Poly(vinyl chloride) Films Incorporated with Antioxidant ZnO-Flavonoid Nanoparticles: A Strategy for Food Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
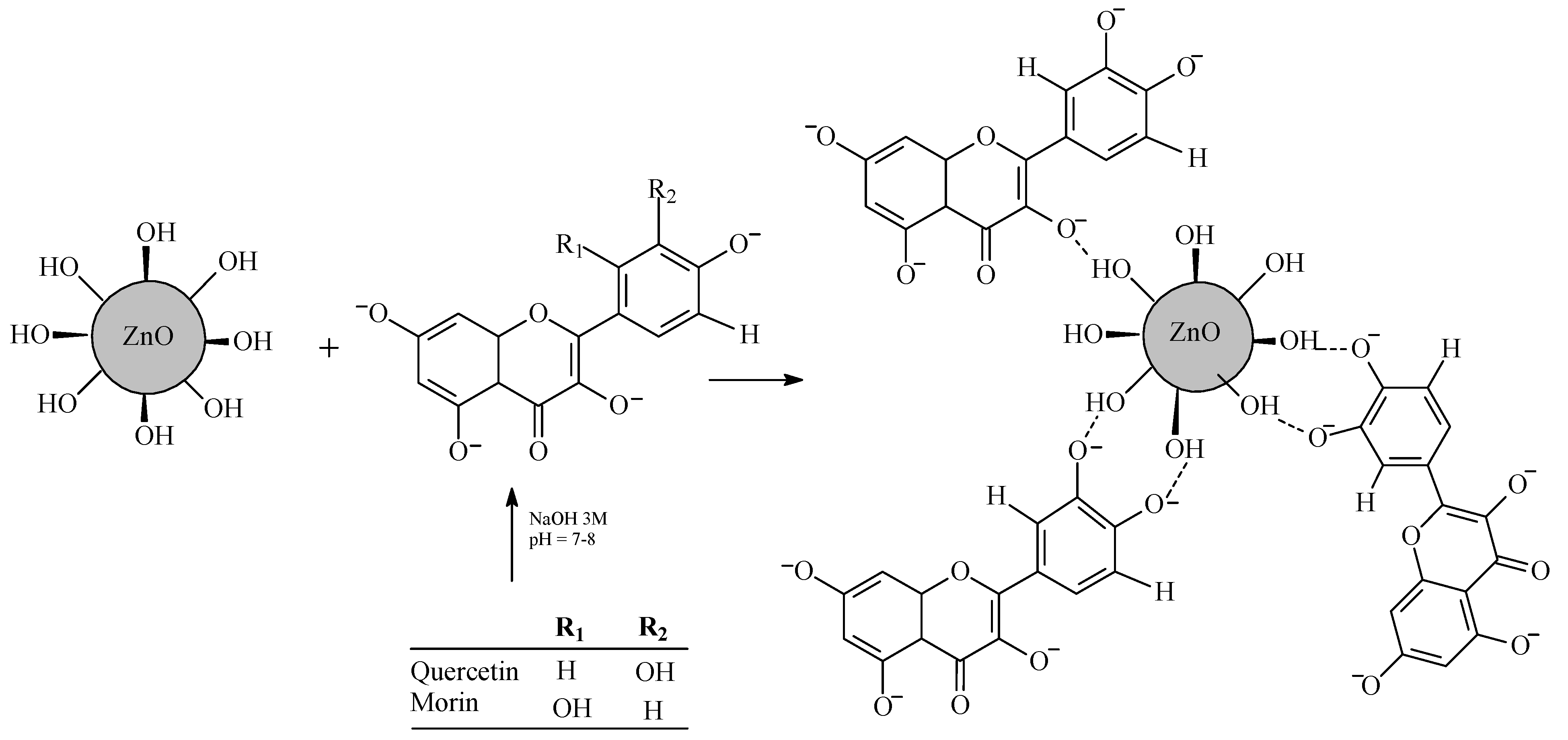
2.2. Synthesis of ZnO-Flavonoid NPs
2.3. Production of NP-Incorporating Films
2.4. Caracterization of ZnO-Flavonoid-Containing NPs
2.5. Antioxidant Activity
3. Results and Discussion
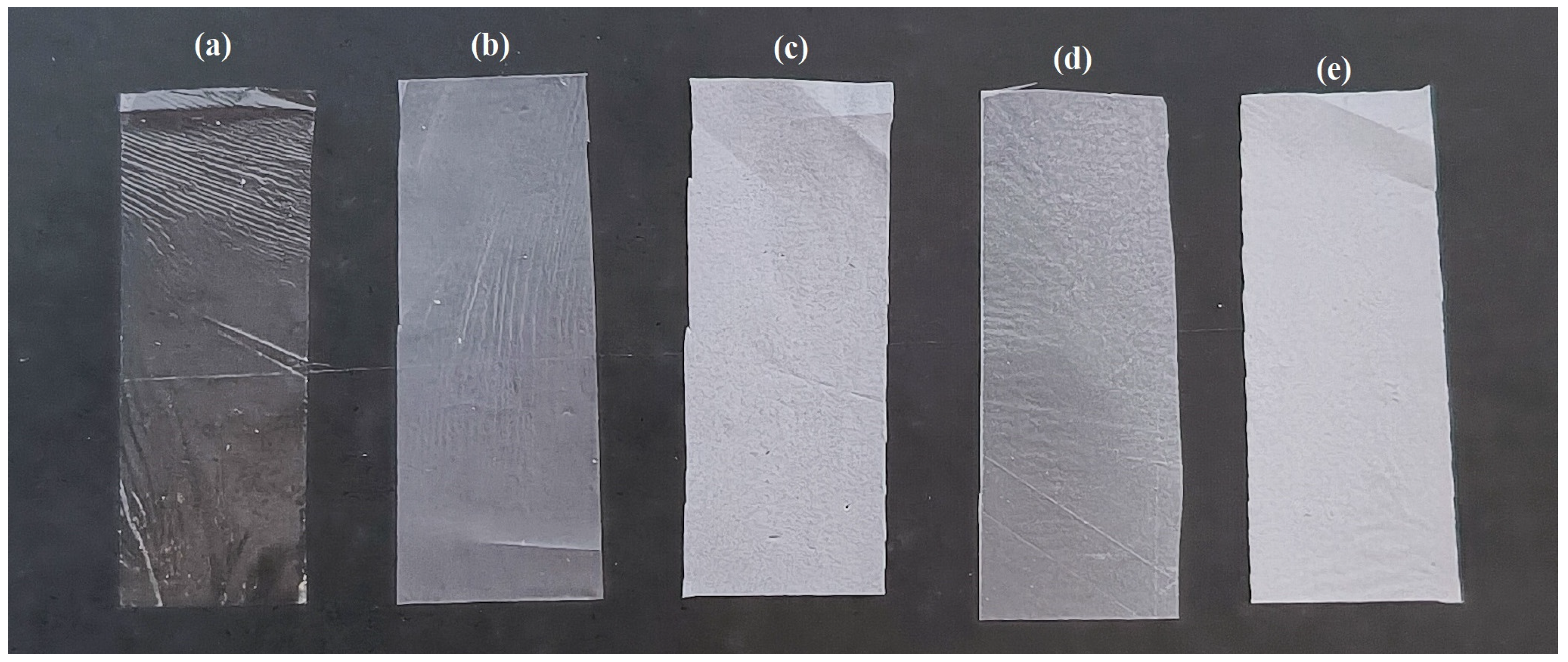
3.1. Thickness, UV Light Transmission, and Transparency
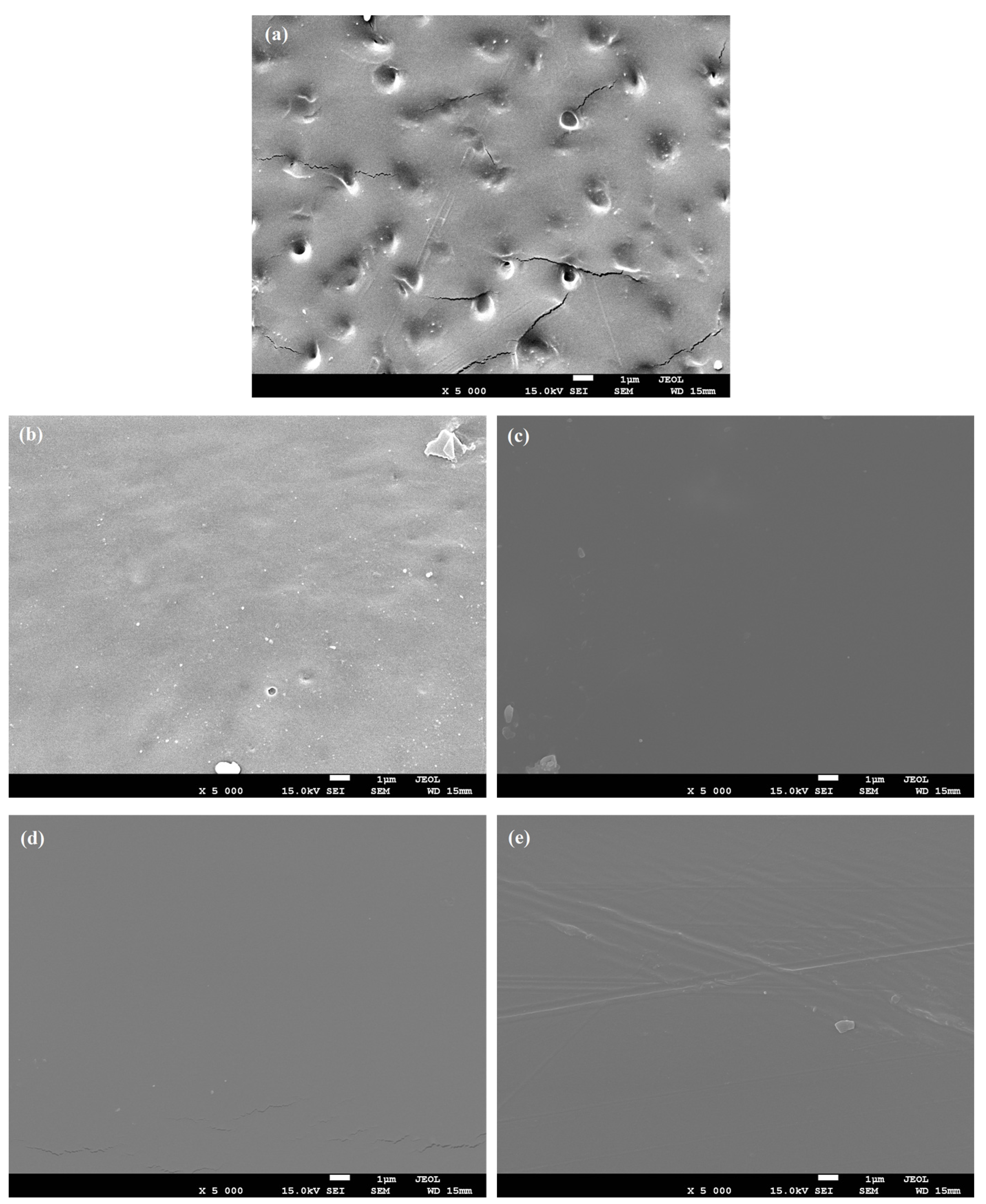
3.2. Morphological Characterization
3.3. FTIR-ATR Analysis
3.4. Mechanical Properties
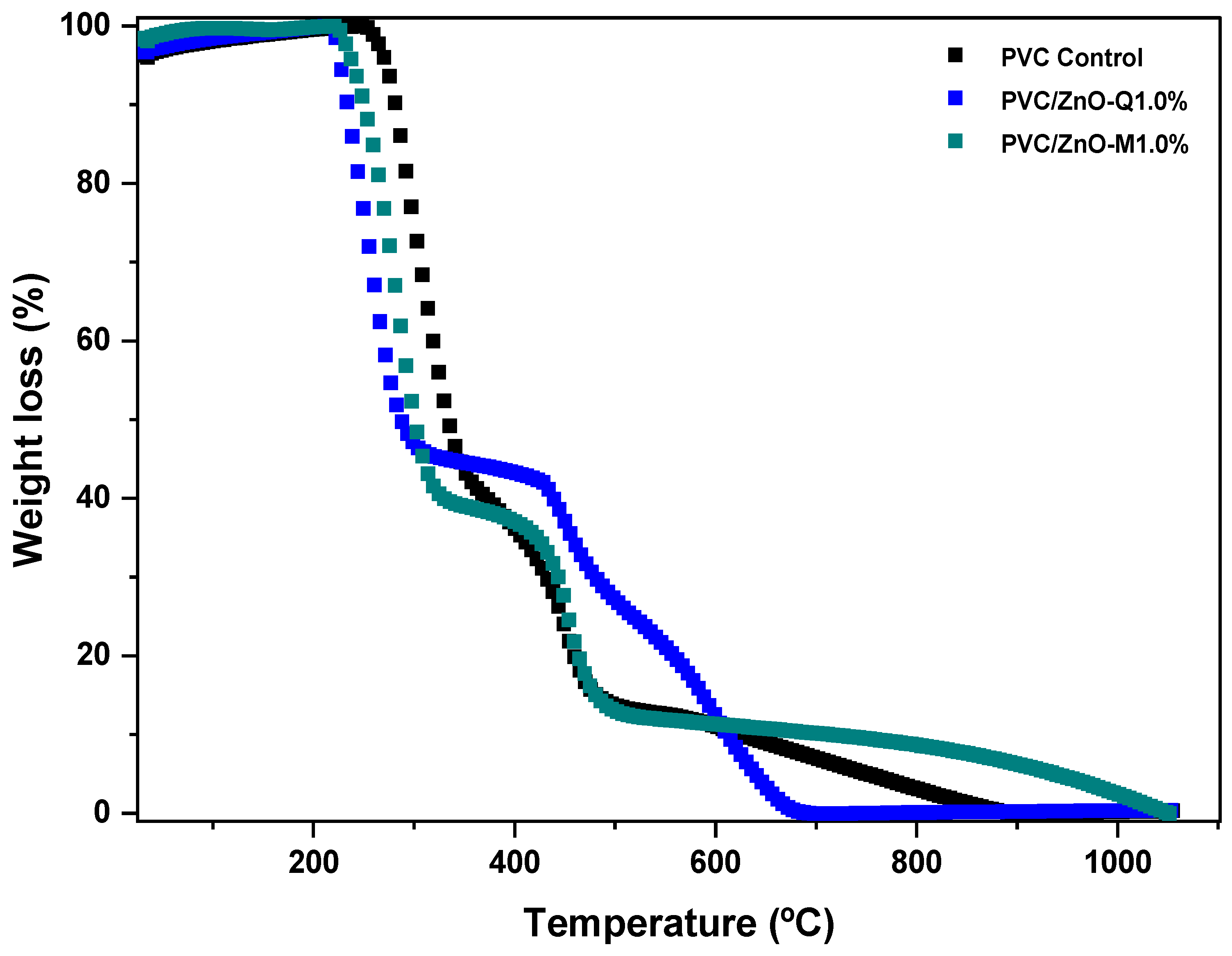
3.5. Thermogravimetric Analysis
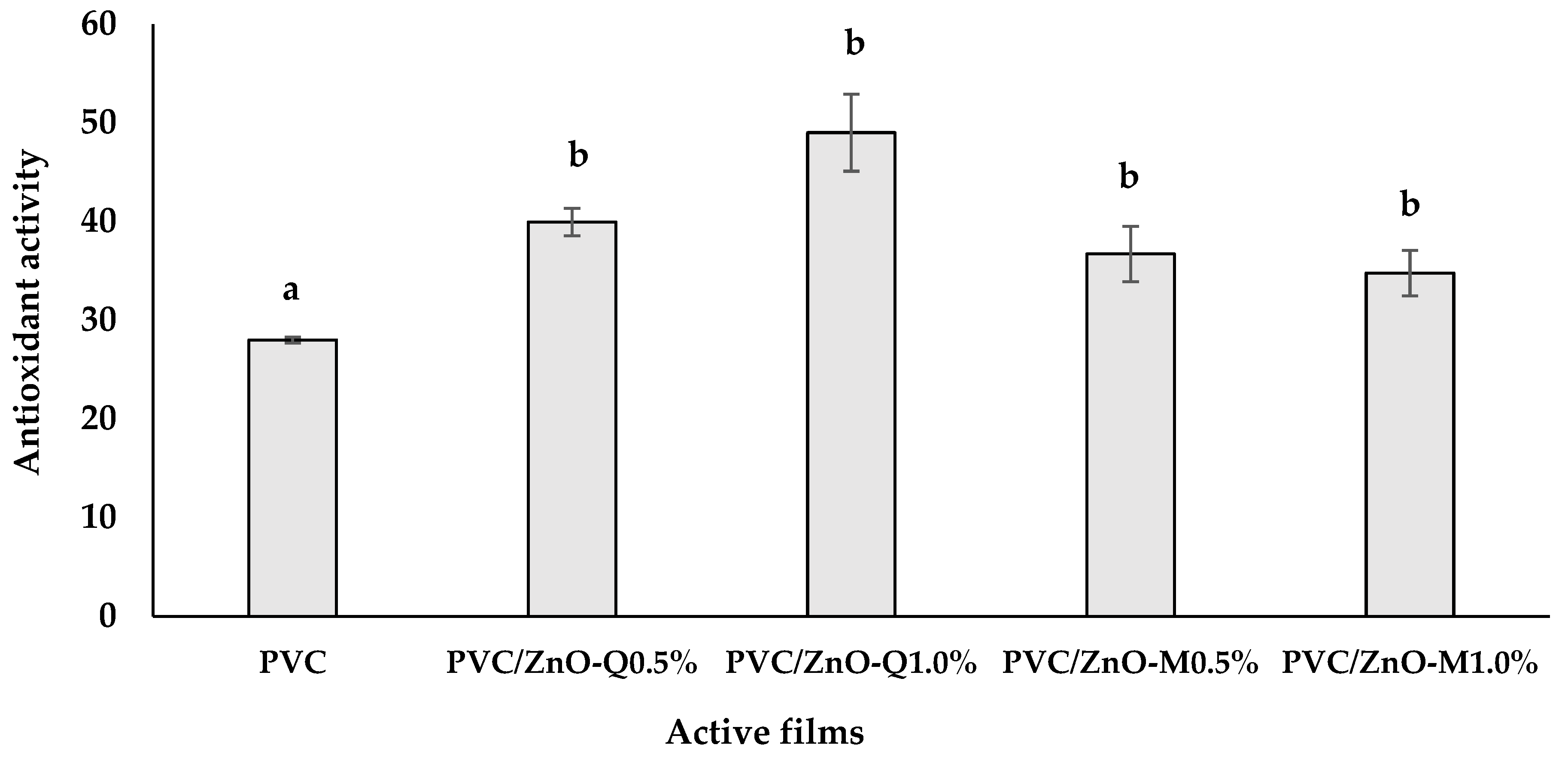
3.6. Antioxidant Activity of Active Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geng, L.; Liu, K.; Zhang, H. Lipid Oxidation in Foods and Its Implications on Proteins. Front. Nutr. 2023, 10, 1192199. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A Comprehensive Review on the Application of Active Packaging Technologies to Muscle Foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Sun, L.; Lu, L.; Pan, L.; Wang, Q.; Qiu, X. Characterization of α-Tocopherol-Loaded MCM-41 Mesoporous Silica with Different Pore Sizes and Antioxidant Active Packaging Films. Packag. Technol. Sci. 2021, 34, 77–89. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active Packaging Films with Natural Antioxidants to Be Used in Meat Industry: A Review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Sirait, M.S.; Warsiki, E.; Setyaningsih, D. Potential of Red Fruit Oil (Pandanus Conoideus Lam.) as an Antioxidant Active Packaging: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 749, 012008. [Google Scholar] [CrossRef]
- Wrona, M.; Manso, S.; Silva, F.; Cardoso, L.; Salafranca, J.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. New Active Packaging Based on Encapsulated Carvacrol, with Emphasis on Its Odour Masking Strategies. Food Packag. Shelf Life 2023, 40, 101177. [Google Scholar] [CrossRef]
