Black and Red Currant Pomaces as Raw Materials to Create Smoothies with In Vitro Health-Promoting Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Physical Parameters
2.3.1. CIEL*a*b* Color Measurement
2.3.2. Dynamic Viscosity
2.3.3. Turbidity Stability
2.4. Chemical Ingredients
2.4.1. Basic Chemical Composition
2.4.2. Sugar Content Using HPLC-ELSD Method
2.4.3. Organic Acid Content
2.4.4. Content of Polyphenolic Compounds, Including Polymers Procyanidins, Using UPLC
2.5. Analysis of Health-Promoting Potential Using In Vitro Methods
2.5.1. Antioxidant Activity
2.5.2. Ability to Inhibit α-Amylase and α-Glucosidase
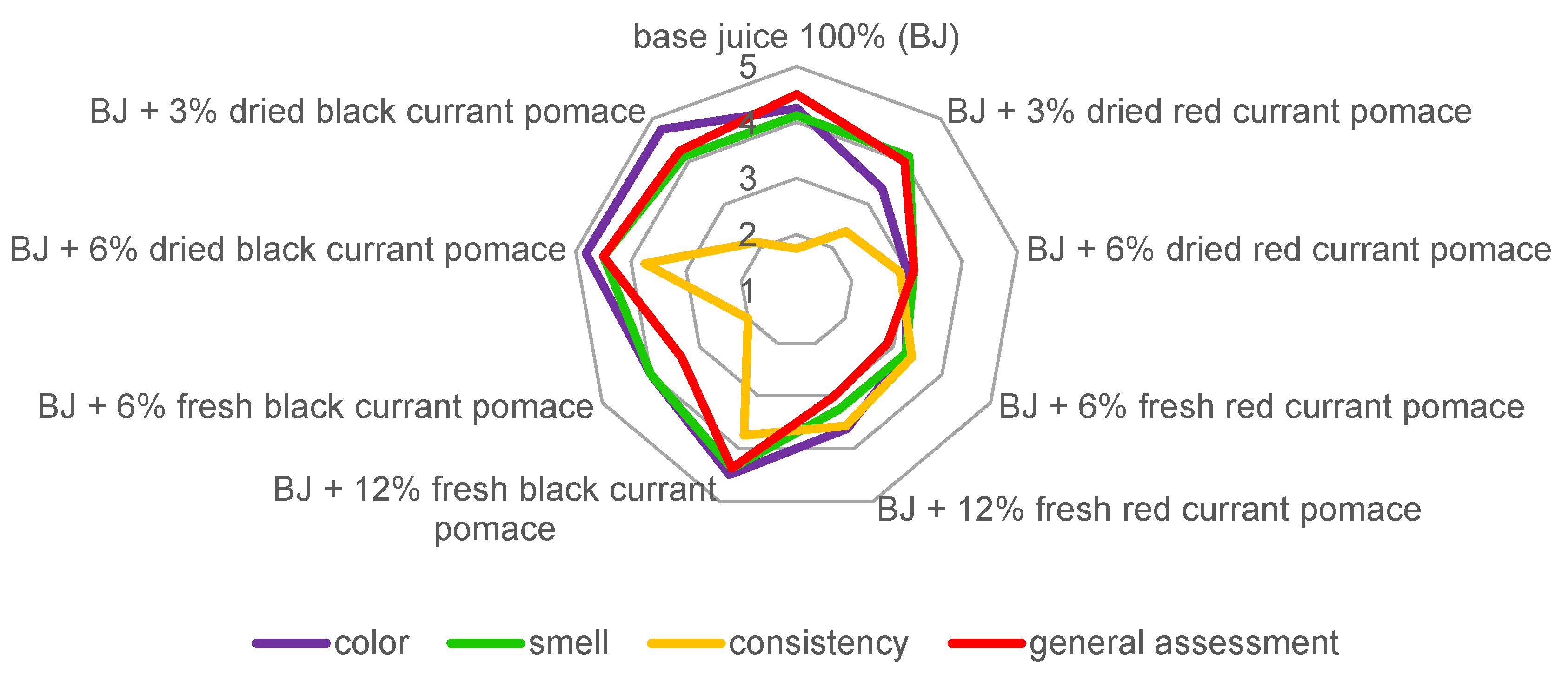
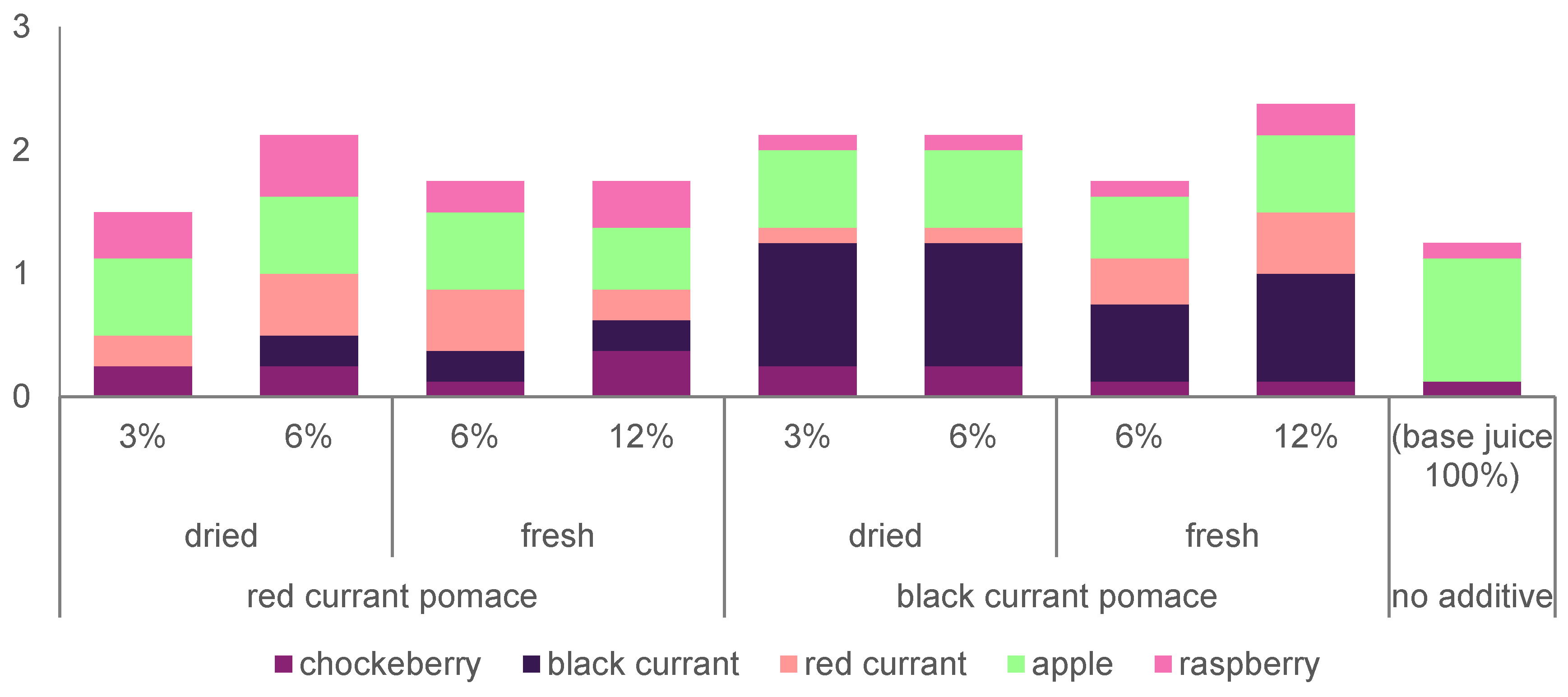
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Basic Physical Properties of the Obtained Smoothies
3.2. Basic Chemical Properties
3.3. Sugar and Organic Acid Contents Using HPLC
3.4. Bioactive Compound Contents in the Obtained Smoothies
3.5. Health-Promoting Potential
3.6. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oszmiański, J.; Sożyński, J. Przewodnik do Ćwiczeń z Technologii Przetwórstwa Owoców i Warzyw; Wydawnictwo Akademii Rolniczej we Wrocławiu: Wrocław, Poland, 2001. [Google Scholar]
- Oszmiański, J. Technologia i Analiza Produktów z Owoców i Warzyw: Wybrane Zagadnienia; Wydawnictwo Akademii Rolniczej: Wrocław, Poland, 2002. [Google Scholar]
- Jara-Palacios, M.J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F.J.; Escudero-Gilete, M.L. Comparative study of red berry pomaces (blueberry, red raspberry, red currant and blackberry) as source of antioxidants and pigments. Eur. Food Res. Technol. 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Zegartowska, P.; Korus, A.; Skoczeń-Słupska, R. Wartość odżywcza i prozdrowotna wytłoków z owoców i warzyw. In Składniki Bioaktywne Surowców i Produktów Roślinnych, Kraków, Polska, 24–25 September 2018; Słupski, J., Tarko, T., Drożdż, I., Eds.; Oddział Małopolski Polskiego Towarzystwa Technologów Żywności: Kraków, Poland, 2018; pp. 79–89. [Google Scholar]
- Piotrowska, A.; Góralczyk, M.; Żebrowska-Krasuska, M. Owoce jagodowe i ich przetwory jako źródła przeciwutleniaczy®. Postępy Przetwórstwa Spożywczego 2013, 2, 98–103. [Google Scholar]
- Kapci, B.; Neradová, E.; Čížková, H.; Voldřich, M.; Rajchl, A.; Capanoglu, E. Investigating the antioxidant potential of chokeberry (Aronia melanocarpa) products. J. Food Nutr. Res. 2013, 4, 219–229. [Google Scholar]
- Wiza, P.L.; Czubak, W.; Kowalczewski, P. Wytłoki winogronowe jako dodatek do produkcji cydru domowego®. Postępy Tech. Przetwórstwa Spożywczego 2018, 2, 9–14. [Google Scholar]
