In Vitro Antioxidant, Anti-Inflammatory Activity and Bioaccessibility of Ethanolic Extracts from Mexican Moringa oleifera Leaf
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Pressurized Liquid Extraction (PLE)
2.3. Determination of Total Phenol Content (TPC) and Total Flavonoid Content (TFC)
2.4. Determination of Antioxidant Activity (ABTS•+ and DPPH•)
2.5. Analysis of Phenolic Compounds by HPLC-DAD
2.6. Cell Culture
2.7. Cytotoxicity Assays
2.8. Anti-Inflammatory Activity
2.9. In Vitro Gastrointestinal Digestion
2.10. Statistical Analysis
3. Results
3.1. Yield Results of the Hydroalcoholic Extracts Obtained
3.2. Total Phenol Content (TPC) and Total Flavonoid Content (TFC)
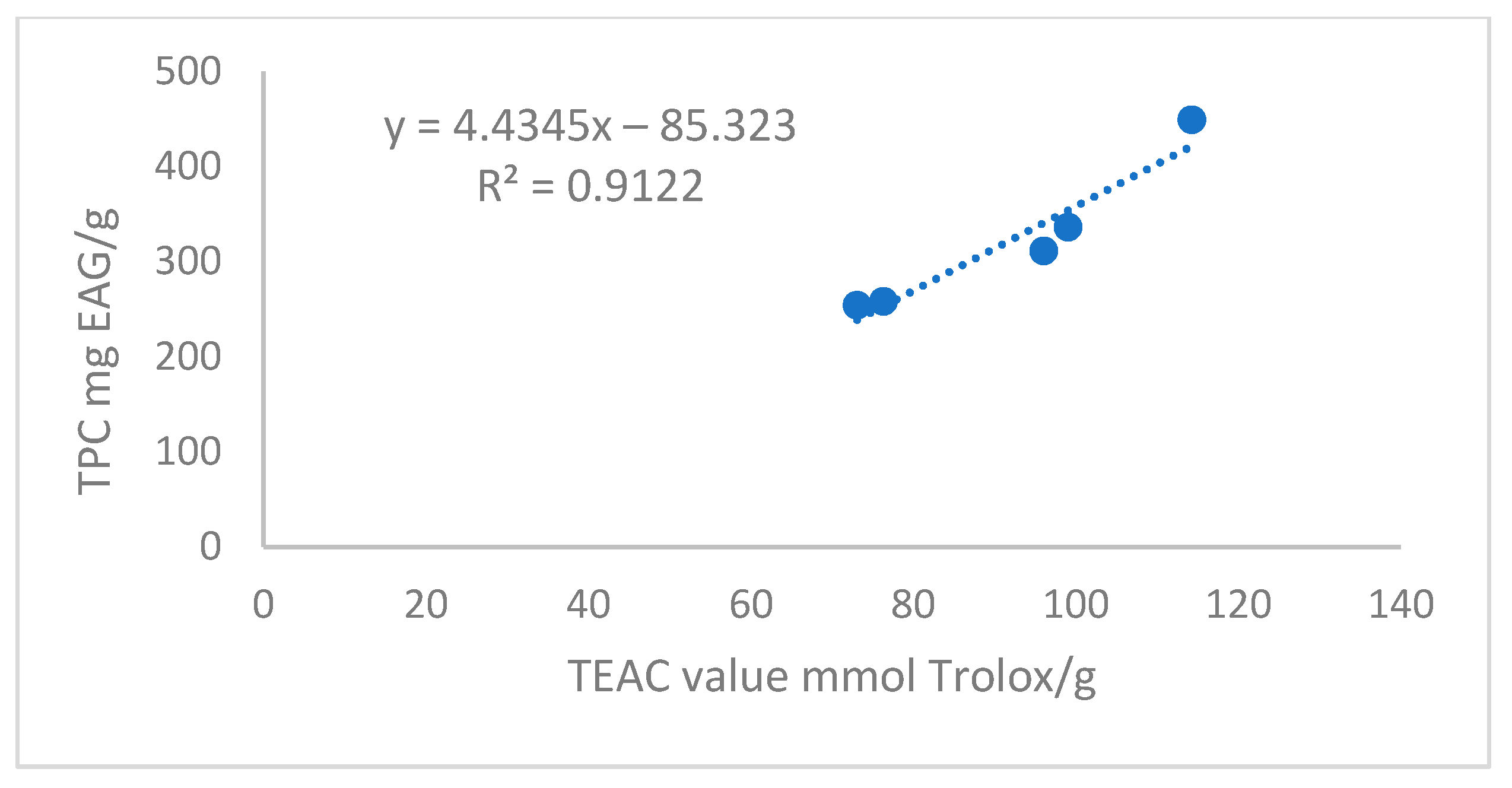
3.3. Antioxidant Activities (ABTS•+ and DPPH•)
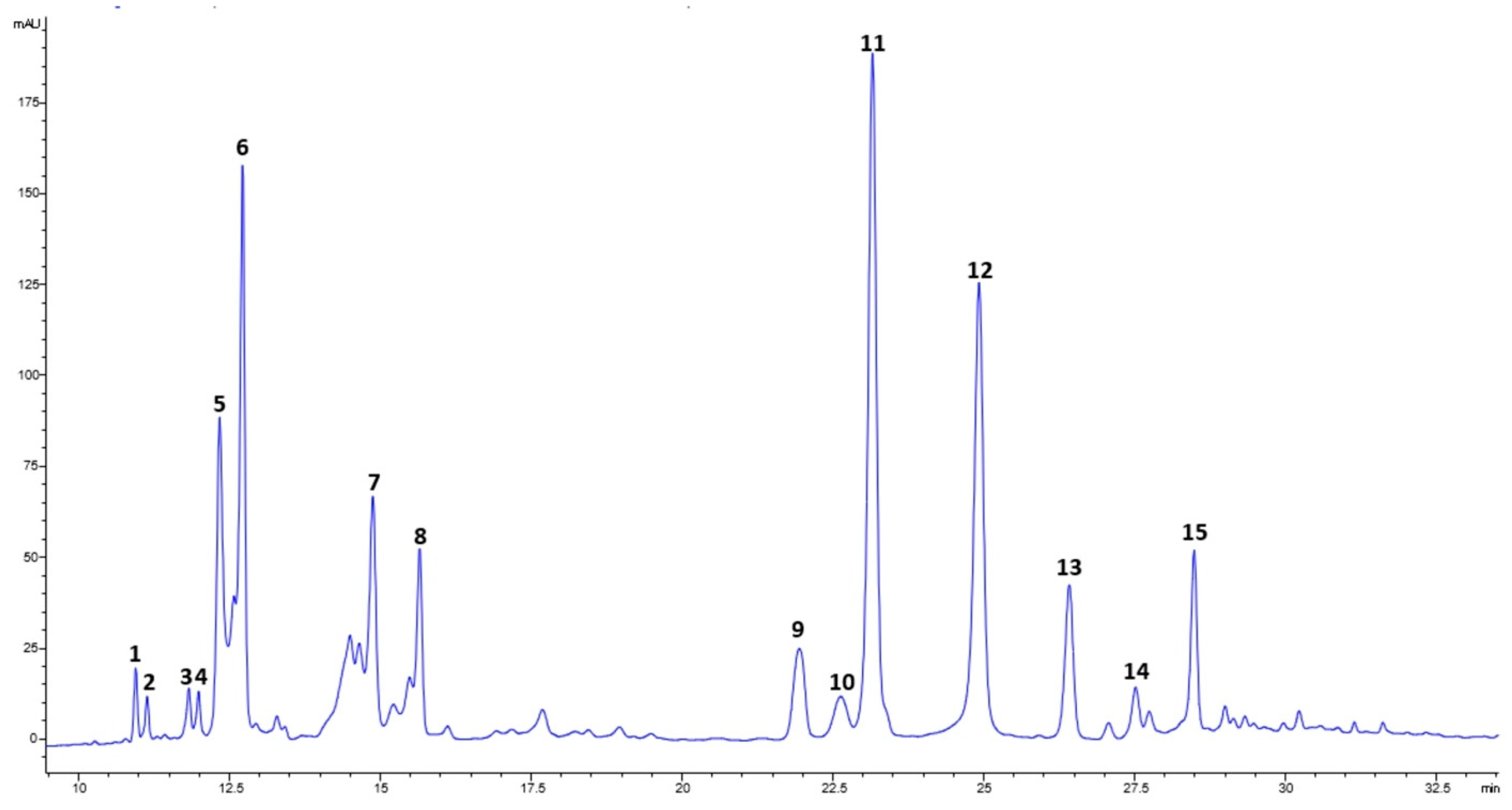
3.4. Phenolic Composition of the Extracts
3.5. Evaluation of Cytotoxicity and Anti-Inflammatory Activity of Moringa Extracts
3.5.1. Cytotoxicity Evaluation
3.5.2. Anti-Inflammatory Activity
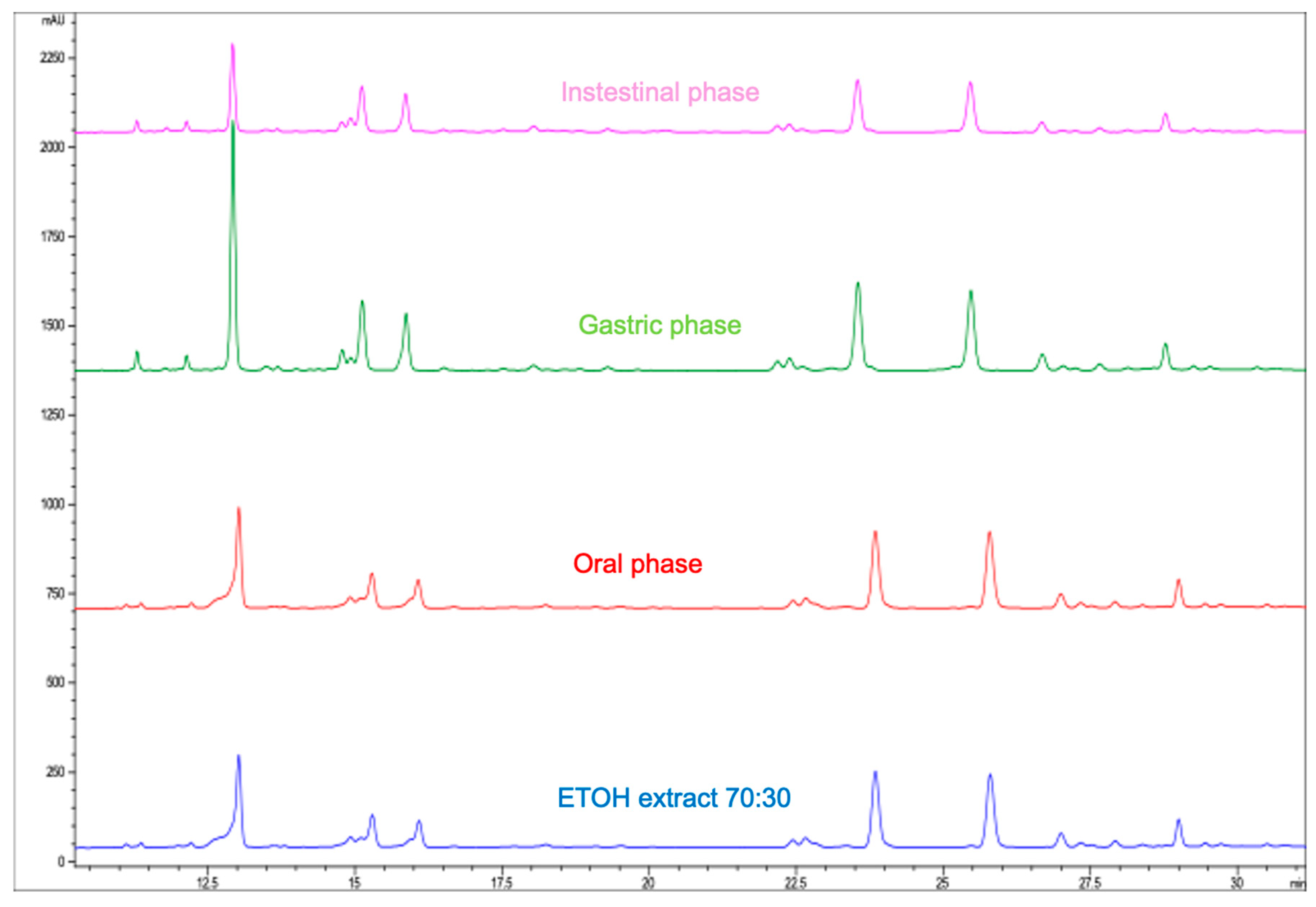
3.6. Effect of In Vitro Digestion on the Phenolic Composition of Moringa Extract
Influence of In Vitro Digestion on Total Phenolic Compounds and Antioxidant Activity of Moringa Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzmán-Maldonado, S.H.; López-Manzano, M.J.; Madera-Santana, T.J.; Núñez-Colín, C.A.; Grijalva-Verdugo, C.P.; Villa-Lerma, A.G.; Rodríguez-Núñez, J.R. Nutritional characterization of Moringa oleifera leaves, seeds, husks and flowers from two regions of Mexico. Agron. Colomb. 2020, 38, 287–297. [Google Scholar] [CrossRef]
- Sreeja, M.; Jayasri, P.; Keerthi, N.; Yeshashwini, J.; Praveen, J. Moringa oleifera: A review on nutritive importance and its potential use as nutraceutical plant. J. Med. Plants Stud. 2021, 9, 15–17. [Google Scholar]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Martínez-González, C.L.; Martínez, L.E.; Martínez-Ortiz, J.; González-Trujano, M.E.; Déciga-Campos, M.; Ventura-Martínez, R.; Díaz-Reval, I. Moringa oleifera, a species with potential analgesic and anti-inflammatory activities. Biomed. Pharmacother. 