- Parhi, B.; Bharatiya, D.; Swain, S.K. Application of Quercetin Flavonoid Based Hybrid Nanocomposites: A Review. Saudi Pharm. J. 2020, 28, 1719–1732. [Google Scholar] [CrossRef]
- Vimalraj, S.; Rajalakshmi, S.; Raj Preeth, D.; Vinoth Kumar, S.; Deepak, T.; Gopinath, V.; Murugan, K.; Chatterjee, S. Mixed-Ligand Copper(II) Complex of Quercetin Regulate Osteogenesis and Angiogenesis. Mater. Sci. Eng. C 2018, 83, 187–194. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid–Metal Ion Complexes: A Novel Class of Therapeutic Agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lee, H.-S. Evaluation of Antioxidant and Antibacterial Activities of Morin Isolated from Mulberry Fruits (Morus Alba L.). J. Korean Soc. Appl. Biol. Chem. 2012, 55, 485–489. [Google Scholar] [CrossRef]
- Nafisi, S.; Hashemi, M.; Rajabi, M.; Tajmir-Riahi, H.A. DNA Adducts with Antioxidant Flavonoids: Morin, Apigenin, and Naringin. DNA Cell Biol. 2008, 27, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Dowling, D.P.; Monahan, F.J. Development of Active Packaging Containing Natural Antioxidants. Procedia Food Sci. 2011, 1, 224–228. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Xu, H.; Long, J.; Zhao, J.; Xu, Z.; Meng, M.; Jin, Z. Recent Advances in the Application of Nanotechnology to Create Antioxidant Active Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2024, 64, 2890–2905. [Google Scholar] [CrossRef]
- Mallakpour, S.; Javadpour, M. Design and Characterization of Novel Poly(Vinyl Chloride) Nanocomposite Films with Zinc Oxide Immobilized with Biocompatible Citric Acid. Colloid Polym. Sci. 2015, 293, 2565–2573. [Google Scholar] [CrossRef]
- Hannon, J.C.; Kerry, J.; Cruz-Romero, M.; Morris, M.; Cummins, E. Advances and Challenges for the Use of Engineered Nanoparticles in Food Contact Materials. Trends Food Sci. Technol. 2015, 43, 43–62. [Google Scholar] [CrossRef]
- Braga, L.R.; Pérez, L.M.; Soazo, M.d.V.; Machado, F. Evaluation of the Antimicrobial, Antioxidant and Physicochemical Properties of Poly(Vinyl Chloride) Films Containing Quercetin and Silver Nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef]
