Recent Advances in Physical Processing Techniques to Enhance the Resistant Starch Content in Foods: A Review
Abstract
:1. Introduction
2. Modification of Resistant Starch
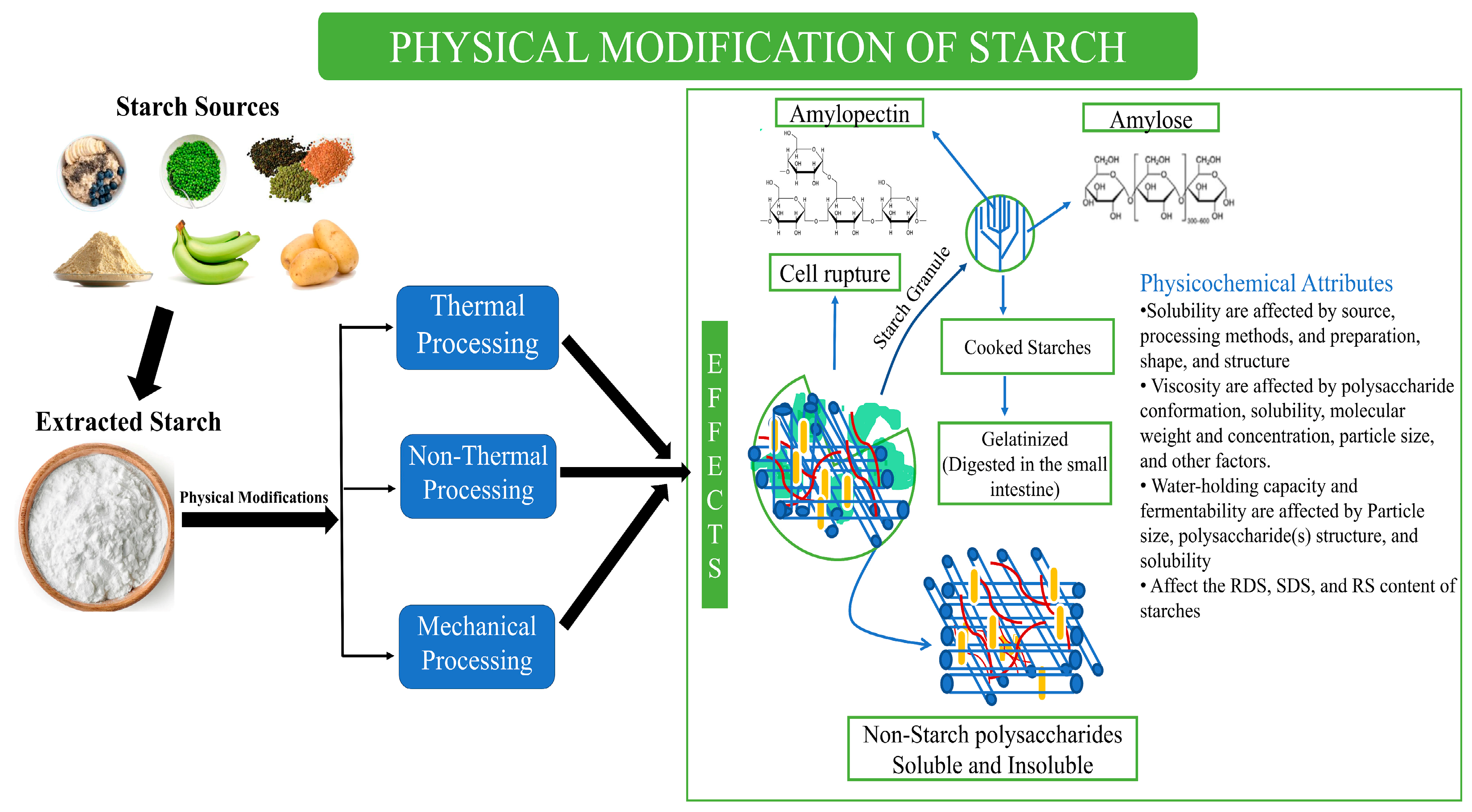
2.1. Physical Modification
2.1.1. Mechanical Processing
Milling
Grinding
Extrusion
2.2. Thermal Treatments
2.2.1. Heat–Moisture Treatment (HMT)
2.2.2. Annealing
2.2.3. Roasting
2.2.4. Microwave
2.3. Non-Thermal Processing
2.3.1. High-Pressure Processing (HPP)
2.3.2. Irradiation
2.3.3. Ultrasonication
2.3.4. Pulsed Electric Field (PEF)
3. Differences among Modification Methods and Their Effects on RS
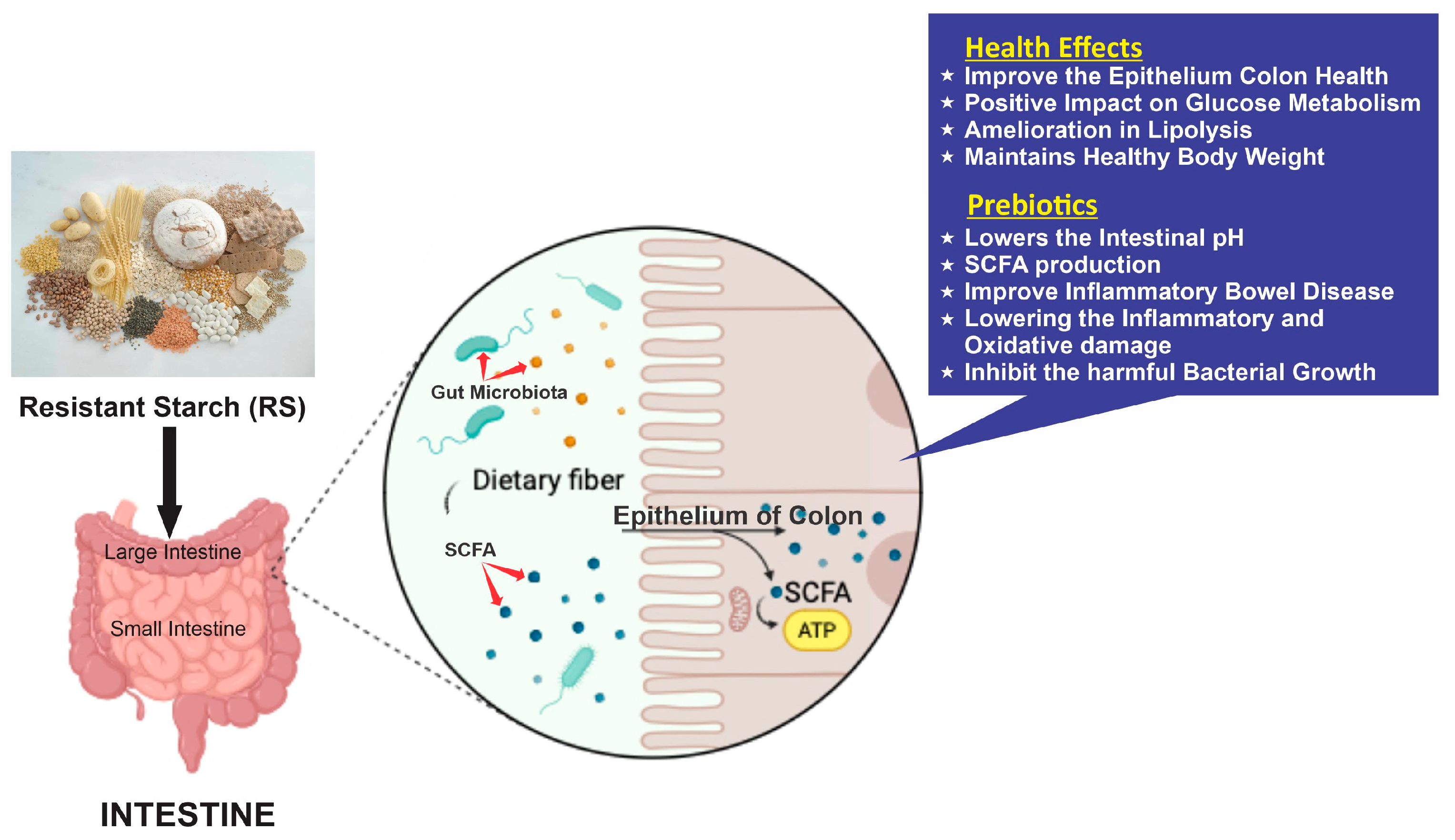
4. Health Benefits of Resistant Starch
5. Applications of Modified Resistant Starch
6. Conclusions
7. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2020, 61, 66–71. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Xu, Q.; Kong, Q.; Li, F.; Lu, L.; Xu, Y.; Wei, Y. Synthesis and functions of resistant starch. Adv. Nutr. 2023, 14, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Bojarczuk, A.; Skąpska, S.; Khaneghah, A.M.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Lockyer, S.; Nugent, A. Health effects of resistant starch. Nutr. Bull. 2017, 42, 10–41. [Google Scholar] [CrossRef]
- Jiali, L.; Wu, Z.; Liu, L.; Yang, J.; Wang, L.; Li, Z.; Liu, L. The research advance of resistant starch: Structural characteristics, modification method, immunomodulatory function, and its delivery systems application. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef]
- Punia, S. Barley starch modifications: Physical, chemical and enzymatic—A review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef]
- Chibuogwu, C.; Amadi, B.; Anyaegbunam, Z.; Emesiani, B.; Ofoefule, S. Application of starch and starch derivatives in pharmaceutical formulation. In Chemical Properties of Starch; IntechOpen: London, UK, 2020; pp. 1–10. [Google Scholar]
- Akbarian, M.; Ghasemi, Y.; Uversky, V.N.; Yousefi, R. Chemical modifications of insulin: Finding a compromise between stability and pharmaceutical performance. Int. J. Pharm. 2018, 547, 450–468. [Google Scholar] [CrossRef]
- Hong, J.; Zeng, X.A.; Brennan, C.S.; Brennan, M.; Han, Z. Recent advances in techniques for starch esters and the applications: A review. Foods 2016, 53, 50. [Google Scholar] [CrossRef] [PubMed]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: A review. Enzym. Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef]
- Tran, T.T.; Shelat, K.J.; Tang, D.; Li, E.; Gilbert, R.G.; Hasjim, J. Milling of rice grains. The degradation on three structural levels of starch in rice flour can be independently controlled during grinding. J. Agric. Food Chem. 2011, 59, 3964–3973. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Flanagan, B.M.; Hasjim, J.; Gidley, M.J. Cryo-milling of starch granules leads to differential effects on molecular size and conformation. Carbohydr. Polym. 2011, 84, 1133–1140. [Google Scholar] [CrossRef]
- Stark, J.; Yin, X. The effect of physical damage on large and small barley starch granules. Starch-Stärke 1986, 38, 369–374. [Google Scholar] [CrossRef]
- Morrison, W.; Tester, R.; Gidley, M. Properties of damaged starch granules. II. Crystallinity, molecular order and gelatinisation of ball-milled starches. J. Cereal Sci. 1994, 19, 209–217. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Y.; Chen, X.; Zhang, L.; Li, H.; Sui, Z.; Corke, H. Polishing conditions in rice milling differentially affect the physicochemical properties of waxy, low-and high-amylose rice starch. J. Cereal Sci. 2021, 99, 103183. [Google Scholar] [CrossRef]
- Li, F.; Guan, X.; Li, C. Effects of degree of milling on the starch digestibility of cooked rice during (in vitro) small intestine digestion. Int. J. Biol. Macromol. 2021, 188, 774–782. [Google Scholar] [CrossRef]
- Situ, W.; Song, X.; Luo, S.; Yang, J. Digestibility and structures of vinasse starches with different types of raw rice and fermented leaven. Food Chem. 2019, 294, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Shrestha, A.K.; Gidley, M.J. Effect of cryo-milling on starches: Functionality and digestibility. Food Hydrocoll. 2010, 24, 152–163. [Google Scholar] [CrossRef]
- Fu, Z.; Luo, S.J.; Liu, W.; Liu, C.M.; Zhan, L.j. Structural changes induced by high speed jet on in vitro digestibility and hydroxypropylation of rice starch. Int. J. Food Sci. Technol. 2016, 51, 1034–1040. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Wang, J.; Li, C.; Xin, Q.; Huang, W.; Zhang, Y.; He, Z.; Wang, S. Effect of laboratory milling on properties of starches isolated from different flour millstreams of hard and soft wheat. Food Chem. 2015, 172, 504–514. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; dos Santos Pereira, T.; de Andrade Freire, V.; Santiago, Â.M.; Oliveira, H.M.L.; de Sousa Conrado, L.; de Gusmão, R.P. Influence of enzymatic hydrolysis on the properties of red rice starch. Int. J. Biol. Macromol. 2019, 141, 1210–1219. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, L.; Li, M.; He, X.; Hao, L.; Dai, Y. Physicochemical properties and digestibility of potato starch treated by ball milling with tea polyphenols. Int. J. Biol. Macromol. 2019, 129, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guo, Y.; Zhao, S.; Kong, J.; Qiao, D.; Lin, L.; Lin, Q.; Zhang, B. Amylose content and molecular-order stability synergistically affect the digestion rate of indica rice starches. Int. J. Biol. Macromol. 2020, 144, 373–379. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Guo, X.; Liang, Y.; Xie, F. Understanding the multi-scale structure and digestibility of different waxy maize starches. Int. J. Biol. Macromol. 2020, 144, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gao, F.; Yin, D.-M.; Luo, Q.; Fu, Z.-Q.; Zhou, Y.-G.J. Processing of superfine grinding corn straw fiber-reinforced starch film and the enhancement on its mechanical properties. Polymers 2018, 10, 855. [Google Scholar] [CrossRef]
- Zhang, K.; Dai, Y.; Hou, H.; Li, X.; Dong, H.; Wang, W.; Zhang, H. Influences of grinding on structures and properties of mung bean starch and quality of acetylated starch. Food Chem. 2019, 294, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xing, F.; Dai, Y.; Hou, H.; Wang, W.; Wang, B.; Zhang, H.; Li, C.J. Preparation of starch-lipid complexes under wet grinding and its mechanism analysis. Cereal Chem. 2023, 100, 1059–1070. [Google Scholar] [CrossRef]
- Alam, M.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and extruded products: Changes in quality attributes as affected by extrusion process parameters: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef]
- Shelar, G.A.; Gaikwad, S.T. Extrusion in food processing: An overview. Pharma Innov. J. 2019, 8, 562–568. [Google Scholar]
- Cappa, C.; Masseroni, L.; Ng, P.K.; Alamprese, C. Effect of extrusion conditions on the physical and chemical properties of bean powders. J. Food Process. Preserv. 2020, 44, e14608. [Google Scholar] [CrossRef]
- Neder-Suárez, D.; Amaya-Guerra, C.A.; Pérez-Carrillo, E.; Quintero-Ramos, A.; Mendez-Zamora, G.; Sánchez-Madrigal, M.Á.; Barba-Dávila, B.A.; Lardizábal-Gutiérrez, D. Optimization of an extrusion cooking process to increase formation of resistant starch from corn starch with addition of citric acid. Starch-Starke 2020, 72, 1900150. [Google Scholar] [CrossRef]
- Al-Rabadi, G.J.; Torley, P.J.; Williams, B.A.; Bryden, W.L.; Gidley, M.J. Effect of extrusion temperature and pre-extrusion particle size on starch digestion kinetics in barley and sorghum grain extrudates. Anim. Feed. Sci. Technol. 2011, 168, 267–279. [Google Scholar] [CrossRef]
