Optimization and Detection of Freshness Biomarkers of Atlantic Salmon Subjected to Different Vacuum Packaging Conditions during Storage at 0 °C by Metabolomics and Molecular Docking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Salmon Samples and Storage Conditions
2.3. Extraction Method Optimized by the PB Experimental Design
2.4. Metabolite Analysis via UHPLC-QTRAP/MS
2.5. Method Performance
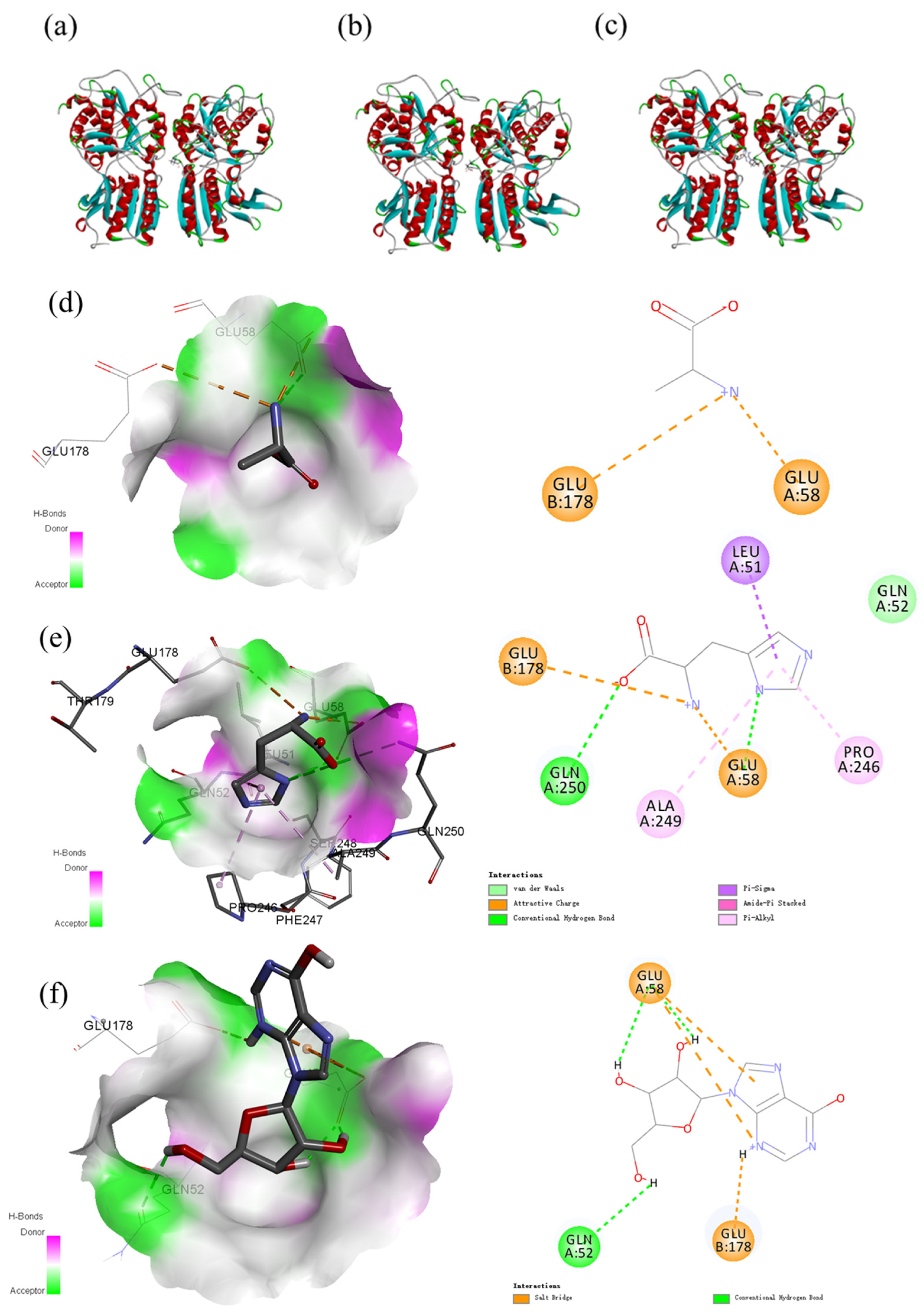
2.6. Homology Modeling of the Umami Receptor T1R1/T1R3 and Molecular Docking with Low Molecular Weight Compounds
2.7. Data Processing, Statistical Analysis, and Metabolic Pathway Analysis
3. Results and Discussion
3.1. Optimization of the Extraction Step
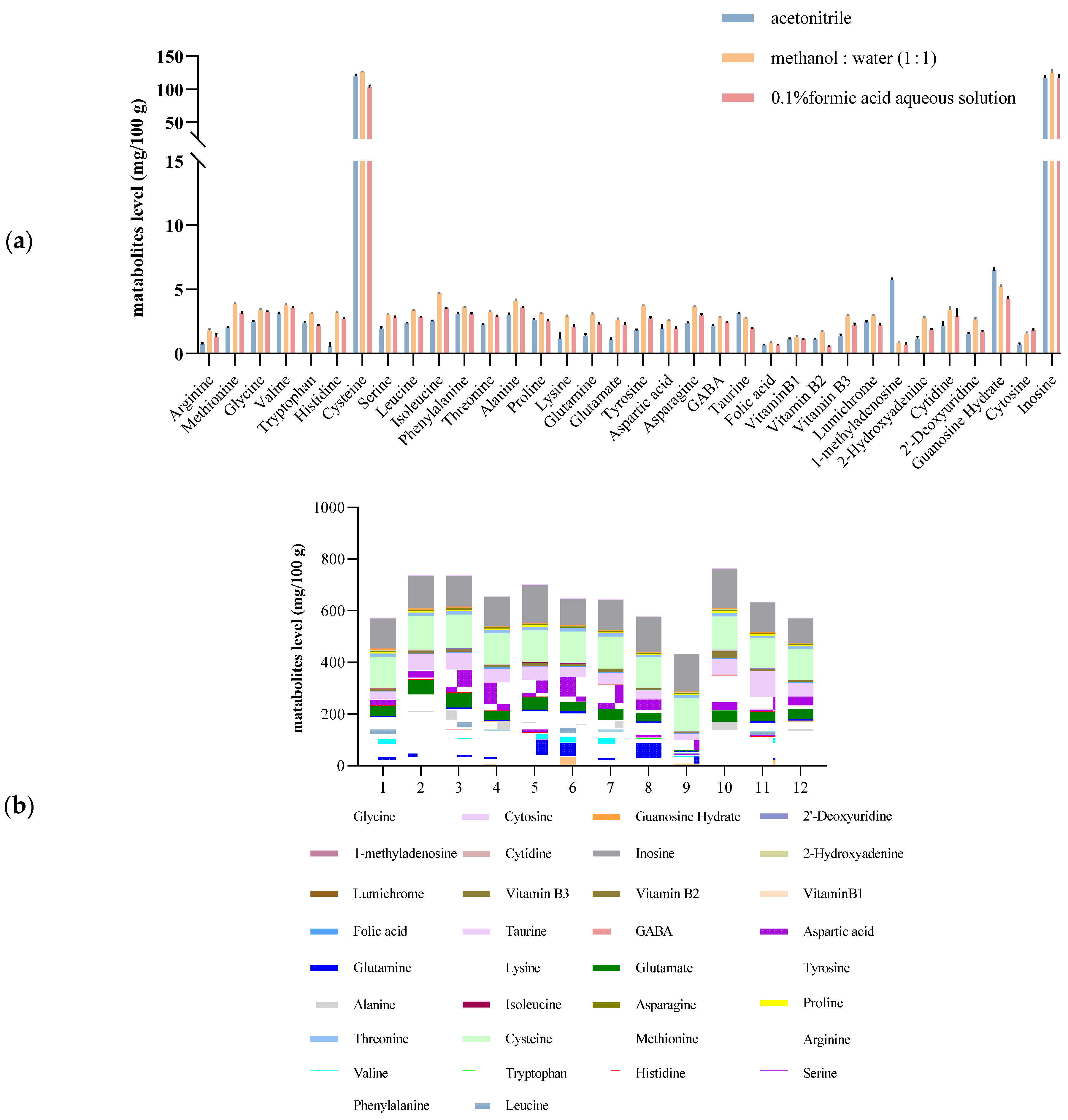
3.2. Method Optimization and Quality Control
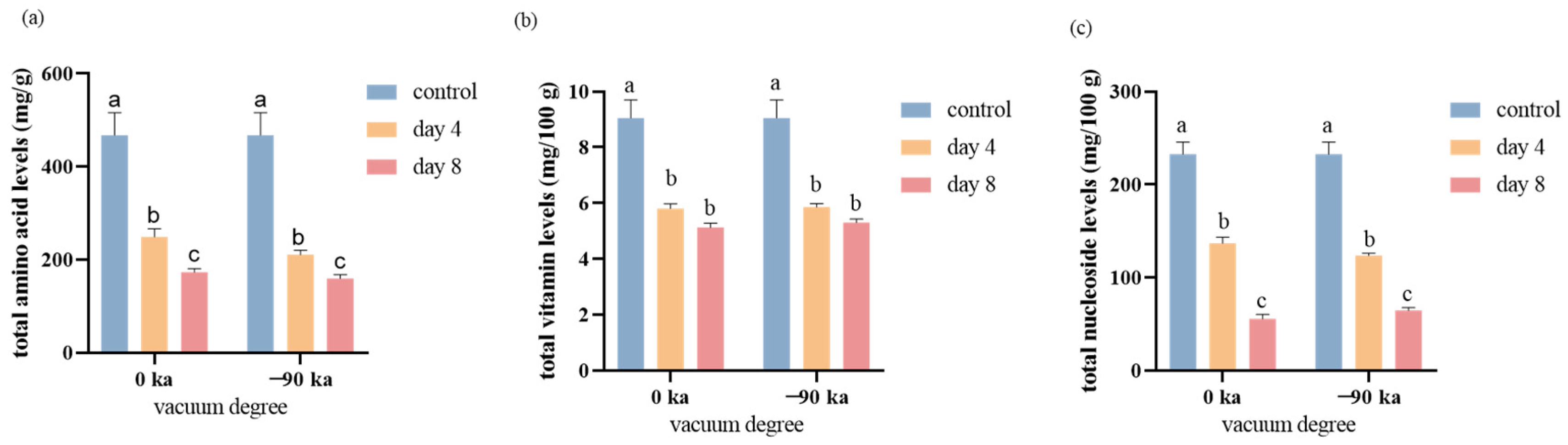
3.3. Variations in Total Nutrient Metabolism during Storage
3.4. Changes of Free Amino Acids during Storage
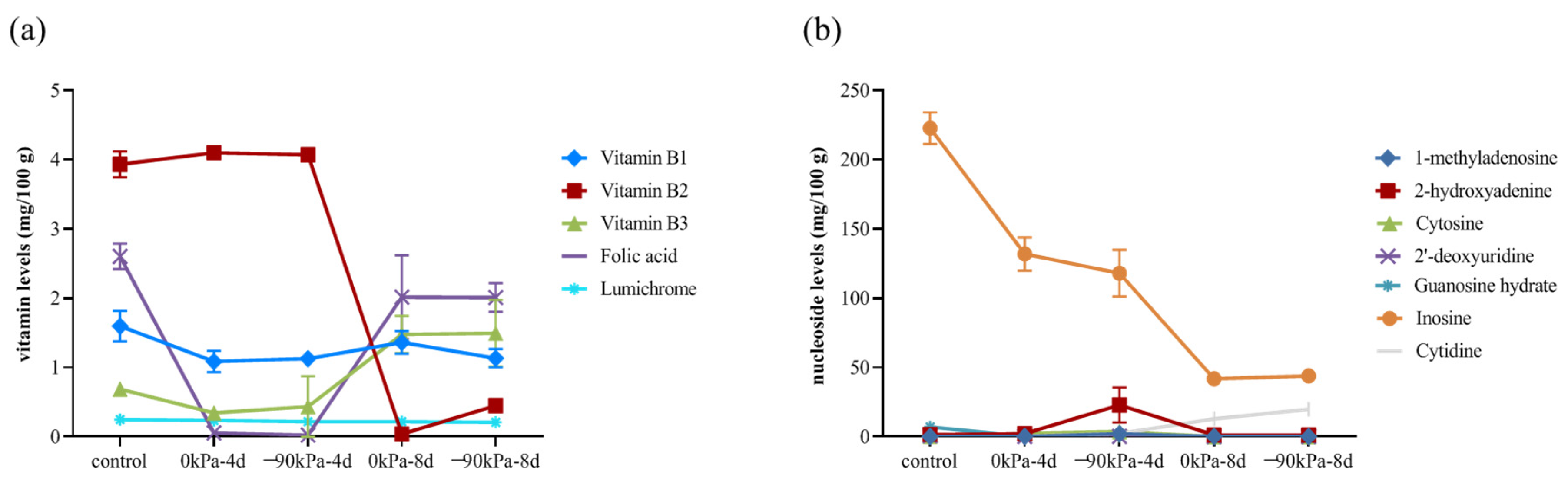
3.5. Changes in Vitamins and Nucleosides during Storage
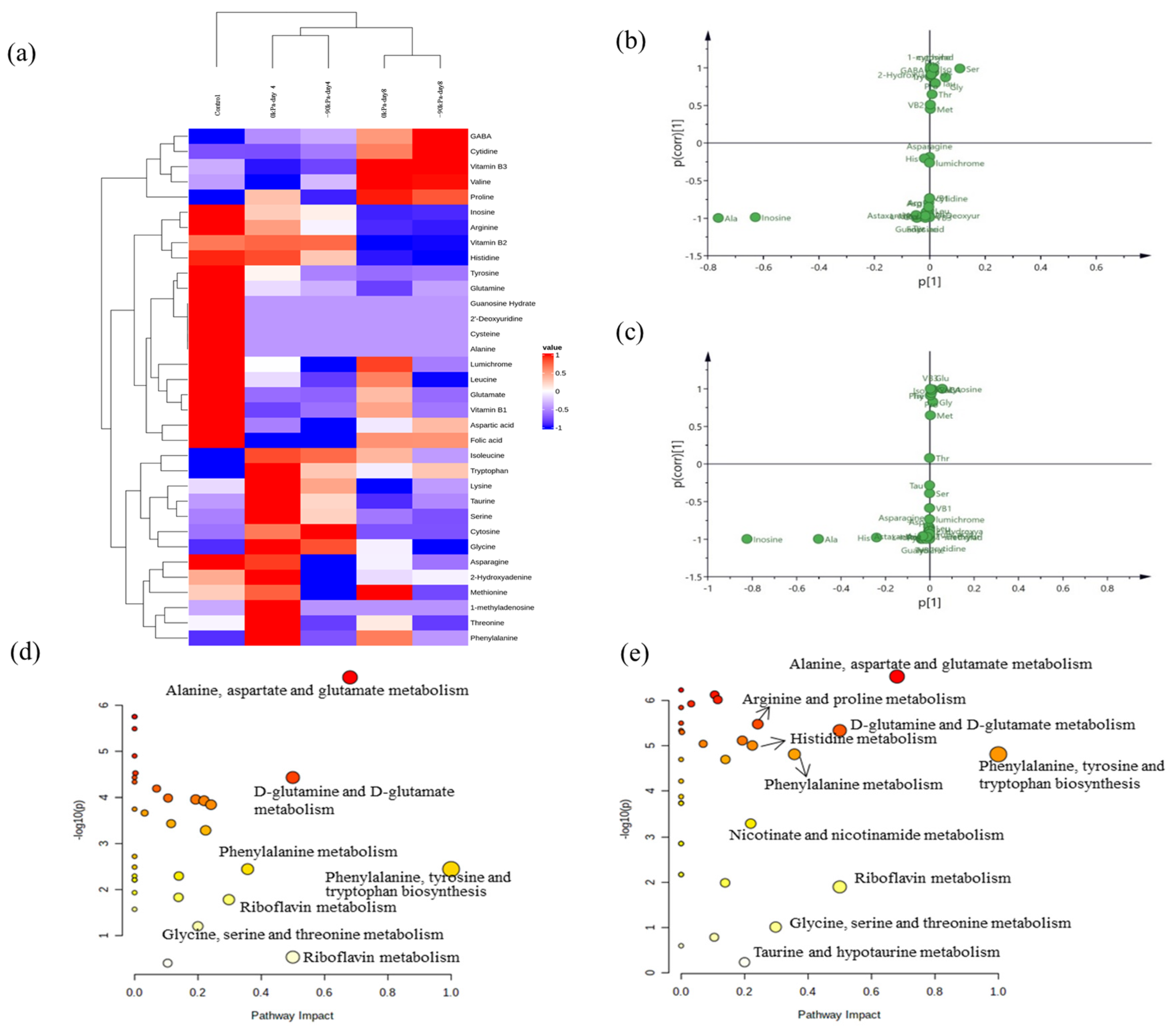
3.6. Unique Biomarkers for Salmon during Storage
3.7. Changes in Metabolic Pathways Associated with Different Packages and Durations of Salmon According to Targeted Profiling as Metabolite Growth Occurs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naghdi, S.; Rezaei, M.; Heidari, M.G.; Tahergorabi, R.; Lorenzo, J.M.; Mirzaei, F. Insights into fishery by-product application in aquatic feed and food: A review. Aquac. Int. 2024, 1–60. [Google Scholar] [CrossRef]
- Sun, J. Nutrition Taste Difference between Fish and Freshwater Fish. Modern Food 2016, 2, 11–12. [Google Scholar]
- Haq, M.; Chun, B.-S. Microencapsulation of omega-3 polyunsaturated fatty acids and astaxanthin-rich salmon oil using particles from gas saturated solutions (PGSS) process. LWT 2018, 92, 523–530. [Google Scholar] [CrossRef]
- Lin, L.; Tao, N.; Su, H.; Zhang, J.; Zhong, J. Migration of nutrients and formation of micro/nano-sized particles in Atlantic salmon (Salmo salar) and bighead carp (Aristichthys nobilis) head soups. Food Biosci. 2020, 36, 100646. [Google Scholar] [CrossRef]
