Investigation of the Lipid-Lowering Activity and Mechanism of Three Extracts from Astragalus membranaceus, Hippophae rhamnoides L., and Taraxacum mongolicum Hand. Mazz Based on Network Pharmacology and In Vitro and In Vivo Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
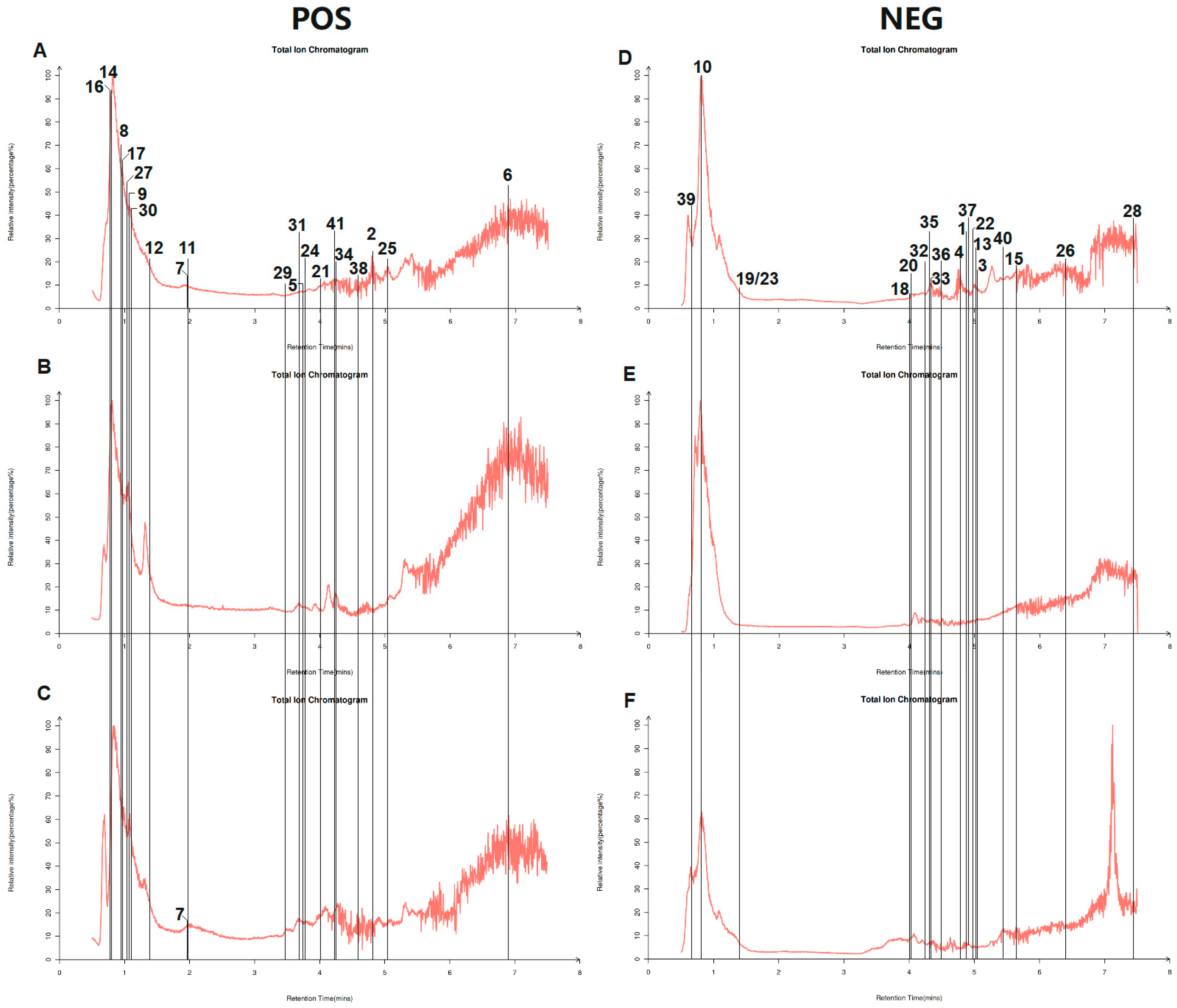
2.2. LC-MS Analysis Conditions
2.3. AHT Chemical Composition Screening and Target Prediction
2.4. Prediction of Targets for Hyperlipidemia
2.5. Construction of Drug Target Network and Pathways
2.6. Pancreatic Lipase Inhibition Test and Combined Index Analysis
2.7. Cell Culture and MTT Assay
2.8. Establishment of High-Fat Model and Administration Regimen
2.9. Oil Red O Staining
2.10. Determination of Lipid-Lowering Levels in HepG2 Cells by AHT
2.11. Western Blot Analysis
2.12. Animal Experiments
2.13. Determination of Serum Biochemical Indicators
2.14. Statistical Analysis
3. Results
3.1. Analysis of AHT Active Ingredients
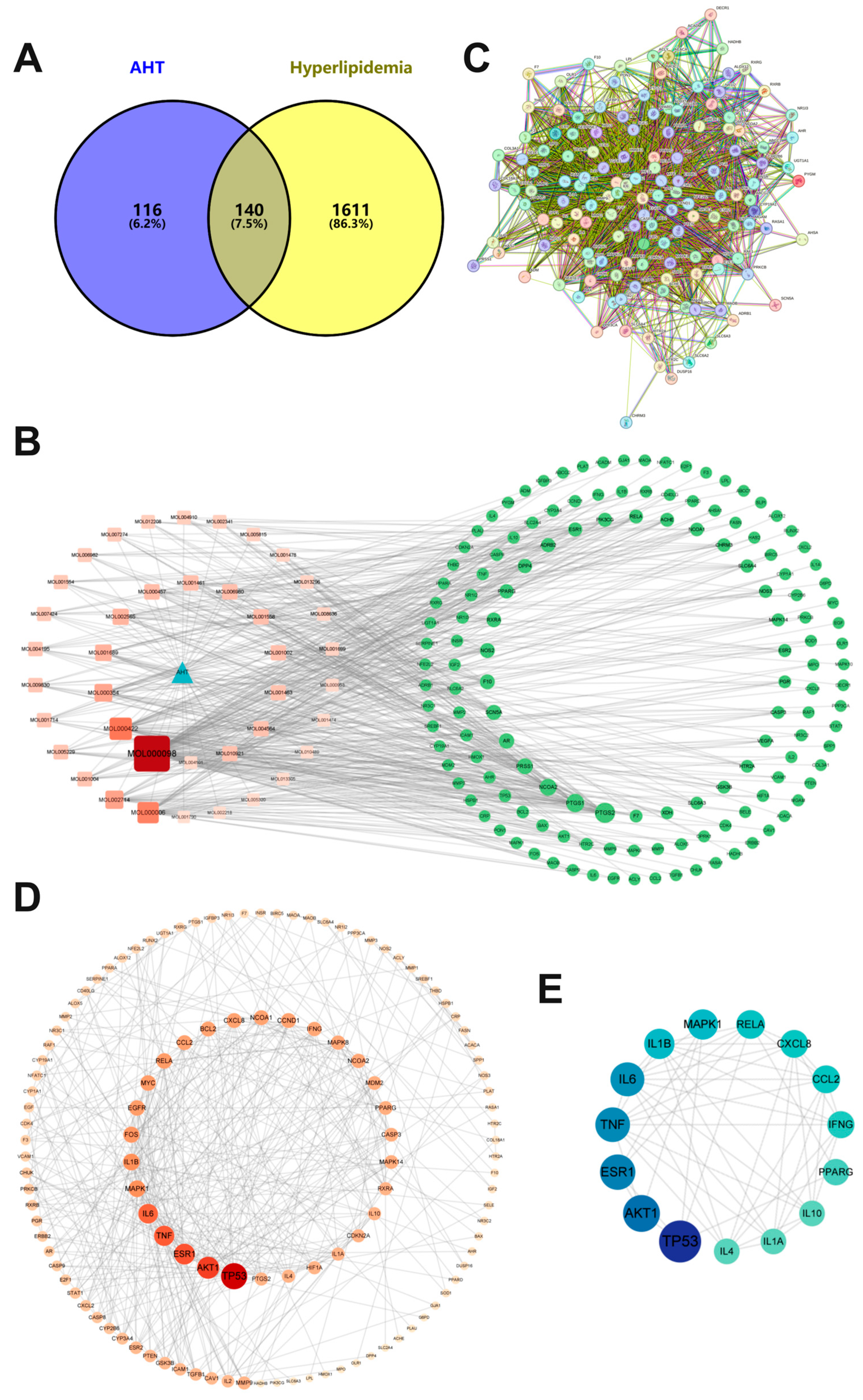
3.2. Potential Target Prediction of AHT and HLP
3.3. Target Network Construction for AHT and HLP
3.4. Construction of PPI Network
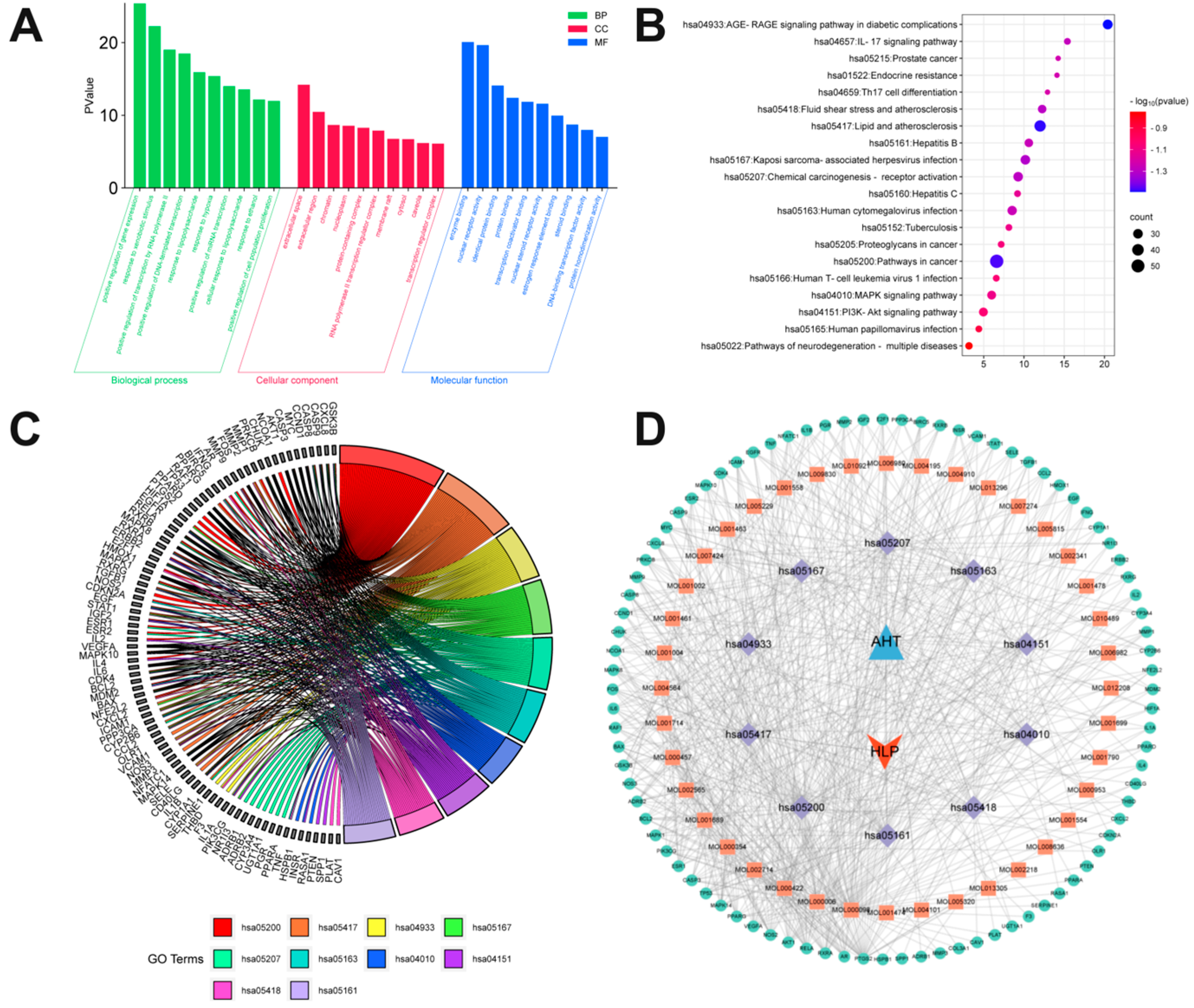
3.5. Enrichment Analysis of GO and KEGG Pathways
3.6. Determination of AHT Compounding Ratio
3.7. Combination Index Analysis of AHT, AME, HRE, and TME
3.8. The Effects of AHT, AME, HRE, and TME on HepG2 Cell Proliferation and Oleic Acid Staining
3.9. The Lipid-Lowering Effect of AHT on Oleic Acid-Induced HepG2 Cells
3.10. The Effect of AHT on the Lipid-Lowering Mechanism Induced by Oleic Acid in HepG2 Cells
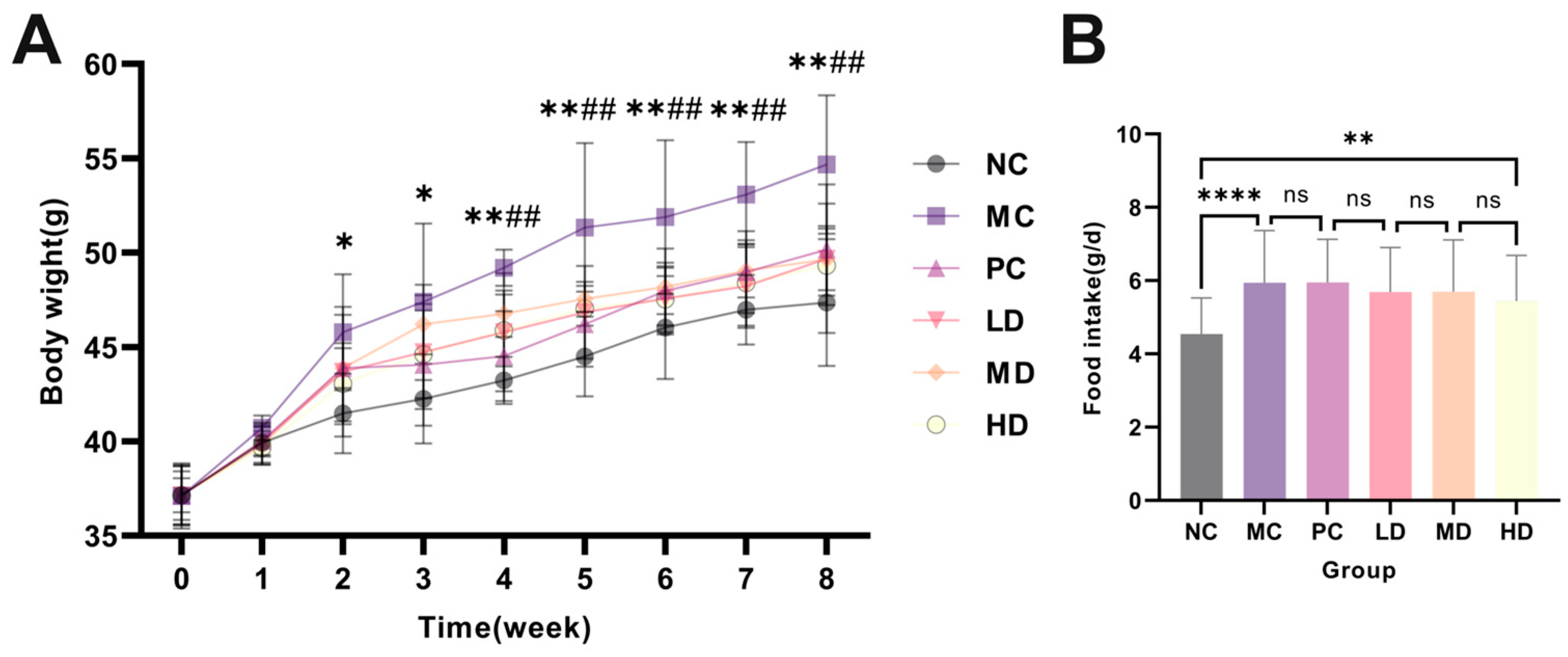
3.11. The Effect of AHT on Body Weight, Food Intake, and Organ Index in Mice
3.12. The Effect of AHT on Serum Biochemical Indicators in High-Fat Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| AME | Astragalus membranaceus extract |
| AHT | Astragalus membranaceus extract: Hippophae rhamnoides L. extract: Taraxacum mongolicum Hand. Mazz extract = 3:1:2 |
| HDL-C | High-density lipoprotein cholesterol |
| HRE | Hippophae rhamnoides L. extract |
| LC-MS | Liquid chromatography–mass spectrometry |
| LDL-C | Low-density lipoprotein cholesterol |
| OMIM | Online Mendelian Inheritance in Man |
| PPI | Protein–protein interaction |
| SOD | Superoxide dismutase |
| TME | Taraxacum mongolicum Hand. Mazz extract |
| TCMSP | Traditional Chinese Medicine System Pharmacology Analysis Platform |
| T-AOC | Total antioxidant capacity |
| TC | Total cholesterol |
| TG | Triglycerides |
References
- Zhou, H.; Wang, X.; She, Z.; Huang, L.; Wei, H.; Yang, S.; Wei, Z.; Chen, H.; Yang, B.; Hu, Z.; et al. Combining bioinformatics and multiomics strategies to investigate the key microbiota and active components of Liupao tea ameliorating hyperlipidemia. J. Ethnopharmacol. 2024, 333, 118438. [Google Scholar] [CrossRef]
- Edward, O.C.; Jeong, D.-Y.; Yang, H.-J.; Han, A.; Cha, Y.-S. Doenjang Ameliorates Diet-Induced Hyperlipidemia and Hepatic Oxidative Damage by Improving Lipid Metabolism, Oxidative Stress, and Inflammation in ICR Mice. Foods 2024, 13, 1471. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Dong, R.; Liu, P.; Zhang, X.; Li, Y.; Lai, X.; Cheong, H.-F.; Wu, Y.; Wang, Y.; et al. Rutin ameliorated lipid metabolism dysfunction of diabetic NAFLD via AMPK/SREBP1 pathway. Phytomedicine 2024, 126, 155437. [Google Scholar] [CrossRef]
