Polyphenolic Content and Antimicrobial Effects of Plant Extracts as Adjuncts for Craft Herbal Beer Stabilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Lyophilization of Leaves
2.2. Microwave Assisted Extraction
2.3. Total Polyphenol Content
2.4. HPLC-PDA-ESI-MS2 for Annotation and Quantification of Individual Polyphenolic Compounds
2.5. UHPLC–QTOF/MS Analysis on M. communis Extract
2.6. Antimicrobial Activity of Plant Extracts
2.7. Agar Disk Diffusion Method
2.8. Minimum Inhibitory Concentration (MIC) of Myrtus communis Extract
2.9. Antimicrobial Effect of Myrtus communis Extract in Wort and Beer
2.10. Statistical Analysis
3. Results
3.1. Chemical Characterization of Plant Extracts
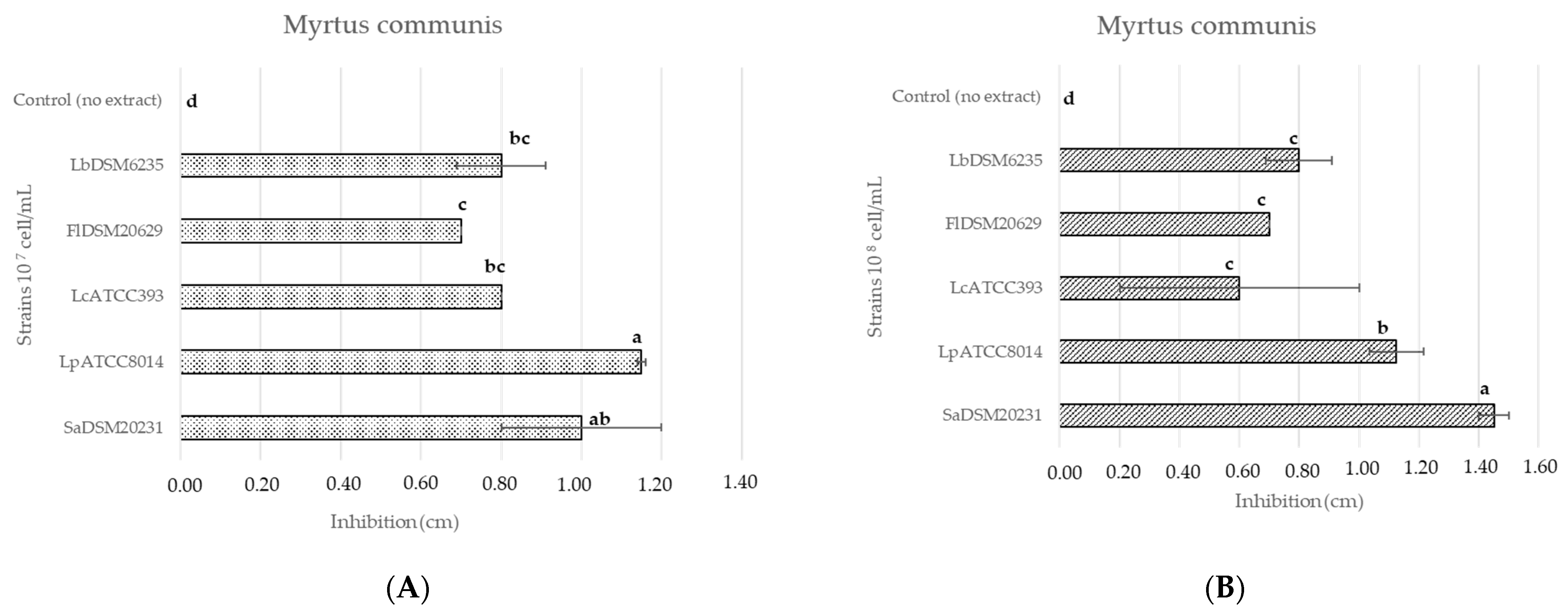
3.2. Antimicrobial Activity of Plant Extracts
3.3. Liquid Chromatography–Quadrupole-Time of Flight–Mass Spectrometry Analysis of the M. communis Extract
3.4. Minimum Inhibitory Concentration (MIC) Myrtus communis Extract
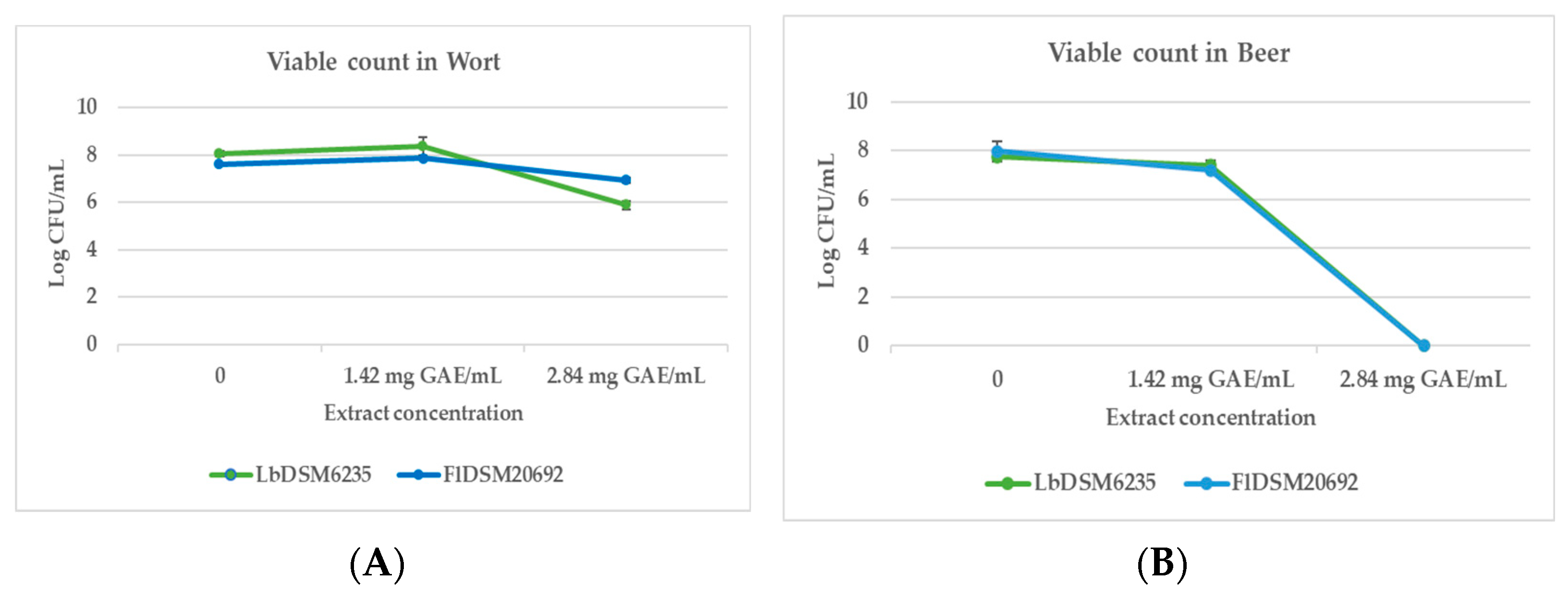
3.5. Antimicrobial Activity of M. communis Extract in Beer and Wort
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kordialik-Bogacka, E. Biopreservation of beer: Potential and constraints. Biotechnol. Adv. 2022, 58, 107910. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Dawson, P. Historical Perspective and Current Challenges for Microbreweries on Bacterial Spoilage of Beer. Eur. J. Agric. Food Sci. 2023, 5, 80–84. [Google Scholar] [CrossRef]
- Rodríguez-Saavedra, M.; González de Llano, D.; Moreno-Arribas, M.V. Beer spoilage lactic acid bacteria from craft brewery microbiota: Microbiological quality and food safety. Food Res. Int. 2020, 138, 109762. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, Y.; Mao, Y.; Peng, R.; Chen, J.; Soteyome, T.; Bai, C.; Chen, L.; Liang, Y.; Su, J.; et al. Spoilage lactic acid bacteria in the brewing industry. J. Microbiol. Biotechnol. 2020, 30, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M. Update on Biogenic Amines in Fermented and Non-Fermented Beverages. Foods 2022, 11, 353. [Google Scholar] [CrossRef]
- Djordjević, S.; Popović, D.; Despotović, S.; Veljović, M.; Atanacković, M.; Cvejić, J.; Nedović, V.; Leskošek-Čukalović, I. Extracts of medicinal plants as functional beer additives. Chem. Ind. Chem. Eng. Q. 2016, 22, 301–308. [Google Scholar] [CrossRef]
- Buhner, S.H. Sacred and Herbal Healing Beers: The Secrets of Ancient Fermentation; Siris Books; University of Cornell: Ithaca, NY, USA, 2002; ISBN 80-7207-484-9. [Google Scholar]
- Klimczak, K.; Cioch-Skoneczny, M.; Poreda, A. Physicochemical characterization of spontaneously fermented gruit beer: Historic revival and analysis. Eur. Food. Res. Technol. 2024, 250, 1123–1133. [Google Scholar] [CrossRef]
