Rice (Oryza sativa) Stem Cells as a Novel Promising Active Ingredient with Anti-Proliferative Effects for Potential Skin Cancer Prevention and Skin Whitening Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Callus and Extraction
2.3. Evaluating Total Phenols and Flavonoids of Callus Extracts
2.4. Radical Scavenging Activity
2.5. Evaluating Total Carbohydrates
2.6. Measuring Total Protein
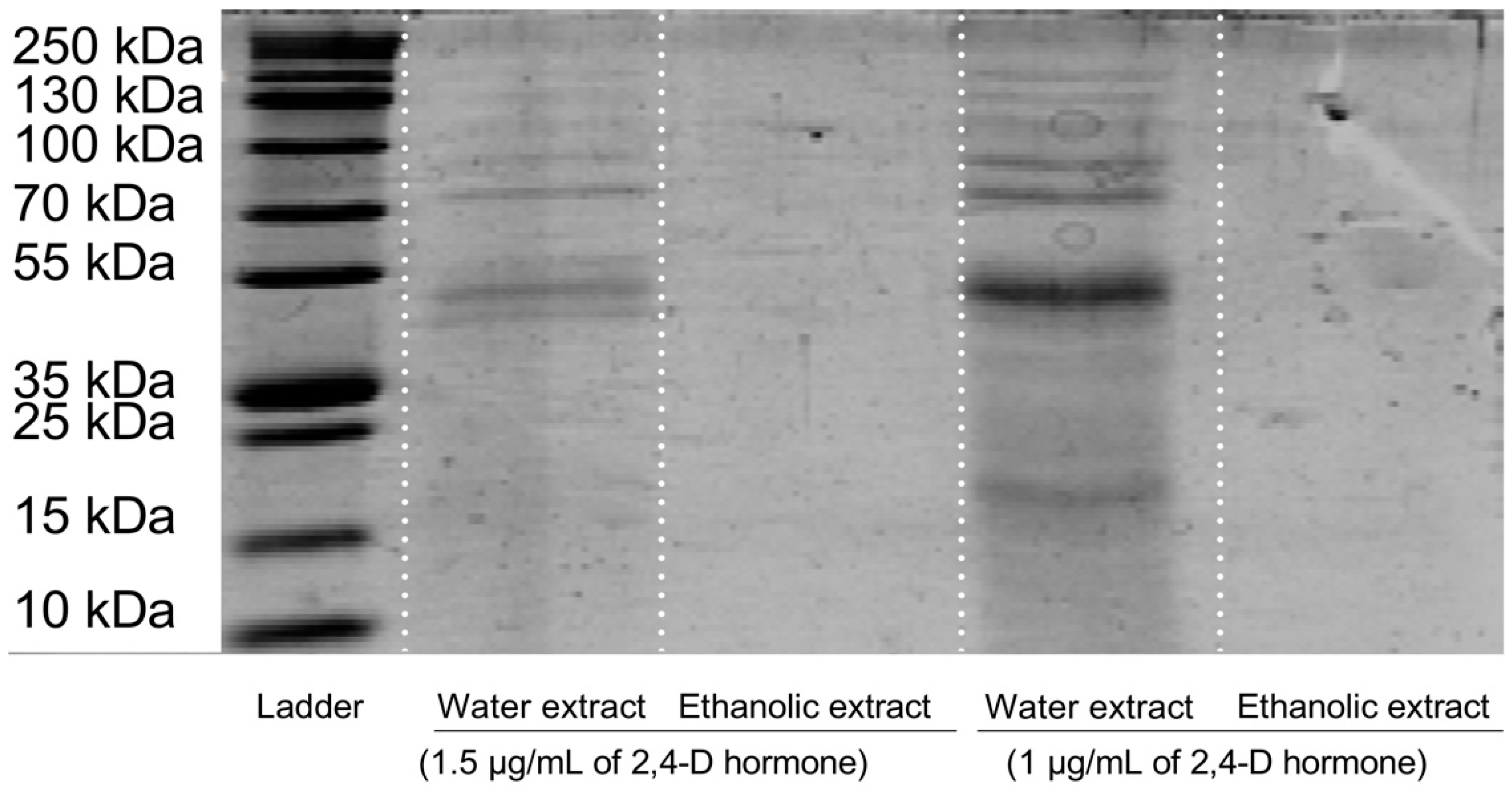
2.7. Investigation of Presence of Proteins
2.8. Cell Culture
2.9. Detection of Cellular ROS
2.10. Cell Viability Study
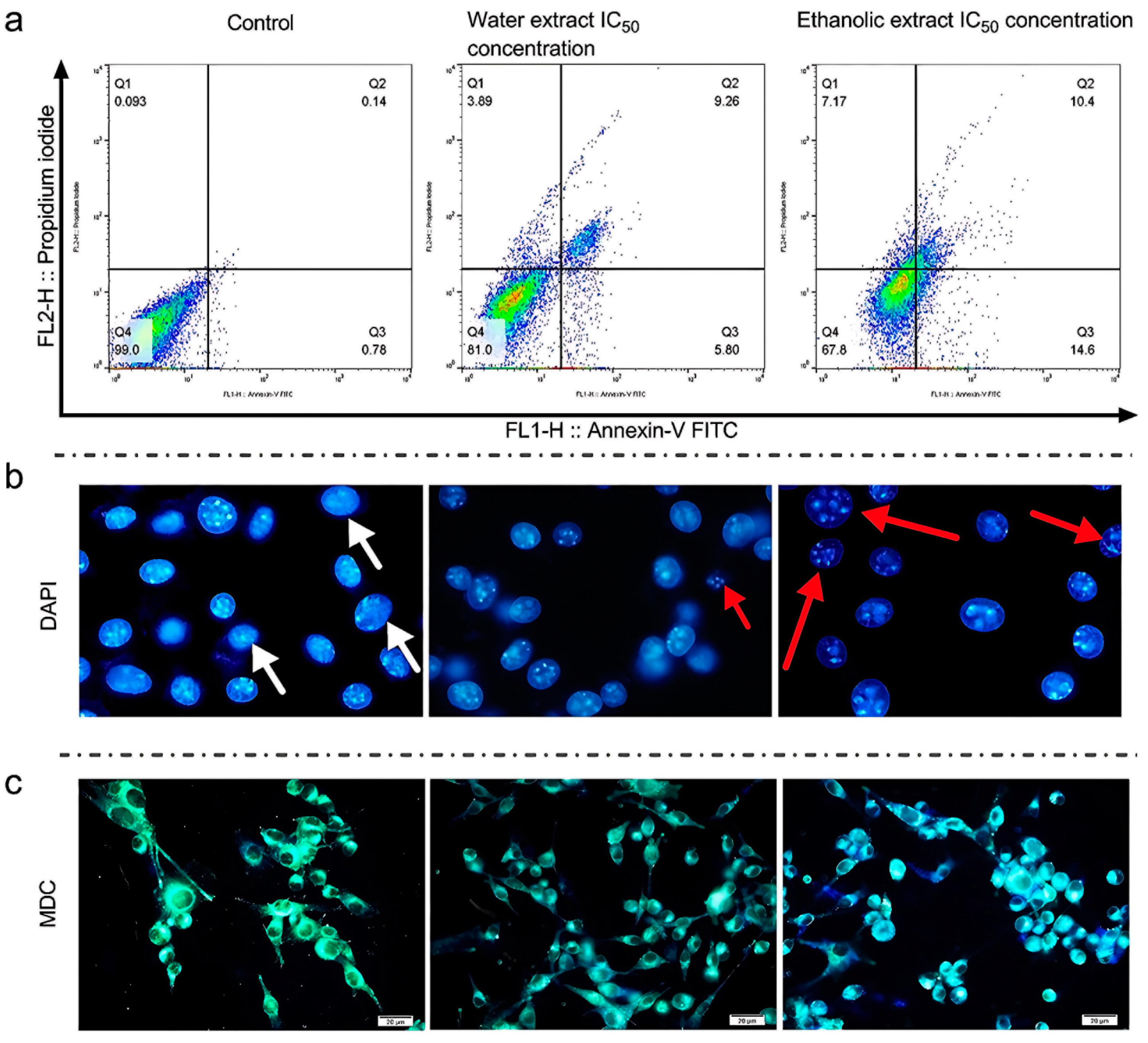
2.11. Apoptosis Assay
2.12. DAPI Staining
2.13. Discovery of Autophagic Vacuoles
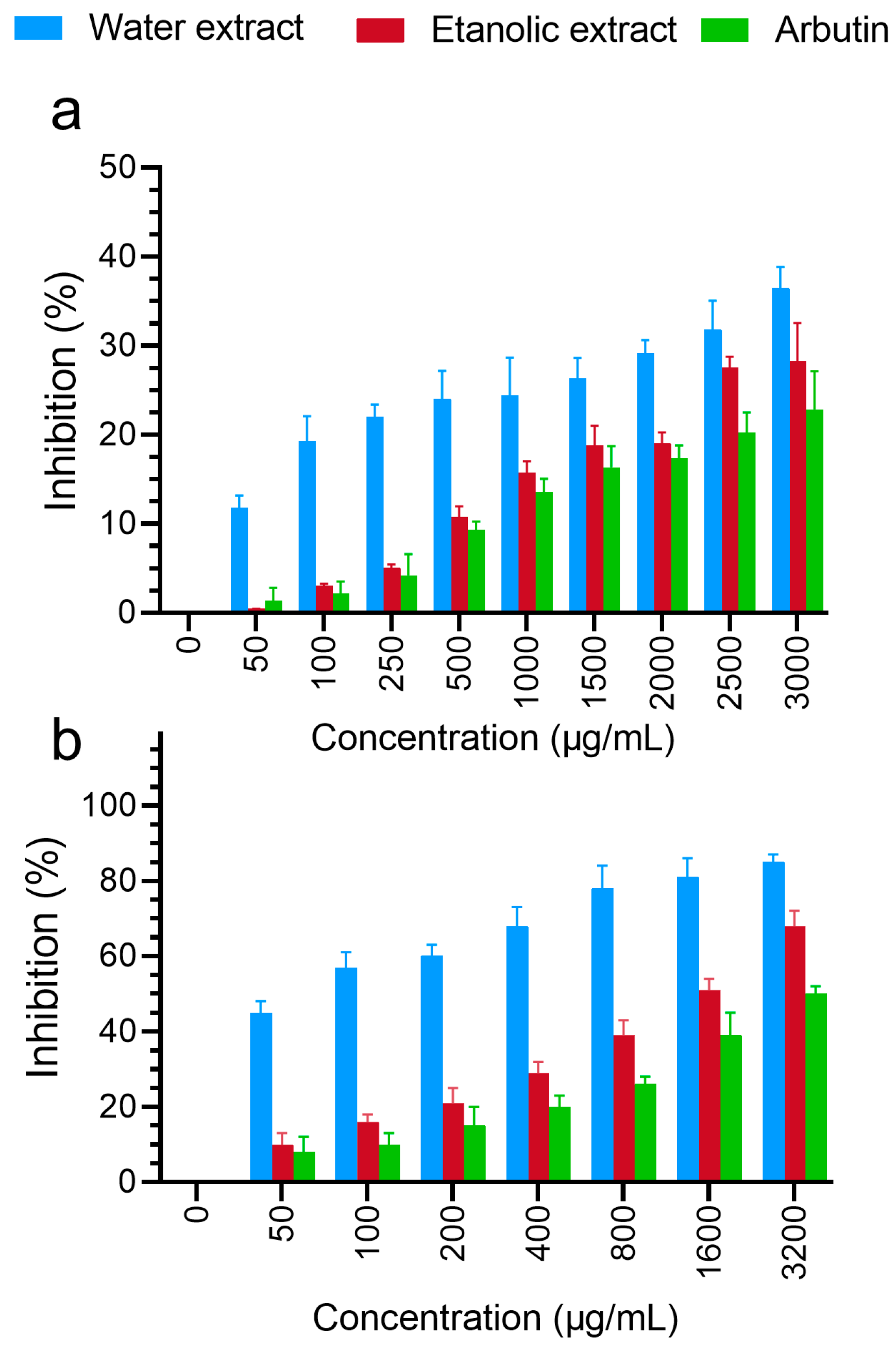
2.14. Prevention of Levodopa Oxidation
2.15. Melanin Biosynthesis Inhibition in Melanocytes
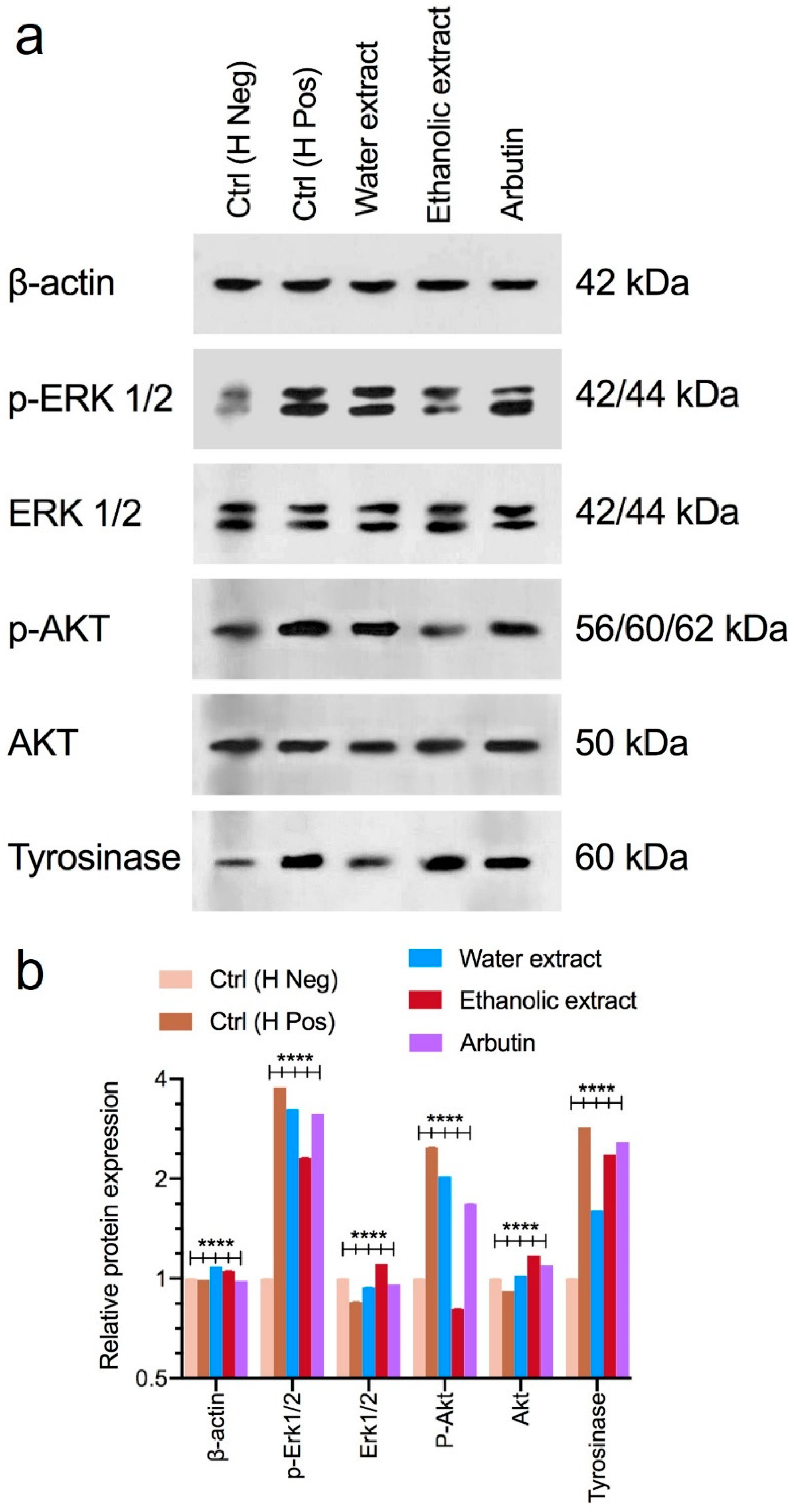
2.16. Western Blot Analysis
2.17. Statistical Analysis
3. Results
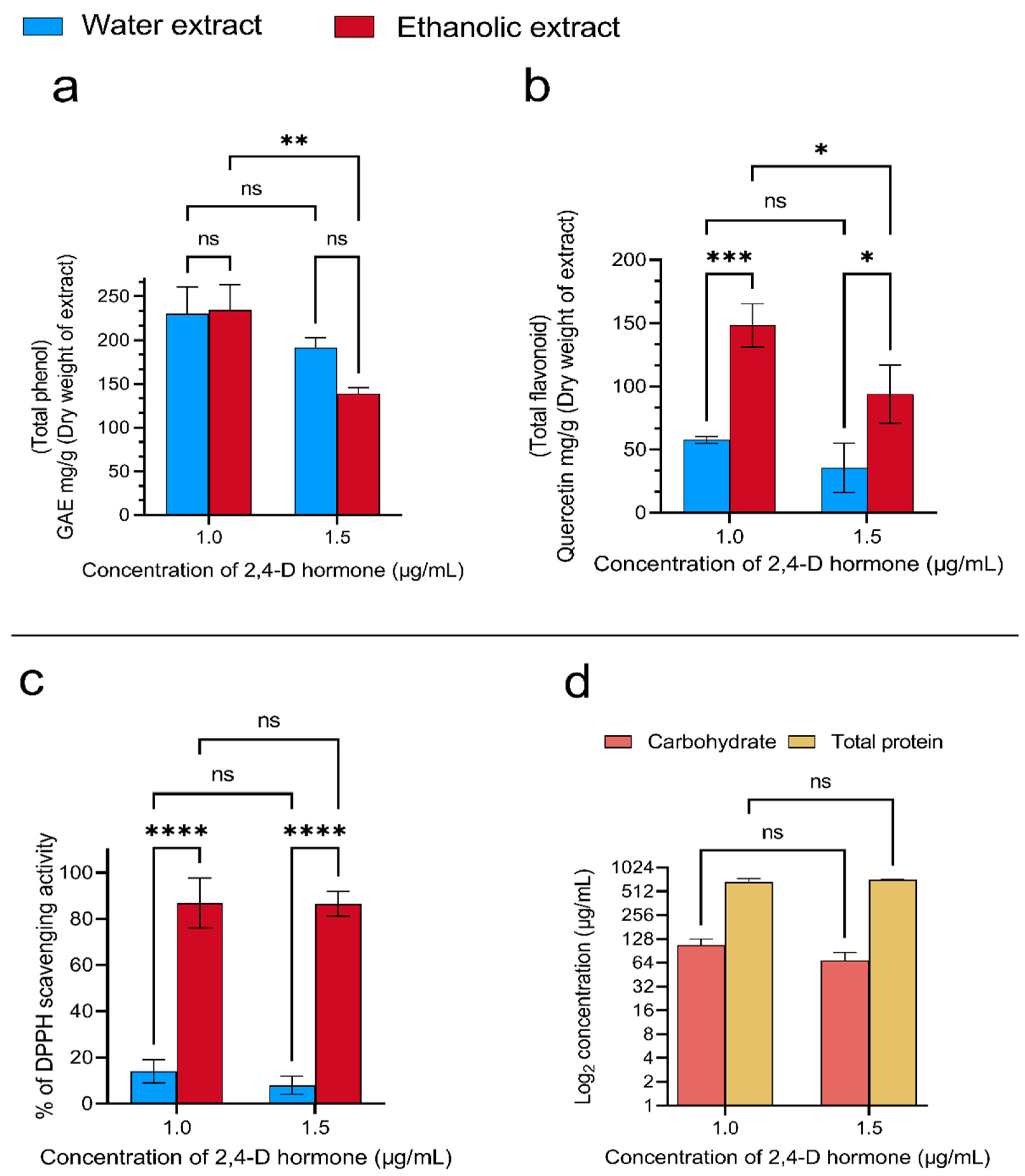
3.1. Preparation and Characterization of Rice Callus Extract
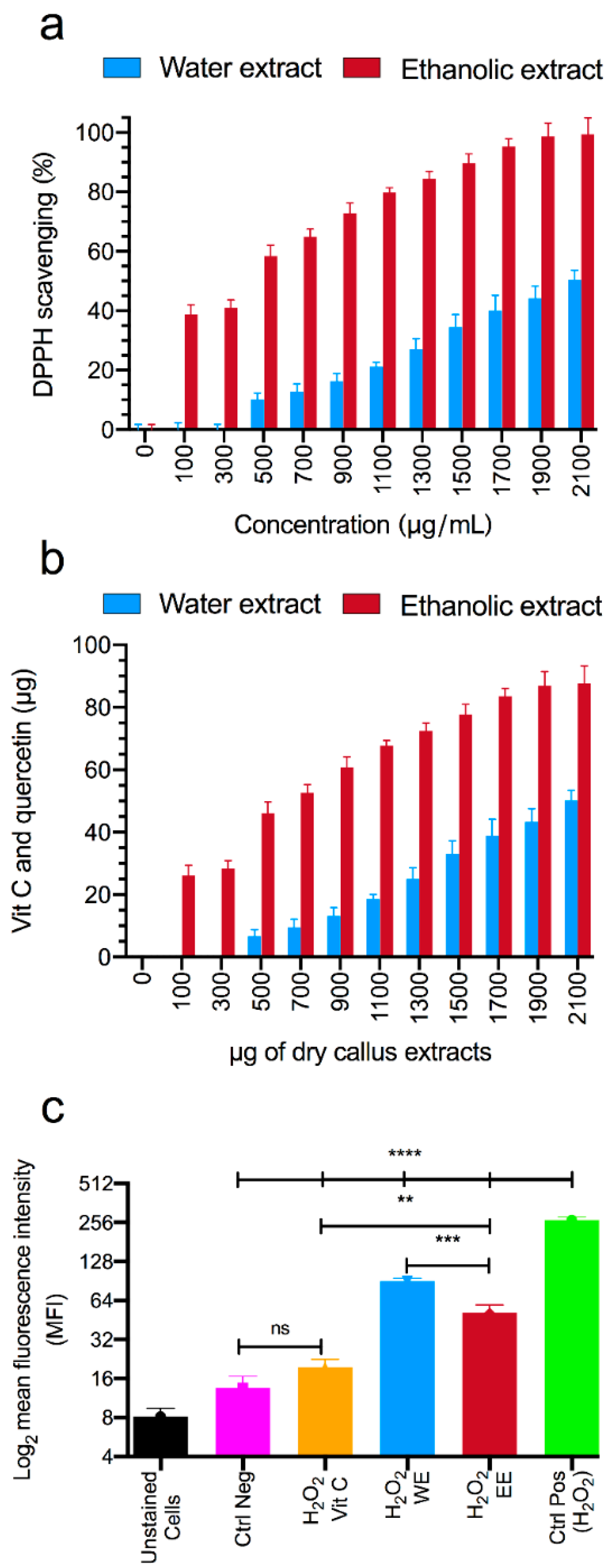
3.2. Antioxidant Capacity Studies
3.3. Studies of Cellular Cytotoxicity of Rice Callus Extracts
3.4. Skin-Whitening Assays
3.5. Western Blot Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortonne, J.P.; Nordlund, J.J. Mechanisms That Cause Abnormal Skin Color. In The Pigmentary System: Physiology and Pathophysiology, 2nd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 521–537. [Google Scholar]
- Im, S.; Kim, J.; Kang, W. Increased expression of α-melanocyte-stimulating hormone in the lesional skin of melasma. Br. J. Dermatol. 2002, 146, 165–167. [Google Scholar] [PubMed]
- Kang, W.; Yoon, K.; Lee, E.S.; Kim, J.; Lee, K.; Yim, H.; Sohn, S.; Im, S. Melasma: Histopathological characteristics in 56 Korean patients. Br. J. Dermatol. 2002, 146, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K.; Zampeli, V.A.; Makrantonaki, E.; Zouboulis, C.C. Discovering the link between nutrition and skin aging. Derm. Endocrinol. 