- Xie, J.; Luo, M.; Chen, Q.; Zhang, Q.; Qin, L.; Wang, Y.; Zhao, Y.; He, Y. Hypolipidemic effect and gut microbiota regulation of Gypenoside aglycones in rats fed a high-fat diet. J. Ethnopharmacol. 2024, 328, 118066. [Google Scholar] [CrossRef]
- Ma, M.; Gu, F.; Yue, Z.; Gao, L.; Chen, C.; Lin, Q.; Huang, K.; Li, X.; Dai, J.; Han, B. Dendrobium huoshanense Improves Lipid Metabolism Disorder by Restoring Gut Flora and Metabolites in Mice Fed a High-Fat Diet. J. Food Biochem. 2024, 2024, 6245499. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Ji, B.; Cai, S.; Wang, R.; Zhou, F.; Yang, J.; Liu, H. A diet formula of Puerariae radix, Lycium barbarum, Crataegus pinnatifida, and Polygonati rhizoma alleviates insulin resistance and hepatic steatosis in CD-1 mice and HepG2 cells. Food Funct. 2014, 5, 1038–1049. [Google Scholar] [CrossRef]
- Cristina, R.T.; Mohamed, E.A.; Tulcan, C.; Dumitrescu, E.; Muselin, F.; Orasan, S.A.; Mateoc-Sirb, T.; Vlad, D. The Oxidative Stress Markers’ Protective Influence of Sea Buckthorn and Grape Extracts in Atorvastatin-Treated Hyperlipidemic Rats. Nutrients 2024, 16, 1954. [Google Scholar] [CrossRef]
- Tian, B.; Pan, Y.; Zhou, X.; Jiang, Y.; Zhang, X.; Luo, X.; Yang, K. Yellow leaf green tea modulates the AMPK/ACC/SREBP1c signaling pathway and gut microbiota in high-fat diet-induced mice to alleviate obesity. J. Sci. Food Agric. 2024, 104, 5882–5895. [Google Scholar] [CrossRef]
- Yang, X.; Chi, C.; Li, W.; Zhang, Y.; Yang, S.; Xu, R.; Liu, R. Metabolomics and lipidomics combined with serum pharmacochemistry uncover the potential mechanism of Huang-Lian-Jie-Du decoction alleviates atherosclerosis in ApoE−/− mice. J. Ethnopharmacol. 2024, 324, 117748. [Google Scholar] [CrossRef]
- Xiao, P.-T.; Liu, S.-Y.; Kuang, Y.-J.; Jiang, Z.-M.; Lin, Y.; Xie, Z.-S.; Liu, E.-H. Network pharmacology analysis and experimental validation to explore the mechanism of sea buckthorn flavonoids on hyperlipidemia. J. Ethnopharmacol. 2021, 264, 113380. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, H.; Zhu, W.; Li, Y.; Li, H. Astragalus polysaccharide attenuates nonalcoholic fatty liver disease through THDCA in high-fat diet-fed mice. J. Ethnopharmacol. 2024, 320, 117401. [Google Scholar] [CrossRef]
- Zhou, X.; Ge, B.; Zhang, X.; Wang, K.; Zhou, C.; Fu, D. Metabolomics Analysis Reveals the Effects of Compound Fuzhuan Brick Tea (CFBT) on Regulating Dyslipidemia and Metabolic Disorders in Mice Induced by High-Fat Diet. Nutrients 2022, 14, 1128. [Google Scholar] [CrossRef]
- Azman, A.N.S.S.; Tan, J.J.; Abdullah, M.N.H.; Bahari, H.; Lim, V.; Yong, Y.K. Network Pharmacology and Molecular Docking Analysis of Active Compounds in Tualang Honey against Atherosclerosis. Foods 2023, 12, 1779. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a Robust and Repeatable UPLC-MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef]