- Pluháčková, H.; Gregor, T.; Boško, R.; Běláková, S.; Svoboda, Z.; Benešová, K. Fortification of beer with extracts of the selected Czech medicinal herbs and plants. Kvas. Prum. 2020, 66, 314–319. [Google Scholar] [CrossRef]
- Habschied, K.; Živković, A.; Krstanović, V.; Mastanjević, K. Functional beer—A review on possibilities. Beverages 2020, 6, 51. [Google Scholar] [CrossRef]
- Borșa, A.; Muntean, M.V.; Salanță, L.C.; Tofană, M.; Socaci, S.A.; Mudura, E.; Pop, A.; Pop, C.R. Effects of botanical ingredients addition on the bioactive compounds and quality of non-alcoholic and craft beer. Plants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Pereira, P.; Bernardo-Gil, M.G.; Cebola, M.J.; Mauricio, E.; Romano, A. Supercritical fluid extracts with antioxidant and antimicrobial activities from myrtle (Myrtus communis L.) leaves. Response surface optimization. J. Supercrit. Fluids 2013, 83, 57–64. [Google Scholar] [CrossRef]
- Yemmen, M.; Landolsi, A.; Hamida, J.; Mégraud, F.; Trabelsi Ayadi, M. Antioxidant activities, anticancer activity and polyphenolics profile, of leaf, fruit and stem extracts of Pistacia lentiscus from Tunisia. Cell. Mol. Biol. 2017, 63, 87–95. [Google Scholar] [CrossRef]
- Bellachioma, L.; Marini, E.; Magi, G.; Pugnaloni, A.; Facinelli, B.; Rocchetti, G.; Martinelli, E.; Lucini, L.; Morresi, C.; Bacchetti, T.; et al. Phytochemical profiling, antibacterial and antioxidant properties of Crocus sativus flower: A comparison between tepals and stigmas. Open Chem. 2022, 20, 431–443. [Google Scholar] [CrossRef]
- Jadouali, S.M.; Atifi, H.; Bouzoubaa, Z.; Majourhat, K.; Gharby, S.; Achemchem, F.; Elmoslih, A.; Laknifli, A.; Mamouni, R. Chemical characterization, antioxidant and antibacterial activity of Moroccan Crocus sativus L. petals and leaves. J. Mater. Environ. Sci. 2018, 9, 113–118. [Google Scholar] [CrossRef]
- Martínez-Graciá, C.; González-Bermúdez, C.A.; Cabellero-Valcárcel, A.M.; Santaella-Pascual, M.; Frontela-Saseta, C. Use of herbs and spices for food preservation: Advantages and limitations. Curr. Opin. Food Sci. 2015, 6, 38–43. [Google Scholar] [CrossRef]
- Coppo, E.; Marchese, A. Antibacterial Activity of Polyphenols. Current. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Nayik, G.A.; Gupta, S.D.; Areche, F.O.; Jagdale, Y.D.; Ansari, M.J.; Hemeg, H.A.; AL-Farga, A.; Alotaibi, S.S. Chemical aspects of polyphenol-protein interactions and their antibacterial activity. Crit. Rev. Food Sci. Nutr. 2022, 63, 9482–9505. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Tran, T.T.; Bui, T.L.P.; Pham, T.H.V. Herbal Extracts: Importance, Classification, Quality Characteristics, and Control. VNU J. Sci. Med. Pharm. Sci. 2022, 38, 10–25. