The Anti-Diabetic Effect of Non-Starch Polysaccharides Extracted from Wheat Beer on Diet/STZ-Induced Diabetic Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Kits
2.2. Extraction of NSPs
2.3. Characterization of NSPs
2.4. Enzymatic Assays
2.4.1. α-Amylase Inhibition
2.4.2. Lipase Inhibition
2.4.3. Determination of Fat-Binding Capacity
2.4.4. Determination of Cholesterol-Binding Capacity Assay
2.5. Animal Experimental Design
2.6. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
2.7. Serum Biochemical Analysis
2.8. Determination of Liver Lipid and Glycogen Content
2.8.1. Lipid Content in Liver Tissue
2.8.2. Glycogen Content in Liver Tissue
2.9. Histological Analysis
2.10. Statistical Analysis
3. Results
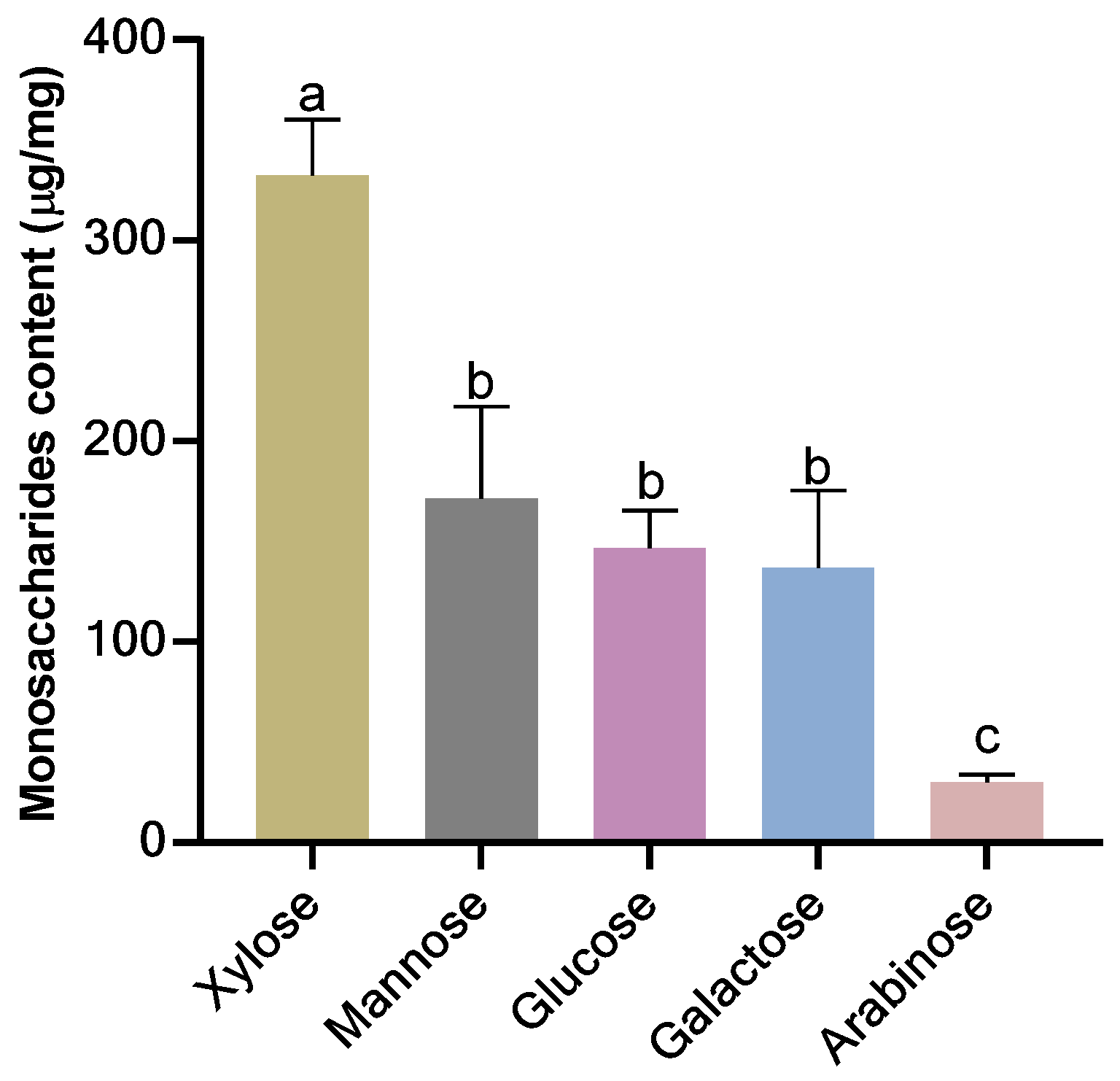
3.1. Monosaccharides Composition of Extracted NSPs
3.2. Enzyme Activity Inhibition Effect of NSPs
3.3. Lipid-Binding Capacity of NSPs Extract
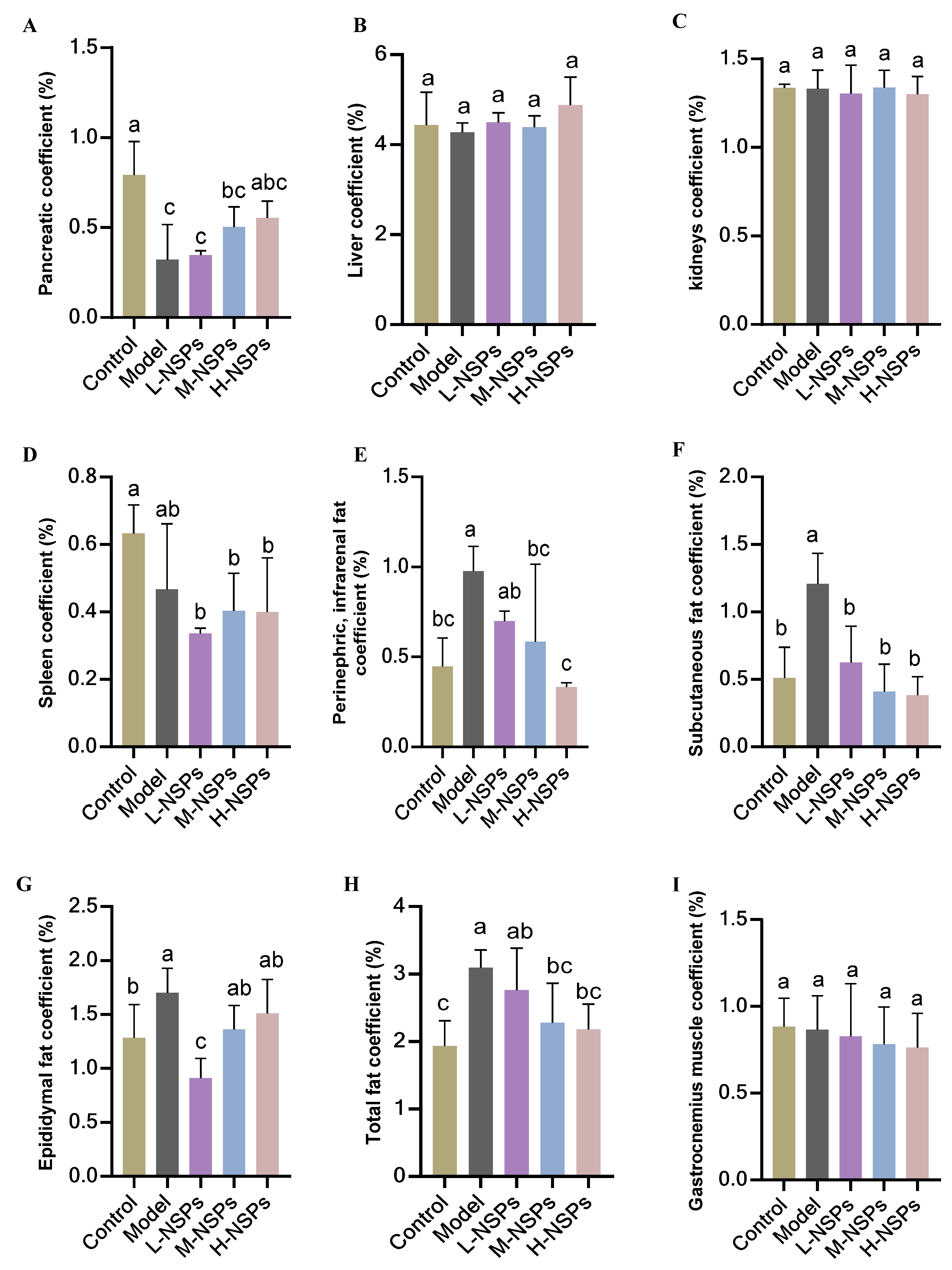