- Wu, B.; Li, C.; Kan, H.; Zhang, Y.; Rao, X.; Liu, Y.; Zhao, P. Hypolipidemic and Antithrombotic Effect of 6′-O-Caffeoylarbutin from Vaccinium dunalianum Based on Zebrafish Model, Network Pharmacology, and Molecular Docking. Molecules 2024, 29, 780. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, Y.; Zhang, L.; Li, Y.; Zhang, H.; Jin, Q.; Wu, G.; Wang, X. Screening of fish oil fatty acids for antihyperlipidemic activity based on network pharmacology and validation of synergistic efficacy in vitro. Food Biosci. 2024, 58, 103745. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Xu, X.; Guo, W.; Yang, J.; Liu, Y.; Xie, S.; Piao, G.; Xu, T.; Wang, Y.; et al. Integrated metabolomics and network pharmacology to reveal the lipid-lowering mechanisms of Qizha Shuangye granules in hyperlipidemic rats. J. Sci. Food Agric. 2024, 104, 3265–3274. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Yu, J.J.J.; Kang, J.; Hou, S.; Cheng, M.; Xu, L.; Gong, L.; Li, Y. DiDang decoction improves mitochondrial function and lipid metabolism via the HIF-1 signaling pathway to treat atherosclerosis and hyperlipidemia. J. Ethnopharmacol. 2023, 308, 116289. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, X.; He, C.; Xiao, C.; Chen, Z.; Chen, Q.; Chen, J.; Bo, H. Sanhuang xiexin decoction synergizes insulin/PI3K-Akt/FoxO signaling pathway to inhibit hepatic glucose production and alleviate T2DM. J. Ethnopharmacol. 2023, 306, 116162. [Google Scholar] [CrossRef]
- Jia, M.J.; Yang, X.; Bao, Y.H.; Huo, J.W. Anti-osteoporosis effect of Mori Follum-Portulaca oleracea L.-Lycium barbarum L. extract based on network pharmacology and experimental validation and its utilization in functional yogurt. Food Biosci. 2024, 59, 103974. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Xin, W.; Wang, L.; Zhang, Y.; Xu, H.; Wang, H.; Zhao, H.; Yang, H.; Si, N.; et al. Gypenosides and capsaicinoids in combination ameliorates high-fat-diet-induced rat hyperlipidemia via the PPARγ-LXRa-ABCA1/ABCG1 pathway. J. Funct. Foods 2023, 108, 105714. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Zhou, Y.; He, J. Camellia nitidissima Chi leaf as pancreatic lipasen inhibitors: Inhibition potentials and mechanism. J. Food Biochem. 2021, 45, e13837. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.K.; Seo, S.Y. Screening of some medicinal plants for anti-lipase activity. J. Ethnopharmacol. 2005, 97, 453–456. [Google Scholar] [CrossRef]
- Falcao, S.I.; Duarte, D.; Diallo, M.; Santos, J.; Ribeiro, E.; Vale, N.; Vilas-Boas, M. Improvement of the In Vitro Cytotoxic Effect on HT-29 Colon Cancer Cells by Combining 5-Fluorouacil and Fluphenazine with Green, Red or Brown Propolis. Molecules 2023, 28, 3393. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, X.; Jin, Z.; Ni, R.; Pan, Y.; Yang, G. Zhuidu Formula suppresses the migratory and invasive properties of triple-negative breast cancer cells via dual signaling pathways of RhoA/ROCK and CDC42/MRCK. J. Ethnopharmacol. 2023, 315, 116644. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Pineiro, M.; Martinez-Peinado, N.; Barrera, P.; Sosa, M.; Bastida, J.; Alonso-Padilla, J.; Feresin, G.E. Candimine from Hippeastrum escoipense (Amaryllidaceae): Anti-Trypanosoma cruzi activity and synergistic effect with benznidazole. Phytomedicine 2023, 114, 154788. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Z.; Li, P.; Wang, Y.; Wang, X.; Piao, C.; Yuan, L. Lipid-lowering capacity of GABA-rich supernatant from fermented okara in OA-induced HepG2 cells. Food Biosci. 2024, 58, 103659. [Google Scholar] [CrossRef]
