Phytochemical Profiling and Antimicrobial Properties of Various Sweet Potato (Ipomoea batatas L.) Leaves Assessed by RP-HPLC-DAD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Materials
2.3. Moisture Content Analysis
2.4. Sample Preparation and Preservation
2.5. Spectrophotometric Assay for Total Phenolic Content
2.6. Total Flavonoids Content
2.7. ABTS and DPPH Radical Scavenging Assay
2.8. FRAP Assay
2.9. Antimicrobial Assay Determination
2.9.1. Preparation of Inocula
2.9.2. Evaluation of Antimicrobial Activity
2.10. High-Performance Liquid Chromatography (HPLC)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Phenotypic Characteristics
3.2. Moisture Contents
3.3. Extraction Yield Efficiency of Various Solvents
3.4. Evaluation of Total Phenolic Content (TFC) and Total Flavonoid Content (TFC)
3.5. Antioxidant Activity (AA) of Sweet Potato Leaves
3.6. Outcomes of the Antimicrobial Assay Analysis
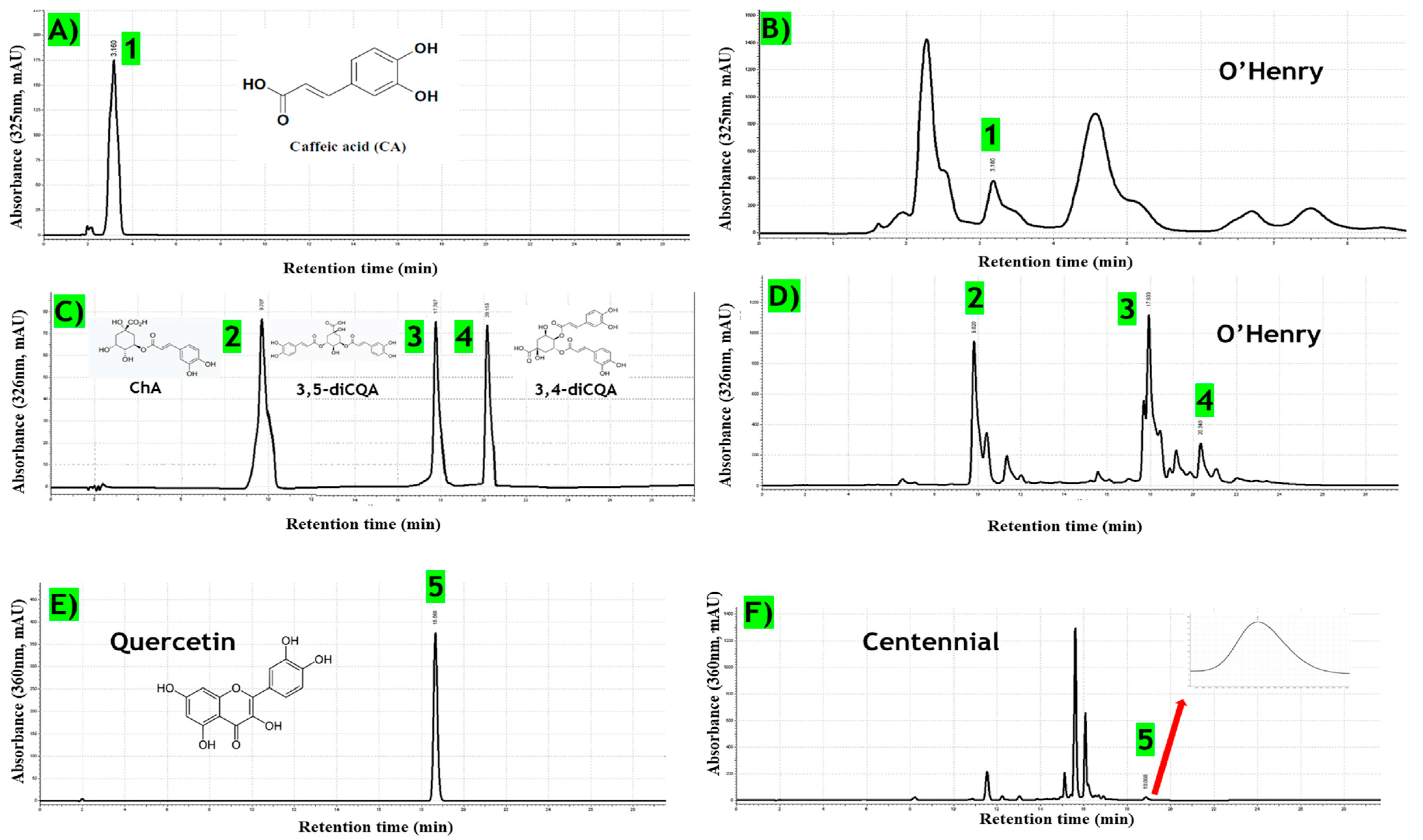
3.7. RP-HPLC-DAD Analysis
3.7.1. Specificity
3.7.2. HPLC Method Validation
Linearity
Limits of Detection (LOD) and Limits of Quantification (LOQ)
3.7.3. Optimization of Extraction Efficacy
3.7.4. Analysis of Phenolic Compounds in Sweet Potato Leaf
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar-Puentes, A.A.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Olivas-Aguirre, F.J.; Wall-Medrano, A. Sweet Potato (Ipomoea batatas L.) Phenotypes: From Agroindustry to Health Effects. Foods 2022, 11, 1058. [Google Scholar] [CrossRef]
- Katayama, K.; Kobayashi, A.; Sakai, T.; Kuranouchi, T.; Kai, Y. Recent progress in sweetpotato breeding and cultivars for diverse applications in Japan. Breed. Sci. 2017, 67, 3–14. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet potato is not simply an abundant food crop: A comprehensive review of its phytochemical constituents, biological activities, and the effects of processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qin, L. Growth and photosynthetic characteristics of sweet potato (Ipomoea batatas) leaves grown under natural sunlight with supplemental LED lighting in a tropical greenhouse. J. Plant Physiol. 2020, 252, 153239. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary polyphenols as natural inhibitors of α-Amylase and α -Glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic Acids–Ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Slam, M.S.; Yoshimoto, M.; Yahara, S.; Okuno, S.; Ishiguro, K.; Yamakawa, O. Identification and Characterization of Foliar Polyphenolic Composition in Sweetpotato (Ipomoea batatas L.) Genotypes. J. Agric. Food Chem. 2002, 50, 3718–3722. [Google Scholar] [CrossRef]