- Sobolev, N.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, D.G.; Nieboer, E.; Varakina, Y.; Veselkina, E.; Kotsur, D.; Thomassen, Y. Essential and non-essential trace elements in fish consumed by indigenous peoples of the European Russian Arctic. Environ. Pollut. 2019, 253, 966–973. [Google Scholar] [CrossRef]
- Esaiassen, M.; Jensen, T.K.; Edvinsen, G.K.; Otnæs, C.H.A.; Ageeva, T.N.; Mæhre, H.K. Nutritional value and storage stability in commercially produced organically and conventionally farmed Atlantic salmon L.) in Norway. Appl. Food Res. 2022, 2, 100033. [Google Scholar] [CrossRef]
- Leshno, M.; Goldbourt, U.; Pinchuk, I.; Lichtenberg, D. The cardiovascular benefits of indiscriminate supplementation of omega-3 fatty acids; meta-analysis and decision-making approach. Int. J. Food Sci. Nutr. 2018, 69, 549–556. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In Vitro Antithrombotic Properties of Salmon (Salmo salar) Phospholipids in a Novel Food-Grade Extract. Mar. Drugs 2019, 17, 62. [Google Scholar] [CrossRef]
- Duan, H.; Song, W.; Zhao, J.; Yan, W.J. Polyunsaturated Fatty Acids (PUFAs): Sources, Digestion, Absorption, Application and Their Potential Adjunctive Effects on Visual Fatigue. Nutrients 2023, 15, 2633. [Google Scholar] [CrossRef]
- Colombo, S.M.; Mazal, X. Investigation of the nutritional composition of different types of salmon available to Canadian consumers. J. Agric. Food Res. 2020, 2, 100056. [Google Scholar] [CrossRef]
- Miks-Krajnik, M.; Yoon, Y.J.; Ukuku, D.O.; Yuk, H.G. Volatile chemical spoilage indexes of raw Atlantic salmon (Salmo salar) stored under aerobic condition in relation to microbiological and sensory shelf lives. Food Microbiol. 2016, 53, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Zeng, X.A.; Liu, D. Recent advances in methods and techniques for freshness quality determination and evaluation of fish and fish fillets: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1012–1225. [Google Scholar] [CrossRef]