- Wu, X.; Li, B.; Lu, H.; Ling, X.; Hu, Z.; Luo, Y.; Qin, D.; Yang, F.; Tang, Y.; Xie, T.; et al. Parboiled rice extracts ameliorate oleic acid-induced steatosis of HepG2 cell and its molecular mechanism. J. Funct. Foods 2023, 110, 105853. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Z.; Qin, B.; Ju, X.; Wang, L. Evaluation of the intracellular lipid-lowering effect of polyphenols extract from highland barley in HepG2 cells. Food Sci. Hum. Wellness 2024, 13, 454–461. [Google Scholar] [CrossRef]
- Ta, N.; Lisha, A.; Erdunduleng, E.; Qi, R.; Mu, X.; Feng, L.; Ba, G.; Li, Y.; Zhang, J.; Bai, L.; et al. Metabolomics analysis reveals amelioration effects of yellowhorn tea extract on hyperlipidemia, inflammation, and oxidative stress in high-fat diet-fed mice. Front. Nutr. 2023, 10, 1087256. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Ma, Y.; Zhang, H.; Chen, W. Prediction and verification of the active ingredients and potential targets of Erhuang Quzhi Granules on non-alcoholic fatty liver disease based on network pharmacology. J. Ethnopharmacol. 2023, 311, 116435. [Google Scholar] [CrossRef]
- Zhang, P.-P.; Zhuo, B.-Y.; Duan, Z.-W.; Li, X.; Huang, S.-L.; Cao, Q.; Zhao, T.; Wei, S.-L.; Hub, X.-H.; Zhang, Y. Marein reduces lipid levels via modulating the PI3K/AKT/mTOR pathway to induce lipophagy. J. Ethnopharmacol. 2023, 312, 116523. [Google Scholar] [CrossRef]
- Duan, Y.; Guo, F.; Li, C.; Xiang, D.; Gong, M.; Yi, H.; Chen, L.; Yan, L.; Zhang, D.; Dai, L.; et al. Aqueous extract of fermented Eucommia ulmoides leaves alleviates hyperlipidemia by maintaining gut homeostasis and modulating metabolism in high-fat diet fed rats. Phytomedicine 2024, 128, 155291. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Liu, J.; Li, M.; Lu, J.; Zhou, J.; Zhang, M.; Ferri, N.; Chen, H. Mulberry leaf and its effects against obesity: A systematic review of phytochemistry, molecular mechanisms and applications. Phytomedicine 2024, 128, 155528. [Google Scholar] [CrossRef]
- Teng, H.; He, Z.; Hong, C.; Xie, S.; Zha, X. Extraction, purification, structural characterization and pharmacological activities of polysaccharides from sea buckthorn (Hippophae rhamnoides L.): A review. J. Ethnopharmacol. 2024, 324, 117809. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Ye, Y.L.; Kong, L.M.; Xu, A.L.; Liu, L.H.; Li, Z.B. Regulatory effects of Astragalus membranaceus polysaccharides on lipid metabolism disorders induced by a high-fat diet in spotted sea bass (Lateolabrax maculatus). Int. J. Biol. Macromol. 2024, 271, 132584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.F.; Li, W.; Xu, G.Y.; Wang, K.R.; Li, L.; Luo, H.; Zou, L.; Wu, J.S. Updates and advances on pharmacological properties of Taraxacum mongolicum Hand.-Mazz and its potential applications. Food Chem. 2022, 373, 131380. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.; Mei, X.; Huang, R.; Zhang, S.; Tang, Y.; Dong, Q.; Zhou, C. Effect of the polyphenol-rich extract from Allium cepa on hyperlipidemic sprague-dawley rats. J. Food Biochem. 2021, 45, e13565. [Google Scholar] [CrossRef]