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Saravanabavan, N.; Salwe, K.J.; Codi, R.S.; Kumarappan, M. Herbal extraction procedures: Need of the hour. Int. J. Basic Clin. Pharmacol. 2020, 9, 1135–1139. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Fancello, F.; Bianco, A.; Niccolai, M.; Zara, G.; Coronas, R.; Serra, E.; D’Hallewin, G.; Valentoni, A.; Santoru, A.; Pretti, L.; et al. Fruit Microbial Communities of the Bisucciu Sardinian Apricot Cultivar (Prunus armeniaca L.) as a Reservoir of New Brewing Starter Strains. Fermentation 2022, 8, 364. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Hoff, J.E.; Singleton, K.I. A method for determination of tannins in foods by means of immobilized protein. J. Food Sci. 1977, 42, 1566–1569. [Google Scholar] [CrossRef]
- Fiehn, O.; Robertson, D.; Griffin, J.; van der Werf, M.; Nikolau, B.; Morrison, N.; Sumner, L.W.; Goodacre, R.; Hardy, N.W.; Taylor, C.; et al. The Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 175–178. [Google Scholar] [CrossRef]
- Wang, F.; Allen, D.; Tian, S.; Oler, E.; Gautam, V.; Greiner, R.; Metz, T.O.; Wishart, D.S. CFM-ID 4.0—A web server for accurate MS-based metabolite identification. Nucleic Acids Res. 2022, 5, W165–W174. [Google Scholar] [CrossRef]
- Parekh, P.; Serra, M.; Allaw, M.; Perra, M.; Marongiu, J.; Tolle, G.; Pinna, A.; Casu, M.A.; Manconi, M.; Caboni, P.; et al. Characterization of Nasco grape pomace-loaded nutriosomes and their neuroprotective effects in the MPTP mouse model of Parkinson’s disease. Front. Pharmacol. 2022, 13, 935784. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crops Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of polyphenolic profile and antioxidant activity of Pistacia lentiscus L. Leaves and fruit extract obtained by optimized microwave-assisted extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef]
- Sehaki, C.; Jullian, N.; Ayati, F.; Fernane, F.; Gontier, E. A Review of Pistacia lentiscus Polyphenols: Chemical Diversity and Pharmacological Activities. Plants 2023, 12, 279. [Google Scholar] [CrossRef]
- Boucheffa, S.; Sobhi, W.; Attoui, A.; Selli, S.; Kelebek, H.; Semmeq, A.; Benguerba, Y. Effect of the main constituents of Pistacia lentiscus leaves against the DPPH radical and xanthine oxidase: Experimental and theoretical study. J. Biomol. Struc. Dyn. 2022, 40, 9870–9884. [Google Scholar] [CrossRef]
- Benhammou, N.; Bekkara, F.A.; Panovska, T.K. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. Afr. J. Pharm. Pharmacol. 2008, 2, 22–28. [Google Scholar]