- Islam, S.; Adam, Z.; Akanda, J.H. Quinic and caffeic acids derivatives: Affecting antioxidant capacities and phenolics contents of certain therapeutic and specialty crops employing water and ethanolic extracts. Food Chem. Adv. 2024, 4, 100693. [Google Scholar] [CrossRef]
- De Souza Farias, S.A.; Da Costa, K.S.; Martins, J.B.L. Analysis of conformational, structural, magnetic, and electronic properties related to antioxidant activity: Revisiting flavan, anthocyanidin, flavanone, flavonol, isoflavone, flavone, and flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef]
- Chang, W.; Hu, S.; Huang, Y.; Yeh, T.; Liu, J. Effect of purple sweet potato leaves consumption on exercise-induced oxidative stress and IL-6 and HSP72 levels. J. Appl. Physiol. 2010, 109, 1710–1715. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Chen, C.; Lin, K.; Chao, P.; Lin, H.; Huang, M. Bioactive compounds, antioxidants, and health benefits of sweet potato leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef] [PubMed]
- Fanmoe, M.J.M.; Ngoune, L.T.; Ndjouenkeu, R. Ipomea batatas Leaf Powder from Cameroon: Antioxidant Activity and Antihyperlipidemic Effect in Rats Fed with a High-Fat Diet. J. Lipids 2021, 2021, 5539878. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Yahara, S.; Okuno, S.; Islam, M.S.; Ishiguro, K.; Yamakawa, O. Antimutagenicity of Mono-, Di-, and Tricaffeoylquinic Acid Derivatives Isolated from Sweetpotato (Ipomoea batatas L.) Leaf. Biosci. Biotechnol. Biochem. 2002, 66, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Islam, S. Sweetpotato (Ipomoea batatas L.) Leaf: Its Potential Effect on Human Health and Nutrition. J. Food Sci. 2006, 71, R13–R121. [Google Scholar] [CrossRef]
- Jin, X.; Shi, C.; Yu, C.Y.; Yamada, T.; Sacks, E.J. Determination of leaf water content by Visible and Near-Infrared spectrometry and multivariate calibration in miscanthus. Front. Plant Sci. 2017, 8, 721. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Naqvi, S.A.R.; Rasool, M.H.; Noureen, A.; Mubarik, M.S.; Tareen, R.B. Phytochemical analysis, antioxidant and antimicrobial screening of Seriphidium oliverianum plant extracts. Dose-Response 2021, 19, 155932582110047. [Google Scholar] [CrossRef]
- Sultana, T.; Islam, I.; Rahman, A.; Jahurul, A. Antimicrobial and antioxidant properties of the acetone extracts of the leaves of Lagenaria siceraria and Cucurbita pepo. Food Chem. Adv. 2023, 3, 100556. [Google Scholar] [CrossRef]
- Munhoz, V.M.; Longhini, R.; Souza, J.R.; Zequi, J.A.; Mello EV, L.; Lopes, G.C.; Mello, J.C. Extraction of flavonoids from Tagetes patula: Process optimization and screening for biological activity. Rev. Bras. Farmacogn. 2014, 24, 576–583. [Google Scholar] [CrossRef]