- Faraj, A.; Vasanthan, T.; Hoover, R. The effect of extrusion cooking on resistant starch formation in waxy and regular barley flours. Food Res. Int. 2004, 37, 517–525. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Zhang, R.; Zhong, Y.; Luo, Y.; Xu, S.; Liu, J.; Xue, J.; Guo, D. Effects of extrusion treatment on physicochemical properties and in vitro digestion of pregelatinized high amylose maize flour. J. Cereal Sci. 2016, 68, 108–115. [Google Scholar] [CrossRef]
- Robin, F.; Heindel, C.; Pineau, N.; Srichuwong, S.; Lehmann, U. Effect of maize type and extrusion-cooking conditions on starch digestibility profiles. Int. J. Food Sci. Technol. 2016, 51, 1319–1326. [Google Scholar] [CrossRef]
- Colonna, P.; Mercier, C. Macromolecular modifications of manioc starch components by extrusion-cooking with and without lipids. Carbohydr. Polym. 1983, 3, 87–108. [Google Scholar] [CrossRef]
- Kamau, E.H.; Nkhata, S.G.; Ayua, E.O. Extrusion and nixtamalization conditions influence the magnitude of change in the nutrients and bioactive components of cereals and legumes. Food Sci. Nutr. 2020, 8, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Björck, I.; Ostrowska, S.; Eliasson, A.-C.; Asp, N.-G.; Larsson, K.; Lundquist, I. Digestibility of Amylose-Lipid Complexes in-vitro and in-vivo. Starch—Stärke 1983, 35, 294–297. [Google Scholar] [CrossRef]
- Morales-Sánchez, E.; Cabrera-Ramírez, A.H.; Gaytán-Martínez, M.; Mendoza-Zuvillaga, A.L.; Velázquez, G.; Méndez-Montealvo, M.G.; Rodríguez-García, M.E. Heating-cooling extrusion cycles as a method to improve the physicochemical properties of extruded corn starch. Int. J. Biol. Macromol. 2021, 188, 620–627. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Hanna, M. Amylose-lipid complex formation during single-screw extrusion of various corn starches. Cereal Chem. 1994, 71, 582–587. [Google Scholar]
- Wang, S.; Wang, J.; Yu, J.; Wang, S. Effect of fatty acids on functional properties of normal wheat and waxy wheat starches: A structural basis. Food Chem. 2016, 190, 285–292. [Google Scholar] [CrossRef]
- Thachil, M.T.; Chouksey, M.K.; Gudipati, V. Amylose-lipid complex formation during extrusion cooking: Effect of added lipid type and amylose level on corn-based puffed snacks. Int. J. Food Sci. Technol. 2014, 49, 309–316. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M.; Brennan, C. Effect of guar gum content on some physical and nutritional properties of extruded products. J. Food Eng. 2011, 103, 324–332. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Hoover, R.; Warkentin, T.D.; Vandenberg, B. In vitro starch digestibility, expected glycemic index, and thermal and pasting properties of flours from pea, lentil and chickpea cultivars. Food Chem. 2008, 111, 316–321. [Google Scholar] [CrossRef]
- Aarathi, A.; Urooj, A.; Puttaraj, S. In vitro Starch Digestibility and Nutritionally Important Starch Fractions in Cereals and Their Mixtures. Starch—Stärke 2003, 55, 94–99. [Google Scholar] [CrossRef]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of Extrusion Cooking on Functional Properties and in vitro Starch Digestibility of Barley-Based Extrudates from Fruit and Vegetable By-Products. J. Food Sci. 2009, 74, E77–E86. [Google Scholar] [CrossRef] [PubMed]
- Karkle, E.L.; Keller, L.; Dogan, H.; Alavi, S. Matrix transformation in fiber-added extruded products: Impact of different hydration regimens on texture, microstructure and digestibility. J. Food Eng. 2012, 108, 171–182. [Google Scholar] [CrossRef]
- Pratiwi, M.; Faridah, D.N.; Lioe, H.N. Structural changes to starch after acid hydrolysis, debranching, autoclaving-cooling cycles, and heat moisture treatment (HMT): A review. Starch-Starke 2018, 70, 1700028. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and potential applications: A review of the modification of starches by heat-moisture treatment (HMT). Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. Recent advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2021, 344, 128700. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. A review of the hydrothermal treatments impact on starch based systems properties. Crit. Rev. Food Sci. Nutr. 2020, 60, 3890–3915. [Google Scholar] [CrossRef]
- Khatun, A.; Waters, D.L.; Liu, L. A review of rice starch digestibility: Effect of composition and heat-moisture processing. Starch-Stärke 2019, 71, 1900090. [Google Scholar] [CrossRef]
- Liu, H.; Lv, M.; Wang, L.; Li, Y.; Fan, H.; Wang, M. Comparative study: How annealing and heat-moisture treatment affect the digestibility, textural, and physicochemical properties of maize starch. Starch-Stärke 2016, 68, 1158–1168. [Google Scholar] [CrossRef]
- Chung, H.-J.; Cho, D.-W.; Park, J.-D.; Kweon, D.-K.; Lim, S.-T. In vitro starch digestibility and pasting properties of germinated brown rice after hydrothermal treatments. J. Cereal Sci. 2012, 56, 451–456. [Google Scholar] [CrossRef]
- Brahma, B.; Sit, N. Physicochemical properties and digestibility of heat moisture–treated potato starches for different treatment conditions. Potato Res. 2020, 63, 367–383. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, B.; Su, C.; Gong, B.; Zheng, J.; Jiang, H.; Zhang, G.; Li, W. Repeated heat-moisture treatment: A more effectiveway for structural and physicochemical modification of mung bean starch compared with continuous way. Food Bioprocess Technol. 2020, 13, 452–461. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Chen, L.; Li, X. Understanding the structure and digestibility of heat-moisture treated starch. Int. J. Biol. Macromol. 2016, 88, 1–8. [Google Scholar] [CrossRef]
- Ashogbon, A. Current Research Addressing Physical Modification of Starch from Various Botanical Sources. Glob. Nutr. Diet. 2018, 1, 001. [Google Scholar]
- Zhong, Y.; Xiang, X.; Zhao, J.; Wang, X.; Chen, R.; Xu, J.; Luo, S.; Wu, J.; Liu, C. Microwave pretreatment promotes the annealing modification of rice starch. Food Chem. 2020, 304, 125432. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, S.; Wang, S. Annealing improves paste viscosity and stability of starch. Food Hydrocoll. 2017, 62, 203–211. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Lu, P.; Miao, S.; Zhang, Y.; Chen, L. Dry heating and annealing treatment synergistically modulate starch structure and digestibility. Int. J. Biol. Macromol. 2019, 137, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Anugerah, M.P.; Faridah, D.N.; Afandi, F.A.; Hunaefi, D.; Jayanegara, A. Annealing processing technique divergently affects starch crystallinity characteristic related to resistant starch content: A literature review and meta-analysis. Int. J. Food Sci. Technol. 2022, 57, 2535–2544. [Google Scholar] [CrossRef]
- Chang, R.; Lu, H.; Bian, X.; Tian, Y.; Jin, Z. Ultrasound assisted annealing production of resistant starches type 3 from fractionated debranched starch: Structural characterization and in-vitro digestibility. Food Hydrocoll. 2021, 110, 106141. [Google Scholar] [CrossRef]
- Fonseca, L.M.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, C.; Sunooj, K.V.; Anjali, K.; Aaliya, B.; Navaf, M.; Kumar, S.; Sajeevkumar, V.A.; George, J. Effect of lysine incorporation, annealing and heat moisture treatment alone and in combination on the physico-chemical, retrogradation, rheological properties and in vitro digestibility of kithul (Caryota urens L.) starch. Int. J. Food Sci. Technol. 2020, 55, 2391–2398. [Google Scholar] [CrossRef]
- Song, H.Y.; Lee, S.Y.; Choi, S.J.; Kim, K.M.; Kim, J.S.; Han, G.J.; Moon, T.W. Digestibility and physicochemical properties of granular sweet potato starch as affected by annealing. Food Sci. Biotechnol. 2014, 23, 23–31. [Google Scholar] [CrossRef]