- Howgate, P. A History of the Development of Sensory Methods for the Evaluation of Freshness of Fish. J. Aquat. Food Prod. Technol. 2015, 24, 516–532. [Google Scholar] [CrossRef]
- Li, D.P.; Li, Q.; Zhang, Y.M.; Liu, X.C.; Hong, H.; Luo, Y.K. Quality changes and microbiological spoilage analysis of air-packed and vacuum-packed silver carp (Hypophthalmichthys molitrix) fillets during chilled storage. J. Food Process Pres. 2018, 42, e13389. [Google Scholar] [CrossRef]
- Limbo, S.; Sinelli, N.; Torri, L.; Riva, M. Freshness decay and shelf life predictive modelling of European sea bass (Dicentrarchus labrax) applying chemical methods and electronic nose. LWT-Food Sci. Technol. 2009, 42, 977–984. [Google Scholar] [CrossRef]
- Jääskeläinen, E.; Jakobsen, L.M.A.; Hultman, J.; Eggers, N.; Bertram, H.C.; Björkroth, J. Metabolomics and bacterial diversity of packaged yellowfin tuna (Thunnus albacares) and salmon (Salmo salar) show fish species-specific spoilage development during chilled storage. Int. J. Food Microbiol. 2019, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, A.; Aska, I.; Ekonomou, S.; Mallouchos, A.; Parlapani, F.F.; Haroutounian, S.A.; Boziaris, I.S. Volatilome of Chill-Stored European Seabass (Dicentrarchus labrax) Fillets and Atlantic Salmon (Salmo salar) Slices under Modified Atmosphere Packaging. Molecules 2020, 25, 1981. [Google Scholar] [CrossRef]
- Pattarapon, P.; Zhang, M.; Bhandari, B.; Gao, Z.X. Effect of vacuum storage on the freshness of grass carp (Ctenopharyngodon idella) fillet based on normal and electronic sensory measurement. J. Food Process Pres. 2018, 42, e13418. [Google Scholar] [CrossRef]
- Pan, Z.; Lin, H. Comparative research on the effect of controlled freezing-point storage preservation and cold storage preservation on the quality of raw Salmo salar. J. Food Saf. Qual. 2019, 10, 2232–2239. [Google Scholar]