- Everette, J.D.; Islam, S. Effect of Extraction Procedures, Genotypes and Screening Methods to Measure the Antioxidant Potential and Phenolic Content of Orange-fleshed Sweetpotatoes (Ipomoea batatas L.). Am. J. Food Technol. 2012, 7, 50–61. [Google Scholar] [CrossRef]
- Nithianantham, K.; Shyamala, M.; Chen, Y.; Latha, L.Y.; Jothy, S.L.; Sasidharan, S. Hepatoprotective Potential of Clitoria ternatea Leaf Extract Against Paracetamol Induced Damage in Mice. Molecules 2011, 16, 10134–10145. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yuan, Y.; Tao, J. Antioxidant and antibacterial activities of 13 ornamental herbaceous peony cultivars: A comparative study with stems and leaves. N. Z. J. Crop Hortic. Sci. 2021, 50, 326–340. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby WM, M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.; McFeeters, R.; Thompson, R.; Dean, L.; Shofran, B. Phenolic Acid Content and Composition in Leaves and Roots of Common Commercial Sweetpotato (Ipomea batatas L.) Cultivars in the United States. J. Food Sci. 2007, 72, C343–C349. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2012, 51, 883–890. [Google Scholar] [CrossRef]
- Fernandes, F.H.; De A Batista, R.S.; De Medeiros, F.D.; Santos, F.S.; Medeiros, A.C. Development of a rapid and simple HPLC-UV method for determination of gallic acid in Schinopsis brasiliensis. Rev. Bras. Farmacogn. 2015, 25, 208–211. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Harrison, S.P.; Prentice, I.C. Leaf morphological traits as adaptations to multiple climate gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef]
- Hong, J.; Mu, T.; Sun, H.; Richel, A.; Blecker, C. Valorization of the green waste parts from sweet potato (Impoea batatas L.): Nutritional, phytochemical composition, and bioactivity evaluation. Food Sci. Nutr. 2020, 8, 4086–4097. [Google Scholar] [CrossRef]
- Yolcu, S.; Alavilli, H.; Ganesh, P.; Panigrahy, M.; Song, K. Salt and Drought Stress Responses in Cultivated Beets (Beta vulgaris L.) and Wild Beet (Beta maritima L.). Plants 2021, 10, 1843. [Google Scholar] [CrossRef]
- Arawande, J.O.; Orimoloye, O.R.; Adeleke, A.R.; Afolabi, F.O.; Adesuyi, A.T.; Imoukhuede, B.; Ayodele, C.O. Study on Solvent Extraction Values and Antioxidant Properties of Bioactive Extracts Obtained from Leaves, Tuber Peels and Tubers of Sweet Potato. Biomed. J. Sci. Tech. Res. 2023, 48, 39856–39861. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.; Wu, L.; Zhang, J.; Li, X.; Wu, W. Chemical Characterization and Antioxidant Properties of Ethanolic Extract and Its Fractions from Sweet Potato (Ipomoea batatas L.) Leaves. Foods 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Yoshimoto, M.; Yamakawa, O. Distribution and physiological functions of caffeoylquinic acid derivatives in leaves of sweetpotato genotypes. J. Food Sci. 2003, 68, 111–116. [Google Scholar] [CrossRef]