- Dash, K.K.; Deka, P.; Bangar, S.P.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of Inorganic Nanoparticles in Food Packaging: A Comprehensive Review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef]
- Beigmohammadi, F.; Peighambardoust, S.H.; Hesari, J.; Azadmard-Damirchi, S.; Peighambardoust, S.J.; Khosrowshahi, N.K. Antibacterial Properties of LDPE Nanocomposite Films in Packaging of UF Cheese. LWT 2016, 65, 106–111. [Google Scholar] [CrossRef]
- Paidari, S.; Ibrahim, S.A. Potential Application of Gold Nanoparticles in Food Packaging: A Mini Review. Gold Bull. 2021, 54, 31–36. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.-S.; Hu, X.-H.; Chu, L.-Q. Recent Advances in Antimicrobial Hydrogels Containing Metal Ions and Metals/Metal Oxide Nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef]
- Braga, L.R.; Rangel, E.T.; Suarez, P.A.Z.; Machado, F. Simple Synthesis of Active Films Based on PVC Incorporated with Silver Nanoparticles: Evaluation of the Thermal, Structural and Antimicrobial Properties. Food Packag. Shelf Life 2018, 15, 122–129. [Google Scholar] [CrossRef]
- Panea, B.; Ripoll, G.; González, J.; Fernández-Cuello, Á.; Albertí, P. Effect of Nanocomposite Packaging Containing Different Proportions of ZnO and Ag on Chicken Breast Meat Quality. J. Food Eng. 2014, 123, 104–112. [Google Scholar] [CrossRef]
- Petkova, P.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Simultaneous Sonochemical-Enzymatic Coating of Medical Textiles with Antibacterial ZnO Nanoparticles. Ultrason. Sonochem. 2016, 29, 244–250. [Google Scholar] [CrossRef]
- Stuparu-Cretu, M.; Braniste, G.; Necula, G.A.; Stanciu, S.; Stoica, D.; Stoica, M. Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health. Foods 2023, 12, 1882. [Google Scholar] [CrossRef]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory Landscape of Nanotechnology and Nanoplastics from a Global Perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpai, S.K. Preparation, Characterization and Antibacterial Applications of ZnO-Nanoparticles Coated Polyethylene Films for Food Packaging. Colloids Surf. B Biointerfaces 2012, 90, 16–20. [Google Scholar] [CrossRef]