2017, 87, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Nuapia, Y.; Cukrowska, E.; Tutu, H.; Chimuka, L. Statistical comparison of two modeling methods on pressurized hot water extraction of vitamin C and phenolic compounds from Moringa oleifera leaves. S. Afr. J. Bot. 2020, 129, 9–16. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, S.M.; Ahmed, A.I.; Awad, A.; Mohammed, W.A.; Metwally, M.M.; Almeer, R.; Khalil, S.R. Moringa oleifera ethanolic extract attenuates tilmicosin-induced renal damage in male rats via suppression of oxidative stress, inflammatory injury, and intermediate filament proteins mRNA expression. Biomed. Pharmacother. 2021, 133, 110997. [Google Scholar] [CrossRef]
- Martín, C.; Martín, G.; García, A.; Fernández, T.; Hernández, E.; Puls, J. Potenciales aplicaciones de Moringa oleifera. Una revisión crítica. Pastos y Forrajes 2013, 36, 137–149. [Google Scholar]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Saini, R.K.; Sivanesan, I.; Keum, Y.S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016, 6, 203. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Jaime, L.; Santoyo, S. The health benefits of the bioactive compounds in foods. Foods 2021, 10, 325. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorization of grape stems as a source of phenolic antioxidants by using a sustainable extraction methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C. Standardized static in vitro digestion method suitable for food -an international consensus. Food Funct. 2014, 5, 1113. [Google Scholar] [CrossRef] [PubMed]
- Biehler, E.; Bohn, T. Methods for assessing aspects of carotenoid bioavailability. Curr. Nut. Food Sci. 2010, 6, 44–69. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Noguer, M.; Cereza, A.B.; Rentzsch, M.; Winterhalter, P.; Troncoso, A.M.; García-Parrilla, M.C. Simulated Digestion and Antioxidant Activity of Red Wine Fractions Separated by High Speed Countercurrent Chromatography. J. Agric. Food Chem. 2008, 56, 8879–8884. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Marín, F.R.; Santoyo, S.; García-Risco, M.R.; Señoráns, F.J.; Reglero, G. Testing and Enhancing the in vitro Bioaccessibility and Bioavailability of Rosmarinus officinalis Extracts with a High Level of Antioxidant Abietanes. J. Agric. Food Chem. 2010, 58, 1144–1152. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the Flavan-3-Ols Epicatechin and Catechin and Related Dimeric Procyanidins Derived from Cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef]