3.4. Effect of NSPs on Mice Body Weight and Organ Coefficient
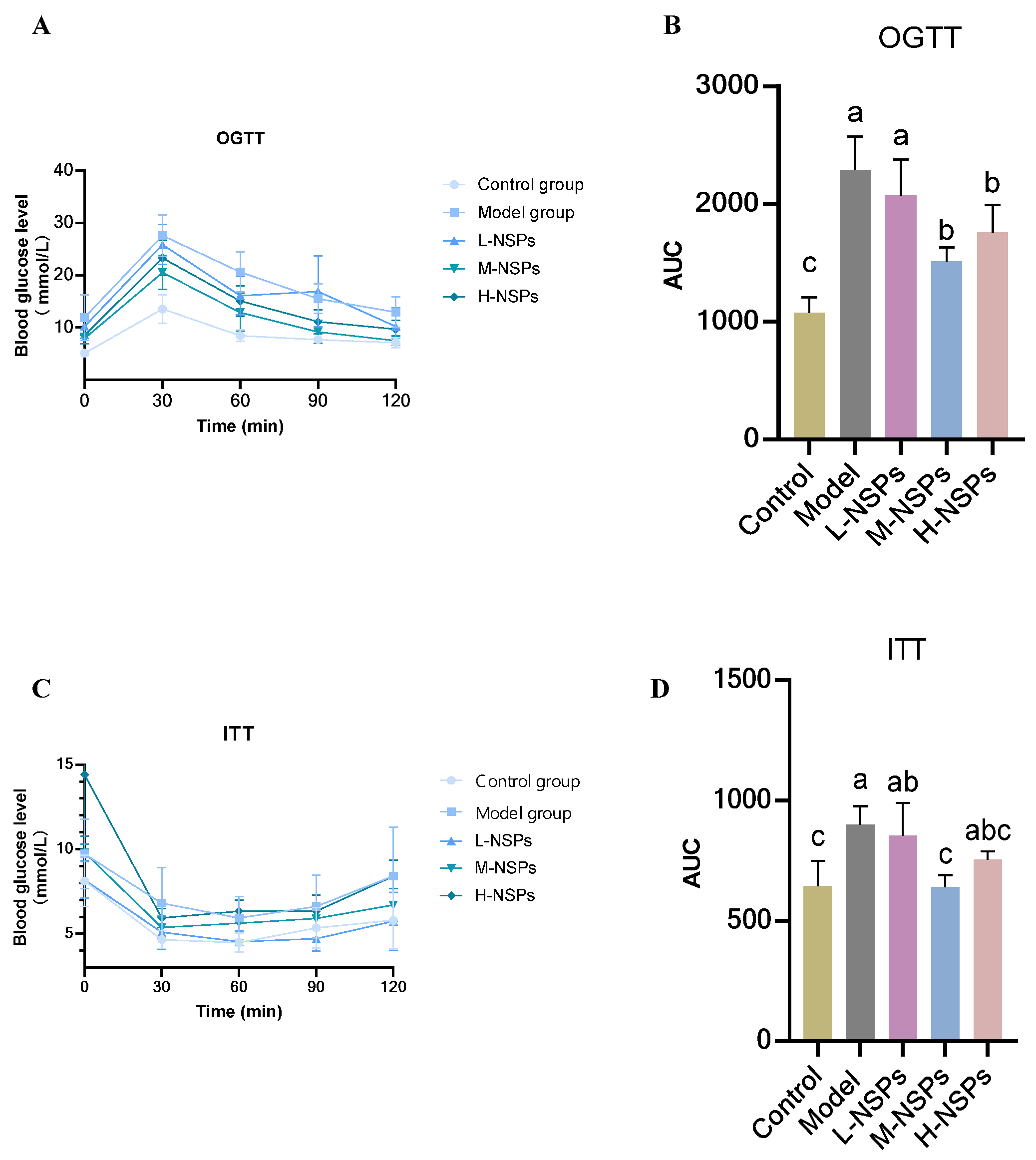
3.5. The Effect of NSPs on OGTT and ITT in the Diabetic Mice
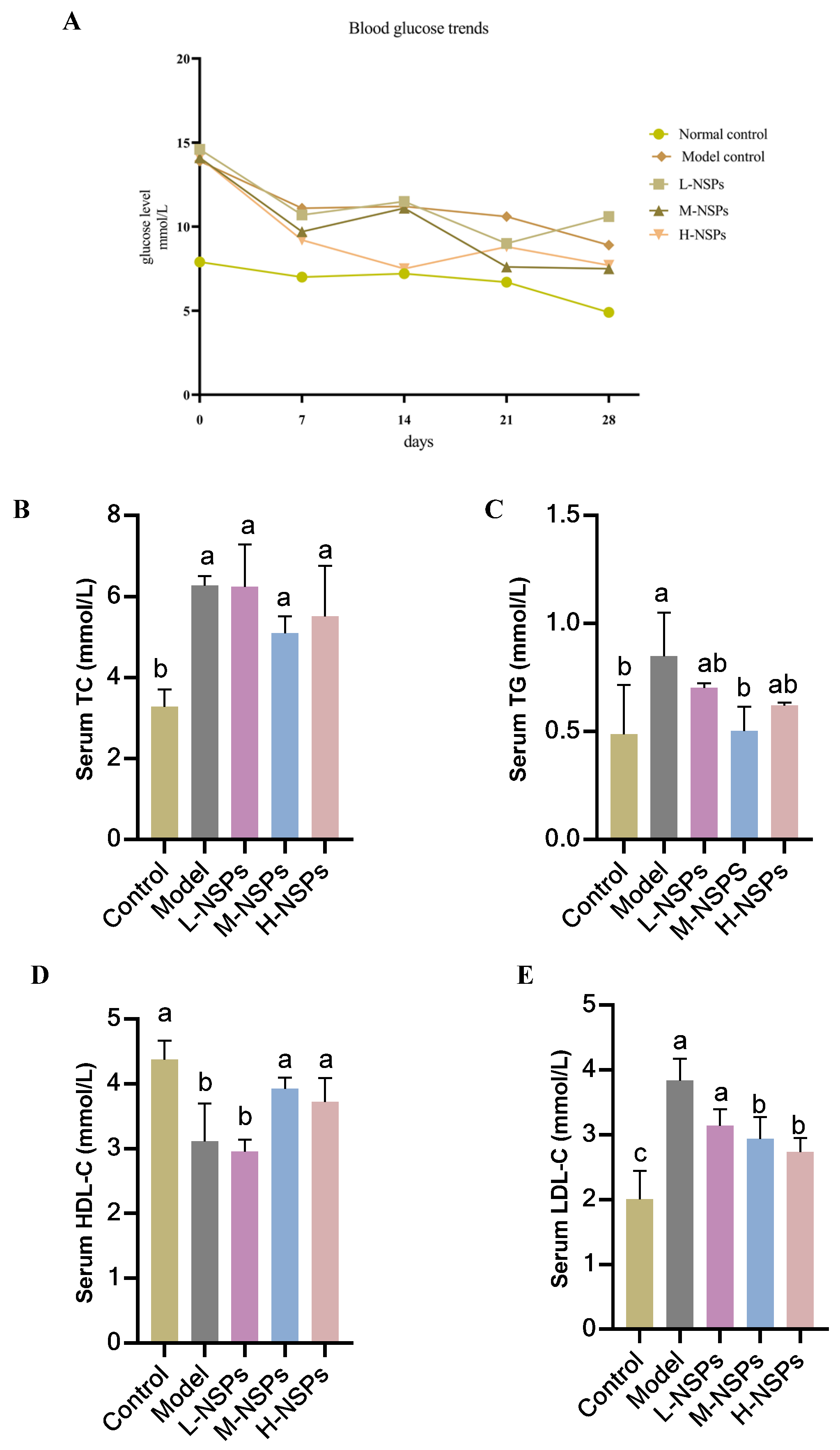
3.6. The Effect of NSPs on Mice Fasting Blood Glucose
3.7. The Effect of NSPs on Serum Lipid Profile
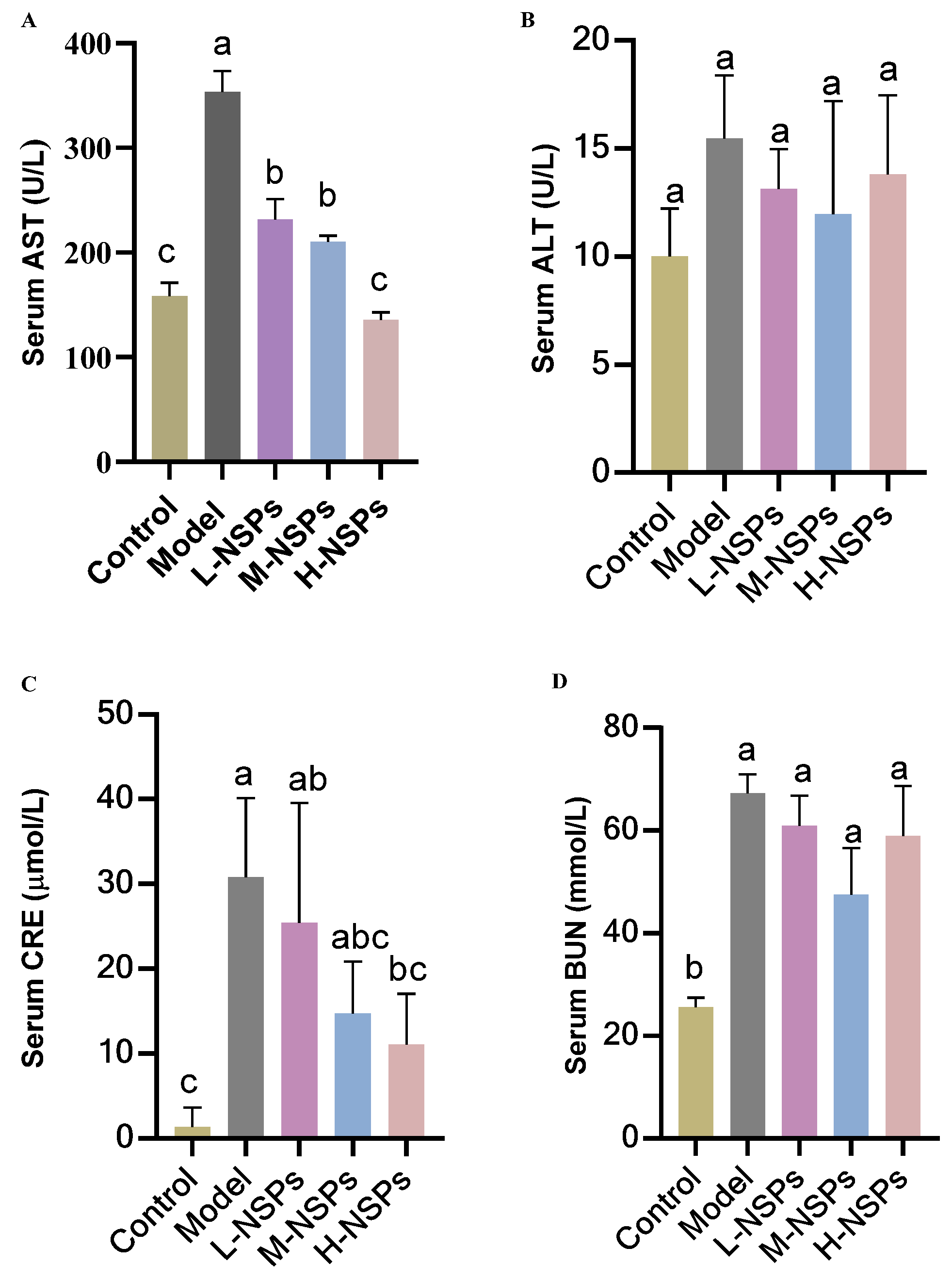
3.8. The Effect of NSPs on Hepatic Enzyme Levels
3.9. The Effect of NSPs on Renal Enzyme Levels
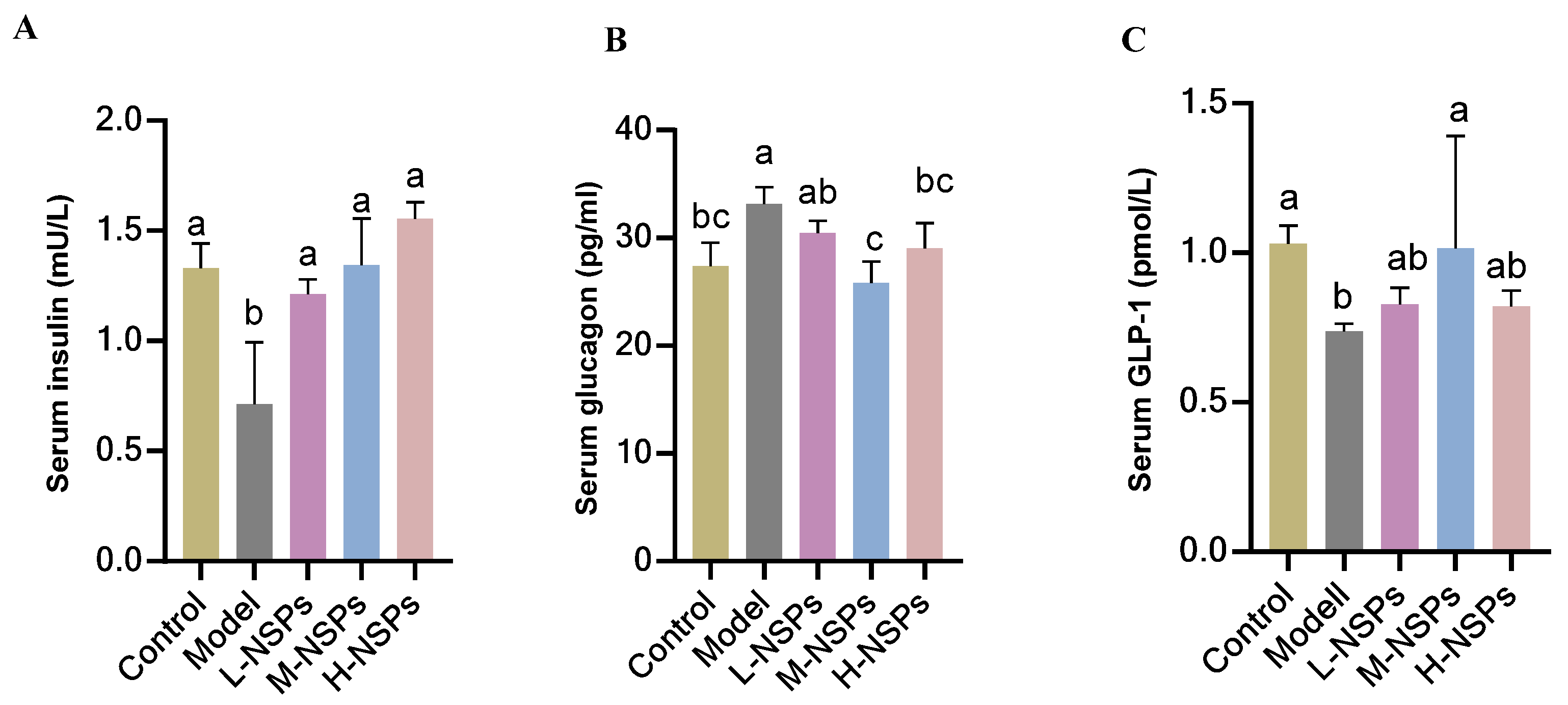