- Shi, X.; Ding, Y.; Wan, J.; Liu, C.; Prakash, S.; Xia, X. Effect of annealing on structural, physicochemical, and in vitro digestive properties of starch from castanopsis sclerophylla. Starch-Stärke 2021, 73, 2100005. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yu, J.; Wang, S. A comparative study of annealing of waxy, normal and high-amylose maize starches: The role of amylose molecules. Food Chem. 2014, 164, 332–338. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Premjit, Y.; Pandiselvam, R.; Kothakota, A.; Ramesh, S.V. An overview of conventional and emerging techniques of roasting: Effect on food bioactive signatures. Food Chem. 2021, 348, 129088. [Google Scholar] [CrossRef]
- Frisullo, P.; Barnabà, M.; Navarini, L.; Del Nobile, M.A. Coffea arabica beans microstructural changes induced by roasting: An X-ray microtomographic investigation. J. Food Eng. 2012, 108, 232–237. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Zhao, B.; Shang, J.; Liu, L.; Tong, L.; Zhou, X.; Wang, S.; Zhang, Y.; Wang, L.; Zhou, S. Effect of roasting process on enzymes inactivation and starch properties of highland barley. Int. J. Biol. Macromol. 2020, 165, 675–682. [Google Scholar] [CrossRef]
- Torbica, A.; Pećinar, I.; Lević, S.; Belović, M.; Jovičić, M.; Stevanović, Z.D.; Nedović, V. Insight in changes in starch and proteins molecular structure of non-wheat cereal flours influenced by roasting and extrusion treatments. Food Hydrocoll. 2023, 140, 108591. [Google Scholar] [CrossRef]
- Platel, K.; Shurpalekar, K. Resistant starch content of Indian foods. Plant Foods Hum. Nutr. 1994, 45, 91–95. [Google Scholar] [CrossRef]
- Holm, J.; Lundquist, I.; Björck, I.; Eliasson, A.-C.; Asp, N.-G. Degree of starch gelatinization, digestion rate of starch in vitro, and metabolic response in rats. Am. J. Clin. Nutr. 1988, 47, 1010–1016. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, K. Changes in the characteristics of indica rice on the process of flaking. J. Food Eng. 2018, 6, 2310–2317. [Google Scholar]
- Kanagaraj, S.P.; Ponnambalam, D.; Antony, U. Effect of dry heat treatment on the development of resistant starch in rice (Oryza sativa) and barnyard millet (Echinochloa furmantacea). J. Food Process. Preserv. 2019, 43, e13965. [Google Scholar] [CrossRef]
- Wen, H. Research Progress on the Impact of Nuclear Radiation in Food on Human Health. Highlights Sci. Eng. Technol. 2024, 91, 105–117. [Google Scholar] [CrossRef]
- Tao, Y.; Yan, B.; Fan, D.; Zhang, N.; Ma, S.; Wang, L.; Wu, Y.; Wang, M.; Zhao, J.; Zhang, H. Structural changes of starch subjected to microwave heating: A review from the perspective of dielectric properties. Trends Food Sci. Technol. 2020, 99, 593–607. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Zailani, M.A. Microwave Irradiation of Starch. In Starch: Advances in Modifications, Technologies and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 361–384. [Google Scholar]
- Yi, M.; Tang, X.; Liang, S.; He, R.; Huang, T.; Lin, Q.; Zhang, R. Effect of microwave alone and microwave-assisted modification on the physicochemical properties of starch and its application in food. Food Chem. 2024, 446, 138841. [Google Scholar] [CrossRef]
- Yılmaz, A.; Tugrul, N. Effect of ultrasound-microwave and microwave-ultrasound treatment on physicochemical properties of corn starch. Ultrason. Sonochemistry 2023, 98, 106516. [Google Scholar] [CrossRef]
- Lewandowicz, G.; Jankowski, T.; Fornal, J. Effect of microwave radiation on physico-chemical properties and structure of cereal starches. Carbohydr. Polym. 2000, 42, 193–199. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.R. Behaviour of starch exposed to microwave radiation treatment. Starch-Stärke 2014, 66, 3–14. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, L.; Luo, Z.; Kong, X.; Xiao, Z.; Wang, P.; Peng, X. Effect of microwave irradiation on internal molecular structure and physical properties of waxy maize starch. Food Hydrocoll. 2017, 69, 473–482. [Google Scholar] [CrossRef]
- Fan, D.; Wang, L.; Chen, W.; Ma, S.; Ma, W.; Liu, X.; Zhao, J.; Zhang, H. Effect of microwave on lamellar parameters of rice starch through small-angle X-ray scattering. Food Hydrocoll. 2014, 35, 620–626. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Park, S.S.; Lim, S.-T. Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Deka, D.; Sit, N. Dual modification of taro starch by microwave and other heat moisture treatments. Int. J. Biol. Macromol. 2016, 92, 416–422. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, X.; Lin, S.; Zeng, H.; Lu, X.; Zhang, Y.; Zheng, B. Structural characteristics and physicochemical properties of lotus seed resistant starch prepared by different methods. Food Chem. 2015, 186, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Palav, T.; Seetharaman, K. Impact of microwave heating on the physico-chemical properties of a starch–water model system. Carbohydr. Polym. 2007, 67, 596–604. [Google Scholar] [CrossRef]
- Bilbao-Sáinz, C.; Butler, M.; Weaver, T.; Bent, J. Wheat starch gelatinization under microwave irradiation and conduction heating. Carbohydr. Polym. 2007, 69, 224–232. [Google Scholar] [CrossRef]
- Mutlu, S.; Kahraman, K.; Öztürk, S. Optimization of resistant starch formation from high amylose corn starch by microwave irradiation treatments and characterization of starch preparations. Int. J. Biol. Macromol. 2017, 95, 635–642. [Google Scholar] [CrossRef]
- Li, R.; Dai, L.; Peng, H.; Jiang, P.; Liu, N.; Zhang, D.; Wang, C.; Li, Z. Effects of microwave treatment on sorghum grains: Effects on the physicochemical properties and in vitro digestibility of starch. J. Food Process Eng. 2021, 44, e13804. [Google Scholar] [CrossRef]
- Gulzar, S.; Narciso, J.O.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Recent developments in the application of novel technologies for the modification of starch in light of 3D food printing. Curr. Opin. Food Sci. 2023, 52, 101067. [Google Scholar] [CrossRef]
- Vishvaa, A.; Preethi, R.; Shweta, D.; Jayan, H.; Moses, J.; Anandharamakrishnan, C. High-Pressure Processing of Foods. In Emerging Technologies for the Food Industry; Apple Academic Press: Williston, VT, USA, 2024; pp. 1–47. [Google Scholar]
- Nath, K.G.; Pandiselvam, R.; Sunil, C. High-pressure processing: Effect on textural properties of food-A review. J. Food Eng. 2023, 351, 111521. [Google Scholar] [CrossRef]
- Houška, M.; Silva, F.V.M.; Evelyn; Buckow, R.; Terefe, N.S.; Tonello, C. High pressure processing applications in plant foods. Foods 2022, 11, 223. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Cao, R.; Blanchard, C.; Wang, M. Physicochemical properties and in vitro digestibility of sorghum starch altered by high hydrostatic pressure. Int. J. Biol. Macromol. 2016, 92, 753–760. [Google Scholar] [CrossRef]
- Colussi, R.; Kaur, L.; da Rosa Zavareze, E.; Dias, A.R.G.; Stewart, R.; Singh, J. High pressure processing and retrogradation of potato starch: Influence on functional properties and gastro-small intestinal digestion in vitro. Food Hydrocoll. 2018, 75, 131–137. [Google Scholar] [CrossRef]
- Bajaj, R.; Singh, N.; Ghumman, A.; Kaur, A.; Mishra, H.N. Effect of high pressure treatment on structural, functional, and in-vitro digestibility of starches from tubers, cereals, and beans. Starch-Stärke 2022, 74, 2100096. [Google Scholar] [CrossRef]
- Zhi-Guang, C.; Hong-Hui, Z.; Keipper, W.; Hua-Yin, P.; Qi, Y.; Chen-Lu, F.; Guo-Wei, S.; Jun-Rong, H. The analysis of the effects of high hydrostatic pressure (HHP) on amylose molecular conformation at atomic level based on molecular dynamics simulation. Food Chem. 2020, 327, 127047. [Google Scholar] [CrossRef]
- Zhiguang, C.; Junrong, H.; Huayin, P.; Keipper, W. The effects of temperature on starch molecular conformation and hydrogen bonding. Starch-Stärke 2022, 74, 2100288. [Google Scholar] [CrossRef]
- Hsiao, Y.-T.; Wang, C.-Y. Microbial shelf-life, starch physicochemical properties, and in vitro digestibility of pigeon pea Milk altered by high pressure processing. Molecules 2020, 25, 2516. [Google Scholar] [CrossRef]