- Bernard, L.; Décaudin, B.; Lecoeur, M.; Richard, D.; Bourdeaux, D.; Cueff, R.; Sautou, V. Analytical Methods for the Determination of DEHP Plasticizer Alternatives Present in Medical Devices: A Review. Talanta 2014, 129, 39–54. [Google Scholar] [CrossRef]
- Krehula, L.K.; Papic, A.; Krehula, S.; Gilja, V.; Foglar, L.; Hrnjak-murgic, Z. Properties of UV Protective Films of Poly(Vinyl-Chloride)/TiO2 Nanocomposites for Food Packaging. Polym. Bull. 2017, 74, 1387–1404. [Google Scholar] [CrossRef]
- Afzal, A.B.; Akhtar, M.J. Effects of Silver Nanoparticles on Thermal Properties of DBSA-Doped Polyaniline/PVC Blends. Iran. Polym. J. 2012, 21, 489–496. [Google Scholar] [CrossRef]
- Amar, A.; Parisi, A.V. Spectral Response of Solvent-Cast Polyvinyl Chloride (PVC) Thin Film Used as a Long-Term UV Dosimeter. J. Photochem. Photobiol. B Biol 2013, 125, 115–120. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of Whey Protein Purity and Glycerol Content upon Physical Properties of Edible Films Manufactured Therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Pérez, L.M.; Piccirilli, G.N.; Delorenzi, N.J.; Verdini, R.A. Effect of Different Combinations of Glycerol and/or Trehalose on Physical and Structural Properties of Whey Protein Concentrate-Based Edible Films. Food Hydrocoll. 2016, 56, 352–359. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical Properties and Antioxidant Activity of an Active Film from Chitosan Incorporated with Green Tea Extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Kumar, S.; Aswal, D.K. Thin Film and Significance of Its Thickness. In Recent Advances in Thin Films. Materials Horizons: From Nature to Nanomaterials; Kumar, S., Aswal, D., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. Engineering Evaluation of Thickness and Type of Packaging Materials Based on the Modified Atmosphere Packaging Requirements of Guava (Cv. Baruipur). LWT 2017, 78, 273–280. [Google Scholar] [CrossRef]