3.10. The Effect of NSPs on Serum Levels of Insulin, Glucagon, and Glucagon-like Peptide 1
3.11. The Effect of NSPs on Serum MDA Levels
3.12. The Effect of NSPs on the Inflammatory Response in Diabetic Mice
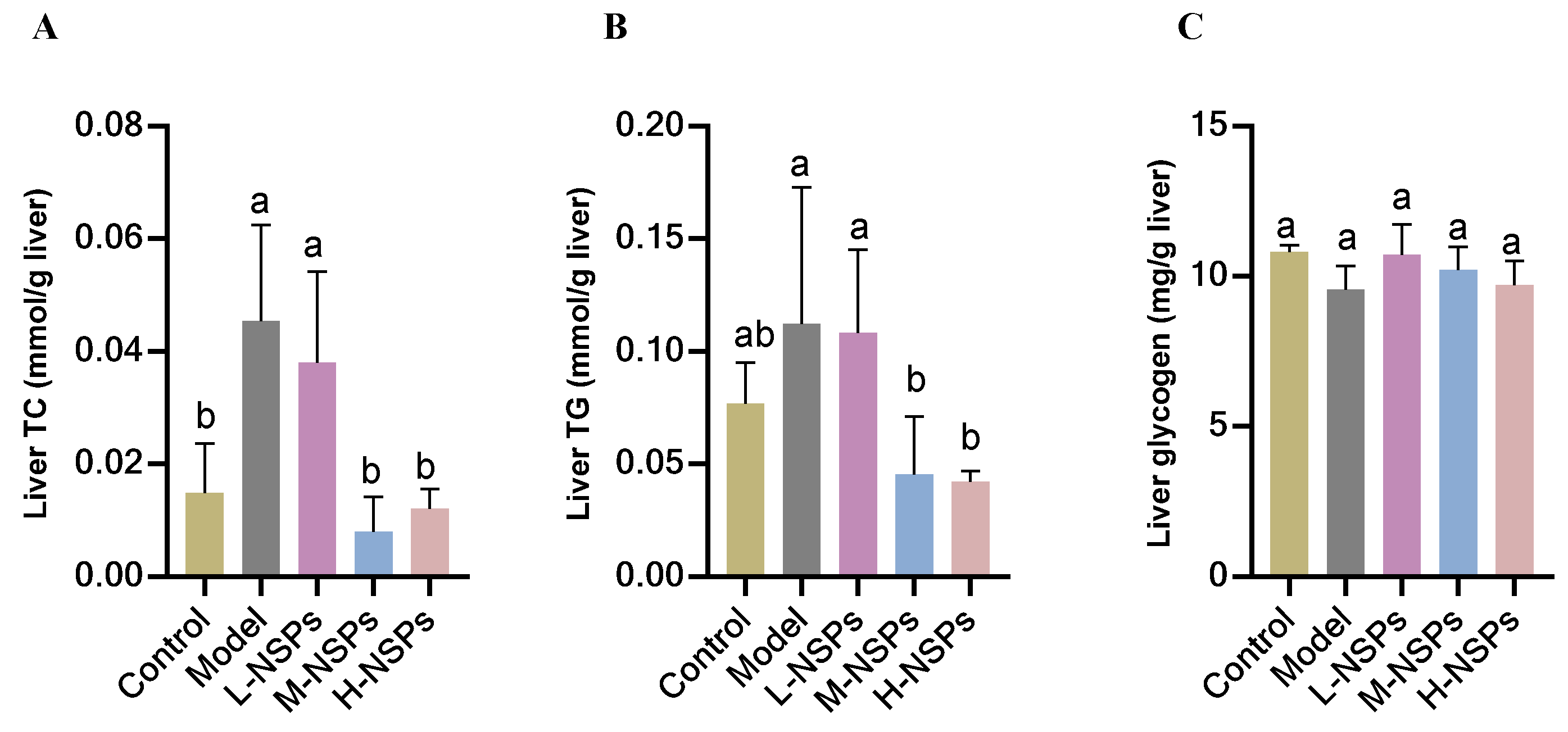
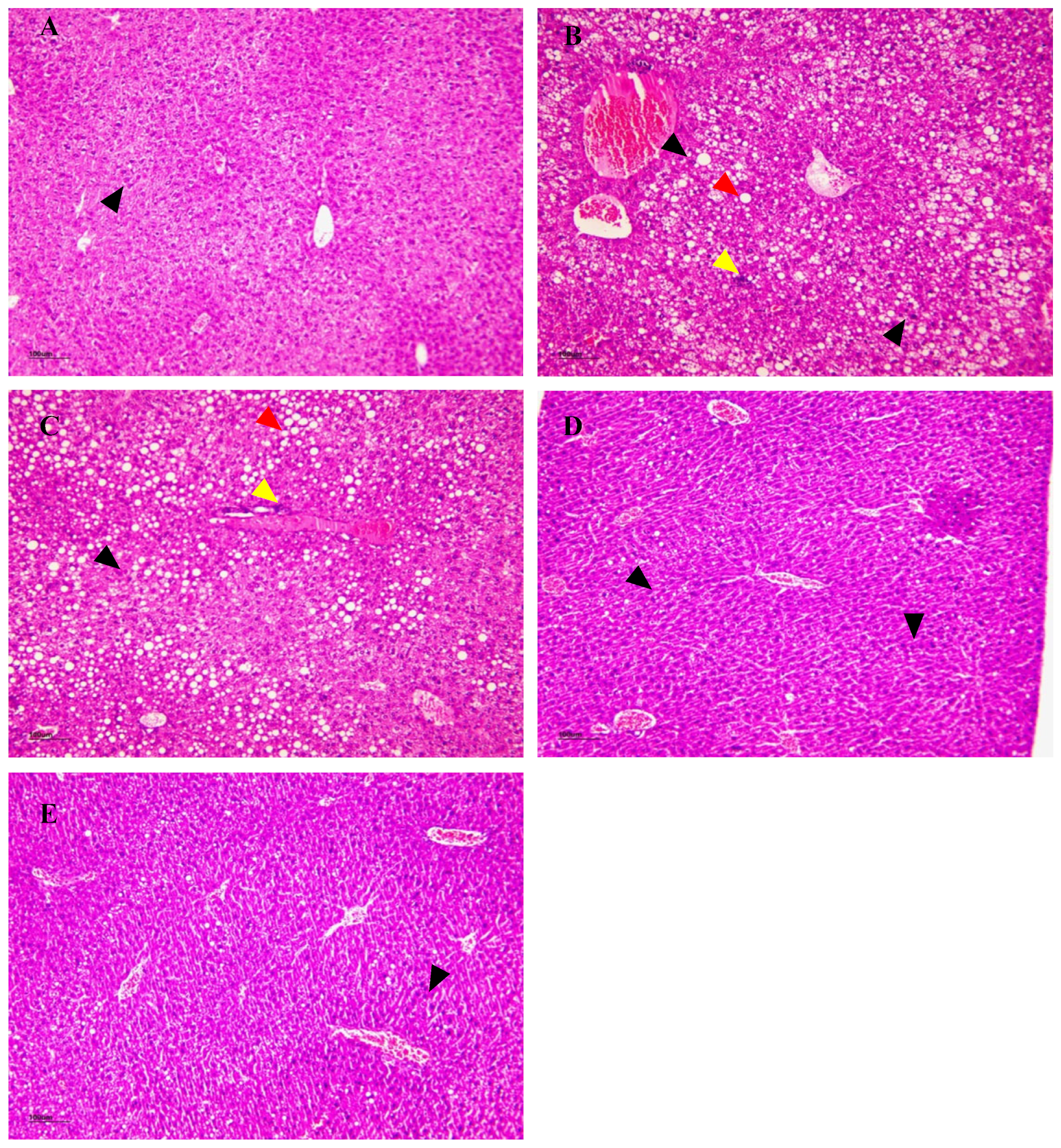
3.13. The Effect of NSPs on Liver Lipid Accumulation of Diabetic Mice
3.14. The Effect of NSPs on Liver Glycogen Content of Diabetic Mice
3.15. The Effect of NSPs on Liver Histology
3.15.1. H&E Staining