- Fidalgo, L.G.; Simoes, M.M.Q.; Casal, S.; Lopes-da-Silva, J.A.; Carta, A.M.S.; Delgadillo, I.; Saraiva, J.A. Physicochemical parameters, lipids stability, and volatiles profile of vacuum-packaged fresh Atlantic salmon (Salmo salar) loins preserved by hyperbaric storage at 10 degrees C. Food Res. Int. 2020, 127, 10. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, S.; Chen, L.; Ding, H.; Wu, P.; Zhang, G.; Xie, K.; Dai, G.; Wang, J. UHPLC–MS/MS-Based Nontargeted Metabolomics Analysis Reveals Biomarkers Related to the Freshness of Chilled Chicken. Foods 2020, 9, 1326. [Google Scholar] [CrossRef]
- Feng, Z.; Ding, C.; Li, W.; Wang, D.; Cui, D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020, 310, 125914. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Barba, F.J.; Lorenzo, J.M.; Munekata, P.E.S.; Bernardo, L.; Tomasevic, I.; Pateiro, M.; Lucini, L. Untargeted metabolomics to explore the oxidation processes during shelf life of pork patties treated with guarana seed extracts. Int. J. Food Sci. Technol. 2019, 55, 1002–1009. [Google Scholar] [CrossRef]
- Johnson, A.E.; Sidwick, K.L.; Pirgozliev, V.R.; Edge, A.; Thompson, D.F. Metabonomic Profiling of Chicken Eggs during Storage Using High-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. Anal. Chem. 2018, 90, 7489–7494. [Google Scholar] [CrossRef] [PubMed]
- Aru, V.; Pisano, M.B.; Savorani, F.; Engelsen, S.B.; Cosentino, S.; Cesare Marincola, F. Metabolomics analysis of shucked mussels’ freshness. Food Chem. 2016, 205, 58–65. [Google Scholar] [CrossRef]
- Syukri, D.; Thammawong, M.; Naznin, H.A.; Kuroki, S.; Tsuta, M.; Yoshida, M.; Nakano, K. Identification of a freshness marker metabolite in stored soybean sprouts by comprehensive mass-spectrometric analysis of carbonyl compounds. Food Chem. 2018, 269, 588–594. [Google Scholar] [CrossRef]
- Fan, H.B.; Liu, X.C.; Hong, H.; Shen, S.; Xu, Q.; Feng, L.G.; Luo, Y.K. Quality Changes and Biogenic Amines Accumulation of Black Carp (Mylopharyngodon piceus) Fillets Stored at Different Temperatures. J. Food Prot. 2016, 79, 635–645. [Google Scholar] [CrossRef]