- Neilson, A.P.; Hopf, A.S.; Cooper, B.R.; Pereira, M.A.; Bomser, J.A.; Ferruzzi, M.G. Catechin degradation with concurrent formation of homo-and heterocatechin dimers during in vitro digestion. J. Agric. Food Chem. 2007, 55, 8941–8949. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic Composition and Antioxidant Activity of Aqueous Infusions from Capparis spinosa L. and Crithmum maritimum L. before and after Submission to a Two-Step in vitro Digestion Model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Zając, N. Digestion and absorption of phenolic compounds assessed by in vitro simulation methods. A Review. Rocz. Państwowego Zakładu Hig. 2013, 64, 79–84. [Google Scholar]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid test to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Takashiba, S.; Van Dyke, T.E.; Amar, S.; Murayama, Y.; Soskolne, A.W.; Shapira, L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappa B. Infect. Immun. 1999, 67, 5573–5578. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival-application to proliferation and cytotoxicity assays. J. Inmmunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kim, J.A.; Kong, C.S.; Pyun, S.Y.; Kim, S.K. Phosphorylated glucosamine inhibits the inflammatory response in LPS-stimulated PMA-differentiated THP-1 cells. Carb. Res. 2010, 345, 1851–1855. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Ding, H.; Liu, S.; Han, X.; Gui, J.; Liu, D. Subcritical ethanol extraction of flavonoids from Moringa oleifera leaf and evaluatin of antioxidant activity. Food Chem. 2015, 208, 152–158. [Google Scholar]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Braham, F.; Carvalho, D.O.; Almeida, C.M.R.; Zaidi, F.; Magalhães, J.M.C.S.; Guido, L.F.; Gonçalves, M.P. Online HPLC-DPPH screening method for evaluation of radical scavenging phenols extracted from Moringa oleifera leaves. S. Afr. J. Bot. 2020, 129, 146–154. [Google Scholar] [CrossRef]