- Amensour, M.; Sendra, E.; Abrini, J.; Bouhdid, S.; Pérez-Alvarez, J.A.; Fernández-López, J. Natural Product Communications Total Phenolic Content and Antioxidant Activity of Myrtle. Nat. Prod. Commun. 2009, 4, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Stelluti, S.; Caser, M.; Demasi, S.; Scariot, V. Sustainable processing of floral bio- residues of saffron (Crocus sativus L.) for valuable biorefinery products. Plants 2021, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Current Understanding of Modes of Action of Multicomponent Bioactive Phytochemicals: Potential for Nutraceuticals and Antimicrobials. Annu. Rev. Food Sci. Technol. 2022, 13, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Bianchi, F.; Minassi, A.; Sterner, O.; Ballero, M.; Gibbons, S. Oligomeric acylphloroglucinols from myrtle (Myrtus communis). J. Nat. Prod. 2002, 65, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Coinu, R.; Carta, S.; Pinelli, P.; Galardi, C.; Vincieri, F.F.; Franconi, F. Evaluation of antioxidant effect of different extracts of Myrtus communis L. Free Radic. Res. 2004, 38, 97–103. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Tabatabaei Yazdi, F.; Noorbakhsh, H.; Riazi, F.; Jajarmi, A.; Tabatabaei Yazdi, F. Study of the Antibacterial Activity of Methanolic and Aqueous Extracts of Myrtus communis on Pathogenic Strains Causing Infection. Zahedan J. Res. Med. Sci. 2016, 18, e5989. [Google Scholar] [CrossRef]
- Dib, K.; Cherrah, Y.; Rida, S.; Filali-Maltouf, A.; Ennibi, O. In Vitro Antibacterial Activity of Myrtus communis L. and Marrubium vulgare L. Leaves against Aggregatibacter actinomycetemcomitans and Eikenella corrodens. J. Evid. Based Complement. Altern. Med. 2021, 1, 8351332. [Google Scholar] [CrossRef]
- Mansouri, S.; Foroumadi, A.; Ghaneie, T.; Najar, A.G. Antibacterial activity of the crude extracts and fractionated constituents of Myrtus communis. Pharm. Biol. 2001, 39, 399–401. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, S.; Chen, S.; Zhou, Y.; Gong, C.; Xue, W. Synthesis, antibacterial and antifungal activity of myricetin derivatives containing piperidine and amide fragments. Pest Manag. Sci. 2023, 79, 4795–4808. [Google Scholar] [CrossRef]
- Kiousi, D.E.; Bucka-Kolendo, J.; Wojtczak, A.; Sokołowska, B.; Doulgeraki, A.I.; Galanis, A. Genomic Analysis and In Vitro Investigation of the Hop Resistance Phenotype of Two Novel Loigolactobacillus backii Strains, Isolated from Spoiled Beer. Microorganisms 2023, 11, 280. [Google Scholar] [CrossRef]
- Peña-Gómez, N.; Ruiz-Rico, M.; Pérez-Esteve, É.; Fernández-Segovia, I.; Barat, J.M. Microbial stabilization of craft beer by filtration through silica supports functionalized with essential oil components. LWT 2020, 117, 108626. [Google Scholar] [CrossRef]
- Taylor, T.M. Natural food antimicrobials: Recent trends in their use, limitations, and opportunities for their applications in food preservation. In Natural and Bio-Based Antimicrobials for Food Applications; Fan, X., Ngo, H., Wu, C., Eds.; American Chemical Society: Washington, DC, USA, 2018; pp. 25–43. Available online: https://pubs.acs.org/doi/abs/10.1021/bk-2018-1287.ch002 (accessed on 22 August 2024).