2012, 4, 298–307. [Google Scholar] [CrossRef]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F. Essential fatty acid metabolism and its modification in atopic eczema. Am. J. Clin. Nutr. 2000, 71, 367s–372s. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Kim, Y.G.; Sumiyoshi, M.; Sakanaka, M.; Kimura, Y. Effects of ginseng saponins isolated from red ginseng on ultraviolet B-induced skin aging in hairless mice. Eur. J. Pharmacol. 2009, 602, 148–156. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Reuter, J.; Wölfle, U.; Korting, H.C.; Schempp, C. Which plant for which skin disease? Part 2: Dermatophytes, chronic venous insufficiency, photoprotection, actinic keratoses, vitiligo, hair loss, cosmetic indications. JDDG J. Dtsch. Dermatol. Ges. 2010, 8, 866–873. [Google Scholar] [CrossRef]
- Qiao, H.; Bell, J.; Juliao, S.; Li, L.; May, J.M. Ascorbic acid uptake and regulation of type I collagen synthesis in cultured vascular smooth muscle cells. J. Vasc. Res. 2009, 46, 15–24. [Google Scholar] [CrossRef]
- Singh, R.P.; Agarwal, R. Cosmeceuticals and silibinin. Clin. Dermatol. 2009, 27, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Trauer, S.; Patzelt, A.; Otberg, N.; Knorr, F.; Rozycki, C.; Balizs, G.; Büttemeyer, R.; Linscheid, M.; Liebsch, M.; Lademann, J. Permeation of topically applied caffeine through human skin—A comparison of in vivo and in vitro data. Br. J. Clin. Pharmacol. 2009, 68, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-P.; Lou, Y.-R.; Peng, Q.-Y.; Nghiem, P.; Conney, A.H. Caffeine decreases phospho-Chk1 (Ser317) and increases mitotic cells with cyclin B1 and caspase 3 in tumors from UVB-treated mice. Cancer Prev. Res. 2011, 4, 1118–1125. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, H.; Huang, J.; Jiang, L.; Chen, J.; Zeng, Q. Traditional Asian Herbs in Skin Whitening: The Current Development and Limitations. Front. Pharmacol. 2020, 11, 982. [Google Scholar] [CrossRef]