- Saleem, A.; Saleem, M.; Akhtar, M.F. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. S. Afr. J. Bot. 2020, 128, 246–256. [Google Scholar] [CrossRef]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Torres-Castillo, J.A.; Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Ngangyo-Heya, M.; Martínez-Ávila, G.C.G. Moringa plants: Bioactive compounds and promising applications in food products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Udechukwu, M.C.; Abbey, L.; Nwodo, U.; Udenigwe, C.C. Potential of Moringa oleifera seeds and leaves as functional food ingredients for human health promotion. J. Food Nutr. Res. 2018, 57, 1–14. [Google Scholar]
- Hossain, M.A.; Disha, N.K.; Shourove, J.H.; Dey, P. Determination of antioxidant activity and total tannin from drumstick (Moringa oleifera lam.) leaves using different solvent extraction methods. Turk. J. Agric. Food Sci. Technol. 2020, 8, 2749–2755. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Jahan, I.A.; Jahan, F.; Hossain, H. Antioxidant activities and simultaneous HPLC-DAD profiling of polyphenolic compounds from Moringa oleifera Lam. Leaves grown in Bangladesh. Food Res. 2021, 5, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Oyeniran, O.H.; Ademiluyi, A.O.; Oboh, G. Comparative study of the phenolic profile, antioxidant properties, and inhibitory effects of Moringa (Moringa oleifera Lam.) and Almond (Terminalia catappa Linn.) leaves on acetylcholinesterase and monoamine oxidase activities in the head region of Fruitfly (Drosophila melanogaster Meigen) in vitro. J. Food Biochem. 2021, 45, e13401. [Google Scholar]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Optimization of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.), and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011, 126, 339–346. [Google Scholar] [CrossRef]