3.15.2. Oil Red O Staining
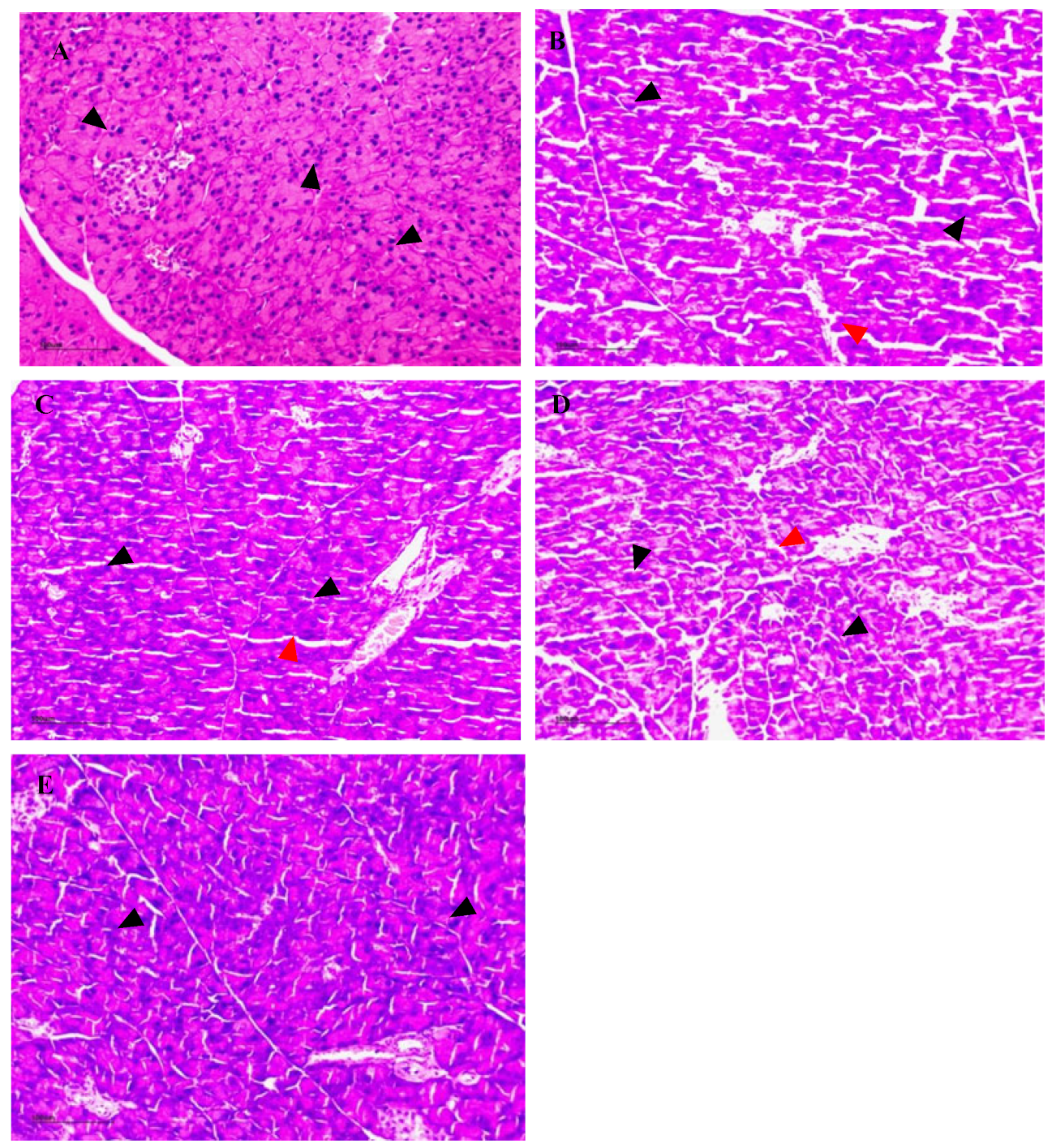
3.16. The Effect of NSPs on Pancreas Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ahmad, L.A.; Crandall, J.P. Type 2 diabetes prevention: A review. Clin. Diabetes 2010, 28, 53–59. [Google Scholar] [CrossRef]
- International Diabetes Federation. Diabetes Around the World|2021. Available online: https://idf.org/news/diabetes-now-affects-one-in-10-adults-worldwide/ (accessed on 6 August 2024).