- Wei, J.; Meng, Z.; Yang, L.; Jinlong, W. Research on the digestibility of glutinous rice starch based on high hydrostatic pressure technology. IOP Conf. Ser. Earth Environ. Sci. 2019, 295, 042096. [Google Scholar] [CrossRef]
- Shen, X.; Shang, W.; Strappe, P.; Chen, L.; Li, X.; Zhou, Z.; Blanchard, C. Manipulation of the internal structure of high amylose maize starch by high pressure treatment and its diverse influence on digestion. Food Hydrocoll. 2018, 77, 40–48. [Google Scholar] [CrossRef]
- Hu, X.-P.; Zhang, B.; Jin, Z.-Y.; Xu, X.-M.; Chen, H.-Q. Effect of high hydrostatic pressure and retrogradation treatments on structural and physicochemical properties of waxy wheat starch. Food Chem. 2017, 232, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Al-Attar, H. Structural properties of high-pressure-treated chestnut flour dispersions. Int. J. Food Prop. 2017, 20, S766–S778. [Google Scholar] [CrossRef]
- Papathanasiou, M.; Reineke, K.; Gogou, E.; Taoukis, P.; Knorr, D. Impact of high pressure treatment on the available glucose content of various starch types: A case study on wheat, tapioca, potato, corn, waxy corn and resistant starch (RS3). Innov. Food Sci. Emerg. Technol. 2015, 30, 24–30. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, X.; Wang, F.; Li, J.; Si, X.; Cao, R.; Yang, R.; Strappe, P.; Blanchard, C. High pressure processing manipulated buckwheat antioxidant activity, anti-adipogenic properties and starch digestibility. J. Cereal Sci. 2015, 66, 31–36. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, Y.; Luo, Y.; Zhong, Y.; Yue, J.; Song, X.; Zhao, Y. Impact of continuous or cycle high hydrostatic pressure on the ultrastructure and digestibility of rice starch granules. J. Cereal Sci. 2014, 60, 302–310. [Google Scholar] [CrossRef]
- Linsberger-Martin, G.; Lukasch, B.; Berghofer, E. Effects of high hydrostatic pressure on the RS content of amaranth, quinoa and wheat starch. Starch-Stärke 2012, 64, 157–165. [Google Scholar] [CrossRef]
- Lei, X.; Yu, J.; Hu, Y.; Bai, J.; Feng, S.; Ren, Y. Comparative investigation of the effects of electron beam and X-ray irradiation on potato starch: Structure and functional properties. Int. J. Biol. Macromol. 2023, 236, 123909. [Google Scholar] [CrossRef]
- Habib, M.; Jan, K.; Qureshi, I.; Rani, S.; Bashir, K. Gamma Irradiation of Starch. In Starch: Advances in Modifications, Technologies and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 385–407. [Google Scholar]
- Kong, X. Gamma irradiation of starch. In Physical Modifications of Starch; Springer: Berlin/Heidelberg, Germany, 2023; pp. 103–143. [Google Scholar]
- Barroso, A.G.; del Mastro, N.L. Physicochemical characterization of irradiated arrowroot starch. Radiat. Phys. Chem. 2019, 158, 194–198. [Google Scholar] [CrossRef]
- Ben Bettaïeb, N.; Jerbi, M.T.; Ghorbel, D. Gamma radiation influences pasting, thermal and structural properties of corn starch. Radiat. Phys. Chem. 2014, 103, 1–8. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Sinha, S.K.; George, J.; Kumar, S.; Murugesan, P.; Arumugam, S.; Ashwath Kumar, K.; Sajeev Kumar, V.A. Impact of energetic neutral nitrogen atoms created by glow discharge air plasma on the physico-chemical and rheological properties of kithul starch. Food Chem. 2019, 294, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Liu, Q. Effect of gamma irradiation on molecular structure and physicochemical properties of corn starch. J. Food Sci. 2009, 74, C353–C361. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Liu, Q. Molecular structure and physicochemical properties of potato and bean starches as affected by gamma-irradiation. Int. J. Biol. Macromol. 2010, 47, 214–222. [Google Scholar] [CrossRef]
- Chung, H.-J.; Lee, S.-Y.; Kim, J.-H.; Lee, J.-W.; Byun, M.-W.; Lim, S.-T. Pasting characteristics and in vitro digestibility of γ-irradiated RS4 waxy maize starches. J. Cereal Sci. 2010, 52, 53–58. [Google Scholar] [CrossRef]
- Lee, J.-S.; Ee, M.-L.; Chung, K.-H.; Othman, Z. Formation of resistant corn starches induced by gamma-irradiation. Carbohydr. Polym. 2013, 97, 614–617. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Yoo, J.-Y.; Kim, J.-H.; Lee, J.-W.; Byun, M.-W.; Baik, B.-K.; Lim, S.-T. In vitro digestibility of gamma-irradiated corn starches. Carbohydr. Polym. 2010, 81, 961–963. [Google Scholar] [CrossRef]
- Yang, J.; Pan, M.; Han, R.; Yang, X.; Liu, X.; Yuan, S.; Wang, S. Food irradiation: An emerging processing technology to improve the quality and safety of foods. Food Rev. Int. 2023, 1–23. [Google Scholar] [CrossRef]
- Lima, F.F.; Andrade, C.T. Effect of melt-processing and ultrasonic treatment on physical properties of high-amylose maize starch. Ultrason. Sonochemistry 2010, 17, 637–641. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Rokita, B.; Lotfy, S.; Ulanski, P.; Rosiak, J.M. Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr. Polym. 2005, 60, 175–184. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Chen, L.; Li, X. Study on supramolecular structural changes of ultrasonic treated potato starch granules. Food Hydrocoll. 2012, 29, 116–122. [Google Scholar] [CrossRef]
- Izidoro, D.R.; Sierakowski, M.-R.; Haminiuk, C.W.I.; De Souza, C.F.; de Paula Scheer, A. Physical and chemical properties of ultrasonically, spray-dried green banana (Musa cavendish) starch. J. Food Eng. 2011, 104, 639–648. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Herceg, Z.; Šubarić, D.; Babić, J.; Brnčić, M.; Brnčić, S.R.; Bosiljkov, T.; Čvek, D.; Tripalo, B.; Gelo, J. Ultrasound effect on physical properties of corn starch. Carbohydr. Polym. 2010, 79, 91–100. [Google Scholar] [CrossRef]
- Bonto, A.P.; Tiozon, R.N., Jr.; Sreenivasulu, N.; Camacho, D.H. Impact of ultrasonic treatment on rice starch and grain functional properties: A review. Ultrason. Sonochemistry 2021, 71, 105383. [Google Scholar] [CrossRef]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research progress and application of ultrasonic-and microwave-assisted food processing technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef]
- Zhu, F. Impact of ultrasound on structure, physicochemical properties, modifications, and applications of starch. Trends Food Sci. Technol. 2015, 43, 1–17. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, S.; Lv, C.; Chen, Z. Preparation and physicochemical properties of Cyperus esculentus starch from its tubers using ultrasoundassisted alkali method. BioResources 2023, 18. [Google Scholar]
- Kumar, G.; Le, D.T.; Durco, J.; Cianciosi, S.; Devkota, L.; Dhital, S. Innovations in legume processing: Ultrasound-based strategies for enhanced legume hydration and processing. Trends Food Sci. Technol. 2023, 139, 104122. [Google Scholar] [CrossRef]
- Castro, L.M.G.; Caço, A.I.; Pereira, C.F.; Sousa, S.C.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Structure and properties of Quercus robur acorn starch extracted by pulsed electric field technology. Int. J. Biol. Macromol. 2024, 260, 129328. [Google Scholar] [CrossRef]
- de Castro, M.; Baptista, J.; Matos, C.; Valente, A.; Briga-Sá, A. Energy efficiency in winemaking industry: Challenges and opportunities. Sci. Total Environ. 2024, 930, 172383. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Q.-Y.; Jiang, W.; Qian, J.-Y.; Zhang, L.; Wu, M.; Rao, S.-Q.; Wu, C.-S. Effect of pulsed electric field on structural properties and digestibility of starches with different crystalline type in solid state. Carbohydr. Polym. 2019, 207, 362–370. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Q.-Y.; Wu, M.; Jiang, W.; Qian, J.-Y.; Rao, S.-Q.; Zhang, L.; Li, Q.; Zhang, C. Effect of pulsed electric field on properties and multi-scale structure of japonica rice starch. LWT 2019, 116, 108515. [Google Scholar] [CrossRef]