- Shumilina, E.; Ciampa, A.; Capozzi, F.; Rustad, T.; Dikiy, A. NMR approach for monitoring post-mortem changes in Atlantic salmon fillets stored at 0 and 4 degrees C. Food Chem. 2015, 184, 12–22. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. The European Green Deal. Published Brussels.11.12.2019, COM(2019) 640 Final; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- NY/T 788–2018; Guideline for the Testing of Pesticide Residues in Crops. China Agriculture Press: Beijing, China, 2018.
- Cheng, K.; Wang, S.; Wang, Y.; Bao, Y.; Gao, P.; Lei, L.; Liang, H.; Zhang, S.; Dong, L. Modification of a Novel Umami Octapeptide with Trypsin Hydrolysis Sites via Homology Modeling and Molecular Docking. J. Agric. Food Chem. 2023, 71, 5326–5336. [Google Scholar] [CrossRef]
- Li, X.R.; Chen, F.L.; Li, S.Y.; Jia, J.; Gu, H.Y.; Yang, L. An efficient homogenate-microwave-assisted extraction of flavonols and anthocyanins from blackcurrant marc: Optimization using combination of Plackett-Burman design and Box-Behnken design. Ind. Crop. Prod. 2016, 94, 834–847. [Google Scholar] [CrossRef]
- Li, T.T.; Ren, L.K.; Wang, D.F.; Song, M.J.; Li, Q.Y.; Li, J.R. Optimization of extraction conditions and determination of purine content in marine fish during boiling. Peerj 2019, 7, e6690. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.Y.; Li, X.; Wang, H.; Mehmood, W.; Zhang, C.H.; Blecker, C. Effects of Frozen Storage Temperature and Duration on Changes in Physicochemical Properties of Beef Myofibrillar Protein. J. Food Quality 2021, 2021, 8836749. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Xie, Q.; Wang, Y.; Yu, J.; Ma, X. Physicochemical and structural changes of myofibrillar proteins in muscle foods during thawing: Occurrence, consequences, evidence, and implications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3444–3477. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Konno, Y.; Konno, K. Below-zero storage of fish to suppress loss of freshness. Nippon. Suisan Gakk 2020, 86, 253. [Google Scholar] [CrossRef]