- Shen, Z.; Gao, X.; Huang, D.; Xu, X.; Shen, J. The potential of Gynostemma pentaphyllum in the treatment of hyperlipidemia and its interaction with the LOX1-PI3K-AKT-eNOS pathway. Food Sci. Nutr. 2024, 8, 25. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, W.; Yang, J.; Ali, A.; Qin, H. Mechanism of Astragalus membranaceus Alleviating Acquired Hyperlipidemia Induced by High-Fat Diet through Regulating Lipid Metabolism. Nutrients 2022, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Rong, P.; Wang, J.; Yu, X.; Wang, N.; Wang, S.; Xue, Z.; Chen, J.; Meng, W.; Peng, X. Anti-osteoporosis effects and regulatory mechanism of Lindera aggregata based on network pharmacology and experimental validation. Food Funct. 2022, 13, 6419–6432. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Liu, Y.; Huang, C.; Feng, W.; Cui, H.; Li, M. Xanthoceras sorbifolium leaves alleviate hyperuricemic nephropathy by inhibiting the PI3K/AKT signaling pathway to regulate uric acid transport. J. Ethnopharmacol. 2024, 327, 117946. [Google Scholar] [CrossRef]
- Meng, H.; Song, J.; Fan, B.; Li, Y.; Zhang, J.; Yu, J.; Zheng, Y.; Wang, M. Monascus vinegar alleviates high-fat-diet-induced inflammation in rats by regulating the NF-KB and PI3K/AKT/mTOR pathways. Food Sci. Hum. Wellness 2022, 11, 943–953. [Google Scholar] [CrossRef]
- Shen, B.; Wen, Y.; Li, S.; Zhou, Y.; Chen, J.; Yang, J.; Zhao, C.; Wang, J. Paeonol ameliorates hyperlipidemia and autophagy in mice by regulating Nrf2 and AMPK/mTOR pathways. Phytomedicine 2024, 132, 155839. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, W.; Zhu, Z.; Li, S.; Chen, X.; Cravotto, G.; Sui, Y.; Zhou, L. Complexes of Soluble Dietary Fiber and Polyphenols from Lotus Root Regulate High-Fat Diet-Induced Hyperlipidemia in Mice. Antioxidants 2024, 13, 466. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, M.; Zheng, Y.; Wang, M.; Gao, Y.; Wang, X.; Liu, X.; Lv, W.; Zeng, X.; Belosludtsev, K.N.; et al. α-Ketoglutarate prevents hyperlipidemia-induced fatty liver mitochondrial dysfunction and oxidative stress by activating the AMPK-pgc-1α/Nrf2 pathway. Redox Biol. 2024, 74, 103230. [Google Scholar] [CrossRef]
- Yang, J.-H.; Bai, T.-C.; Shi, L.-L.; Hou, B.; Tang, R.; Zhang, R.-P.; Chen, X.-L. Antihyperlipidemic effect of Vaccinium dunalianum buds based on biological activity screening and LC-MS. J. Ethnopharmacol. 2023, 306, 116190. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, F.; Yan, Y.; Jin, J.; Quan, Z.; Tong, H.; Du, J. Fucoidan derived from Sargassum pallidum alleviates metabolism disorders associated with improvement of cardiac injury and oxidative stress in diabetic mice. Phytother. Res. 2023, 37, 4210–4223. [Google Scholar] [CrossRef]





| No. | Name | Molecule ID | Molecule Name | OB (%) | DL |
|---|---|---|---|---|---|
| 1 | Luteolin | MOL000006 | Luteolin | 36.16 | 0.25 |
| 2 | Quercetin | MOL000098 | Quercetin | 46.43 | 0.28 |
| 3 | Isorhamnetin | MOL000354 | Isorhamnetin | 49.6 | 0.31 |
| 4 | Kaempferol | MOL000422 | Kaempferol | 41.88 | 0.24 |
| 5 | Phaseollidin | MOL000457 | Phaseollidin | 52.04 | 0.53 |
| 6 | Cholesterol | MOL000953 | CLR | 37.87 | 0.68 |
| 7 | Ellagic acid | MOL001002 | Ellagic acid | 43.06 | 0.43 |
| 8 | Pelargonidin | MOL001004 | Pelargonidin | 37.99 | 0.21 |
| 9 | Dihydrochelerythrine | MOL001461 | Dihydrochelerythrine | 32.73 | 0.81 |
| 10 | Dihydrosanguinarine | MOL001463 | Dihydrosanguinarine | 59.31 | 0.86 |
| 11 | Sanguinarine | MOL001474 | Sanguinarine | 37.81 | 0.86 |
| 12 | Chelerythrine | MOL001478 | Toddaline | 25.99 | 0.81 |
| 13 | Scopolamine | MOL001554 | Scopolamine | 67.97 | 0.27 |
| 14 | (+)-Sesamin | MOL001558 | Sesamin | 56.55 | 0.83 |