- Gaspar, J.; Couto, F.; Salomão, L.C.C.; Finger, F.L.; Cardoso, A. Effect of low temperature and plastic films on post-harvest life of guava (Psidium Guajava L.). ISHS Acta Hortic. 1997, 452, 107–114. [Google Scholar] [CrossRef]
- Pereira, L.M.; Rodrigues, A.C.C.; Sarantópoulos, C.I.G.L.; Junqueira, V.C.A.; Cunha, R.L.; Hubinger, M.D. Influence of Modified Atmosphere Packaging and Osmotic Dehydration on the Quality Maintenance of Minimally Processed Guavas. J. Food Sci. 2004, 69, FEP172–FEP177. [Google Scholar] [CrossRef]
- Cannarsi, M.; Baiano, A.; Marino, R.; Sinigaglia, M.; Del Nobile, M.A. Use of Biodegradable Films for Fresh Cut Beef Steaks Packaging. Meat Sci. 2005, 70, 259–265. [Google Scholar] [CrossRef]
- Qi, Y.; Yin, X.; Zhang, J. Transparent and Heat-Insulation Plasticized Polyvinyl Chloride (PVC) Thin Film with Solar Spectrally Selective Property. Sol. Energy Mater. Sol. Cells 2016, 151, 30–35. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Rawdkuen, S. Effect of Protein Concentrations on the Properties of Fish Myofibrillar Protein Based Film Compared with PVC Film. J. Food Sci. Technol. 2016, 53, 2083–2091. [Google Scholar] [CrossRef]
- Othón-Díaz, E.D.; Fimbres-García, J.O.; Flores-Sauceda, M.; Silva-Espinoza, B.A.; López-Martínez, L.X.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Antioxidants in Oak (Quercus Sp.): Potential Application to Reduce Oxidative Rancidity in Foods. Antioxidants 2023, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Bher, A.; Jacob, H.; Auras, R. Compression Molded LLDPE Films Loaded with Bimetallic (Ag-Cu) Nanoparticles and Cinnamon Essential Oil for Chicken Meat Packaging Applications. LWT 2018, 93, 329–338. [Google Scholar] [CrossRef]
- Emamifar, A.; Mohammadizadeh, M. Preparation and Application of LDPE/ZnO Nanocomposites for Extending Shelf Life of Fresh Strawberries. Food Technol. Biotechnol. 2015, 53, 488–495. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of Polymeric Food Packaging Materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef] [PubMed]