- Gong, Y.; Li, X.; He, W.; Xu, H.; Yuan, F.; Gao, Y. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Cioloca, M.; Bădărău, C.L.; Tican, A.; Popa, M. Antioxidant potential, Total Vitamin C, phenolic and flavonoids content of sweet potato leaves. Rom. Biotechnol. Lett. 2021, 26, 2441–2447. [Google Scholar] [CrossRef]
- Phahlane, C.J.; Laurie, S.M.; Shoko, T.; Manhivi, V.E.; Sivakumar, D. An evaluation of phenolic compounds, carotenoids, and antioxidant properties in leaves of South African cultivars, Peruvian 199062.1 and USA’s Beauregard. Front. Nutr. 2021, 8, 773550. [Google Scholar] [CrossRef]
- Ghasemzadeh, N.A.; Omidvar, V.; Jaafar, H.Z. Polyphenolic content and their antioxidant activity in leaf extract of sweet potato (Ipomoea batatas). J. Med. Plant Res. 2012, 6, 2971–2976. [Google Scholar] [CrossRef]
- Sahidur Islam, S.; Jahurul, M. Garlic (Allium sativum) as a natural antidote or a protective agent against diseases and toxicities: A critical review. Food Chem. Adv. 2023, 3, 100353. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef]
- Suárez, S.; Mu, T.; Sun, H.; Añón, M.C. Antioxidant activity, nutritional, and phenolic composition of sweet potato leaves as affected by harvesting period. Int. J. Food Prop. 2020, 23, 178–188. [Google Scholar] [CrossRef]
- Yang, R.Y.; Tsou, S.C.S.; Lee, T.C.; Hanson, P.M.; Lai, P.Y. Antioxidant Capacities and Daily Antioxidant Intake from Vegetables Consumed in Taiwan. In Proceedings of the Symposium on Taiwan-America Agricultural Cooperative Projects, Taipei, Taiwan, 15 November 2005. [Google Scholar]
- Adsull, V.B.; Khatiwora, E.; Rasika, T.; Deshpande, N.R. Antimicrobial activities of Ipomoea carnea leaves. J. Nat. Prod. Plant Resour. 2012, 2, 597–600. [Google Scholar]
- Mbaeyi-Nwaoha, I.E.; Emejulu, V.N. Evaluation of Phytochemical Composition and Antimicrobial Activity of Sweet Potato (Ipomoea batatas) Leaf. Pak. J. Nutr. 2013, 12, 575–586. [Google Scholar] [CrossRef]
- Pochapski, M.T.; Fosquiera, E.C.; Esmerino, L.A.; Santos EB, D.; Farago, P.V.; Santos, F.A.B.D.; Groppo, F.C. Phytochemical screening, antioxidant, and antimicrobial activities of the crude leaves′ extract from Ipomoea batatas (L.) Lam. Pharmacogn. Mag. 2011, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D. Antimicrobial Chemotherapy; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Takenaka, M.; Nanayama, K.; Isobe, S.; Murata, M. Changes in Caffeic Acid Derivatives in Sweet Potato (Ipomoea batatas L.) during Cooking and Processing. Biosci. Biotechnol. Biochem. 2006, 70, 172–177. [Google Scholar] [CrossRef]
- El-Askary, H.; Salem, H.H.; Motaal, A.A. Potential Mechanisms Involved in the Protective Effect of Dicaffeoylquinic Acids from Artemisia annua, L. Leaves against Diabetes and Its Complications. Molecules 2022, 27, 857. [Google Scholar] [CrossRef]