- Leyden, J.J.; Shergill, B.; Micali, G.; Downie, J.; Wallo, W. Natural options for the management of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1140–1145. [Google Scholar] [CrossRef]
- Dildar, M.; Akram, S.; Irfan, M.; Khan, H.U.; Ramzan, M.; Mahmood, A.R.; Alsaiari, S.A.; Saeed, A.H.M.; Alraddadi, M.O.; Mahnashi, M.H. Skin cancer detection: A review using deep learning techniques. Int. J. Environ. Res. Public Health 2021, 18, 5479. [Google Scholar] [CrossRef]
- Nandagopal, K.; Halder, M.; Dash, B.; Nayak, S.; Jha, S. Biotechnological approaches for production of anti-cancerous compounds resveratrol, podophyllotoxin and zerumbone. Curr. Med. Chem. 2018, 25, 4693–4717. [Google Scholar] [CrossRef]
- Bibi, A.; Khan, M.A.; Adil, M.; Mashwani, Z.U.R. Production of callus biomass and antioxidant secondary metabolites in black cumin. J. Anim. Plant Sci. 2018, 28, 1321–1328. [Google Scholar]
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern Trends in the In Vitro Production and Use of Callus, Suspension Cells and Root Cultures of Medicinal Plants. Molecules 2020, 25, 5805. [Google Scholar] [CrossRef]
- Arumugam, S.; Kavimani, S.; Kadalmani, B.; Ahmed, A.B.A.; Akbarsha, M.A.; Rao, M.V. Antidiabetic activity of leaf and callus extracts of Aegle marmelos in rabbit. Sci. Asia 2008, 34, 317–321. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef]
- Byrne, M.E.; Kidner, C.A.; Martienssen, R.A. Plant stem cells: Divergent pathways and common themes in shoots and roots. Curr. Opin. Genet. Dev. 2003, 13, 551–557. [Google Scholar] [CrossRef]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Trehan, S.; Michniak-Kohn, B.; Beri, K. Plant stem cells in cosmetics: Current trends and future directions. Futur. Sci. OA 2017, 3, FSO226. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Schurch, C.; Blum, P.; Belser, E.; Zulli, F. Plant stem cell extract for longevity of skin and hair. SOFW J. Seifen Ole Fette Wachse 2008, 134, 30–35. [Google Scholar]