- Zhao, B.; Deng, J.; Li, H.; He, Y.; Lan, T.; Wu, D.; Gong, H.; Zhang, Y.; Chen, Z. Optimization of phenolic compound extraction from Chinese Moringa oleifera leaves and antioxidant activities. J. Food Qual. 2019, 2019, 5346279. [Google Scholar] [CrossRef]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of solvent evaporation method on phenolic compounds and the antioxidant activity of Moringa oleifera cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Pollini, L.; Tringaniello, C.; Ianni, F.; Blasi, F.; Manes, J.; Cossignani, L. Impact of ultrasound extraction parameters on the antioxidant properties of Moringa oleifera leaves. Antioxidants 2020, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, L.; Wang, X.; Yao, L.; Wang, L. Ultrasonic-assisted extraction for flavonoid compounds content and antioxidant activities of India Moringa oleifera L. leaves: Simultaneous optimization, HPLC characterization and comparison with other methods. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100284. [Google Scholar] [CrossRef]
- Koheil, M.A.; Hussein, M.A.; Othman, S.M.; El-Haddad, A. Anti-inflammatory and antioxidant activities of Moringa peregrina seeds. Free Rad. Antiox. 2011, 1, 49–61. [Google Scholar] [CrossRef]
- Onsare, J.G.; Kaur, H.; Arora, D.S. Antimicrobial activity of Moringa oleifera from different locations against some human pathogens. Acad. J. Med. Plants 2013, 1, 80–91. [Google Scholar]
- Prabakaran, M.; Kim, S.H.; Sasireka, A.; Chandrasekaran, M.; Chung, I.M. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Charoensin, S. Antioxidant and anticancer activities of Moringa oleifera leaves. J. Med. Plants Res. 2014, 8, 318–325. [Google Scholar]
- Ferreira, P.M.P.; Farias, D.F.J.; Oliveira, T.A.; Carvalho, A.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. J. Nutr. 2008, 21, 431–437. [Google Scholar] [CrossRef]
- Zhang, L.; Ravipati, A.S.S.; Koyyalamudi, R.S.; Jeong, C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef]
- Goulas, V.; Hadjisolomou, A. Dynamic changes in targeted phenolic compounds and antioxidant potency of carob fruit (Ceratonia siliqua L.) products during in vitro digestion. LWT-Food Sci. Technol. 2019, 101, 269–275. [Google Scholar] [CrossRef]