- Ojong, P.B.; Njiti, V.; Guo, Z.; Gao, M.; Besong, S.; Barnes, S.L. Variation of flavonoid content among sweetpotato accessions. J. Am. Soc. Hortic. Sci. 2008, 133, 819–824. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmiański, J.; Kaszuba, J.; Żurek, N. The Content of Phenolic Acids and Flavonols in the Leaves of Nine Varieties of Sweet Potatoes (Ipomoea batatas L.) Depending on Their Development, Grown in Central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef]



| Varieties | Internode Distance (cm) | Petiole Length (cm) | Leaf Length (cm) | Leaf Width (cm) |
|---|---|---|---|---|
| O’Henry | 7.37 ± 0.78 a | 14.00 ± 2.00 a | 6.00 ± 0.44 c | 6.63 ± 0.60 d |
| Covington | 4.07 ± 0.40 b | 6.93 ± 2.48 b | 8.63 ± 0.40 b | 10.50 ± 0.50 b,c |
| Vardaman | 8.33 ± 0.76 a | 15.33 ± 1.04 a | 9.33 ± 1.26 b | 12.77 ± 1.50 a,b |
| Beauregard | 4.17 ± 0.76 b | 8.50 ± 2.18 b | 8.50 ± 0.87 b | 8.33 ± 0.58 c,d |
| Georgia Jet | 3.83 ± 0.76 b | 16.00 ± 1.50 a | 10.50 ± 1.00 b | 11.67 ± 2.52 a,b,c |
| Centennial | 3.10 ± 0.82 b | 13.60 ± 0.53 a | 12.73 ± 0.38 a | 14.20 ± 0.53 a |
| Sample | Moisture Content (%) | Extraction Yield (%) | |||
|---|---|---|---|---|---|
| Methanol | Ethanol | Acetone | Hexane | ||
| O’Henry | 72.89 ± 1.71 a | 18.79 ± 1.71 f | 12.31 ± 1.69 d,e | 6.46 ± 1.24 a,b,c,d | 3.25 ± 2.00 a,b,c |
| Covington | 80.00 ± 3.00 b,c | 18.19 ± 2.87 e,f | 9.49 ± 0.57 b,c,d | 5.61 ± 0.86 a,b,c | 3.81 ± 2.79 a,b,c |
| Vardaman | 81.40 ± 1.99 b,c | 16.94 ± 1.81 e,f | 8.85 ± 0.84 b,c,d | 5.90 ± 0.92 a,b,c,d | 2.25 ± 0.12 a |
| Beauregard | 77.31 ± 2.03 a,b | 18.46 ± 2.65 e,f | 9.33 ± 1.41 b,c,d | 5.42 ± 0.35 a,b,c | 3.06 ± 1.49 a,b |
| Georgia jet | 79.29 ± 1.16 b,c | 18.44 ± 2.51 e,f | 9.63 ± 1.47 c,d | 6.25 ± 0.59 a,b,c,d | 3.04 ± 1.36 a,b |
| Centennial | 84.14 ± 2.43 c | 16.68 ± 1.55 e,f | 8.69 ± 1.69 b,c,d | 5.21 ± 0.29 a,b,c | 1.81 ± 0.04 a |
| Varieties | Total Phenol (mg TAE/g Dw) | Total Flavonoid (mg QE/g Dw) |
|---|---|---|
| O’Henry | 10.50 ± 1.04 a | 4.26 ± 0.23 a |
| Covington | 9.62 ± 0.80 a | 3.07 ± 1.02 a |
| Vardaman | 8.29 ± 0.83 a | 3.88 ± 0.13 a |
| Beauregard | 7.96 ± 0.89 a | 3.38 ± 0.12 a |
| Georgia Jet | 7.63 ± 0.49 a | 3.05 ± 0.14 a |
| Centennial | 7.29 ± 0.62 a | 2.30 ± 0.04 a |
| Varieties | ABTS Assay | DPPH Assay | FRAP Value | ||
|---|---|---|---|---|---|
| Total Antioxidant Capacity (mg TE/g Dw) | IC50 Value (µg/mL) | Total Antioxidant Capacity (mg AAE/g Dw) | IC50 Value (µg/mL) | ||
| O’Henry | 0.81 ± 0.30 b | 88.80 ± 15.30 b | 125.04 ± 0.73 a | 88.83 ± 1.94 c | 2.83 ± 0.07 a |
| Covington | 0.66 ± 0.82 a | 115.17 ± 7.65 a | 122.56 ± 1.59 a | 95.17 ± 4.77 b,c | 2.64 ± 0.11 a |