- Umar, A.H.; Ratnadewi, D.; Rafi, M.; Sulistyaningsih, Y.C.; Hamim, H. Callus of Curculigo latifolia Dryand. ex W.T. Aiton: Initiation, regeneration, secretory structure and histochemistry. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Lodon, UK, 2021; Volume 948, p. 012051. [Google Scholar]
- Tiwari, S.; Agrawal, P.; Pande, V.; Gupta, H. Callus induction and whole plant regeneration in sub-tropical maize (Zea mays L.) using mature embryos as explants. Indian J. Genet. Plant Breed. 2015, 75, 330–335. [Google Scholar] [CrossRef]
- Ji, S.H.; Akter, M.; Ko, E.Y.; Choi, E.H.; Keum, Y.S.; Han, I. Enhancing Antioxidant Activities and Anti-Aging Effect of Rice Stem Cell Extracts by Plasma Treatment. Appl. Sci. 2022, 12, 2903. [Google Scholar] [CrossRef]
- Deshpande, A.; Dhadi, S.R.; Hager, E.J.; Ramakrishna, W. Anticancer activity of rice callus suspension culture. Phytother. Res. 2012, 26, 1075–1081. [Google Scholar] [CrossRef]
- Kumari, A.; Ramakrishna, W. Anticancer Activity of Rice Callus Suspension Cultures from Aromatic Varieties and Metabolite Regulated in Treated Cancer Cell Lines. Rice Sci. 2024, 31, 449–462. [Google Scholar] [CrossRef]
- Saewan, N.; Vichit, W.; Prinyarux, T. Anti-aging efficacy of Thai red rice callus cosmetic product. J. Appl. Sci. 2018, 17, 63–72. [Google Scholar]
- Khan, M.; Park, S.; Kim, H.J.; Lee, K.J.; Kim, D.H.; Baek, S.H.; Hong, S.T. The resveratrol rice DJ526 callus significantly increases the lifespan of drosophila (resveratrol rice DJ526 callus for longevity). Nutrients 2019, 11, 983. [Google Scholar] [CrossRef]
- Widowati, W.; Fauziah, N.; Herdiman, H.; Afni, M.; Afifah, E.; Kusuma, H.S.W.; Nufus, H.; Arumwardana, S.; Rihibiha, D.D. Antioxidant and anti aging assays of Oryza sativa extracts, vanillin and coumaric acid. J. Nat. Remedies 2016, 16, 88–99. [Google Scholar] [CrossRef]