- Białek, M.; Rutkowska, J. Potencjalne wykorzystanie prozdrowotnych surowców roślinnych do zwiększania wartości odżywczej wyrobów ciastkarskich. Episteme 2013, 2, 187–206. [Google Scholar]
- Karaağaç, H.E.; Sahan, Y. Comparison of phenolics, antioxidant capacity and total phenol bioaccessibility of Ribes spp. grown in Turkey. Food Sci. Technol. 2020, 40 (Suppl. S2), 512–520. [Google Scholar] [CrossRef]
- Törrönen, R.; Sarkkinen, E.; Niskanen, T.; Tapola, N.; Kilpi, K.; Niskanen, L. Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects. Br. J. Nutr. 2012, 107, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, N.; Kupe, M.; Gundogdu, M.; Ilhan, G.; Ercisli, S. Phytochemical and Antioxidant Diversity in Fruits of Currant (Ribes spp.). Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 381–387. [Google Scholar] [CrossRef]
- Borowiec, K.; Stachniuk, A.; Szwajgier, D.; Trzpil, A. Polyphenols composition and the biological effects of six selected small dark fruits. Food Chem. 2022, 391, 133281. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K. Profiling of polyphenols by LC-QTOF/ESI-MS, characteristics of nutritional compounds and in vitro effect on pancreatic lipase, α-glucosidase, α-amylase, cholinesterase and cyclooxygenase activities of sweet (Prunus avium) and sour (P. cerasus) cherries leaves and fruits. Ind. Crops Prod. 2021, 174, 114214. [Google Scholar] [CrossRef]
- Oczkowski, M. Health-promoting effects of bioactive compounds in blackcurrant (Ribes nigrum L.) berries. Rocz. Panstw. Zakl. Hig. 2021, 72, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, K.; Mańka, A.; Krzywonos, M. Możliwości wykorzystania owoców krajowych do produkcji win owocowych. Nauk. Inżynierskie I Technol. 2015, 1, 27–35. [Google Scholar]
- Jurgiel-Małecka, G.; Buchwał, A. Charakterystyka składu chemicznego owoców porzeczki uprawianej w regionie pomorza zachodniego. Żywn. Nauka Technol. Jakość. 2016, 6, 90–101. [Google Scholar]
- Krzepiłko, A.; Prażak, R.; Skwaryło-Bednarz, B.; Święciło, A. Pąki, liście i nasiona porzeczki czarnej—Źródło substancji bioaktywnych o prozdrowotnych właściwościach. Żywn. Nauka Technol. Jakość. 2018, 2, 24–33. [Google Scholar] [CrossRef]
- Piątkowska, E.; Kopeć, A.; Leszczyńska, T. Antocyjany charakterystyka, występowanie i oddziaływanie na organizm człowieka. Żywn. Nauka Technol. Jakość. 2011, 4, 24–35. [Google Scholar]
- Sterczyńska, M. Wpływ różnych szczepów szlachetnych drożdży winiarskich na wybrane składniki chemiczne win owocowych z koncentratu z czarnej porzeczki. Inżynieria Przetwórstwa Spożywczego 2015, 1, 28–33. [Google Scholar]