- Zeng, F.; Gao, Q.-Y.; Han, Z.; Zeng, X.-A.; Yu, S.-J. Structural properties and digestibility of pulsed electric field treated waxy rice starch. Food Chem. 2016, 194, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yu, Q.; Zeng, X.A.; Luo, D.H.; Yu, S.J.; Zhang, B.S.; Chen, X.D. Studies on the microstructure and thermal properties of pulsed electric fields (PEF)-treated maize starch. Int. J. Food Eng. 2012, 8, 1–17. [Google Scholar] [CrossRef]
- Abduh, S.B.; Leong, S.Y.; Agyei, D.; Oey, I. Understanding the properties of starch in potatoes (Solanum tuberosum var. Agria) after being treated with pulsed electric field processing. Foods 2019, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Máthé, E.; Csapó, J.; Sipos, P. Modifying effects of physical processes on starch and dietary fiber content of foodstuffs. Processes 2020, 9, 17. [Google Scholar] [CrossRef]
- Bangar, S.P.; Singh, A.; Ashogbon, A.O.; Bobade, H. Ball-milling: A sustainable and green approach for starch modification. Int. J. Biol. Macromol. 2023, 237, 124069. [Google Scholar] [CrossRef]
- Bulgakov, V.; Pascuzzi, S.; Ivanovs, S.; Kaletnik, G.; Yanovich, V. Angular oscillation model to predict the performance of a vibratory ball mill for the fine grinding of grain. Biosyst. Eng. 2018, 171, 155–164. [Google Scholar] [CrossRef]
- Li, W.; Pagán-Jiménez, J.R.; Tsoraki, C.; Yao, L.; Van Gijn, A. Influence of grinding on the preservation of starch grains from rice. Archaeometry 2020, 62, 157–171. [Google Scholar] [CrossRef]
- Rojas-Molina, I.; Mendoza-Avila, M.; Cornejo-Villegas, M.d.l.Á.; Real-López, A.D.; Rivera-Muñoz, E.; Rodríguez-García, M.; Gutiérrez-Cortez, E. Physicochemical properties and resistant starch content of corn tortilla flours refrigerated at different storage times. Foods 2020, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, B.; Hussain, S.Z.; Naseer, B.; Naik, H.R. Enhancement of resistant starch content in modified rice flour using extrusion technology. Cereal Chem. 2021, 98, 634–641. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, C.; McClements, D.J. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Bao, J.; Zhou, X.; Hu, Y.; Zhang, Z. Resistant starch content and physicochemical properties of non-waxy rice starches modified by pullulanase, heat-moisture treatment, and citric acid. J. Cereal Sci. 2022, 105, 103472. [Google Scholar] [CrossRef]
- Faridah, D.N.; Anugerah, M.P.; Hunaefi, D.; Afandi, F.A.; Jayanegara, A. The effect of annealing on resistant starch content of different crop types: A systematic review and meta-analysis study. Int. J. Food Sci. Technol. 2022, 57, 2026–2038. [Google Scholar] [CrossRef]
- Yang, R.; Tang, J.; Zhao, Q.; Piao, Z.; Lee, G.; Wan, C.; Bai, J. Starch Properties of Roasting Rice from Naturally High-Resistant Starch Rice Varieties. Molecules 2023, 28, 6408. [Google Scholar] [CrossRef]
- Park, J.; Oh, S.-K.; Chung, H.-J.; Shin, D.S.; Choi, I.; Park, H.-J. Effect of steaming and roasting on the quality and resistant starch of brown rice flour with high amylose content. LWT 2022, 167, 113801. [Google Scholar] [CrossRef]
- Lam, N.D.; Quynh, T.M.; Diep, T.B.; Binh, P.T.; Lam, T.D. Effect of gamma irradiation and pyrolysis on indigestible fraction, physicochemical properties, and molecular structure of rice starch. J. Food Process. Preserv. 2021, 45, e15880. [Google Scholar] [CrossRef]
- Noor, N.; Gani, A.; Jhan, F.; Jenno, J.; Dar, M.A. Resistant starch type 2 from lotus stem: Ultrasonic effect on physical and nutraceutical properties. Ultrason. Sonochem. 2021, 76, 105655. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Li, J.; Wang, W.; Hu, A.; Zheng, J. Structural and physicochemical properties of resistant starch under combined treatments of ultrasound, microwave, and enzyme. Int. J. Biol. Macromol. 2023, 232, 123331. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.M.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M. Starch extraction and modification by pulsed electric fields. Food Rev. Int. 2023, 39, 2161–2182. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Wang, X.; Meng, Q. Recent advances in processing food powders by using superfine grinding techniques: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2222–2255. [Google Scholar] [CrossRef]
- Shi, L.; Li, W.; Sun, J.; Qiu, Y.; Wei, X.; Luan, G.; Hu, Y.; Tatsumi, E. Grinding of maize: The effects of fine grinding on compositional, functional and physicochemical properties of maize flour. J. Cereal Sci. 2016, 68, 25–30. [Google Scholar] [CrossRef]
- Qiu, C.; Li, P.; Li, Z.; Corke, H.; Sui, Z. Combined speed and duration of milling affect the physicochemical properties of rice flour. Food Hydrocoll. 2019, 89, 188–195. [Google Scholar] [CrossRef]
- Štěrbová, L.; Bradová, J.; Sedláček, T.; Holasová, M.; Fiedlerová, V.; Dvořáček, V.; Smrčková, P. Influence of technological processing of wheat grain on starch digestibility and resistant starch content. Starch-Stärke 2016, 68, 593–602. [Google Scholar] [CrossRef]
- Asmeda, R.; Noorlaila, A.; Norziah, M. Relationships of damaged starch granules and particle size distribution with pasting and thermal profiles of milled MR263 rice flour. Food Chem. 2016, 191, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ashwar, B.A.; Gani, A.; Shah, A.; Wani, I.A.; Masoodi, F.A. Preparation, health benefits and applications of resistant starch—A review. Starch-Stärke 2016, 68, 287–301. [Google Scholar] [CrossRef]
- Liu, Q.; Jiao, A.; Yang, Y.; Wang, Y.; Li, J.; Xu, E.; Yang, G.; Jin, Z. The combined effects of extrusion and recrystallization treatments on the structural and physicochemical properties and digestibility of corn and potato starch. LWT 2021, 151, 112238. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Hoover, R. Effect of single and dual hydrothermal treatments on the crystalline structure, thermal properties, and nutritional fractions of pea, lentil, and navy bean starches. Food Res. Int. 2010, 43, 501–508. [Google Scholar] [CrossRef]
- Zhou, S.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Effect of heat-moisture treatment on the in vitro digestibility and physicochemical properties of starch-hydrocolloid complexes. Food Hydrocoll. 2020, 104, 105736. [Google Scholar] [CrossRef]
- de la Rosa-Millán, J. Physicochemical, molecular, and digestion characteristics of annealed and heat–moisture treated starches under acidic, neutral, or alkaline pH. Cereal Chem. 2017, 94, 770–779. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Fu, X.; Huang, Q. In vitro digestion and physicochemical properties of wheat starch/flour modified by heat-moisture treatment. J. Cereal Sci. 2015, 63, 109–115. [Google Scholar] [CrossRef]
- Yi, D.; Maike, W.; Yi, S.; Xiaoli, S.; Dianxing, W.; Wenjian, S. Physiochemical Properties of Resistant Starch and Its Enhancement Approaches in Rice. Rice Sci. 2021, 28, 31–42. [Google Scholar] [CrossRef]
- Li, S.; Ward, R.; Gao, Q. Effect of heat-moisture treatment on the formation and physicochemical properties of resistant starch from mung bean (Phaseolus radiatus) starch. Food Hydrocoll. 2011, 25, 1702–1709. [Google Scholar] [CrossRef]
- Van Hung, P.; Binh, V.T.; Nhi, P.H.Y.; Phi, N.T.L. Effect of heat-moisture treatment of unpolished red rice on its starch properties and in vitro and in vivo digestibility. Int. J. Biol. Macromol. 2020, 154, 1–8. [Google Scholar] [CrossRef]
- Tester, R.; Debon, S.; Karkalas, J. Annealing of wheat starch. J. Cereal Sci. 1998, 28, 259–272. [Google Scholar] [CrossRef]
- Babu, A.S.; Mohan, R.J.; Parimalavalli, R. Effect of single and dual-modifications on stability and structural characteristics of foxtail millet starch. Food Chem. 2019, 271, 457–465. [Google Scholar] [CrossRef]
- Xu, M.; Saleh, A.S.; Liu, Y.; Jing, L.; Zhao, K.; Wu, H.; Zhang, G.; Yang, S.O.; Li, W. The changes in structural, physicochemical, and digestive properties of red adzuki bean starch after repeated and continuous annealing treatments. Starch-Stärke 2018, 70, 1700322. [Google Scholar] [CrossRef]