- Karakayaa, G.; Ercanb, A.; Onculb, S.; Aytemira, M.D. Synthesis and cytotoxic evaluation of kojic acid derivatives with inhibitory activity on melanogenesis in human melanoma cells. Anti-Cancer Agents Med. Chem. 2018, 18, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Abbas, M.S.; El-Shabrawi, H.M.; Soliman, A.S.; Selim, M.A. Optimization of germination, callus induction, and cell suspension culture of African locust beans Parkia biglobosa (Jacq.) Benth. J. Genet. Eng. Biotechnol. 2018, 16, 191–201. [Google Scholar] [CrossRef]
- Pourebad, N.; Motafakkerazad, R.; Kosari-Nasab, M.; Farsad Akhtar, N.; Movafeghi, A. The influence of TDZ concentrations on in vitro growth and production of secondary metabolites by the shoot and callus culture of Lallemantia iberica. Plant Cell Tissue Organ Cult. 2015, 122, 331–339. [Google Scholar] [CrossRef]
- Lee, B.S.; So, H.M.; Kim, S.; Kim, J.K.; Kim, J.-C.; Kang, D.-M.; Ahn, M.-J.; Ko, Y.-J.; Kim, K.H. Comparative evaluation of bioactive phytochemicals in Spinacia oleracea cultivated under greenhouse and open field conditions. Arch. Pharm. Res. 2022, 45, 795–805. [Google Scholar] [CrossRef]
- Zeinali, M.; Abbaspour-Ravasjani, S.; Soltanfam, T.; Paiva-Santos, A.C.; Babaei, H.; Veiga, F.; Hamishehkar, H. Prevention of UV-induced skin cancer in mice by gamma oryzanol-loaded nanoethosomes. Life Sci. 2021, 283, 119759. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [CrossRef]
- Asadollahi, L.; Mahoutforoush, A.; Dorreyatim, S.S.; Soltanfam, T.; Paiva-Santos, A.C.; Peixoto, D.; Veiga, F.; Hamishehkar, H.; Zeinali, M.; Abbaspour-Ravasjani, S. Co-Delivery of erlotinib and resveratrol via nanostructured lipid Carriers: A synergistically promising approach for cell proliferation prevention and ROS-Mediated apoptosis activation. Int. J. Pharm. 2022, 624, 122027. [Google Scholar] [CrossRef]