- Jessa, J.; Hozyasz, K.K. Czarna porzeczka i olej z jej nasion—Fitoterapeutyczne panaceum? Probl. Hig. Epidemiol. 2016, 97, 14–23. [Google Scholar]
- Wawrzyniak, A.; Krotki, M.; Stoparczyk, B. Właściwości antyoksydacyjne owoców i warzyw. Med. Rodz. 2011, 1, 19–23. [Google Scholar]
- Frum, A.; Georgescu, C.; Gigor, F.; Dobrea, C.; Tita, O. Identification and quantification of phenolic compounds from red currant (Ribes rubrum L.) and raspberries (Rubus idaeus L.). Int. J. Pharmacol. Phytochem. Ethnomed. 2017, 6, 30–37. [Google Scholar]
- PN-EN 12145:2001; Soki Owocowe i Warzywne. Oznaczanie Całkowitej Suchej Substancji. Metoda Grawimetryczna Oznaczania Ubytku Masy w Wyniku Suszenia. Polish Committee for Standardization: Warsaw, Poland, 2001.
- PN-EN 12143:2000; Soki Owocowe i Warzywne. Oznaczanie Zawartości Substancji Rozpuszczalnych Metodą Refraktometryczną. Polish Committee for Standardization: Warsaw, Poland, 2000.
- PN-EN 12147:2000; Soki Owocowe i Warzywne. Oznaczanie Kwasowości Miareczkowej. Polish Committee for Standardization: Warsaw, Poland, 2000.
- PN-EN 1135:1999; Soki Owocowe i Warzywne. Oznaczanie Zawartości Popiołu. Polish Committee for Standardization: Warsaw, Poland, 1999.
- Pijanowski, E.; Mrożewski, S.; Horubała, A.; Jarczyk, A. Technologia Produktów Owocowych i Warzywnych; PWRiL: Warsaw, Poland, 1973; Volume 1. [Google Scholar]
- Wojdyło, A.; Nowicka, P.; Carbonell-Barrachina, Á.A.; Hernández, F. Phenolic compounds, antioxidant and antidiabetic activity of different cultivars of Ficus carica L. fruits. J. Funct. Foods 2016, 25, 421–432. [Google Scholar] [CrossRef]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, Á.A.; Oszmiański, J.; Golis, T. Variability of phytochemical properties and content of bioactive compounds in Lonicera caerulea L. var. kamtschatica berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A.; Tkacz, K.; Turkiewicz, I.P. Quantitative and qualitative determination of carotenoids and polyphenolics compounds in selected cultivars of Prunus persica L. and their ability to in vitro inhibit lipoxygenase, cholinoesterase, α-amylase, α-glucosidase and pancreatic lipase. Food Chem. X 2023, 17, 100619. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of „antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagang, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- EN ISO 8589:2009; Sensory Analysis—General Guidelines for the Design of Sensory Analysis Laboratories. ISO: Geneva, Switzerland, 2009.