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A systematic review of plants with antibacterial activities: A taxonomic and phylogenetic perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Seddiek, A.S.; Hamad, G.M.; Zeitoun, A.A.; Zeitoun, M.A.M.; Ali, S. Antimicrobial and antioxidant activity of some plant extracts against different food spoilage and pathogenic microbes. Eur. J. Nutr. Food Saf. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Lyumugabe Loshima, F.; Uyisenga, J.P.; Bayingana, C.; Songa, E.B. Antimicrobial activity and phytochemicals analysis of Vernonia aemulans, Vernonia amygdalina, Lantana camara and Markhamia lutea leaves as natural beer preservatives. Am. J. Food Technol. 2017, 12, 35–42. [Google Scholar] [CrossRef]
- Rodriguez Vaquero, M.J.; Aredes Fernandez, P.A.; Manca de Nadra, M.C.; Strasser de Saad, A.M. Phenolic compound combinations on Escherichia coli viability in a meat system. J. Agric. Food Chem. 2010, 58, 6048–6052. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, D.; Petrovic, J.; Sokovic, M.; Glamoclija, J.; Kukic-Markovic, J.; Petrovic, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z. Characteristics of cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem. 2020, 312, 922–935. [Google Scholar] [CrossRef]
- Guglielmotti, M.; Passaghe, P.; Buiatti, S. Use of olive (Olea europaea L.) leaves as beer ingredient, and their influence on beer chemical composition and antioxidant activity. J. Food Sci. 2020, 85, 2278–2285. [Google Scholar] [CrossRef]
- Oldham, R.C.; Held, M.A. Methods for detection and identification of beer-spoilage microbes. Front. Microbiol. 2023, 14, 1217704. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Demirci, T.; Akın, N. Production of functional probiotic ice creams with white and dark blue fruits of Myrtus communis: The comparison of the prebiotic potentials on Lactobacillus casei 431 and functional characteristics. LWT 2018, 90, 339–345. [Google Scholar] [CrossRef]
| Standard for Quantification | Q3 * (m/z) | Polarity |
|---|---|---|
| Catechin | 289 | negative |
| Chlorogenic acid | 353 | negative |
| Coumaric acid | 163 | negative |
| Apigenin glucoside | 431 | negative |
| Quercetin | 303 | positive |
| Kaempferol | 285 | negative |
| Isorhamnetin | 315 | negative |
| Micro-Organism | Abbreviation | Medium and Growth Condition |
|---|---|---|
| Levilactobacillus brevis | LbDSM6235 | MRS agar and broth 30 °C (48 h) anaerobic |
| Fructilactibacillus lindneri | FlDSM20692 | MRS agar and broth 30 °C (48 h) anaerobic |