- Yu, J.S.; Jeong, S.Y.; Li, C.; Oh, T.; Kwon, M.; Ahn, J.S.; Ko, S.-K.; Ko, Y.-J.; Cao, S.; Kim, K.H. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2, 3-dioxygenase 1 (IDO1). Arch. Pharm. Res. 2022, 45, 105–113. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Abedi-Gaballu, F.; Abbaspour, S.; Ghasabi, M.; Yekta, R.; Shirjang, S.; Dehghan, G.; Hamblin, M.R.; Baradaran, B. Hyaluronic acid-decorated liposomal nanoparticles for targeted delivery of 5-fluorouracil into HT-29 colorectal cancer cells. J. Cell. Physiol. 2020, 235, 6817–6830. [Google Scholar] [CrossRef]
- Mahoutforoush, A.; Abbaspour-Ravasjani, S.; Nazarpak, M.H.; Hamishehkar, H.; Solouk, A. Novel decorated nanostructured lipid carrier for simultaneous active targeting of three anti-cancer agents. Life Sci. 2021, 279, 119576. [Google Scholar] [CrossRef]
- Adhikari, D.; Panthi, V.K.; Pangeni, R.; Kim, H.J.; Park, J.W. Preparation, characterization, and biological activities of topical anti-aging ingredients in a Citrus junos callus extract. Molecules 2017, 22, 2198. [Google Scholar] [CrossRef]
- Sakuma, K.; Ogawa, M.; Sugibayashi, K.; Yamada, K.I.; Yamamoto, K. Relationship between tyrosinase inhibitory action and oxidation-reduction potential of cosmetic whitening ingredients and phenol derivatives. Arch. Pharmacal Res. 1999, 22, 335–339. [Google Scholar] [CrossRef]
- Chen, H.J.; Dai, F.J.; Chen, C.Y.; Fan, S.L.; Zheng, J.H.; Huang, Y.C.; Chau, C.F.; Lin, Y.S.; Chen, C.S. Evaluating the antioxidants, whitening and antiaging properties of rice protein hydrolysates. Molecules 2021, 26, 3605. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kang, J.H.; Seo, J.O.; Baek, S.H.; Moh, S.H.; Chae, J.K.; Park, Y.U.; Ko, Y.T.; Kim, S.Y. Anti-melanogenic potentials of nanoparticles from calli of resveratrol-enriched rice against UVB-induced hyperpigmentation in guinea pig skin. Biomol. Ther. 2016, 24, 85–93. [Google Scholar] [CrossRef]