- Teleszko, M.; Kolniak, J.; Oszmiański, J. Wpływ odmiany jabłek na zmętnienie i barwę naturalnie mętnych soków. In Jakość i Prozdrowotne Cechy Żywności; Wojtatowicz, M., Kawa-Rygielska, J., Eds.; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2010; pp. 27–38. [Google Scholar]
- Kucharska, A.; Kowalczyk, K.; Nawirska-Olszańska, A.; Sokół-Łętowska, A. Wpływ dodatku aronii, truskawek i malin na skład fizykochemiczny przecieru dereniowego. Żywn. Nauka Technol. Jakość. 2010, 4, 95–106. [Google Scholar]
- Ochmian, I.; Dobrowolska, A.; Strzelecki, R.; Kozos, K. Porównanie jakości owoców trzech odmian porzeczki czarnej (Ribes nigrum L.) w zależności od ich wielkości. Folia Pomeranae Univ. Technol. Stetinensis. Agric. Aliment. Piscaria Zootech. 2013, 26, 97–106. [Google Scholar]
- Robert, P.; Fredes, C. The encapsulation of anthocyanins from berry-type fruits. Trends in foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef]
- Mot, P.R.; Gurbina, D.; Stoin, D.; Cozma, A. Biophysical and nutritional characterization of some natural fruit and vegetable juices. J. Agroaliment. Proc. Technol. 2019, 25, 222–226. [Google Scholar]
- Sitkiewicz, I.; Kalinowska, R.; Kamiński, B. Właściwości reologiczne klarowanych soków: Aroniowego i jabłkowego. Postępy Tech. Przetwórstwa Spożywczego 2008, 2, 17–21. [Google Scholar]
- Szymański, L.; Witkowska, D. Wpływ preparatu enzymatycznego z Trichoderma reesei m7-1 na jakość i wydajność soków przecierowych z jabłek. Żywn. Nauka Technol. Jakość. 2003, 1, 39–47. [Google Scholar]
- Tajner-Czopek, A.; Kita, A. Analiza Żywności Jakość Produktów Spożywczych; Wydawnictwo Akademii Rolniczej we Wrocławiu: Wrocław, Poland, 2005. [Google Scholar]
- Banaś, A.; Korus, A. Wartości odżywcze i wykorzystanie w żywieniu owoców truskawki i wiśni. Med. Rodz. 2016, 19, 158–162. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Zawartość wybranych wyróżników chemicznych w owocach trzech odmian jabłoni z uprawy ekologicznej i konwencjonalnej. J. Res. Appl. Agric. Eng. 2010, 55, 173–177. [Google Scholar]
- Wichrowska, D.; Żary-Sikorska, E. Właściwości prozdrowotne jabłkowych wytłoków poprasowych. Inż. Apar. Chem. 2015, 5, 286–287. [Google Scholar]
- Szajdek, A.; Borowska, E.J.; Borowski, J.; Saczuk, B. Musy owocowe jako źródło naturalnych przeciwutleniaczy. Żywn. Nauka Technol. Jakość. 2007, 6, 100–108. [Google Scholar]
- Michalak-Majewska, M.; Żukiewicz-Sobczak, W.; Kalbarczyk, J. Ocena składu i właściwości soków owocowych preferowanych przez konsumentów. Bromat. Chem. Toksykol. 2009, 3, 836–841. [Google Scholar]
- Biernat, J. Wybrane Zagadnienia z Nauki o Żywieniu Człowieka; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2009. [Google Scholar]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Skotnicka, M.; Duraj, N. Rola składników odżywczych w regulacji sytości organizmu. Ann. Acad. Med. Gedan. 2015, 45, 79–87. [Google Scholar]
- Paunović, M.; Maskovic, P.; Milinkovic, M.; Karaklajić-Stajić, Ż.; Tomic, J.; Rilak, B. Seasonal Changes in Black Currant Fruit Quality. J. Agric. Sci. Technol. 2023, 25, 1155–1166. [Google Scholar]
- Lebiedzińska, A.; Czaja, J.; Brodkowska, K.; Woźniak, A.; Szefer, P. Ocena zawartości cukrów prostych i sacharozy w sokach owocowych z wykorzystaniem HPLC. Bromat. Chem. Toksykol. 2011, 3, 326–330. [Google Scholar]
- Giryn, H.; Szteke, B.; Szymczyk, K. Wpływ procesu technologicznego i przechowywania na zawartość kwasów organicznych w zagęszczonych sokach jabłkowych. Żywn. Nauka Technol. Jakość. 2004, 2, 92–107. [Google Scholar]