| Lactiplantibacillus plantarum | LpATCC8014 | MRS agar and broth 30 °C (48 h) anaerobic |
| Lacticaseibacillus casei | LcATCC393 | MRS agar and broth 30 °C (48 h) anaerobic |
| Staphylococcus aureus | SaDSM20231 | BHI agar and broth 30 °C (48 h) aerobic |
| Saccharomyces cerevisiae | F2 (Commercial yeast) | YPD agar and broth 25 °C (24 h) aerobic |
| Saccharomyces cerevisiae | S04 (Commercial yeast) | YPD agar and broth 25 °C (24 h) aerobic |
| Saccharomyces cerevisiae | S33 (Commercial yeast) | YPD agar and broth 25 °C (24 h) aerobic |
| Saccharomyces cerevisiae | WB06 (Commercial yeast) | YPD agar and broth 25 °C (24 h) aerobic |
| Sample | TPC (mg GAE/g) |
|---|---|
| Crocus sativus | 32.80 ± 3.20 |
| Myrtus communis | 56.80 ± 2.80 |
| Artemisia arborescens | 8.80 ± 0.60 |
| Pistacia lentiscus | 111.20 ± 2.90 |
| Compound | Concentration (mg/g) n = 3 | Qualitative Parameters (Parent > Fragment m/z) | Quant. Method | Quant. Std. |
|---|---|---|---|---|
| Crocus sativus | ||||
| Coumaric Acid | 0.08 | Analytical Standard | MS: SIM | Coumaric Acid |
| Quercetin | 0.02 | Analytical Standard | MS: SIM | Quercetin |
| Kaempferol | 0.28 | Analytical Standard | MS: SIM | Kaempferol |
| Isorhamnetin | 0.06 | Analytical Standard | MS: SIM | Isorhamnetin |
| Quercetin-3-O-glucoside | 2.38 | (−) 463.01 > 301.1 (-glu) | PDA 365 nm | Quercetin |
| Quercetin 3-O-galactoside 7-O-rhamnoside | 25.94 | (−) 609.1 > 463.1 (-rha) 609.1 > 299.1 (-rha -glu) | PDA 365 nm | Quercetin |
| Isorhamnetin 3-O-glucoside 7-O-rhamnoside | 2.63 | (−) 623.16 > 477.0 (-rha) 623.16 > 315.0 (-rha -glu) | PDA 365 nm | Isorhamnetin |
| Kaempferol 3-O-glucoside | 0.66 | (−) 447.1 > 285.03 (-glu) | PDA 365 nm | Kaempferol |
| Myrtus communis | ||||
| Apigenin glucoside | 0.05 | Analytical Standard | MS: SIM | Apigenin glucoside |
| Quercetin | 0.00 | Analytical Standard | MS: SIM | Quercetin |
| Myricetin 3-(2″-Galloyl-Beta-D- Glucopyranoside) | 1.91 | (−) 631.4 > 317.1 | PDA 365 nm | Apigenin glucoside |
| Myricetin 3-beta-D-glucopyranoside | 2.21 | (−) 479.1 > 315.03 (-glupyr) | PDA 365 nm | Apigenin glucoside |
| Myricetin 3-rhamnoside | 9.31 | (−) 463.1 > 317.04 (-rha) | PDA 365 nm | Apigenin glucoside |
| Quercetin Caprylate | 0.14 | (−) 443.1 > 301.1 | PDA 365 nm | Quercetin |
| Myricetin 3′-Xyloside | 0.22 | (−) 449.3 > 317.04 (-xyl) | PDA 365 nm | Apigenin glucoside |
| Isorhamnetin-3-O-galactoside * | 3.58 | (−) 477.1 > 315.03 (-gal) | PDA 280 nm | Apigenin glucoside |
| Isorhamnetin-3-O-glucoside * | 4.92 | (−) 477.1 > 315.03 (-glu) | PDA 280 nm | Apigenin glucoside |
| Artemisia arborescens | ||||
| Chlorogenic acid | 0.28 | Analytical Standard | MS: SIM | Chlorogenic acid |
| Coumaric acid | 0.07 | Analytical Standard | MS: SIM | Coumaric acid |