- Bao, Y.L.; Wang, K.Y.; Yang, H.X.; Regenstein, J.M.; Ertbjerg, P.; Zhou, P. Protein degradation of black carp (Mylopharyngodon piceus) muscle during cold storage. Food Chem. 2020, 308, 125576. [Google Scholar] [CrossRef]
- Rotzoll, N.; Dunkel, A.; Hofmann, T. Quantitative studies, taste reconstitution, and omission experiments on the key taste compounds in Morel mushrooms (Morchella deliciosa fr.). J. Agric. Food Chem. 2006, 54, 2705–2711. [Google Scholar] [CrossRef]
- Tissier, M.L.; Kraus, S.; Gomez-Moracho, T.; Lihoreau, M. Supplementation in vitamin B3 counteracts the negative effects of tryptophan deficiencies in bumble bees. Conserv. Physiol. 2023, 11, coac084. [Google Scholar] [CrossRef]
- Green, J.M.; Matthews, R.G. Folate Biosynthesis, Reduction, and Polyglutamylation and the Interconversion of Folate Derivatives. EcoSal Plus 2007, 2, 1–18. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Cheng, J.X.; Xia, Y.Q.; Li, X.H.; Liu, Y.; Liu, P.F. Response mechanism of gut microbiome and metabolism of European seabass (Dicentrarchus labrax) to temperature stress. Sci. Total Environ. 2022, 813, 151786. [Google Scholar] [CrossRef]
- Wen, D.L.; Liu, Y.; Yu, Q. Metabolomic approach to measuring quality of chilled chicken meat during storage. Poultry Sci. 2020, 99, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, H.; Chen, L.; Zhang, S.S.; Wu, P.F.; Xie, K.Z.; Pan, Z.M.; Zhang, G.X.; Dai, G.J.; Wu, H.Q.; et al. Characterization of chilled chicken spoilage using an integrated microbiome and metabolomics analysis. Food Res. Int. 2021, 144, 110328. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Z.; Wang, H.P.; Huang, X.; Wang, X.; Qin, L. Discrimination and evaluation of commercial salmons by low-molecule-weight compounds: Oligopeptides and phosphatides. Food Chem. 2024, 455, 139777. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Y.Y.; Huang, X.H.; Xin, R.; Dong, X.P.; Konno, K.; Zhu, B.W.; Fisk, I.; Qin, L. Dynamic sensations of fresh and roasted salmon (Salmo salar) during chewing. Food Chem. 2022, 368, 130844. [Google Scholar] [CrossRef]