- Piekut, J.; Kowczyk-Sadowy, M.; Łozowicka, B.; Dec, D.; Wołejko, E.; Obidziński, S. Ocena zawartości związków fenolowych w materiale z upraw prowadzonych metodami integrowanymi. In Trendy w Żywieniu Człowieka; Karwowska, M., Gustaw, W., Eds.; Polskie Towarzystwo Technologów Żywności, Wydawnictwo Naukowe PTTŻ: Kraków, Poland, 2015; pp. 245–256. [Google Scholar]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Gryszczyńska, B.; Iskra, M.; Gryszczyńska, A.; Budzyń, M. Aktywność przeciwutleniająca wybranych owoców jagodowych. Post. Fitoter. 2011, 4, 265–274. [Google Scholar]
- Szajdek, A.; Borowska, J. Właściwości przeciwutleniające żywności pochodzenia roślinnego. Żywn. Nauka Technol. Jakość. 2004, 11, 5–28. [Google Scholar]
- Szot, I.; Lipa, T.; Sosnowska, B. Jagoda kamczacka—Właściwości prozdrowotne owoców i możliwość ich zastosowania. Żywn. Nauka Technol. Jakość. 2014, 4, 18–29. [Google Scholar]
- Skąpska, S.; Sieliwanowicz, B.; Jasińska, U.; Owczarek, L.; Lipowski, J.; Trzcińska, M.; Hałasińska, A. Zmiany zawartości naturalnych przeciwutleniaczy oraz pojemności przeciw utleniającej zachodzące w surowcu w trakcie procesu otrzymywania soku zagęszczonego z jabłek. Żywn. Nauka Technol. Jakość. 2006, 1 (Suppl. S46), 152–160. [Google Scholar]


| Parameter | Storage Time | Apple Juice with | Apple Juice | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dried Red Currant Pomace | Fresh Red Currant Pomace | Dried Black Currant Pomace | Fresh Black Currant Pomace | |||||||
| 3% | 6% | 6% | 12% | 3% | 6% | 6% | 12% | 100% | ||
| L* (lightness of color) | 0 months | 40.64 ± 0.31 a | 43.11 ± 0.38 a | 50.20 ± 0.11 a | 50.86 ± 0.01 a | 37.50 ± 0.01 a | 35.31 ± 0.01 a | 37.13 ± 0.01 a | 34.80 ± 0.02 a | 37.48 ± 0.05 a |
| 3 months | 39.76 ± 1.63 a | 43.64 ± 0.12 a | 49.40 ± 0.29 a | 51.50 ± 0.17 a | 39.22 ± 0.41 a | 37.16 ± 0.04 a | 38.35 ± 0.08 a | 36.30 ± 0.05 a | 37.48 ± 0.13 a | |
| a* (intensity of red color) | 0 months | 13.98 ± 0.15 c | 16.60 ± 0.28 a | 12.50 ± 0.08 d | 15.12 ± 0.01 b | 13.84 ± 0.02 c | 13.88 ± 0.00 c | 15.64 ± 0.01 b | 15.27 ± 0.00 b | 0.32 ± 0.01 h |
| 3 months | 8.24 ± 0.71 g | 14.12 ± 0.05 c | 9.88 ± 0.35 f | 12.50 ± 0.16 d | 10.98 ± 0.49 e | 12.28 ± 0.15 d | 11.38 ± 0.25 e | 13.48 ± 0.19 c | 0.12 ± 0.02 h | |
| b* (intensity of yellow color) | 0 months | 10.39 ± 0.34 a | 11.47 ± 0.24 a | 9.60 ± 0.03 a | 9.85 ± 0.00 a | 4.81 ± 0.03 a | 3.88 ± 0.01 a | 4.22 ± 0.01 a | 3.73 ± 0.01 a | 3.70 ± 0.04 a |
| 3 months | 7.86 ± 1.99 a | 12.25 ± 0.07 a | 9.70 ± 0.36 a | 10.95 ± 0.20 a | 6.36 ± 0.38 a | 5.14 ± 0.04 a | 5.43 ± 0.20 a | 4.14 ± 0.14 a | 4.60 ± 0.11 a | |
| Dynamic viscosity (mPas) | 0 months | 6.0 ± 1.7 a | 24.6 ± 0.8 a | 137.4 ± 5.9 a | 434.3 ± 1.7 a | 90.0 ± 1.7 a | 350.3 ± 18.7 a | 76.2 ± 2.5 a | 345.5 ± 8.5 a | 5.4 ± 0.8 a |
| 3 months | 6.0 ± 0.0 a | 33.0 ± 2.5 a | 154.8 ± 1.7 a | 451.1 ± 23.8 a | 102.0 ± 5.1 a | 352.1 ± 9.3 a | 85.8 ± 5.9 a | 329.3 ± 21.2 a | 1.2 ± 0.0 a | |
| Turbidity stability (% NTU) | 0 months | 10.98 ± 0.23 f | 4.35 ± 0.30 i | 3.15 ± 0.01 j | 4.82 ± 0.37 i | 15,98 ± 0.06 c | 18.95 ± 0.55 b | 18.42 ± 0.11 b | 13.27 ± 0.37 e | 19.04 ± 0.09 b |
| 3 months | 7.88 ± 0.77 g | 6.43 ± 0.01 h | 2.30 ± 0.07 jk | 1.66 ± 0.18 k | 13.68 ± 0.37 e | 22.82 ± 0.50 a | 13.52 ± 0.78 e | 11.72 ± 1.22 f | 14.84 ± 0.27 d | |
| Dry matter (g/100 g) | 0 months | 15.41 ± 0.13 fg | 18.72 ± 0.27 a | 15.37 ± 0.04 fg | 17.01 ± 0.17 d | 15.52 ± 0.09 f | 17.76 ± 0.03 b | 14.22 ± 0.16 i | 16.05 ± 0.01 e | 13.28 ± 0.11 j |
| 3 months | 15.10 ± 0.03 h | 17.91 ± 0.16 b | 15.25 ± 0.05 gh | 17.11 ± 0.01 d | 15.11 ± 0.18 h | 17.50 ± 0.11 c | 14.36 ± 0.16 i | 15.84 ± 0.17 e | 13.03 ± 0.04 j | |