| Vardaman | 0.80 ± 0.63 b | 94.60 ± 2.76 a,b | 119.40 ± 0.69 b | 99.50 ± 4.92 b,c | 2.21 ± 0.07 b,c |
| Beauregard | 0.78 ± 2.10 b | 100.47 ± 5.37 a,b | 115.83 ± 1.32 c | 110.67 ± 14.75 b | 1.98 ± 0.14 c |
| Georgia Jet | 0.67 ± 1.67 a | 104.33 ± 10.60 a,b | 110.05 ± 0.73 d | 147.60 ± 1.22 a | 2.08 ± 0.07 b,c |
| Centennial | 0.68 ± 2.08 a | 99.9 ± 6.50 a,b | 112.86 ± 0.93 d | 143.63 ± 2.80 a | 2.28 ± 0.07 b |
| Varieties | Extracted Solvent | S. aureus | S. epidermidis | S. mutans | B. subtilis | L. monocytogenes | E. coli | S. dysentriae | K. aerogenes | C. albicans | A. niger |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O’Henry | Methanol | 14.67 ± 1.53 | - | 21.67 ± 3.21 | - | 12.67 ± 2.08 | 19.67 ± 1.53 | 19.83 ± 1.53 | - | - | 17.67 ± 0.58 |
| Ethanol | - | - | 21.33 ± 0.58 | - | - | 18 ± 1.00 | - | - | 18.33 ± 1.15 | - | |
| Acetone | - | - | - | - | - | 18.33 ± 0.58 | 18.17 ± 1.04 | - | 16.83 ± 1.26 | - | |
| Hexane | - | - | 12 ± 1.00 | - | - | 11.33 ± 0.58 | - | - | - | - | |
| Covington | Methanol | 16.67 ± 1.53 | - | 21.33 ± 2.08 | - | - | 22.33 ± 2.52 | - | - | 11.5 ± 1.32 | 14.33 ± 2.08 |
| Ethanol | - | - | - | - | - | 13.67 ± 1.53 | - | - | 20.83 ± 0.76 | - | |
| Acetone | - | - | 13.33 ± 1.53 | - | 13 ± 1.00 | - | 20.5 ± 1.50 | - | - | - | |
| Hexane | - | - | - | - | - | 11.33 ± 0.58 | - | - | 15 ± 3.60 | - | |
| Vardaman | Methanol | 19.33 ± 0.58 | - | 32.33 ± 1.53 | - | - | 13 ± 1.00 | - | - | 13.5 ± 0.50 | 15.33 ± 3.51 |
| Ethanol | - | - | 20.33 ± 0.58 | - | - | 12.67 ± 1.55 | 11.67 ± 0.58 | - | 10.83 ± 0.29 | 13.00 ± 0.58 | |
| Acetone | - | - | - | - | 12.67 ± 0.58 | - | 15.33 ± 1.53 | - | 19.17 ± 1.04 | - | |
| Hexane | - | - | - | - | - | 13.33 ± 0.58 | - | - | 11.17 ± 0.76 | 24.67 ± 3.51 | |
| Beauregard | Methanol | - | - | 27.33 ± 1.53 | - | 12.33 ± 0.58 | - | 18.33 ± 0.76 | - | - | 15 ± 1.00 |
| Ethanol | - | - | 16.33 ± 1.53 | - | 14 ± 1.00 | 11.33 ± 0.58 | 16.67 ± 1.15 | - | 17.33 ± 1.53 | 19 ± 3.06 | |
| Acetone | - | - | - | - | 11.33 ± 0.58 | - | 11.33 ± 0.58 | - | 12.5 ± 0.50 | - | |
| Hexane | - | - | - | - | - | 11.33 ± 0.58 | - | - | - | - | |
| Georgia Jet | Methanol | 13.33 ± 0.58 | - | 26.67 ± 1.53 | - | 11.33 ± 0.58 | - | - | - | - | 13.33 ± 1.53 |
| Ethanol | - | - | 18.67 ± 1.53 | - | - | 12.67 ± 1.55 | - | - | 13.33 ± 1.53 | - | |
| Acetone | - | - | - | - | 11.33 ± 0.58 | - | - | - | - | - | |
| Hexane | - | - | - | - | - | 10.83 ± 0.29 | - | - | 13.83 ± 0.76 | - | |
| Centennial | Methanol | 13.67 ± 0.58 | - | 19.67 ± 1.15 | - | - | - | - | - | - | 17.33 ± 0.58 |
| Ethanol | - | - | - | - | - | - | 12.33 ± 0.58 | - | - | - | |
| Acetone | - | - | 18.67 ± 1.53 | - | - | - | 18.03 ± 0.29 | - | - | - | |