- Elashmawi, I.S.; Hakeem, N.A.; Marei, L.K.; Hanna, F.F. Structure and Performance of ZnO/PVC Nanocomposites. Phys. B Condens. Matter 2010, 405, 4163–4169. [Google Scholar] [CrossRef]
- Tang, E.; Tian, B.; Zheng, E.; Fu, C.; Cheng, G. Preparation of Zinc Oxide Nanoparticle via Uniform Precipitation Method and Its Surface Modification by Methacryloxypropyltrimethoxysilane. Chem. Eng. Commun. 2008, 195, 479–491. [Google Scholar] [CrossRef]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, Characterization and Antioxidant Activity Copper-Quercetin Complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 1901–1906. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Wang, Y.; Huang, J.; Li, K.; Nie, X.; Jiang, J. Synthesis and Application of Environmental Soybean Oil-Based Epoxidized Glycidyl Ester Plasticizer for Poly(Vinyl Chloride). Eur. J. Lipid Sci. Technol. 2017, 119, 1600216. [Google Scholar] [CrossRef]
- Zeddam, C.; Belhaneche-Bensemra, N. Application of FT-IR Spectroscopy to the Specific Migration Study of an Organotin Heat Stabilizer from Rigid Poly(Vinyl Chloride) into Food Simulants. Polimery 2011, 56, 657–661. [Google Scholar] [CrossRef]
- Bueno-Ferrer, C.; Garrigós, M.C.; Jiménez, A. Characterization and Thermal Stability of Poly(Vinyl Chloride) Plasticized with Epoxidized Soybean Oil for Food Packaging. Polym. Degrad. Stab. 2010, 95, 2207–2212. [Google Scholar] [CrossRef]
- Mallakpour, S.; Javadpour, M. Antimicrobial, Mechanical, Optical and Thermal Properties of PVC/ZnO-EDTA Nanocomposite Films. Polym. Adv. Technol. 2017, 28, 393–403. [Google Scholar] [CrossRef]
- Das, P.; Roy, A.; Chakrabarti, S. Photocatalytic Degradation of the Nanocomposite Film Comprising Polyvinyl Chloride (PVC) and Sonochemically Synthesized Iron-Doped Zinc Oxide: A Comparative Study of Performances Between Sunlight and UV Radiation. J. Polym. Environ. 2017, 25, 1231–1241. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Patil, H.; Marathe, K. Studies in Synthesis of Nano-Zinc Oxide Mixed PVC Matrix Membrane and Its Application for Ibuprofen Drug Removal. J. Hazard. Mater. Adv. 2023, 9, 100247. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Santacruz-Ortega, H.; Balandrán-Quintana, R.R. Relationship between Structure, Properties, and the Radical Scavenging Activity of Morin. J. Mol. Struct. 2011, 995, 134–141. [Google Scholar] [CrossRef]
- Mariño-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; Rodríguez-Bernaldo de Quirós, A.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of Active Films Utilizing Antioxidant Compounds Obtained from Tomato and Lemon By-Products for Use in Food Packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- Kubo, M.T.K.; Baicu, A.; Erdogdu, F.; Poças, M.F.; Silva, C.L.M.; Simpson, R.; Vitali, A.A.; Augusto, P.E.D. Thermal Processing of Food: Challenges, Innovations and Opportunities. A Position Paper. Food Rev. Int. 2023, 39, 3344–3369. [Google Scholar] [CrossRef]
- Kurek, M.; Benaida-Debbache, N.; Garofulić, I.E.; Galić, K.; Avallone, S.; Voilley, A.; Waché, Y. Antioxidants and Bioactive Compounds in Food: Critical Review of Issues and Prospects. Antioxidants 2022, 11, 742. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E. Effect of UV-A Irradiation and Temperature on the Antioxidant Activity of Quercetin Studied Using ABTS, DPPH and Electrochemistry Methods. Int. J. Electrochem. Sci. 2015, 10, 5276–5290. [Google Scholar] [CrossRef]
- Kongpichitchoke, T.; Hsu, J.L.; Huang, T.C. Number of Hydroxyl Groups on the B-Ring of Flavonoids Affects Their Antioxidant Activity and Interaction with Phorbol Ester Binding Site of PKCδ C1B Domain: In Vitro and in Silico Studies. J. Agric. Food Chem. 2015, 63, 4580–4586. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Chérif, I.; Ben Hlima, H.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc Oxide Nanoparticles in Meat Packaging: A Systematic Review of Recent Literature. Food Packag. Shelf Life 2023, 36, 101045. [Google Scholar] [CrossRef]


| Films | Thickness (µm) | Wavelength (nm) | ||||
|---|---|---|---|---|---|---|
| 250 | 300 | 350 | 400 | A600 | ||