| General extract (°Brix) | 0 months | 13.6 ± 0.0 e | 14.9 ± 0.1 a | 12.5 ± 0.0 j | 12.7 ± 0.0 i | 13.0 ± 0.0 g | 13.9 ± 0.0 d | 12.5 ± 0.0 j | 12.1 ± 0.0 l | 12.3 ± 0.0 k |
| 3 months | 13.6 ± 0.0 e | 14.7 ± 0.0 b | 12.5 ± 0.0 j | 12.9 ± 0.0 h | 13.2 ± 0.0 f | 14.3 ± 0.0 c | 12.3 ± 0.0 k | 11.6 ± 0.1 m | 12.5 ± 0.0 j | |
| pH | 0 months | 3.188 ± 0.004 def | 3.206 ± 0.060 def | 3.328 ± 0.079 c | 3.238 ± 0.023 de | 3.141 ± 0.010 f | 3.061 ± 0.015 g | 3.187 ± 0.030 def | 3.134 ± 0.016 f | 3.330 ± 0.023 bc |
| 3 months | 3.255 ± 0.017 bd | 3.325 ± 0.074 c | 3.407 ± 0.013 a | 3.427 ± 0.047 a | 3.406 ± 0.041 a | 3.166 ± 0.016 ef | 3.181 ± 0.011 ef | 3.130 ± 0.008 f | 3.409 ± 0.023 a | |
| Total acidity (g malic acid/100 g) | 0 months | 0.54 ± 0.03 a | 0.70 ± 0.01 a | 0.51 ± 0.03 a | 0.64 ± 0.03 a | 0.56 ± 0.00 a | 0.74 ± 0.02 a | 0.53 ± 0.00 a | 0.69 ± 0.00 a | 0.37 ± 0.05 a |
| 3 months | 0.55 ± 0.02 a | 0.69 ± 0.00 a | 0.50 ± 0.01 a | 0.59 ± 0.01 a | 0.53 ± 0.00 a | 0.69 ± 0.06 a | 0.53 ± 0.00 a | 0.67 ± 0.06 a | 0.35 ± 0.02 a | |
| Ash content (g/100 g) | 0 months | 0.26 ± 0.04 a | 0.29 ± 0.02 a | 0.22 ± 0.01 a | 0.31 ± 0.01 a | 0.24 ± 0.06 a | 0.30 ± 0.08 a | 0.23 ± 0.01 a | 0.30 ± 0.10 a | 0.18 ± 0.02 a |
| 3 months | 0.25 ± 0.01 a | 0.30 ± 0.01 a | 0.23 ± 0.01 a | 0.35 ± 0.27 a | 0.22 ± 0.03 a | 0.27 ± 0.01 a | 0.21 ± 0.03 a | 0.22 ± 0.08 a | 0.17 ± 0.06 a | |
| Pectin content (%) | 0 months | 0.08 ± 0.01 a | 0.10 ± 0.00 a | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.09 ± 0.01 a | 0.04 ± 0.03 a | 0.07 ± 0.01 a | 0.00 ± 0.00 a |
| 3 months | 0.03 ± 0.00 a | 0.06 ± 0.01 a | 0.03 ± 0.00 a | 0.05 ± 0.00 a | 0.04 ± 0.01 a | 0.08 ± 0.06 a | 0.07 ± 0.00 a | 0.10 ± 0.04 a | 0.02 ± 0.00 a | |
| Parameter | Storage Time | Apple Juice with | Apple Juice | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dried Red Currant Pomace | Fresh Red Currant Pomace | Dried Black Currant Pomace | Fresh Black Currant Pomace | |||||||
| 3% | 6% | 6% | 12% | 3% | 6% | 6% | 12% | 100% | ||
| Fructose (g/100 g) | 0 months | 13.28 ± 0.13 b | 13.97 ± 0.31 a | 12.31 ± 0.12 c | 11.35 ± 0.25 f | 11.70 ± 0.12 de | 11.63 ± 0.26 def | 11.41 ± 0.11 ef | 10.32 ± 0.23 g | 11.85 ± 0.12 d |
| Sorbitol (g/100 g) | 0 months | 0.07 ± 0.00 b | 0.09 ± 0.01 a | 0.09 ± 0.01 a | 0.08 ± 0.01 ab | 0.08 ± 0.01 ab | 0.07 ± 0.00 b | 0.09 ± 0.01 a | 0.00 ± 0.00 c | 0.08 ± 0.01 ab |
| Glucose (g/100 g) | 0 months | 2.69 ± 0.06 b | 2.97 ± 0.07 a | 2.36 ± 0.05 c | 2.36 ± 0.05 c | 2.24 ± 0.05 de | 2.28 ± 0.05 cd | 2.17 ± 0.05 ef | 2.12 ± 0.05 f | 2.18 ± 0.05 ef |
| Sucrose (g/100 g) | 0 months | 0.29 ± 0.01 a | 0.23 ± 0.01 b | 0.00 ± 0.01 e | 0.00 ± 0.00 e | 0.17 ± 0.00 d | 0.20 ± 0.01 c | 0.18 ± 0.01 d | 0.00 ± 0.01 e | 0.22 ± 0.01 bc |
| Total sugar (g/100 g) | 0 months | 16.33 ± 0.20 b | 17.26 ± 0.39 a | 14.76 ± 0.20 c | 13.79 ± 0.31 e | 14.19 ± 0.18 de | 14.18 ± 0.31 de | 13.85 ± 0.18 e | 12.44 ± 0.28 f | 14.33 ± 0.18 cd |
| Oxalic acid (g/100 g) | 0 months | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.01 ± 0.01 bc | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.09 ± 0.00 a | 0.00 ± 0.01 c |
| 3 months | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.00 ± 0.01 c | 0.01 ± 0.01 bc | 0.00 ± 0.01 c | |
| Maleic acid (g/100 g) | 0 months | 0.04 ± 0.00 a | 0.06 ± 0.02 a | 0.04 ± 0.00 a | 0.08 ± 0.00 a | 0.01 ± 0.01 a | 0.02 ± 0.00 a | 0.01 ± 0.01 a | 0.02 ± 0.00 a | 0.00 ± 0.01 a |