| 15 | Acacetin | MOL001689 | Acacetin | 34.97 | 0.24 |
| 16 | Diphyllin | MOL001699 | Diphyllin | 36.23 | 0.75 |
| 17 | Podofilox | MOL001714 | Podophyllotoxin | 59.94 | 0.86 |
| 18 | Linarin | MOL001790 | Linarin | 39.84 | 0.71 |
| 19 | Scopolin | MOL002218 | Scopolin | 56.45 | 0.39 |
| 20 | Hesperetin | MOL002341 | Hesperetin | 70.31 | 0.27 |
| 21 | Medicarpin | MOL002565 | Medicarpin | 49.22 | 0.34 |
| 22 | Baicalein | MOL002714 | Baicalein | 33.52 | 0.21 |
| 23 | Melilotoside | MOL004101 | Melilotoside | 36.85 | 0.26 |
| 24 | Corydaline | MOL004195 | CORYDALINE | 65.84 | 0.68 |
| 25 | Kaempferide | MOL004564 | Kaempferid | 73.41 | 0.27 |
| 26 | Glabranin | MOL004910 | Glabranin | 52.9 | 0.31 |
| 27 | Artemetin | MOL005229 | Artemetin | 49.55 | 0.48 |
| 28 | Arachidonic acid | MOL005320 | Arachidonate | 45.57 | 0.2 |
| 29 | Citromitin | MOL005815 | Citromitin | 86.9 | 0.51 |
| 30 | Papaverine | MOL006980 | Papaverine | 64.04 | 0.38 |
| 31 | Codeine | MOL006982 | Codeine | 45.48 | 0.56 |
| 32 | Cirsimaritin | MOL007274 | Skrofulein | 30.35 | 0.3 |
| 33 | Artemisinin | MOL007424 | Artemisinin | 49.88 | 0.31 |
| 34 | Corynoline | MOL008636 | Corynoline | 30.53 | 0.85 |
| 35 | Camptothecin | MOL009830 | EHD | 61.04 | 0.81 |
| 36 | Leucocyanidin | MOL010489 | Resivit | 30.84 | 0.27 |
| 37 | Estrone | MOL010921 | Estrone | 53.56 | 0.32 |
| 38 | Lobelanine | MOL012208 | Lobelanine | 54.13 | 0.32 |
| 39 | Uridine 5’-monophosphate | MOL012820 | 5’-Uridylic acid | 40.25 | 0.2 |
| 40 | Fustin | MOL013296 | Fustin | 50.91 | 0.24 |
| 41 | Garbanzol | MOL013305 | Garbanzol | 83.67 | 0.21 |
| Peak No. | Proposed Compound | RT/s | Precursor m/z | Error (ppm) | Formula |
|---|---|---|---|---|---|
| 1 | Luteolin | 290 | 285.0761 | 2.606 | C15H10O6 |
| 2 | Quercetin | 287 | 303.0498 | 0.420 | C15H10O7 |
| 3 | Isorhamnetin | 303.7 | 315.0515 | 1.512 | C16H12O7 |
| 4 | Kaempferol | 282 | 285.0391 | 4.770 | C15H10O6 |
| 5 | Phaseollidin | 224 | 325.1379 | 17.003 | C20H20O4 |
| 6 | CLR | 412.7 | 369.3588 | 19.607 | C27H46O |
| 7 | Ellagic acid | 116.6 | 324.9999 | 13.574 | C14H6O8 |
| 8 | Pelargonidin | 57.5 | 543.1263 | 4.175 | C15H11O5 |
| 9 | Dihydrochelerythrine | 63.8 | 332.1285 | 1.169 | C21H19NO4 |
| 10 | Dihydrosanguinarine | 48.1 | 665.1907 | 3.352 | C20H15NO4 |
| 11 | Sanguinarine | 116.9 | 289.1103 | 1.990 | C20H14NO4 |
| 12 | Toddaline | 83.9 | 332.1315 | 10.197 | C21H18NO4 |
| 13 | Scopolamine | 301.9 | 285.1163 | 10.784 | C17H21NO4 |
| 14 | Sesamin | 48.5 | 337.1115 | 13.218 | C20H18O6 |
| 15 | Acacetin | 336.1 | 283.0606 | 2.098 | C16H12O5 |
| 16 | Diphyllin | 46.8 | 381.0936 | 8.594 | C21H16O7 |
| 17 | Podophyllotoxin | 58.8 | 829.2688 | 1.687 | C22H22O8 |
| 18 | Linarin | 240.2 | 653.184 | 17.888 | C28H32O14 |
| 19 | Scopolin | 81.8 | 335.0758 | 4.287 | C16H18O9 |
| 20 | Hesperetin | 241.6 | 301.071 | 2.518 | C16H14O6 |
| 21 | Medicarpin | 240.9 | 254.0728 | 11.289 | C16H14O4 |
| 22 | Baicalein | 297.6 | 269.0448 | 2.767 | C15H10O5 |
| 23 | Melilotoside | 81.8 | 371.0968 | 4.221 | C15H18O8 |
| 24 | CORYDALINE | 226.1 | 387.2221 | 14.781 | C22H27NO4 |
| 25 | Kaempferid | 302.1 | 299.0567 | 1.977 | C16H12O6 |
| 26 | Glabranin | 385.4 | 369.1471 | 4.907 | C20H20O4 |
| 27 | Artemetin | 61.3 | 345.1367 | 9.972 | C20H20O8 |
| 28 | Arachidonate | 447.9 | 303.2351 | 7.121 | C20H32O2 |