- Xu, M.; Saleh, A.S.; Gong, B.; Li, B.; Jing, L.; Gou, M.; Jiang, H.; Li, W. The effect of repeated versus continuous annealing on structural, physicochemical, and digestive properties of potato starch. Food Res. Int. 2018, 111, 324–333. [Google Scholar] [CrossRef]
- Liu, M.; Wu, N.N.; Yu, G.P.; Zhai, X.T.; Chen, X.; Zhang, M.; Tian, X.H.; Liu, Y.X.; Wang, L.P.; Tan, B. Physicochemical properties, structural properties, and in vitro digestibility of pea starch treated with high hydrostatic pressure. Starch-Stärke 2018, 70, 1700082. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Cao, R.; Fan, H.; Wang, M. In vitro digestibility and changes in physicochemical and structural properties of common buckwheat starch affected by high hydrostatic pressure. Carbohydr. Polym. 2016, 144, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Li, Y.; Li, H.; Fan, H.; Wang, M. In vitro digestibility and changes in physicochemical and textural properties of tartary buckwheat starch under high hydrostatic pressure. J. Food Eng. 2016, 189, 64–71. [Google Scholar] [CrossRef]
- Polesi, L.F.; Sarmento, S.B.S.; Canniatti-Brazaca, S.G. Starch Digestibility and Functional Properties of Rice Starch Subjected to Gamma Radiation. Rice Sci. 2018, 25, 42–51. [Google Scholar] [CrossRef]
- Teixeira, B.S.; Garcia, R.H.L.; Takinami, P.Y.I.; del Mastro, N.L. Comparison of gamma radiation effects on natural corn and potato starches and modified cassava starch. Radiat. Phys. Chem. 2018, 142, 44–49. [Google Scholar] [CrossRef]
- Wang, M.; Sun, M.; Zhang, Y.; Chen, Y.; Wu, Y.; Ouyang, J. Effect of microwave irradiation-retrogradation treatment on the digestive and physicochemical properties of starches with different crystallinity. Food Chem. 2019, 298, 125015. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, B.; Wang, L.; Zhao, S.; Qiao, D.; Zhang, L.; Xie, F. Microwave reheating increases the resistant starch content in cooked rice with high water contents. Int. J. Biol. Macromol. 2021, 184, 804–811. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Zhao, S.; Qiao, D.; Jia, C.; Niu, M.; Lin, Q.; Zhang, B. An insight into starch slowly digestible features enhanced by microwave treatment. Food Hydrocoll. 2020, 103, 105690. [Google Scholar] [CrossRef]
- You, Q.; Zhang, X.; Fang, X.; Yin, X.; Luo, C.; Wan, M. Ultrasonic-Assisted Preparation and Characterization of RS3 from Pea Starch. Food Bioprocess Technol. 2019, 12, 1244–1249. [Google Scholar] [CrossRef]
- Vaitkeviciene, R.; Bendoraitiene, J.; Degutyte, R.; Svazas, M.; Zadeike, D. Optimization of the sustainable production of resistant starch in rice bran and evaluation of its physicochemical and technological properties. Polymers 2022, 14, 3662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bao, J. Recent advances in modification approaches, health benefits, and food applications of resistant starch. Starch-Starke 2023, 75, 2100141. [Google Scholar] [CrossRef]
- Kraithong, S.; Wang, S.; Junejo, S.A.; Fu, X.; Theppawong, A.; Zhang, B.; Huang, Q. Type 1 resistant starch: Nutritional properties and industry applications. Food Hydrocoll. 2022, 125, 107369. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Xi, H.; Xu, J.; Deng, D.; Huang, G. Effects of soluble dietary fiber on the crystallinity, pasting, rheological, and morphological properties of corn resistant starch. LWT 2019, 111, 632–639. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Song, Y.-H.; Zhao, R.; Xia, L.; Chen, Y.; Cui, Y.-P.; Rao, Z.-Y.; Zhou, Y.; Zhuang, W.; et al. Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: A systematic review and meta-analysis. Nutr. Diabetes 2019, 9, 19. [Google Scholar] [CrossRef]
- Han, J.; Wu, J.; Liu, X.; Shi, J.; Xu, J. Physiological effects of resistant starch and its applications in food: A review. Food Prod. Process. Nutr. 2023, 5, 48. [Google Scholar] [CrossRef]
- Tekin, T.; Dincer, E. Effect of resistant starch types as a prebiotic. Appl. Microbiol. Biotechnol. 2023, 107, 491–515. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Xiao, Z. Resistant starch prevents tumorigenesis of dimethylhydrazine-induced colon tumors via regulation of an ER stress-mediated mitochondrial apoptosis pathway. Int. J. Mol. Med. 2018, 41, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gutierrez, A.; Tan, L.; Kong, L. Considerations and strategies for optimizing health benefits of resistant starch. Curr. Opin. Food Sci. 2023, 51, 101008. [Google Scholar] [CrossRef]
- Tan, F.P.; Beltranena, E.; Zijlstra, R.T. Resistant starch: Implications of dietary inclusion on gut health and growth in pigs: A review. J. Anim. Sci. Biotechnol. 2021, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tan, L.; Kong, L. Multiple levels of health benefits from resistant starch. J. Agric. Food Res. 2022, 10, 100380. [Google Scholar] [CrossRef]
- Cione, E.; Fazio, A.; Curcio, R.; Tucci, P.; Lauria, G.; Cappello, A.; Dolce, V. Resistant Starches and Non-Communicable Disease: A Focus on Mediterranean Diet. Foods 2021, 10, 2062. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.S.; Yan, T.H.; Saari, N.; Sarbini, S.R. A review: Resistant starch, a promising prebiotic for obesity and weight management. Food Biosci. 2022, 50, 101965. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Śliżewska, K. Efficiency of Resistant Starch and Dextrins as Prebiotics: A Review of the Existing Evidence and Clinical Trials. Nutrients 2021, 13, 3808. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.J.; Giannuzzi, L.; Weisstaub, A.R.; Zuleta, A.; Ferrero, C. Chemically modified resistant starch in breadmaking: Impact on bone, mineral metabolism and gut health of growing Wistar rats. Int. J. Food Sci. Technol. 2020, 55, 239–247. [Google Scholar] [CrossRef]
- Li, X. Resistant starch and its applications. In Functional Starch and Applications in Food; Springer: Berlin/Heidelberg, Germany, 2018; pp. 63–90. [Google Scholar]
- Karunarathna, S.; Wickramasinghe, I.; Truong, T.; Brennan, C.; Navaratne, S.; Chandrapala, J. Development of Low-Calorie Food Products with Resistant Starch-Rich Sources—A Review. Food Rev. Int. 2024, 40, 814–831. [Google Scholar] [CrossRef]
- Jiang, F.; Du, C.; Jiang, W.; Wang, L.; Du, S.-K. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. 2020, 150, 1155–1161. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, D.; Blennow, A.; Zörb, C. Mineral nutrients and crop starch quality. Trends Food Sci. Technol. 2021, 114, 148–157. [Google Scholar] [CrossRef]
- Rashed, A.A.; Saparuddin, F.; Rathi, D.-N.G.; Nasir, N.N.M.; Lokman, E.F. Effects of resistant starch interventions on metabolic biomarkers in pre-diabetes and diabetes adults. Front. Nutr. 2022, 8, 793414. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-T.; Zhang, W.; Zhu, H.; Chao, C.; Guo, Q. Unlocking the potential of high-amylose starch for gut health: Not all function the same. Fermentation 2023, 9, 134. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Liu, Y.-S.; Li, J.-L.; Xing, T.; Jiang, Y.; Zhang, L.; Gao, F. Role of dietary resistant starch in the regulation of broiler immunological characteristics. Br. J. Nutr. 2023, 129, 617–626. [Google Scholar] [CrossRef]
- Kadyan, S.; Park, G.; Singh, P.; Arjmandi, B.; Nagpal, R. Prebiotic mechanisms of resistant starches from dietary beans and pulses on gut microbiome and metabolic health in a humanized murine model of aging. Front. Nutr. 2023, 10, 1106463. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Li, J.; Wu, Q.; Qian, L.; He, J.; Ni, Y.; Kovatcheva-Datchary, P.; Yuan, R.; Liu, S. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat. Metab. 2024, 6, 578–597. [Google Scholar] [CrossRef]