- Lima, K.; Silva, O.; Figueira, M.E.; Pires, C.; Cruz, D.; Gomes, S.; Mauricio, E.M.; Duarte, M.P. Influence of the in vitro gastrointestinal digestion on the antioxidant activity of Artemisia gorgorum Webb and Hyptis pectinata (L.) Poit. Infusions from Cape Verde. Food Res. Int. 2019, 115, 150–159. [Google Scholar] [CrossRef]
- Guo, J.; Giusti, M.M.; De Santiago, E. Stability of various phenolic compounds during simulated digestion. J. Agric. Food Chem. 2018, 66, 12609–12618. [Google Scholar]
- Reboul, E.; Gonçalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.F.; Jourdheuil-Rahmani, D. Digestion of phenolic compounds from different fruits and vegetables during simulated digestion: Impact on phenolic metabolites availability and lipid peroxidation. Food Chem. 2017, 228, 186–192. [Google Scholar]
- Ribeiro, D.; Freitas, V.; Silva, A.M.; Carvalho, F.; Fernandes, E.; Mateus, N. Influence of ester bonds on the stability of phenolic compounds in beverages during simulated digestion. J. Agric. Food Chem. 2016, 64, 3287–3296. [Google Scholar]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.P. Digestion stability of phenolic compounds in virgin olive oil: A review. Comp. Rev. Food Sci. Food Saf. 2014, 13, 180–198. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vázquez-Olivo, G.; Basilio Heredia, J. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]


| Ethanol (%) | Yield (mg Extract/g) |
|---|---|
| 0 | 33.73 ± 1.65 a |
| 30 | 33.89 ± 0.40 a |
| 50 | 31.34 ± 0.33 b |
| 70 | 29.27 ± 0.62 b |
| 100 | 16.00 ± 1.33 c |
| Ethanol (%) | TPC (mg GAE/g Extract) | TFC (mg Q/g Extract) | ABTS•+ (mmol Trolox/g Extract) | DPPH• (mmol Trolox/g Extract) |
|---|---|---|---|---|
| 0 | 73.06 ± 5.80 c | 18.80 ± 1.16 d | 0.36 ± 0.02 c | 0.26 ± 0.01 d |
| 30 | 96.04 ± 7.58 b | 24.90 ± 0.71 c | 0.41 ± 0.01 b | 0.31 ± 0.01 c |
| 50 | 99.07 ± 5.33 b | 29.85 ± 1.36 b | 0.43 ± 0.00 b | 0.34 ± 0.01 b |
| 70 | 111.30 ± 4.43 a | 33.20 ± 2.05 b | 0.52 ± 0.01 a | 0.46 ± 0.00 a |
| 100 | 76.34 ± 3.94 c | 51.00 ± 4.79 a | 0.27 ± 0.02 d | 0.26 ± 0.00 d |
| Peak | Compound | Ethanol (%) | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 50 | 70 | 100 | ||
| 1 | Unidentified | -- | -- | 95.5 ± 0.3 | 90.9 ± 5.8 | 16.3 ± 0.6 |
| 2 | Unidentified | 125.4 ± 0.1 | 152.7 ± 0.3 | 69.8 ± 0.3 | 60.5 ± 2.9 | -- |