| 3 months | 0.00 ± 0.01 a | 0.01 ± 0.01 a | 0.00 ± 0.01 a | 0.00 ± 0.01 a | 0.00 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.00 ± 0.01 a | |
| Citric acid (g/100 g) | 0 months | 0.71 ± 0.02 f | 1.32 ± 0.03 b | 0.82 ± 0.01 e | 2.00 ± 0.02 a | 0.62 ± 0.01 h | 0.99 ± 0.01 d | 0.70 ± 0.02 f | 1.28 ± 0.03 c | 0.05 ± 0.00 m |
| 3 months | 0.23 ± 0.01 l | 0.45 ± 0.01 j | 0.24 ± 0.01 l | 0.44 ± 0.01 j | 0.24 ± 0.01 l | 0.67 ± 0.02 g | 0.35 ± 0.01 k | 0.57 ± 0.02 i | 0.02 ± 0.00 n | |
| Malic acid (g/100 g) | 0 months | 1.42 ± 0.03 f | 1.92 ± 0.05 c | 1.53 ± 0.04 e | 2.51 ± 0.06 a | 1.70 ± 0.04 d | 1.68 ± 0.02 d | 1.68 ± 0.02 d | 2.05 ± 0.02 b | 0.96 ± 0.02 hi |
| 3 months | 0.95 ± 0.02 i | 1.00 ± 0.03 h | 0.86 ± 0.01 j | 0.86 ± 0.02 j | 0.89 ± 0.01 j | 1.15 ± 0.03 g | 1.13 ± 0.02 g | 0.95 ± 0.01 i | 0.85 ± 0.02 j | |
| Quinic acid (g/100 g) | 0 months | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.09 ± 0.01 b | 0.00 ± 0.01 d |
| 3 months | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.00 ± 0.01 d | 0.11 ± 0.01 a | 0.02 ± 0.01 c | 0.00 ± 0.01 d | 0.00 ± 0.01 d | |
| Shikimic acid (g/100 g) | 0 months | 0.02 ± 0.00 a | 0.04 ± 0.00 a | 0.02 ± 0.00 a | 0.06 ± 0.02 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.00 ± 0.01 a |
| 3 months | 0.01 ± 0.01 a | 0.02 ± 0.00 a | 0.01 ± 0.01 a | 0.02 ± 0.01 a | 0.00 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.00 ± 0.00 a | 0.00 ± 0.01 a | |
| Total organic acids (g/100 g) | 0 months | 2.19 ± 0.07 f | 3.34 ± 0.12 c | 2.41 ± 0.07 e | 4.65 ± 0.12 a | 2.35 ± 0.10 e | 2.72 ± 0.04 d | 2.42 ± 0.06 e | 3.54 ± 0.05 b | 1.01 ± 0.06 k |
| 3 months | 1.19 ± 0.06 j | 1.48 ± 0.06 h | 1.11 ± 0.05 jk | 1.32 ± 0.07 i | 1.13 ± 0.05 jk | 1.95 ± 0.09 g | 1.52 ± 0.06 h | 1.54 ± 0.05 h | 0.87 ± 0.06 l | |
| Parameter | Storage Time | Apple Juice with | Apple Juice | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dried Red Currant Pomace | Fresh Red Currant Pomace | Dried Black Currant Pomace | Fresh Black Currant Pomace | |||||||
| 3% | 6% | 6% | 12% | 3% | 6% | 6% | 12% | 100% | ||
| Anthocyanins (mg/100 mL) | 0 months | 1.14 ± 0.03 l | 1.98 ± 0.05 j | 1.67 ± 0.01 k | 2.65 ± 0.08 h | 5.26 ± 0.13 d | 9.65 ± 0.10 b | 6.78 ± 0.07 c | 12.80 ± 0.13 a | 0.34 ± 0.01 n |
| 3 months | 0.22 ± 0.01 o | 0.47 ± 0.01 m | 0.37 ± 0.01 n | 1.07 ± 0.02 l | 2.28 ± 0.06 i | 4.55 ± 0.07 f | 3.41 ± 0.05 g | 4.79 ± 0.12 e | 0.04 ± 0.00 p | |
| Phenolic acids (mg/100 mL) | 0 months | 5.74 ± 0.14 e | 8.07 ± 0.20 a | 8.16 ± 0.20 a | 6.73 ± 0.10 c | 6.23 ± 0.09 d | 6.17 ± 0.01 d | 5.05 ± 0.08 f | 5.11 ± 0.08 f | 5.18 ± 0.08 f |
| 3 months | 6.09 ± 0.09 d | 7.13 ± 0.11 b | 4.33 ± 0.06 g | 6.83 ± 0.10 c | 8.19 ± 0.12 a | 2.48 ± 0.01 h | 1.23 ± 0.03 j | 1.72 ± 0.04 i | 6.70 ± 0.17 c | |
| Flavonols (mg/100 mL) | 0 months | 0.11 ± 0.00 k | 0.32 ± 0.01 h | 0.09 ± 0.01 k | 0.11 ± 0.01 k | 0.69 ± 0.02 e | 0.98 ± 0.01 c | 0.92 ± 0.02 d | 1.72 ± 0.03 a | 0.00 ± 0.01 m |
| 3 months | 0.14 ± 0.00 j | 0.34 ± 0.01 h | 0.03 ± 0.00 l | 0.20 ± 0.01 i | 0.49 ± 0.01 g | 1.05 ± 0.03 b | 0.52 ± 0.01 f | 0.54 ± 0.01 f | 0.00 ± 0.01 m | |
| Flavan-3-ols monomeric & dimric (mg/100 mL) | 0 months | 3.58 ± 0.09 k | 10.83 ± 0.16 a | 6.20 ± 0.09 d | 5.82 ± 0.09 e | 3.12 ± 0.08 mn | 8.22 ± 0.12 b | 3.04 ± 0.01 n | 4.02 ± 0.06 j | 3.39 ± 0.01 l |
| 3 months | 5.63 ± 0.14 f | 7.20 ± 0.11 c | 3.27 ± 0.08 lm | 5.29 ± 0.08 g | 3.94 ± 0.06 j | 7.30 ± 0.18 c | 4.92 ± 0.07 i | 3.35 ± 0.05 l | 5.10 ± 0.13 h | |
| Procyanidin polymers (mg/100 mL) | 0 months | 57.26 ± 0.86 g | 73.10 ± 1.83 e | 53.33 ± 0.80 h | 68.70 ± 1.72 f | 106.16 ± 1.59 c | 133.70 ± 3.34 b | 79.22 ± 1.19 d | 135.92 ± 3.40 b | 39.24 ± 0.59 j |