- Sobczak, A.I.S.; Blindauer, C.A.; Stewart, A.J. Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef]
- Xiao, M.; Jia, X.; Wang, N.; Kang, J.; Hu, X.; Goff, H.D.; Cui, S.W.; Ding, H.; Guo, Q. Therapeutic potential of non-starch polysaccharides on type 2 diabetes: From hypoglycemic mechanism to clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 64, 1177–1210. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Ponnusamy, K. Evaluation of antidiabetic activity of polyherbal formulations on type 2 diabetic patients: A single blinded randomized study. Int. J. Integ. Med. Sci. 2015, 2, 90–98. [Google Scholar]
- Ye, M.; Qiu, T.; Peng, W.; Chen, W.X.; Ye, Y.W.; Lin, Y.R. Purification, characterization and hypoglycemic activity of extracellular polysaccharides from Lachnum calyculiforme. Carbohydr. Polym. 2011, 86, 285–290. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, D.; Geng, L.; Wang, Z.; Xu, C. Production, fractionation, characterization of extracellular polysaccharide from a newly isolated Trametes gibbosa and its hypoglycemic activity. Carbohydr. Polym. 2013, 96, 460–465. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, Q.; Yin, J.J.; Yao, Y.; Zhang, J.L. Anti-diabetic effects of polysaccharides from Talinum triangulare in streptozotocin (STZ)-induced type 2 diabetic male mice. Int. J. Biol. Macromol. 2015, 72, 575–579. [Google Scholar] [CrossRef]
- Liu, C.; Song, J.; Teng, M.; Zheng, X.; Li, X.; Tian, Y.; Pan, M.; Li, Y.; Lee, R.J.; Wang, D. Antidiabetic and antinephritic activities of aqueous extract of Cordyceps militaris fruit body in diet-streptozotocin-induced diabetic Sprague Dawley rats. Oxid. Med. Cell. Longev. 2016, 2016, 9685257. [Google Scholar] [CrossRef]
- Wu, G.J.; Bai, Z.Y.; Wan, Y.J.; Shi, H.F.; Huang, X.J.; Nie, S.P. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway. Food Hydrocoll. 2020, 101, 105456. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, B. Chlorogenic acid and β-glucan from highland barley grain ameliorate β-cell dysfunction via inhibiting apoptosis and improving cell proliferation. Food Funct. 2021, 12, 10040–10052. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Huang, W.; Suo, J.; Chen, X.; Ding, K.; Sun, Q.; Zhang, H. Antioxidant and anti-inflammatory activities of an anti-diabetic polysaccharide extracted from Gynostemma pentaphyllum herb. Int. J. Biol. Macromol. 2020, 145, 484–491. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Wang, Y.; Wang, Z.; Guo, L.; Li, X.; Huang, L. Arctium lappa L. polysaccharide can regulate lipid metabolism in type 2 diabetic rats through the SREBP-1/SCD-1 axis. Carbohydr. Res. 2020, 494, 108055. [Google Scholar] [CrossRef]
- Kumar, B.P.; Singh, R. Antidiabetic activity of Acacia tortilis (Forsk.) Hayne ssp. raddiana polysaccharide on streptozotocin-nicotinamide induced diabetic rats. BioMed. Res. Int. 2014, 2014, 572013. [Google Scholar]
- Kou, L.; Du, M.; Liu, P.; Zhang, B.; Zhang, Y.; Yang, P.; Shang, M.; Wang, X. Anti-diabetic and anti-nephritic activities of Grifola frondosa mycelium polysaccharides in diet-streptozotocin-induced diabetic rats via modulation on oxidative stress. Appl. Biochem. Biotechnol. 2018, 187, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Stephen, A.M. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef]
- Marcotuli, I.; Colasuonno, P.; Hsieh, Y.S.Y.; Fincher, G.B.; Gadaleta, A. Non-Starch Polysaccharides in Durum Wheat: A Review. Int. J. Mol. Sci. 2020, 21, 2933. [Google Scholar] [CrossRef]
- Sztupecki, W.; Rhazi, L.; Depeint, F.; Aussenac, T. Functional and Nutritional Characteristics of Natural or Modified Wheat Bran Non-Starch Polysaccharides: A Literature Review. Foods 2023, 12, 2693. [Google Scholar] [CrossRef]
- Song, Z.; Li, M.; Du, J.; Zhang, K. Effects of non-starch polysaccharides from pure wheat malt beer on beer quality, in vitro antioxidant, prebiotics, hypoglycemic and hypolipidemic properties. Food Biosci. 2022, 47, 101780. [Google Scholar]
- Cardullo, N.; Muccilli, V.; Pulvirenti, L.; Cornu, A.; Pouységu, L.; Deffieux, D.; Quideau, S.; Tringali, C. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: A study of α-glucosidase and α-amylase inhibition. Food Chem. 2020, 313, 126099. [Google Scholar]
- Franco, R.R.; Mota Alves, V.H.; Ribeiro Zabisky, L.F.; Justino, A.B.; Martins, M.M.; Saraiva, A.L.; Goulart, L.R.; Espindola, F.S. Antidiabetic potential of Bauhinia forficata Link leaves: A non-cytotoxic source of lipase and glycoside hydrolases inhibitors and molecules with antioxidant and antiglycation properties. Biomed. Pharmacother. 2020, 123, 109798. [Google Scholar]
- Sangnark, A.; Noomhorm, A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem. 2003, 80, 221–229. [Google Scholar] [CrossRef]
- Piao, Y.; Liu, Y.; Xie, X. Change trends of organ weight background data in sprague dawley rats at different ages. J. Toxicol. Pathol. 2013, 26, 29–34. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, 81–90. [Google Scholar] [CrossRef]
- Akindele, A.J.; Otuguor, E.; Singh, D.; Ota, D.; Benebo, A.S. Hypoglycemic, antilipidemic and antioxidant effects of valproic acid in alloxan-induced diabetic rats. Eur. J. Pharmacol. 2015, 762, 174–183. [Google Scholar] [CrossRef]