| 3 | Unidentified | -- | -- | 70.7 ± 0.7 | 71.6 ± 1.7 | -- |
| 4 | Caffeoylquinic acid | 124.7 ± 0.1 | 136.6 ± 2.5 | 68.9 ± 0.5 | 61.2 ± 2.1 | -- |
| 5 | Cryptochlorogenic acid derivative | 26.9 ± 0.5 | 21.3 ± 0.4 | 408.5 ± 3.7 | 498.7 ± 34.4 | 502.7 ± 109.5 |
| 6 | Neochlorogenic acid | 1230.5 ± 0.8 | 1856.1 ± 2.3 | 1007.7 ± 1.8 | 875.3 ± 49.6 | 483.5 ± 30.9 |
| 7 | Cryptochlorogenic acid | 592.7 ± 0.2 | 797.6 ± 1.8 | 546.7 ± 0.4 | 497.2 ± 43.5 | 276.7 ± 8.2 |
| 8 | Vicenina | 560.5 ± 0.2 | 635.0 ± 1.1 | 694.8 ± 0.7 | 379.2 ± 16.5 | 200.7 ± 13.3 |
| 9 | Vitexin | 254.9 ± 0.6 | 383.7 ± 0.4 | 415.2 ± 0.4 | 406.3 ± 25.4 | 208.1 ± 14.8 |
| 10 | Vitexin derivative | 119.4 ± 0.5 | 124.8 ± 0.4 | 135.3 ± 0.1 | 169.7 ± 8.2 | 220.8 ± 17.1 |
| 11 | Quercetin-O-hexoside | 1314.3 ± 10.8 | 2093.2 ± 12.9 | 2308.6 ± 22.9 | 2627.5 ± 32.9 | 1657.8 ± 48.8 |
| 12 | Quercetin derivative | 1972.8 ± 0.7 | 2976.4 ± 4.6 | 3162.7 ± 3.4 | 2533.0 ± 20.7 | 348.2 ± 20.1 |
| 13 | Kaempferol derivative | 274.8 ± 0.1 | 424.6 ± 0.5 | 470.3 ± 0.1 | 524.8 ± 39.1 | 422.1 ± 13.0 |
| 14 | Isorhamnetin-3-O-glucoside | 60.7 ± 0.6 | 52.0 ± 0.6 | 72.8 ± 0.2 | 157.6 ± 3.3 | 186.3 ± 4.9 |
| 15 | Kaempferol derivative | 388.7 ± 0.2 | 583.8 ± 0.9 | 641.7 ± 0.1 | 543.3 ± 25.7 | 220.3 ± 6.9 |
| Phenolic Compounds | Content (mg/g Extract) | |||
|---|---|---|---|---|
| Extract | Oral | Gastric | Intestinal | |
| Caffeoylquinic acid | 0.28± 0.03 b | 0.33 ± 0.03 b | 0.52 ± 0.01 a | 0.47 ± 0.01 a |
| Neochlorogenic acid | 7.69 ± 0.02 b | 8.29 ± 0.18 ab | 9.58± 0.13 a | 5.28 ± 0.66 c |
| Chlorogenic acid | 3.10 ± 0.02 b | 3.46 ± 0.15 ab | 3.93 ± 0.11 a | 3.68 ± 0.47 a |
| Vicenine | 2.88 ± 0.38 c | 2.88 ± 0.03 c | 4.47 ± 0.26 a | 3.80 ± 0.01 b |
| Luteolin glycoside | 0.09 ± 0.02 a | 0.11 ± 0.02 a | 0.13 ± 0.02 a | 0.13 ± 0.03 a |
| Luteolin glycoside derivative | 0.02 ± 0.01 b | 0.03 ± 0.01 ab | 0.05 ± 0.01 a | 0.01 ± 0.01 b |
| Vitexin isomer | 0.54 ± 0.04 b | 0.58 ± 0.01 b | 0.69 ± 0.08 a | 0.53 ± 0.03 b |
| Vitexin | 0.73 ± 0.04 a | 0.77 ± 0.01 a | 0.72 ± 0.22 a | 0.65 ± 0.04 b |
| Quercetin-O-hexoxide | 13.87 ± 0.47 a | 14.48 ± 0.14 a | 13.44 ±0.79 a | 10.92 ± 0.60 b |