- Meyer, S.; Dunkel, A.; Hofmann, T. Sensomics-Assisted Elucidation of the Tastant Code of Cooked Crustaceans and Taste Reconstruction Experiments. J. Agric. Food Chem. 2016, 64, 1164–1175. [Google Scholar] [CrossRef]
- Mallouchos, A.; Mikrou, T.; Gardeli, C. Gas Chromatography-Mass Spectrometry-Based Metabolite Profiling for the Assessment of Freshness in Gilthead Sea Bream (Sparus aurata). Foods 2020, 9, 464. [Google Scholar] [CrossRef]
- Shumilina, E.; Moller, I.A.; Dikiy, A. Differentiation of fresh and thawed Atlantic salmon using NMR metabolomics. Food Chem. 2020, 314, 126227. [Google Scholar] [CrossRef]
| Tastant | Compound | Content (mg/100 g) | ||||
|---|---|---|---|---|---|---|
| Control | 0 kPa-4d | 0 kPa-8d | −90 kPa-4d | −90 kPa-8d | ||
| Umami-tasting | Glutamine | 7.90 ± 0.14 | 4.86 ± 0.41 | 4.56 ± 0.11 | 3.78 ± 0.43 | 4.45 ± 0.5 |
| Aspartic acid | 3.08 ± 0.48 | 1.35 ± 0.18 | 0.82 ± 0.13 | 1.74 ± 0.11 | 2.13 ± 0.17 | |
| Umami-tasting compounds total content | 10.99 ± 0.62 | 6.21 ± 0.59 | 5.37 ± 0.24 | 5.52 ± 0.54 | 6.58 ± 0.67 | |
| Sweet-tasting | Glycine | 19.45 ± 1.25 | 27.68 ± 3.57 | 26.35 ± 1.34 | 22.75 ± 1.29 | 18.85 ± 1.49 |
| Serine | 5.52 ± 0.31 | 21.35 ± 2.14 | 11.22 ± 2 | 5.25 ± 0.36 | 4.04 ± 0.11 | |
| Threonine | 13.03 ± 0.81 | 14.24 ± 0.64 | 12.45 ± 0.48 | 13.14 ± 1.04 | 12.44 ± 0.16 | |
| Proline | 4.37 ± 0.22 | 5.28 ± 0.28 | 4.51 ± 0.14 | 5.67 ± 0.33 | 5.58 ± 0.48 | |
| Lysine | 3.38 ± 0.25 | 5.25 ± 0.57 | 4.14 ± 0.22 | 2.06 ± 0.13 | 3.06 ± 0.66 | |
| Alanine | 110.44 ± 7.02 | NG | NG | NG | NG | |
| Sweet-tasting compounds total content | 156.19 ± 9.86 | 73.79 ± 7.2 | 58.67 ± 4.18 | 48.88 ± 3.13 | 43.97 ± 2.91 | |
| Bitter-tasting | Arginine | 2.31 ± 0.34 | 1.66 ± 0.11 | 1.26 ± 0.14 | 0.64 ± 0.05 | 0.61 ± 0.11 |
| Valine | 5.75 ± 0.14 | 3.7 ± 0.66 | 6.04 ± 0.55 | 9.15 ± 0.43 | 9.06 ± 0.36 | |
| Tryptophan | 1.31 ± 0.04 | 1.75 ± 0.2 | 1.62 ± 0.04 | 1.56 ± 0.09 | 1.62 ± 0.11 | |
| Leucine | 4.26 ± 0.13 | 3.58 ± 0.22 | 3.27 ± 0.23 | 3.94 ± 0.05 | 3.20 ± 0.14 | |
