphenol Complexes. Foods 2024, 13, 2654. https://doi.org/10.3390/foods13172654
Zhang X, Qin P, Wu D, Huang J, Zhang J, Gong Y, Zou L, Hu Y. Effects of Microwave Treatment on Physicochemical Attributes, Structural Analysis, and Digestive Characteristics of Pea Starch–Tea Polyphenol Complexes. Foods. 2024; 13(17):2654. https://doi.org/10.3390/foods13172654
Chicago/Turabian StyleZhang, Xin, Peiyou Qin, Dingtao Wu, Jingwei Huang, Jiayi Zhang, Yuanyuan Gong, Liang Zou, and Yichen Hu. 2024. "Effects of Microwave Treatment on Physicochemical Attributes, Structural Analysis, and Digestive Characteristics of Pea Starch–Tea Polyphenol Complexes" Foods 13, no. 17: 2654. https://doi.org/10.3390/foods13172654