| Isoramnetin-O-hexoxide | 0.25 ± 0.09 b | 0.21 ± 0.30 b | 0.52 ± 0.26 a | 0.32 ± 0.16 b |
| Quercetin derivative | 14.54 ± 0.68 a | 14.52 ± 0.18 a | 12.97 ± 0.97 b | 12.12 ± 1.58 b |
| Kaempferol derivative | 2.89 ± 0.14 a | 3.01 ± 0.01 a | 2.93 ± 0.15 a | 2.47 ± 0.15 b |
| Isorhammetin-O-hexoside | 0.72 ± 0.01 b | 0.82 ± 0.00 a | 0.58 ± 0.10 c | 0.34 ± 0.09 d |
| Quercetin derivative | 0.52 ± 0.05 a | 0.56 ± 0.01 a | 0.53 ± 0.03 a | 0.42 ± 0.05 b |
| Isorhammetin-O-hexoside | 0.88 ± 0.05 a | 0.90 ± 0.06 a | 0.84 ±0.05 a | 0.74 ± 0.06 b |
| Kaempferol-O- hexoside | 4.35 ± 0.18 a | 4.65 ± 0.11 a | 3.51 ±0.61 b | 3.85 ± 0.29 b |
| Quercetin derivative | 0.33 ± 0.02 a | 0.36 ± 0.03 a | 0.30 ±0.02 a | 0.25 ± 0.05 b |
| Kaempferol derivative | 0.37 ± 0.03 a | 0.39 ± 0.04 a | 0.33 ±0.04 b | 0.38 ± 0.02 a |
| Samples | TPC (mg GAE/g) | DPPH• (mmol Trolox/g) |
|---|---|---|
| Moringa extract | 76.21 ± 2.37 a | 0.308 ± 0.009 a |
| Oral phase | 78.81 ± 2.75 a | 0.314 ± 0.018 a |
| Gastric phase | 78.87 ± 2.17 a | 0.246 ± 0.008 c |
| Intestinal phase | 73.23 ± 1.98 b | 0.283 ± 0.019 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman-Lara, E.; Rodríguez-Miranda, J.; Ávila-Manrique, S.; Dorado-López, C.; Villalva, M.; Jaime, L.; Santoyo, S.; Martínez-Sánchez, C.E. In Vitro Antioxidant, Anti-Inflammatory Activity and Bioaccessibility of Ethanolic Extracts from Mexican Moringa oleifera Leaf. Foods 2024, 13, 2709. https://doi.org/10.3390/foods13172709
Herman-Lara E, Rodríguez-Miranda J, Ávila-Manrique S, Dorado-López C, Villalva M, Jaime L, Santoyo S, Martínez-Sánchez CE. In Vitro Antioxidant, Anti-Inflammatory Activity and Bioaccessibility of Ethanolic Extracts from Mexican Moringa oleifera Leaf. Foods. 2024; 13(17):2709. https://doi.org/10.3390/foods13172709
Chicago/Turabian StyleHerman-Lara, Erasmo, Jesús Rodríguez-Miranda, Stefany Ávila-Manrique, Celia Dorado-López, Marisol Villalva, Laura Jaime, Susana Santoyo, and Cecilia E. Martínez-Sánchez. 2024. "In Vitro Antioxidant, Anti-Inflammatory Activity and Bioaccessibility of Ethanolic Extracts from Mexican Moringa oleifera Leaf" Foods 13, no. 17: 2709. https://doi.org/10.3390/foods13172709