| 29 | Citromitin | 206.6 | 405.1339 | 18.738 | C21H24O8 |
| 30 | Papaverine | 66.5 | 322.127 | 4.678 | C20H21NO4 |
| 31 | Codeine | 219.3 | 300.1587 | 2.372 | C18H21NO3 |
| 32 | Skrofulein | 252 | 359.073 | 11.803 | C17H14O6 |
| 33 | Artemisinin | 260.9 | 343.1382 | 4.764 | C15H22O5 |
| 34 | Corynoline | 254.3 | 350.1458 | 3.569 | C21H21NO5 |
| 35 | EHD | 260.3 | 330.2363 | 11.189 | C20H16N2O4 |
| 36 | Resivit | 267.9 | 288.0443 | 14.498 | C15H14O7 |
| 37 | Estrone | 292.4 | 269.079 | 10.881 | C18H22O2 |
| 38 | Lobelanine | 276 | 336.1978 | 5.963 | C22H25NO2 |
| 39 | 5’-Uridylic acid | 41.1 | 323.0274 | 3.682 | C9H13N2O9P |
| 40 | Fustin | 326.2 | 333.0473 | 15.279 | C15H12O6 |
| 41 | Garbanzol | 252.4 | 273.0805 | 17.407 | C15H12O5 |
| NO. | Symbol ID | Protein Name | Pathways |
|---|---|---|---|
| 1 | TP53 | Tumor protein p53 | hsa05417, hsa04010, hsa04151 |
| 2 | PPARG | Peroxisome proliferator-activated receptor gamma | hsa05417 |
| 3 | ESR1 | Estrogen receptor | hsa05207 |
| 4 | TNF | Tumor necrosis factor | hsa05417, hsa04933, hsa04010 |
| 5 | CCL2 | C-C motif chemokine 2 | hsa05417, hsa04933, hsa05418 |
| 6 | AKT1 | Potassium channel AKT1 | hsa05417, hsa04933, hsa04010, hsa04151 |
| 7 | RELA | Transcription factor p65 | hsa05417, hsa04933, hsa04010, hsa04151 |
| 8 | MAPK1 | Mitogen-activated protein kinase 1 | hsa05417, hsa04933, hsa04010, hsa04151 |
| 9 | IL6 | Interleukin-6 | hsa05417, hsa04933, hsa04151 |
| 10 | CXCL8 | Interleukin-8 | hsa05417, hsa04933 |
| 11 | IL1A | Interleukin-1 alpha | hsa04933, hsa04010, hsa05418 |
| 12 | IL4 | Interleukin-4 | hsa04151 |
| 13 | IL10 | Interleukin-10 | - |
| 14 | IL1B | Interleukin-1 beta | hsa05417, hsa04933, hsa04010 |
| 15 | IFNG | Interferon gamma | hsa05418 |
| ID | Description | p-Value | Gene ID | Count |
|---|---|---|---|---|
| hsa05200 | Pathways in cancer | 29.15 | GSK3B, CXCL8, PTEN, CASP9, CASP8, CCND1, MYC, CASP3, AKT1, NCOA1, CHUK, PRKCB, MMP1, MMP2, FOS, MMP9, AR, IFNG, BIRC5, PPARG, RAF1, TP53, PPARD, PTGS2, HIF1A, EGFR, RELA, RXRB, MAPK8, RXRA, ERBB2, E2F1, HMOX1, MAPK1, RXRG, TGFB1, NOS2, CDKN2A, EGF, STAT1, IGF2, ESR1, ESR2, IL2, VEGFA, MAPK10, IL4, IL6, CDK4, BCL2, MDM2, BAX, NFE2L2 | 53 |
| hsa05417 | Lipid and atherosclerosis | 30.27 | GSK3B, CXCL8, TNF, CXCL2, RELA, ICAM1, CASP9, RXRB, PPP3CA, MAPK8, CASP8, CYP2B6, RXRA, CASP3, CCL2, AKT1, MAPK1, OLR1, RXRG, VCAM1, CHUK, MMP1, NOS3, MMP3, NFATC1, FOS, MAPK14, SELE, MMP9, MAPK10, IL6, CD40LG, IL1B, CYP1A1, BCL2, BAX, PPARG, TP53, NFE2L2 | 39 |
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 31.05 | CXCL8, SERPINE1, TNF, RELA, ICAM1, THBD, MAPK8, CCND1, CASP3, CCL2, AKT1, MAPK1, TGFB1, VCAM1, PRKCB, NOS3, STAT1, MMP2, NFATC1, MAPK14, SELE, F3, VEGFA, MAPK10, IL1A, COL3A1, IL6, CDK4, IL1B, BCL2, BAX | 31 |
| hsa05167 | Kaposi sarcoma-associated herpesvirus infection | 20.65 | GSK3B, CXCL8, PTGS2, HIF1A, CXCL2, RELA, PIK3CG, ICAM1, CASP9, PPP3CA, MAPK8, CASP8, CCND1, MYC, CASP3, E2F1, AKT1, MAPK1, CHUK, STAT1, NFATC1, FOS, MAPK14, VEGFA, MAPK10, IL6, CDK4, BAX, RAF1, TP53 | 30 |
| hsa05207 | Chemical carcinogenesis—receptor activation | 19.50 | NR1I3, ADRB1, AHR, ADRB2, CYP3A4, RELA, EGFR, RXRB, CYP2B6, RXRA, CCND1, MYC, E2F1, AKT1, MAPK1, RXRG, UGT1A1, PRKCB, EGF, FOS, ESR1, ESR2, VEGFA, AR, CYP1A1, BCL2, BIRC5, PGR, RAF1, PPARA | 30 |