- Chisbert, M.; Castell, A.-L.; Vinoy, S.; Nazare, J.-A. The impact of slowly digestible and resistant starch on glucose homeostasis and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 338–343. [Google Scholar] [CrossRef]
- Guo, J.; Brown, P.R.; Tan, L.; Kong, L. Effect of resistant starch consumption on appetite and satiety: A review. J. Agric. Food Res. 2023, 12, 100564. [Google Scholar] [CrossRef]
- Bede, D.; Zaixiang, L. Recent developments in resistant starch as a functional food. Starch-Starke 2021, 73, 2000139. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. A critical review on anti-diabetic and anti-obesity effects of dietary resistant starch. Crit. Rev. Food Sci. Nutr. 2019, 59, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.; Martinez, M.M. Structural basis of resistant starch (RS) in bread: Natural and commercial alternatives. Foods 2019, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Arp, C.G.; Correa, M.J.; Ferrero, C. High-amylose resistant starch as a functional ingredient in breads: A technological and microstructural approach. Food Bioprocess Technol. 2018, 11, 2182–2193. [Google Scholar] [CrossRef]
- Mohebbi, Z.; Homayouni, A.; Azizi, M.H.; Hosseini, S.J. Effects of beta-glucan and resistant starch on wheat dough and prebiotic bread properties. J. Food Sci. Technol. 2018, 55, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Arp, C.G.; Correa, M.J.; Ferrero, C. Resistant starches: A smart alternative for the development of functional bread and other starch-based foods. Food Hydrocoll. 2021, 121, 106949. [Google Scholar] [CrossRef]
- Rojhani, A.; Naranjo, J.; Ouyang, P. Physiochemical properties and sensory characteristics of resistant starch enriched cookies. Nutr. Food Sci. 2022, 52, 791–800. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Aalami, M.; Jafari, S.M.; Raeisi, M.; Nishinari, K.; Rostamabadi, H. Application of multi-criteria decision-making for optimizing the formulation of functional cookies containing different types of resistant starches: A physicochemical, organoleptic, in-vitro and in-vivo study. Food Chem. 2022, 393, 133376. [Google Scholar] [CrossRef]
- Boue, S.M.; Chen, M.H.; Daigle, K.W.; Lea, J.M.; Bett-Garber, K.L. Changes in fried rice batter with increased resistant starch and effects on sensory quality of battered fried onions. Cereal Chem. 2022, 99, 454–466. [Google Scholar] [CrossRef]
- Shaheen, S.; Shorbagi, M.; Lorenzo, J.M.; Farag, M.A. Dissecting dietary melanoidins: Formation mechanisms, gut interactions and functional properties. Crit. Rev. Food Sci. Nutr. 2022, 62, 8954–8971. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Yang, Q.; Kong, X.-P.; Chen, H.-Q. The addition of resistant starch and protein to the batter reduces oil uptake and improves the quality of the fried batter-coated nuts. Food Chem. 2024, 438, 137992. [Google Scholar] [CrossRef] [PubMed]
- Della, K.F.; Pratiwi, M.; Cahyana, P.T.; Gunawan-Puteri, M.D. Evaluation of Resistant Starch Quality from Different Types of Banana in Batter Coating Formulation to Reduce Oil Absorption in Fried Food. Iconiet Proceeding 2018, 2, 115–120. [Google Scholar] [CrossRef]
- Walsh, S.K.; Lucey, A.; Walter, J.; Zannini, E.; Arendt, E.K. Resistant starch—An accessible fiber ingredient acceptable to the Western palate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2930–2955. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y. Influencing factor of resistant starch formation and application in cereal products: A review. Int. J. Biol. Macromol. 2020, 149, 424–431. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Application of heat moisture treatment in wheat pasta production. Food Control 2021, 128, 108176. [Google Scholar] [CrossRef]
- Cervini, M.; Gruppi, A.; Bassani, A.; Spigno, G.; Giuberti, G. Potential application of resistant starch sorghum in gluten-free pasta: Nutritional, structural and sensory evaluations. Foods 2021, 10, 908. [Google Scholar] [CrossRef]
- Cervini, M.; Gabrielli, M.; Spigno, G.; Giuberti, G. Characterization of durum-wheat pasta containing resistant starch from debranched waxy rice starch. Foods 2023, 12, 327. [Google Scholar] [CrossRef]
- Yang, S.; Dhital, S.; Zhang, M.-N.; Wang, J.; Chen, Z.-G. Structural, gelatinization, and rheological properties of heat-moisture treated potato starch with added salt and its application in potato starch noodles. Food Hydrocoll. 2022, 131, 107802. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Research advances on structural characterization of resistant starch and its structure-physiological function relationship: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1059–1083. [Google Scholar] [CrossRef]
- Öztürk, S.; Mutlu, S. Physicochemical properties, modifications, and applications of resistant starches. In Starches for Food Application; Elsevier: Amsterdam, The Netherlands, 2019; pp. 297–332. [Google Scholar]
- Diamantino, V.R.; Costa, M.S.; Taboga, S.R.; Vilamaior, P.S.; Franco, C.M.; Penna, A.L.B. Starch as a potential fat replacer for application in cheese: Behaviour of different starches in casein/starch mixtures and in the casein matrix. Int. Dairy J. 2019, 89, 129–138. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Belanger, N.; Donner, E.; Liu, Q. Debranching of pea starch using pullulanase and ultrasonication synergistically to enhance slowly digestible and resistant starch. Food Chem. 2018, 268, 533–541. [Google Scholar] [CrossRef]
- Palanisamy, A.; Parimalavalli, R. Resistant starch: A functional ingredient in dairy products. J. Food Process. Preserv. 2022, 46, e17126. [Google Scholar] [CrossRef]
- Hajian, N.; Salami, M.; Mohammadian, M.; Moghadam, M.; Emam-Djomeh, Z. Production of low-fat camel milk functional ice creams fortified with camel milk casein and its antioxidant hydrolysates. Appl. Food Biotechnol. 2020, 7, 95–102. [Google Scholar]
- Patel, D.; Pinto, S.; Pal, M. A comprehensive review on the properties of camel milk and milk products. Int. J. Food Sci. Agric. 2022, 6, 200–207. [Google Scholar]
- Azari, A.M.; Khomeiri, M.; Aalami, M. Rheological and textural properties of camel milk ice cream by using resistant starch, Arabic and xanthan gums in a new formulation. Electron. J. Food Process. Preserv. 2019, 10, 81–104. [Google Scholar]
- Arp, C.G.; Correa, M.J.; Ferrero, C. Improving quality: Modified celluloses applied to bread dough with high level of resistant starch. Food Hydrocoll. 2021, 112, 106302. [Google Scholar] [CrossRef]
- Gutiérrez-Luna, K.; Ansorena, D.; Astiasaran, I. Effect of baking conditions on resistant starch: Model systems and cake formulations. Food Chem. 2024, 449, 139174. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Han, P.; Liang, X. Pea resistant starch preparation with cold-active type I pullulanase from Bacillus megaterium and its potential application in rice noodles. LWT 2023, 182, 114799. [Google Scholar] [CrossRef]
- Gong, X.; Li, J.; Liu, Z.; Xu, X.; Wang, A.; Nie, M.; Lin, R.; Tian, Y.; Zhang, X.; Wang, L. Developing high resistant starch content rice noodles with superior quality: A method using modified rice flour and psyllium fiber. Int. J. Biol. Macromol. 2024, 272, 132779. [Google Scholar] [CrossRef] [PubMed]
- Tas, A.; Shah, A. The replacement of cereals by legumes in extruded snack foods: Science, technology and challenges. Trends Food Sci. Technol. 2021, 116, 701–711. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Ignácio, E.O.; Bis-Souza, C.V.; da Silva-Barretto, A.C. Performance of reduced fat-reduced salt fermented sausage with added microcrystalline cellulose, resistant starch and oat fiber using the simplex design. Meat Sci. 2021, 175, 108433. [Google Scholar] [CrossRef] [PubMed]
- Munir, H.; Alam, H.; Nadeem, M.T.; Almalki, R.S.; Arshad, M.S.; Suleria, H.A.R. Green banana resistant starch: A promising potential as functional ingredient against certain maladies. Food Sci. Nutr. 2024, 12, 3787–3805. [Google Scholar] [CrossRef] [PubMed]