| Hexane | - | - | - | - | - | 10.5 ± 0.50 | - | - | - | - | |
| Rifampin | 30.00 | 38.67 ± 0.69 | C/I | 20.33 ± 1.53 | 30.33 ± 0.58 | 13.67 ± 1.53 | 14.67 ± 1.53 | - | Voriconazole-C/I | Voriconazole- C/I | |
| Azithromycin | - | - | C/I | 28.67 ± 1.53 | 22 ± 1.00 | 24.33 ± 2.08 | 20 ± 2.00 | 9.33 ± 0.58 | |||
| Vancomycin | 20.00 | 18 ± 1.00 | C/I | 20.33 ± 0.58 | 23.33 ± 1.53 | - | 12.33 ± 0.58 | - | |||
| Sample | Caffeic Acid | Chlorogenic Acid | 3,5-Dicaffeoylquinic Acid | 3,4-Dicaffeoylquinic Acid | Quercetin | Gallic Acid | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acidified Methanol Extracted | Methanol Extracted | Acidified Methanol Extracted | Methanol Extracted | Acidified Methanol Extracted | Methanol Extracted | Acidified Methanol Extracted | Methanol Extracted | Acidified Methanol Extracted | Methanol Extracted | ||
| O’Henry | 1.25 ± 1.55 c | 0.99 ± 0.85 c | 6.56 ± 0.19 a | 2.88 ± 3.27 a | 14.35 ± 3.86 c | 8.49 ± 8.03 c | 2.18 ± 0.70 a | 1.50 ± 2.24 a | ND | ND | ND |
| Covington | 0.75 ± 0.99 b | 0.48 ± 0.69 b | 4.88 ± 6.72 b | 1.68 ± 1.34 b | 19.92 ± 8.38 b | 11.22 ± 6.87 b | 2.06 ± 1.87 a | 1.33 ± 0.56 b | 0.21 ± 1.74 a | ||
| Vardaman | 0.74 ± 3.49 b | 0.49 ± 0.49 b | 2.24 ± 1.26 e | 1.27 ± 1.20 e | 9.95 ± 3.82 e | 5.33 ± 2.64 e | 1.08 ± 0.85 d | 1.03 ± 2.99 c | 0.08 ± 0.21 b | ||
| Beauregard | 0.72 ± 6.89 b | 0.47 ± 0.07 b | 3.17 ± 5.82 d | 1.51 ± 1.42 c | 12.93 ± 6.35 d | 6.66 ± 10.22 d | 1.63 ± 2.36 c | 1.07 ± 0.40 c | 0.06 ± 0.80 b | ||
| Georgia jet | 0.31 ± 0.77 a | 0.21 ± 3.81 a | 3.04 ± 5.95 d | 1.40 ± 2.30 d | 9.91 ± 7.16 e | 4.41 ± 3.70 f | 0.82 ± 4.56 e | 0.71 ± 0.77 d | 0.04 ± 0.61 b | ||
| Centennial | 0.64 ± 0.96 b | 0.46 ± 2.19 b | 3.55 ± 4.07 c | 1.65 ± 0.94 b | 21.80 ± 13.11 a | 12.47 ± 11.49 a | 1.81 ± 4.90 b | 1.30 ± 3.25 b | 0.20 ± 0.49 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, T.; Islam, S.; Azad, M.A.K.; Akanda, M.J.H.; Rahman, A.; Rahman, M.S. Phytochemical Profiling and Antimicrobial Properties of Various Sweet Potato (Ipomoea batatas L.) Leaves Assessed by RP-HPLC-DAD. Foods 2024, 13, 2787. https://doi.org/10.3390/foods13172787
Sultana T, Islam S, Azad MAK, Akanda MJH, Rahman A, Rahman MS. Phytochemical Profiling and Antimicrobial Properties of Various Sweet Potato (Ipomoea batatas L.) Leaves Assessed by RP-HPLC-DAD. Foods. 2024; 13(17):2787. https://doi.org/10.3390/foods13172787
Chicago/Turabian StyleSultana, Tasbida, Shahidul Islam, Muhammad Abul Kalam Azad, Md Jahurul Haque Akanda, Atikur Rahman, and Md Sahidur Rahman. 2024. "Phytochemical Profiling and Antimicrobial Properties of Various Sweet Potato (Ipomoea batatas L.) Leaves Assessed by RP-HPLC-DAD" Foods 13, no. 17: 2787. https://doi.org/10.3390/foods13172787