| Phenylalanine | 6.31 ± 0.18 | 7.54 ± 0.39 | 6.39 ± 0.27 | 7.13 ± 0.18 | 6.55 ± 0.32 | |
| Tyrosine | 9.05 ± 0.37 | 3.23 ± 0.98 | 1.13 ± 0.15 | 0.81 ± 0.13 | 0.96 ± 0.02 | |
| Isoleucine | 2.53 ± 0.13 | 4.70 ± 0.04 | 4.27 ± 0.18 | 4.60 ± 0.31 | 3.53 ± 0.66 | |
| Histidine | 93.28 ± 8.13 | 90.52 ± 5.23 | 40.14 ± 2.03 | 75.06 ± 1.63 | 35.07 ± 1.92 | |
| Bitter-tasting compounds total content | 124.8 ± 9.46 | 116.67 ± 7.83 | 64.11 ± 3.59 | 102.88 ± 2.87 | 60.61 ± 3.66 | |
| Salty-tasting | Methionine | 2.64 ± 0.14 | 2.86 ± 0.34 | 2.00 ± 0.07 | 2.96 ± 0.28 | 2.21 ± 0.11 |
| Salty-tasting compounds total content | 2.64 ± 0.14 | 2.86 ± 0.34 | 2.00 ± 0.07 | 2.96 ± 0.28 | 2.21 ± 0.11 | |
| Sour-tasting and mouth-drying | GABA | 0.27 ± 0.001 | 0.69 ± 0.1 | 0.77 ± 0.03 | 1.37 ± 0.13 | 1.99 ± 0.19 |
| Pyroglutamic acid | 12.19 ± 0.37 | 9.68 ± 0.32 | 7.44 ± 1.11 | 8.61 ± 0.39 | 8.82 ± 0.5 | |
| Sour-tasting and mouth-drying compounds total content | 12.46 ± 0.37 | 10.37 ± 0.41 | 8.21 ± 1.13 | 9.98 ± 0.51 | 10.81 ± 0.69 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-S.; Yao, G.-X.; Yu, J.; Qiu, J.; Qian, Y.-Z.; Huang, X.-Y.; Xu, Y.-Y. Optimization and Detection of Freshness Biomarkers of Atlantic Salmon Subjected to Different Vacuum Packaging Conditions during Storage at 0 °C by Metabolomics and Molecular Docking. Foods 2024, 13, 2714. https://doi.org/10.3390/foods13172714
Lu Y-S, Yao G-X, Yu J, Qiu J, Qian Y-Z, Huang X-Y, Xu Y-Y. Optimization and Detection of Freshness Biomarkers of Atlantic Salmon Subjected to Different Vacuum Packaging Conditions during Storage at 0 °C by Metabolomics and Molecular Docking. Foods. 2024; 13(17):2714. https://doi.org/10.3390/foods13172714
Chicago/Turabian StyleLu, Yu-Shun, Gui-Xiao Yao, Jiang Yu, Jing Qiu, Yong-Zhong Qian, Xuan-Yun Huang, and Yan-Yang Xu. 2024. "Optimization and Detection of Freshness Biomarkers of Atlantic Salmon Subjected to Different Vacuum Packaging Conditions during Storage at 0 °C by Metabolomics and Molecular Docking" Foods 13, no. 17: 2714. https://doi.org/10.3390/foods13172714