- Kamarul Zaman, M.A.; Azzeme, A.M.; Ramle, I.K.; Normanshah, N.; Ramli, S.N.; Shaharuddin, N.A.; Ahmad, S.; Abdullah, S.N.A. Induction, multiplication, and evaluation of antioxidant activity of Polyalthia bullata callus, a woody medicinal plant. Plants 2020, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant. Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Broun, P.; Liu, Y.; Queen, E.; Schwarz, Y.; Abenes, M.L.; Leibman, M. Importance of transcription factors in the regulation of plant secondary metabolism and their relevance to the control of terpenoid accumulation. Phytochem. Rev. 2006, 5, 27–38. [Google Scholar] [CrossRef]
- Shin, S.-L.; Lee, C.-H. Antioxidant activities of ostrich fern by different extraction methods and solvents. J. Life Sci. 2011, 21, 56–61. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Sari, D.R.T.; Safitri, A.; Cairns, J.R.K.; Fatchiyah, F. Protein profiling of coloring rice (Oryza sativa L.) using SDS-PAGE and experionTM260 analysis. J. Phys. Conf. Ser. 2019, 1146, 012038. [Google Scholar] [CrossRef]
- Wei, C.; Kwon, O.-Y.; Liu, X.-W.; Kim, H.-C.; Yoon, W.-K.; Kim, H.-M.; Kim, M.-R. Protein profiles of major Korean rice cultivars. Prev. Nutr. Food Sci. 2007, 12, 103–110. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Kilci, A.; Gocmen, D. Phenolic acid composition, antioxidant activity and phenolic content of tarhana supplemented with oat flour. Food Chem. 2014, 151, 547–553. [Google Scholar] [CrossRef]
- Qiu, W.; Chuong, C.M.; Lei, M. Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: Biological patterning and therapeutic potentials. Exp. Dermatol. 2019, 28, 395–405. [Google Scholar] [CrossRef]
- Chung, S.Y.; Seo, Y.K.; Park, J.M.; Seo, M.J.; Park, J.K.; Kim, J.W.; Park, C.S. Fermented rice bran downregulates MITF expression and leads to inhibition of α-MSH-induced melanogenesis in B16F1 melanoma. Biosci. Biotechnol. Biochem. 2009, 73, 1704–1710. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lee, S.M.; Lin, C.C.; Liu, C.Y. Hispolon decreases melanin production and induces apoptosis in melanoma cells through the downregulation of tyrosinase and microphthalmia-associated transcription factor (MITF) expressions and the activation of caspase-3, -8 and -9. Int. J. Mol. Sci. 2014, 15, 1201–1215. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, D.; Zhang, Y.; Li, J.; Wu, Z.; Wang, Z. Investigation of the pro-Apoptotic effects of arbutin and its acetylated derivative on murine melanoma cells. Int. J. Mol. Med. 2018, 41, 1048–1054. [Google Scholar] [CrossRef]
- Lee, H.S. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J. Agric. Food Chem. 2002, 50, 1400–1403. [Google Scholar] [CrossRef]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Resveratrol-Enriched Rice Callus Extract Inhibits Oxidative and Cellular Melanogenic Activities in Melan-A Cells. Antioxidants 2024, 13, 625. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asadollahi, L.; Abbaspour-Ravasjani, S.; Kim, K.A.; Maghsoodi, M.; Hamishehkar, H.; Kosari-Nasab, M.; Kim, K.H. Rice (Oryza sativa) Stem Cells as a Novel Promising Active Ingredient with Anti-Proliferative Effects for Potential Skin Cancer Prevention and Skin Whitening Activity. Foods 2024, 13, 2803. https://doi.org/10.3390/foods13172803
Asadollahi L, Abbaspour-Ravasjani S, Kim KA, Maghsoodi M, Hamishehkar H, Kosari-Nasab M, Kim KH. Rice (Oryza sativa) Stem Cells as a Novel Promising Active Ingredient with Anti-Proliferative Effects for Potential Skin Cancer Prevention and Skin Whitening Activity. Foods. 2024; 13(17):2803. https://doi.org/10.3390/foods13172803
Chicago/Turabian StyleAsadollahi, Leila, Soheil Abbaspour-Ravasjani, Kyung Ah Kim, Maryam Maghsoodi, Hamed Hamishehkar, Morteza Kosari-Nasab, and Ki Hyun Kim. 2024. "Rice (Oryza sativa) Stem Cells as a Novel Promising Active Ingredient with Anti-Proliferative Effects for Potential Skin Cancer Prevention and Skin Whitening Activity" Foods 13, no. 17: 2803. https://doi.org/10.3390/foods13172803