| 3 months | 48.71 ± 0.73 i | 47.98 ± 1.20 i | 37.32 ± 0.56 j | 32.13 ± 0.80 k | 58.87 ± 0.88 g | 161.70 ± 4.04 a | 74.23 ± 1.11 e | 80.83 ± 2.02 d | 32.09 ± 0.48 k | |
| Total polyphenolic compounds (mg/100 mL) | 0 months | 67.83 ± 1.13 h | 94.30 ± 2.25 d | 69.45 ± 1.12 h | 84.01 ± 1.99 f | 121.46 ± 1.91 c | 158.72 ± 3.58 b | 95.01 ± 1.36 d | 159.57 ± 3.69 b | 48.15 ± 0.70 j |
| 3 months | 60.79 ± 0.97 i | 63.12 ± 1.43 i | 45.32 ± 0.72 jk | 45.52 ± 1.01 jk | 73.77 ± 1.13 g | 177.08 ± 4.33 a | 84.31 ± 1.28 f | 91.23 ± 2.25 e | 43.93 ± 0.79 k | |
| Parameter | Storage Time | Apple Juice with | Apple Juice | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dried Red Currant Pomace | Fresh Red Currant Pomace | Dried Black Currant Pomace | Fresh Black Currant Pomace | |||||||
| 3% | 6% | 6% | 12% | 3% | 6% | 6% | 12% | 100% | ||
| ABTS (mmol Trolox/100 mL) | 0 months | 1.163 ± 0.056 b | 1.046 ± 0.045 bc | 0.705 ± 0.041 fg | 0.729 ± 0.042 ef | 1.002 ± 0.062 cd | 1.662 ± 0.067 a | 0.950 ± 0.374 cd | 0.868 ± 0.094 de | 0.392 ± 0.043 hi |
| 3 months | 0.724 ± 0.051 ef | 0.549 ± 0.061 gh | 0.339 ± 0.030 i | 0.320 ± 0.017 i | 0.576 ± 0.017 fg | 0.672 ± 0.038 fg | 0.424 ± 0.041 hi | 0.672 ± 0.045 fg | 0.313 ± 0.015 i | |
| FRAP(mmol Trolox/100 mL) | 0 months | 0.662 ± 0.002 a | 0.620 ± 0.013 b | 0.343 ± 0.006 g | 0.401 ± 0.009 f | 0.219 ± 0.002 j | 0.499 ± 0.027 d | 0.273 ± 0.004 i | 0.552 ± 0.009 c | 0.229 ± 0.003 j |
| 3 months | 0.449 ± 0.009 e | 0.357 ± 0.010 g | 0.124 ± 0.008 l | 0.182 ± 0.006 k | 0.314 ± 0.006 h | 0.485 ± 0.004 d | 0.228 ± 0.010 j | 0.453 ± 0.017 e | 0.192 ± 0.003 k | |
| ORAC (mmol Trolox/100 mL) | 0 months | 3.791 ± 0.587 a | 1.359 ± 0.044 cde | 1.770 ± 0.341 bc | 1.483 ± 0.366 cd | 2.368 ± 1.001 b | 2.237 ± 0.842 b | 2.315 ± 0.110 b | 3.719 ± 0.411 a | 0.697 ± 0.034 efgh |
| 3 months | 0.483 ± 0.140 gh | 0.443 ± 0.070 gh | 0.100 ± 0.032 h | 0.257 ± 0.214 h | 0.483 ± 0.390 gh | 0.581 ± 0.429 fgh | 0.051 ± 0.177 h | 1.234 ± 0.336 cdef | 0.961 ± 0.101 defg | |
| Ability to inhibit α-amylase (IC50 as mg/mL) | 0 months | 0.194 ± 0.004 a | 0.920 ± 0.018 b | 4.441 ± 0.089 e | 0.822 ± 0.016 b | 0.932 ± 0.019 b | 1.125 ± 0.023 b | 0.763 ± 0.015 b | 0.628 ± 0.013 ab | 3.387 ± 0.068 d |
| 3 months | 0.890 ± 0.013 b | 0.767 ± 0.015 b | 1.691 ± 0.034 c | 1.902 ± 0.038 c | 1.614 ± 0.032 c | 9.435 ± 0.189 f | 3.013 ± 0.060 d | 1.094 ± 0.031 b | 32.147 ± 0.643 g | |
| Ability to inhibit α-glucosidase (IC50 as mg/mL) | 0 months | 0.072 ± 0.002 b | 0.026 ± 0.001 a | 0.307 ± 0.005 k | 0.222 ± 0.005 i | 0.163 ± 0.004 e | 0.191 ± 0.005 g | 0.202 ± 0.004 h | 0.123 ± 0.002 c | 0.195 ± 0.003 g |
| 3 months | 0.031 ± 0.001 a | 0.132 ± 0.003 d | 0.206 ± 0.003 h | 0.234 ± 0.007 j | 0.238 ± 0.004 j | 0.189 ± 0.004 g | 0.224 ± 0.005 i | 0.175 ± 0.005 f | 0.207 ± 0.003 h | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydłowska, M.; Wojdyło, A.; Nowicka, P. Black and Red Currant Pomaces as Raw Materials to Create Smoothies with In Vitro Health-Promoting Potential. Foods 2024, 13, 2715. https://doi.org/10.3390/foods13172715
Szydłowska M, Wojdyło A, Nowicka P. Black and Red Currant Pomaces as Raw Materials to Create Smoothies with In Vitro Health-Promoting Potential. Foods. 2024; 13(17):2715. https://doi.org/10.3390/foods13172715
Chicago/Turabian StyleSzydłowska, Martyna, Aneta Wojdyło, and Paulina Nowicka. 2024. "Black and Red Currant Pomaces as Raw Materials to Create Smoothies with In Vitro Health-Promoting Potential" Foods 13, no. 17: 2715. https://doi.org/10.3390/foods13172715