| Apigenin glucoside | 0.09 | Analytical Standard | MS: SIM | Apigenin glucoside |
| Isorhamnetin | 0.05 | Analytical Standard | MS: SIM | Isorhamnetin |
| Scopoletin | 0.37 | (+) 193.0 > 132.0 | PDA 280 nm | Coumaric acid |
| Eriodictyol | 0.13 | (−) 287.1 > 135.0 287.1 > 151.1 | PDA 280 nm | Coumaric Acid |
| Gallocathecin | 0.23 | (+) 307.1 > 163.0 307.1 > 139.05 | PDA 365 nm | Chlorogenic Acid |
| Isoschaftoside | 1.19 | (−) 563.1 > 473.1 563.1 > 383.1 | PDA 365 nm | Chlorogenic Acid |
| Cyanidin 3-(6″-succinyl-glucoside) | 0.47 | (+) 549.1 > 287.4 (-glu) | PDA 365 nm | Apigenin glucoside |
| Medioresinol | 0.71 | (−) 387.1 > 259.1 387.1 > 355.1 | PDA 365 nm | Apigenin glucoside |
| Pistacia lentiscus | ||||
| Catechin | 0.44 | Analytical Standard | MS: SIM | Catechin |
| Chlorogenic acid | 0.04 | Analytical Standard | MS: SIM | Chlorogenic acid |
| Apigenin glucoside | 0.21 | Analytical Standard | MS: SIM | Apigenin glucoside |
| Galloylquinic acid_isomer_1 | 0.70 | (−) 343.0 > 169 (-quinic acid) | PDA 280 nm | Chlorogenic acid |
| Galloylquinic acid_isomer_2 | 2.88 | (−) 343.0 > 169 (-quinic acid) | PDA 280 nm | Chlorogenic acid |
| Gallocathechin | 0.10 | (+) 307.1 > 163.0 307.1 > 139.05 | PDA 280 nm | Chlorogenic acid |
| Coumaric Acid glucoside | 0.11 | (−) 325.1 > 163.0 (-glu) | PDA 280 nm | Coumaric Acid |
| Digalloylquinic acid | 0.68 | 495 > 343 (-galloylquinic ac.) | PDA 280 nm | Chlorogenic acid |
| Myricetin-3-O-rutinoside | 1.02 | (−) 625.1 > 317.1 (-rut) 625.1 > 463.1 (-glu) | PDA 365 nm | Apigenin glucoside |
| Myricetin-3-O-glucoronide | 2.30 | (−) 493.3 > 317 (-glucoronide) | PDA 365 nm | Apigenin glucoside |
| Quercetin 3-O-glucoside | 3.78 | (−) 463.01 > 301.1 (-glu) | PDA 365 nm | Apigenin glucoside |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coronas, R.; Bianco, A.; Niccolai, M.; Fancello, F.; Sanna, A.M.L.; Asteggiano, A.; Medana, C.; Caboni, P.; Budroni, M.; Zara, G. Polyphenolic Content and Antimicrobial Effects of Plant Extracts as Adjuncts for Craft Herbal Beer Stabilization. Foods 2024, 13, 2804. https://doi.org/10.3390/foods13172804
Coronas R, Bianco A, Niccolai M, Fancello F, Sanna AML, Asteggiano A, Medana C, Caboni P, Budroni M, Zara G. Polyphenolic Content and Antimicrobial Effects of Plant Extracts as Adjuncts for Craft Herbal Beer Stabilization. Foods. 2024; 13(17):2804. https://doi.org/10.3390/foods13172804
Chicago/Turabian StyleCoronas, Roberta, Angela Bianco, Marta Niccolai, Francesco Fancello, Anna Maria Laura Sanna, Alberto Asteggiano, Claudio Medana, Pierluigi Caboni, Marilena Budroni, and Giacomo Zara. 2024. "Polyphenolic Content and Antimicrobial Effects of Plant Extracts as Adjuncts for Craft Herbal Beer Stabilization" Foods 13, no. 17: 2804. https://doi.org/10.3390/foods13172804