- Zhang, W.N.; Su, R.N.; Gong, L.L.; Yang, W.W.; Chen, J.; Yang, R.; Wang, Y.; Pan, W.J.; Lu, Y.M.; Chen, Y. Structural characterization and in vitro hypoglycemic activity of a glucan from Euryale ferox Salisb. seeds. Carbohydr. Polym. 2019, 209, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Lin, L.; Zhao, M. Sulfated fucan/fucosylated chondroitin sulfate-dominated polysaccharide fraction from low-edible-value sea cucumber ameliorates type 2 diabetes in rats: New prospects for sea cucumber polysaccharide based-hypoglycemic functional food. Int. J. Biol. Macromol. 2020, 159, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Zhang, X.; Liu, H.; Zhang, H.; Zhang, X.; Zhang, X.; Ma, X.; Wang, B.; Xue, T.; et al. A novel polysaccharide of Undaria pinnatifida: Structural characterization, carboxymethylation and hypoglycemic activity in vivo. Food Biosci. 2024, 60, 104479. [Google Scholar] [CrossRef]
- Avwioroko, O.J.; Anigboro, A.A.; Unachukwu, N.N.; Tonukari, N.J. Isolation, identification and in silico analysis of alpha-amylase gene of Aspergillus niger strain CSA35 obtained from cassava undergoing spoilage. Biochem. Biophys. Rep. 2018, 14, 35–42. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef]
- Zhu, G.; Fang, Q.; Zhu, F.; Huang, D.; Yang, C. Structure and Function of Pancreatic Lipase-Related Protein 2 and Its Relationship with Pathological States. Front. Genet. 2021, 12, 693538. [Google Scholar] [CrossRef]
- Cheng, F.; Yan, X.; Zhang, M.; Chang, M.; Yun, S.; Meng, J.; Liu, J.; Feng, C.P. Regulation of RAW 264.7 cell-mediated immunity by polysaccharides from Agaricus blazei Murill via the MAPK signal transduction pathway. Food Funct. 2017, 8, 1475–1480. [Google Scholar]
- Park, H.; Bae, S.H.; Park, Y.; Choi, H.S.; Suh, H.J. Lipase-mediated lipid removal from propolis extract and its antiradical and antimicrobial activity. J. Sci. Food Agric. 2015, 95, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, J.; Zhang, Q.; Qing, D.; Yan, C. Structural elucidation and bioactivities of a novel arabinogalactan from Coreopsis tinctoria. Carbohydr. Polym. 2019, 219, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Mustar, S.; Mustafa Khalid, N.; Rashed, A.A.; Noh, M.F.M.; Wilcox, M.D.; Chater, P.I.; Brownlee, I.A.; Pearson, J.P. Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J. Appl. Phycol. 2013, 25, 1405–1412. [Google Scholar] [CrossRef]
- Qin, H.; Huang, L.; Teng, J.; Wei, B.; Xia, N.; Ye, Y. Purification, characterization, and bioactivity of Liupao tea polysaccharides before and after fermentation. Food Chem. 2021, 353, 129419. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lin, S.; Lu, M.; Gong, J.D.B.; Wang, L.; Zhang, Q.; Lin, D.R.; Qin, W.; Wu, D.T. Characterization, in vitro binding properties, and inhibitory activity on pancreatic lipase of β-glucans from different Qingke (Tibetan hulless barley) cultivars. Int. J. Biol. Macromol. 2018, 120, 2517–2522. [Google Scholar] [CrossRef]
- Sang, H.L.; Jang, G.Y.; Min, Y.K.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Mi, J.L.; Kim, T.J.; Lee, J.; Jeong, H.S. Physicochemical and in vitro binding properties of barley β-glucan treated with hydrogen peroxide. Food Chem. 2016, 192, 729–735. [Google Scholar]
- Sun, Y.; Yang, K.; Zhang, X.; Li, L.; Zhang, H.; Zhou, L.; Liang, J.; Li, X. In Vitro Binding Capacities, Physicochemical Properties and Structural Characteristics of Polysaccharides Fractionated from Passiflora Edulis Peel. Food Biosci. 2022, 50, 102016. [Google Scholar] [CrossRef]
- Friesen, N.T.E.; Büchau, A.S.; Schott-Ohly, P.; Lgssiar, A.; Gleichmann, H. Generation of hydrogen peroxide and failure of antioxidative responses in pancreatic islets of male c57bl/6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia 2004, 47, 676–685. [Google Scholar]
- Mohapatra, S.; Majumder, S.; Smyrk, T.C.; Zhang, L.; Matveyenko, A.; Kudva, Y.C.; Chari, S.T. Diabetes Mellitus Is Associated with an Exocrine Pancreatopathy: Conclusions from a Review of Literature. Pancreas 2016, 45, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Weng, S.; Wang, L.; Mao, Z. Mechanism of Astragalus polysaccharides in attenuating insulin resistance in Rats with type 2 diabetes mellitus via the regulation of liver microRNA-203a-3p. Mol. Med. Rep. 2018, 17, 1617–1624. [Google Scholar] [CrossRef]
- Zhu, K.; Nie, S.; Li, C.; Lin, S.; Xing, M.; Li, W.; Gong, D.; Xie, M. A newly identified polysaccharide from Ganoderma atrum attenuates hyperglycemia and hyperlipidemia. Int. J. Biol. Macromol. 2013, 57, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, C.; Lu, G.; Cui, W.; Mu, Z.; Gao, H.; Wang, Y. Purification, characterization and anti-diabetic activity of a polysaccharide from mulberry leaf. Regul. Toxicol. Pharm. 2014, 70, 687–695. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Boden, G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr. Diab. Rep. 2006, 6, 177–181. [Google Scholar] [CrossRef]
- Huang, M.; Wang, F.Q.; Wang, Y.Z. Hypoglycemic and hypolipidemic properties of polysaccharides from Enterobacter cloacae Z0206 in KKAy mice. Carbohyd. Polym. 2015, 117, 91–98. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F.; Wang, L.; Yang, T.; Shi, L.; Li, X.; Shen, J.; Xu, W.; Guo, T.; Lin, Q. Anti-Hyperlipidemic Effect of Rice Bran Polysaccharide and Its Potential Mechanism in High-Fat Diet Mice. Food Funct. 2017, 11, 4028–4041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.W.; Jin, G.; Yang, X.D.; Zhou, H.L. Regulating dyslipidemia effect of polysaccharides from Pleurotus ostreatus on fat-emulsion-induced hyperlipidemia rats. Int. J. Biol. Macromol. 2017, 101, 107–116. [Google Scholar] [CrossRef]