| PVC control | 25.0 ± 5.3 a | 78.1 ± 7.3 b | 82.0 ± 6.6 b | 83.8 ± 6.4 b | 83.0 ± 6.4 b | 0.30 ± 0.14 a |
| PVC/ZnO-Q0.5% | 32.0 ± 10.3 a | 73.0 ± 5.6 b | 72.0 ± 8.7 b | 75.1 ± 8.1 b | 76.7 ± 8.0 b | 0.29 ± 0.11 a |
| PVC/ZnO-Q1.0% | 32.5 ± 8.2 a | 1.0 ± 0.4 a | 1.8 ± 1.1 a | 1.9 ± 1.0 | 2.6 ± 1.6 a | 4.58 ± 0.80 b |
| PVC/ZnO-M0.5% | 34.0 ± 5.2 a | 76.7 ± 2.0 b | 81.0 ± 1.2 b | 83.0 ± 1.6 b | 82.0 ± 1.4 b | 0.20 ± 0.02 a |
| PVC/ZnO-M1.0% | 32.0 ± 7.9 a | 1.2 ± 0.3 a | 2.3 ± 2.0 a | 3.3 ± 3.0 a | 4.5 ± 3.9 a | 3.11 ± 1.00 b |
| Films | TS (MPa) | EAB (%) | YM (MPa) |
|---|---|---|---|
| PVC control | 10.86 ± 1.50 b | 16.97 ± 3.20 b | 6.03 ± 0.78 b |
| PVC/ZnO-Q0.5% | 9.80 ± 1.20 b | 8.51 ± 1.07 a | 3.74 ± 0.82 a |
| PVC/ZnO-Q1.0% | 5.67 ± 0.45 a | 8.69 ± 3.66 a | 3.50 ± 0.35 a |
| PVC/ZnO-M0.5% | 21.16 ± 3.24 d | 10.52 ± 2.77 a | 11.22 ± 0.69 c |
| PVC/ZnO-M1.0% | 13.77 ± 0.98 c | 6.86 ± 3.06 a | 6.64 ± 1.29 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, L.R.; Oliveira, M.G.; Pérez, L.M.; Rangel, E.T.; Machado, F. Poly(vinyl chloride) Films Incorporated with Antioxidant ZnO-Flavonoid Nanoparticles: A Strategy for Food Preservation. Foods 2024, 13, 2745. https://doi.org/10.3390/foods13172745
Braga LR, Oliveira MG, Pérez LM, Rangel ET, Machado F. Poly(vinyl chloride) Films Incorporated with Antioxidant ZnO-Flavonoid Nanoparticles: A Strategy for Food Preservation. Foods. 2024; 13(17):2745. https://doi.org/10.3390/foods13172745
Chicago/Turabian StyleBraga, Lilian R., Maria Graciele Oliveira, Leonardo M. Pérez, Ellen T. Rangel, and Fabricio Machado. 2024. "Poly(vinyl chloride) Films Incorporated with Antioxidant ZnO-Flavonoid Nanoparticles: A Strategy for Food Preservation" Foods 13, no. 17: 2745. https://doi.org/10.3390/foods13172745
APA StyleBraga, L. R., Oliveira, M. G., Pérez, L. M., Rangel, E. T., & Machado, F. (2024). Poly(vinyl chloride) Films Incorporated with Antioxidant ZnO-Flavonoid Nanoparticles: A Strategy for Food Preservation. Foods, 13(17), 2745. https://doi.org/10.3390/foods13172745