- Raungrusmee, S.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of resistant starch, xanthan gum, inulin and defatted rice bran on the physicochemical, functional and sensory properties of low glycemic gluten-free noodles. LWT 2020, 126, 109279. [Google Scholar] [CrossRef]
- Cervini, M.; Frustace, A.; Garrido, G.D.; Rocchetti, G.; Giuberti, G. Nutritional, physical and sensory characteristics of gluten-free biscuits incorporated with a novel resistant starch ingredient. Heliyon 2021, 7, e06562. [Google Scholar] [CrossRef] [PubMed]
- Žuljević, S.O.; Akagić, A. Flour-based confectionery as functional food. In Functional Foods: Phytochemicals and Health Promoting Potential; IntechOpen: London, UK, 2021; Volume 351. [Google Scholar]
- Lončarević, I.; Pajin, B.; Petrović, J.; Nikolić, I.; Maravić, N.; Ačkar, Đ.; Šubarić, D.; Zarić, D.; Miličević, B. White chocolate with resistant starch: Impact on physical properties, dietary fiber content and sensory characteristics. Molecules 2021, 26, 5908. [Google Scholar] [CrossRef]
| Processing Technique | Description | Mechanism of Action | Effect on Rs Content | References |
|---|---|---|---|---|
| Ball milling | Grinding and crushing balls inside a revolving drum. | Particle size reduction and starch amorphization. | Reduces the RS concentration by fracturing starch’s crystalline structure. | [143,144] |
| Wet grinding | Uses a ball mill where the grinding chamber has been filled with water. | Combination of shear, impact, and attrition forces with water. | Reduces the RS content of starch and encourages gelatinization. | [145,146] |
| Extrusion (barrel screw extrusion) | Single/twin revolving screw to form and transport material through a die inside a heated barrel. | Combination of heat, pressure, and shear to break down and reorganize starch molecules. | Reduces RS level due to significant granule gelatinization and fragmentation. | [147,148] |
| Heat–moisture treatment | Heating starch for a predetermined amount of time at a high temperature and with limited moisture. | Modifies the amylose-to-amylopectin ratio and encourages retrogradation. | Increases RS content, increases RS5 and RS3. | [49,149] |
| Annealing | Heating starch for a prolonged amount of time with a high moisture content. | Enlarges starch granules without losing their integrity with high moisture content. | Rise in RS content. Increases RS3 and RS2. | [63,150] |
| Roasting | Heating starch at high temperatures with little to no water content. | The starch granules undergo both chemical and physical changes at a high temperature. | Boosts the formation of RS4 and increases the concentration of RS. | [151,152] |
| Ionizing radiation | γ-ray or electron beam penetrates into food materials at regulated dosages, causing ionization and excitation of molecules. | Causes the depolymerization and crosslinking of starch molecules by rupturing chemical bonds. | Primarily raises RS4. | [115,153] |
| Ultrasonication | Employs high-frequency sound waves to cure liquid starch. | Causes starch granules to break apart and partially gelatinize. | Boosting the production of RS3 can also improve the formation of RS4. | [154,155] |
| Pulsed electric field | Exposing starch in a liquid media to brief high-voltage bursts. | Enhances the absorption of water and makes starch modification easier. | Improves retrogradation and encourages structural improvements to raise RS content. | [138,156] |
| Type of Resistant Starch | Health Benefits | Description | References |
|---|---|---|---|
| RS2, RS3 | Digestive health | It acts as a prebiotic, bypasses digestion, avoids spiking glucose, and reaches the large intestine’s gut to feed good bacteria. | [3] |
| RS3, RS4 | Blood sugar control | Lowers postprandial blood glucose and insulin levels and improves metabolic health. | [203] |
| RS2, RS3 | Weight management | Increases satiety and reduces overall calorie intake, aids in weight loss by reshaping the gut microbiota. | [196] |
| RS3 | Colon health | Produces short-chain fatty acids like butyrate, which have anti-inflammatory properties and reduce the risk of colorectal cancer. | [204] |
| RS2, RS3 | Cholesterol reduction | Lowers LDL and total cholesterol levels, which improves cardiovascular health. | [2] |
| RS2, RS3 | Improved mineral absorption | Enhances the absorption of minerals such as calcium and magnesium in the colon. | [189] |
| RS3, RS4 | Enhanced immunity | Modulates immune response by promoting microbial-derived metabolites and dampening neutrophil recruitment. | [205] |
| RS3, RS4 | Reduced inflammation | Lowers systemic inflammation; beneficial for conditions like inflammatory bowel disease (IBD). | [206] |
| RS3 | Gut barrier function | Strengthens gut barrier integrity and prevents leaky gut syndrome. | [207] |
| RS2, RS3 | Improved insulin sensitivity | Enhances insulin sensitivity, reducing the risk of type 2 diabetes. | [208] |
| RS2, RS3 | Weight loss and satiety | Promotes feelings of fullness, reducing overall calorie intake. | [209] |
| Applications | Food Products | Types of Resistant Starch | Benefits | References |
|---|---|---|---|---|
| Baked Goods | Bread, Muffins, Cookies, Cakes | RS2, RS3, RS4 | Improved texture, increased dietary fiber content, enhanced shelf life, reduced the glycemic index of products. | [200,236,237] |
| Pasta and Noodles | Spaghetti, Rice Noodles | RS3, RS4 | Lower glycemic index, improved gut health, increased insoluble dietary fiber, increased satiety. | [224,238,239] |
| Snacks | Chips, Crackers | RS2, RS3 | Reduced calorie content, high in fiber, nutrient-dense, lower salt and sugar, prevents non-communicable diseases, suitable alternative for gluten-intolerant people, improved digestibility. | [240] |
| Dairy Products | Yogurt, Cheese, Ice Cream | RS2, RS4 | Prebiotic effects, enhanced creaminess, increased iron and fiber levels, increased viscosity, and sensory properties. | [189,200] |
| Meat Products | Sausages, Meatballs | RS4, RS5 | Improved texture, fat replacement, and increased fiber content; acts as a prebiotic. | [200,241] |
| Breakfast Cereals | Cornflakes, Granola | RS3, RS4 | Higher fiber content, lower glycemic response, reduced risks of colon cancer, coronary heart disease, and enhanced crunchiness. | [223] |
| Beverages | Smoothies, Meal Replacement Drinks | RS2, RS3 | Improved satiety, prebiotic effects, lower glycemic index, good for gut health, regulates glucose homeostasis. | [242] |
| Gluten-Free Products | Gluten-Free Bread, Pizza Crust, Noodles | RS2, RS3 | Improved texture, increased dietary fiber content, better nutritional profile, lower risk of chronic degenerative diseases, low glycemic index, and improved gut health. | [222,243,244] |
| Confectionery | Chocolate, Candy Bars | RS2, RS4 | Reduced sugar content, lower glycemic index, rich in fiber, high in antioxidants. | [245,246] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, M.A.; Yu, J. Recent Advances in Physical Processing Techniques to Enhance the Resistant Starch Content in Foods: A Review. Foods 2024, 13, 2770. https://doi.org/10.3390/foods13172770
Farooq MA, Yu J. Recent Advances in Physical Processing Techniques to Enhance the Resistant Starch Content in Foods: A Review. Foods. 2024; 13(17):2770. https://doi.org/10.3390/foods13172770
Chicago/Turabian StyleFarooq, Muhammad Adil, and Jianmei Yu. 2024. "Recent Advances in Physical Processing Techniques to Enhance the Resistant Starch Content in Foods: A Review" Foods 13, no. 17: 2770. https://doi.org/10.3390/foods13172770
APA StyleFarooq, M. A., & Yu, J. (2024). Recent Advances in Physical Processing Techniques to Enhance the Resistant Starch Content in Foods: A Review. Foods, 13(17), 2770. https://doi.org/10.3390/foods13172770