- Regnell, S.E.; Lernmark, Å. Hepatic steatosis in type 1 diabetes. Rev. Diabet. Stud. 2011, 8, 454–467. [Google Scholar] [CrossRef]
- Moscatiello, S.; Manini, R.; Marchesini, G. Diabetes and liver disease: An ominous association. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- Yan, Z.; Fan, R.; Yin, S.; Zhao, X.; Liu, J.; Li, L.; Zhang, W.; Ge, L. Protective effects of Ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int. J. Biol. Macromol. 2015, 80, 573–580. [Google Scholar] [CrossRef]
- Dewidar, B.; Kahl, S.; Pafili, K.; Roden, M. Metabolic liver disease in diabetes—From mechanisms to clinical trials. Metab. Clin. Exp. 2020, 111S, 154299. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic db/db Mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef]
- Noroozi, K.M.; Khalili, P.F.; Ayoobi, F.; Esmaeili-Nadimi, A.; La Vecchia, C.; Jamali, Z. Serum liver enzymes and diabetes from the Rafsanjan cohort study. BMC Endocr. Disord. 2022, 22, 127. [Google Scholar]
- Gaeini, Z.; Bahadoran, Z.; Mirmiran, P.F.; Azizi, F. The Association Between Liver Function Tests and Some Metabolic Outcomes: Tehran Lipid and Glucose Study. Hepat. Mon. 2020, 20, e98535. [Google Scholar] [CrossRef]
- Shibabaw, T.; Dessie, G.; Molla, M.D.; Zerihun, M.F.; Ayelign, B. Assessment of liver marker enzymes and its association with type 2 diabetes mellitus in Northwest Ethiopia. BMC Res. Notes 2019, 12, 707. [Google Scholar] [CrossRef]
- Uchino, S.; Bellomo, R.; Goldsmith, D. The meaning of the blood urea nitrogen/creatinine ratio in acute kidney injury. Clin. Kidney J. 2012, 5, 187–191. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Jia, R.B.; Li, Z.R.; Ou, Z.R.; Wu, J.; Sun, B.; Lin, L.; Zhao, M. Physicochemical Characterization of Hizikia fusiforme Polysaccharide and Its Hypoglycemic Activity via Mediating Insulin-Stimulated Blood Glucose Utilization of Skeletal Muscle in Type 2 Diabetic Rats. Chem. Biodivers. 2020, 17, e2000367. [Google Scholar] [CrossRef]
- Luke, N.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar]
- Longuet, C.; Sinclair, E.M.; Maida, A.; Baggio, L.L.; Maziarz, M.; Charron, M.J.; Drucker, D.J. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008, 8, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Qiu, X.; Li, J.; Liang, J.; Li, W.; Zhang, C.; Zhang, Z.; Luan, B. Glucagon-induced extracellular cAMP regulates hepatic lipid metabolism. J. Endocrinol. 2017, 234, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, W.; Liao, D.; Chen, D.P.; Zhu, G.; Cai, A. Kiyoshi. Polysaccharides from Enteromorpha prolifera Improve Glucose Metabolism in Diabetic Rats. J. Diabetes Res. 2015, 2015, 675201. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Tirosh, O.; Cohen, G.; Sasson, S.; Zarkovic, N. Reactive aldehydes—Second messengers of free radicals in diabetes mellitus. Free Radic. Res. 2013, 47, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alaniz, M.H.; Takada, J.; Alonso-Vale, M.I.C.; Lima, F.B. Adipose tissue as an endocrine organ: From theory to practice. J. Pediatr. 2007, 83, 192–203. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Hu, C.; Wang, J.; Zhang, J.; Ren, Z.; Song, X.; Jia, L. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The diabetes mellitus–atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pang, H.; Gao, Z.; Zhao, H.; Zhang, J.; Jia, L. Antioxidant and hepatoprotective activities of residue polysaccharides by Pleurotus citrinipileatus. Int. J. Biol. Macromol. 2019, 131, 315–322. [Google Scholar] [CrossRef] [PubMed]

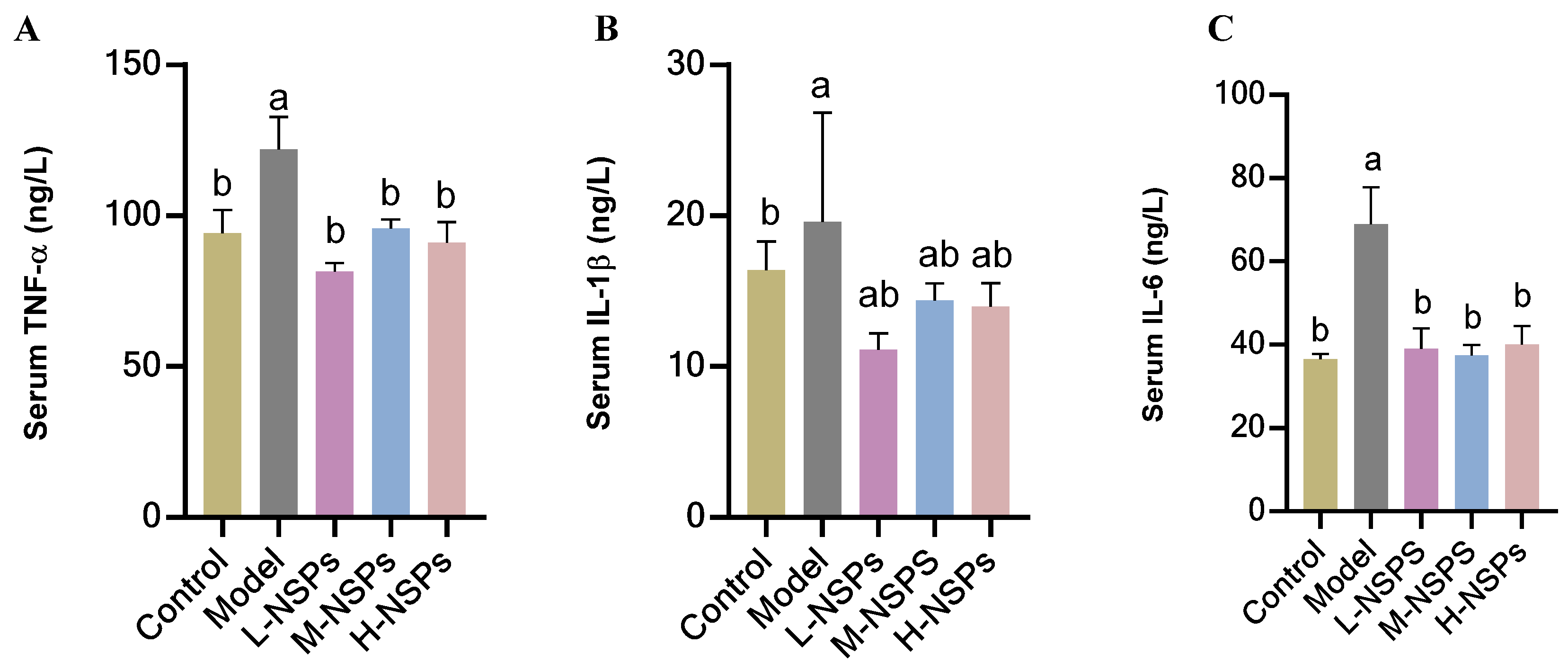

| Sample Concentration (mg/mL) | 0.1 | 0.5 | 1.0 | 1.5 | 2.0 | 3.0 |
|---|---|---|---|---|---|---|
| α-amylase inhibition rate (%) | 11.36 ± 0.18 | 22.73 ± 0.22 | 27.27 ± 0.30 | 43.18 ± 0.27 | 70.45 ± 0.52 | 81.82 ± 0.85 |
| Pancreatic lipase inhibition rate (%) | 12.63 ± 0.11 | 17.79 ± 0.08 | 24.82 ± 0.27 | 27.04 ± 0.33 | 44.35 ± 1.05 | 45.73 ± 1.01 |
| Initial Weight (g) | Final Weight (g) | The Amount of Weight Change (g) | Food Intake (g) | |
|---|---|---|---|---|
| Control group | 27.5667 ± 0.8595 bc | 29.5500 ± 0.6189 a | 1.9833 ± 0.5636 a | 271.6 ± 67.9 a |
| Model group | 26.6750 ± 0.8958 c | 31.1500 ± 2.6211 a | 4.4750 ± 2.5889 a | 302.35 ± 72.58 a |
| Low-dose group | 26.2000 ± 1.5621 c | 30.6000 ± 1.2288 a | 4.4000 ± 2.2517 a | 270.9 ± 65.72 a |
| Medium-dose group | 28.5000 ± 1.6279 ab | 30.7400 ± 2.0070 a | 2.2400 ± 2.3713 a | 291.9 ± 71.65 a |
| High-dose group | 29.3250 ± 0.4992 a | 32.0250 ± 1.6701 a | 2.7000 ± 1.7146 a | 274.1 ± 67.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oumeddour, D.Z.; Lin, W.; Lian, C.; Zhao, L.; Wang, X.; Zhao, L.; Guo, L. The Anti-Diabetic Effect of Non-Starch Polysaccharides Extracted from Wheat Beer on Diet/STZ-Induced Diabetic Mice. Foods 2024, 13, 2692. https://doi.org/10.3390/foods13172692
Oumeddour DZ, Lin W, Lian C, Zhao L, Wang X, Zhao L, Guo L. The Anti-Diabetic Effect of Non-Starch Polysaccharides Extracted from Wheat Beer on Diet/STZ-Induced Diabetic Mice. Foods. 2024; 13(17):2692. https://doi.org/10.3390/foods13172692
Chicago/Turabian StyleOumeddour, Dounya Zad, Wen Lin, Chang Lian, Lei Zhao, Xinyi Wang, Liang Zhao, and Liyun Guo. 2024. "The Anti-Diabetic Effect of Non-Starch Polysaccharides Extracted from Wheat Beer on Diet/STZ-Induced Diabetic Mice" Foods 13, no. 17: 2692. https://doi.org/10.3390/foods13172692