| hsa05163 | Human cytomegalovirus infection | 17.81 | GSK3B, CXCL8, PTGS2, TNF, RELA, EGFR, CASP9, PPP3CA, CASP8, CCND1, MYC, CASP3, E2F1, CCL2, AKT1, MAPK1, CHUK, CDKN2A, PRKCB, NFATC1, MAPK14, VEGFA, IL6, CDK4, IL1B, MDM2, BAX, RAF1, TP53 | 29 |
| hsa04010 | MAPK signaling pathway | 12.69 | HSPB1, TNF, RELA, EGFR, PPP3CA, MAPK8, MYC, CASP3, ERBB2, AKT1, MAPK1, TGFB1, CHUK, PRKCB, EGF, INSR, IGF2, NFATC1, FOS, MAPK14, VEGFA, MAPK10, IL1A, RASA1, IL1B, RAF1, TP53 | 27 |
| hsa04151 | PI3K-Akt signaling pathway | 10.81 | GSK3B, PTEN, RELA, EGFR, PIK3CG, CASP9, RXRA, CCND1, MYC, ERBB2, SPP1, AKT1, MAPK1, CHUK, EGF, NOS3, INSR, IGF2, IL2, VEGFA, IL4, IL6, CDK4, BCL2, MDM2, RAF1, TP53 | 27 |
| hsa05418 | Fluid shear stress and atherosclerosis | 19.75 | PLAT, TNF, RELA, ICAM1, THBD, MAPK8, CCL2, AKT1, HMOX1, VCAM1, CHUK, NOS3, CAV1, MMP2, FOS, MAPK14, SELE, MMP9, VEGFA, MAPK10, IL1A, IFNG, IL1B, BCL2, TP53, NFE2L2 | 26 |
| hsa05161 | Hepatitis B | 18.15 | CXCL8, TNF, RELA, CASP9, MAPK8, CASP8, MYC, CASP3, E2F1, AKT1, MAPK1, TGFB1, CHUK, PRKCB, STAT1, NFATC1, FOS, MAPK14, MMP9, MAPK10, IL6, BCL2, BAX, BIRC5, RAF1, TP53 | 26 |
| Group | AME | HRE | TME | Inhibition Rate% |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 80.71 ± 0.83 |
| 2 | 1 | 2 | 2 | 79.84 ± 0.80 |
| 3 | 1 | 3 | 3 | 72.11 ± 1.94 |
| 4 | 2 | 1 | 2 | 94.25 ± 1.10 |
| 5 | 2 | 2 | 3 | 86.08 ± 0.86 |
| 6 | 2 | 3 | 1 | 89.64 ± 1.23 |
| 7 | 3 | 1 | 3 | 96.72 ± 1.30 |
| 8 | 3 | 2 | 1 | 95.87 ± 0.25 |
| 9 | 3 | 3 | 2 | 96.47 ± 0.32 |
| k1 | 77.55 | 90.56 | 88.74 | |
| k2 | 89.99 | 87.26 | 90.19 | |
| k3 | 96.35 | 86.07 | 84.97 | |
| R | 18.80 | 4.49 | 5.13 | |
| Best combination | A3H1T2 | |||
| Group | NC | MC | PC | LD | MD | HD |
|---|---|---|---|---|---|---|
| Heart (%) | 0.693 ± 0.11 | 0.672 ± 0.11 | 0.602 ± 0.10 | 0.707 ± 0.19 | 0.660 ± 0.09 | 0.613 ± 0.10 |
| Liver (%) | 4.554 ± 0.47 | 4.527 ± 0.35 | 3.847 ± 1.40 | 4.750 ± 0.67 | 4.483 ± 0.66 | 4.548 ± 0.74 |
| Spleen (%) | 0.327 ± 0.03 | 0.309 ± 0.05 | 0.253 ± 0.06 | 0.306 ± 0.08 | 0.282 ± 0.10 | 0.321 ± 0.09 |
| Kidney (%) | 1.462 ± 0.18 | 1.367 ± 0.13 | 1.357 ± 0.17 | 1.463 ± 0.19 | 1.334 ± 0.23 | 1.271 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Jia, M.; Luo, J.; An, Y.; Chen, Z.; Bao, Y. Investigation of the Lipid-Lowering Activity and Mechanism of Three Extracts from Astragalus membranaceus, Hippophae rhamnoides L., and Taraxacum mongolicum Hand. Mazz Based on Network Pharmacology and In Vitro and In Vivo Experiments. Foods 2024, 13, 2795. https://doi.org/10.3390/foods13172795
Yang X, Jia M, Luo J, An Y, Chen Z, Bao Y. Investigation of the Lipid-Lowering Activity and Mechanism of Three Extracts from Astragalus membranaceus, Hippophae rhamnoides L., and Taraxacum mongolicum Hand. Mazz Based on Network Pharmacology and In Vitro and In Vivo Experiments. Foods. 2024; 13(17):2795. https://doi.org/10.3390/foods13172795
Chicago/Turabian StyleYang, Xue, Mingjie Jia, Jiayuan Luo, Yuning An, Zefu Chen, and Yihong Bao. 2024. "Investigation of the Lipid-Lowering Activity and Mechanism of Three Extracts from Astragalus membranaceus, Hippophae rhamnoides L., and Taraxacum mongolicum Hand. Mazz Based on Network Pharmacology and In Vitro and In Vivo Experiments" Foods 13, no. 17: 2795. https://doi.org/10.3390/foods13172795






