PhoP/PhoQ Two-Component System Contributes to Intestinal Inflammation Induced by Cronobacter sakazakii in Neonatal Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Animal Experiment
2.3. Number of C. sakazakii
2.4. Histopathology
2.5. Caspase-3 Activity
2.6. ELISA
2.7. qRT-PCR
2.8. Immunohistochemical Staining
2.9. Western Blot
2.10. Statistical Analysis
3. Results
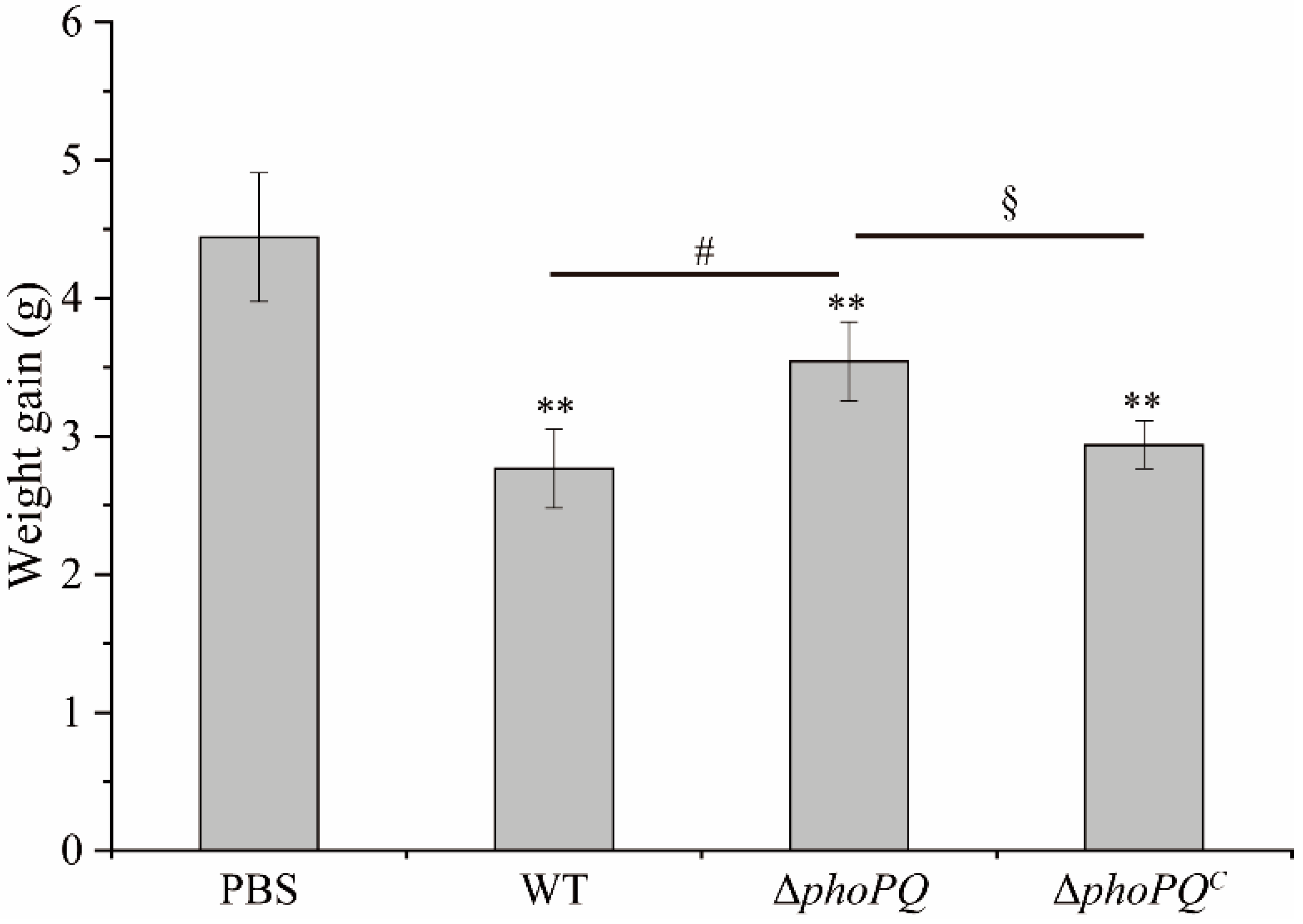
3.1. Deletion of phoPQ Gene Reduced the Count of C. sakazaki in Ileum
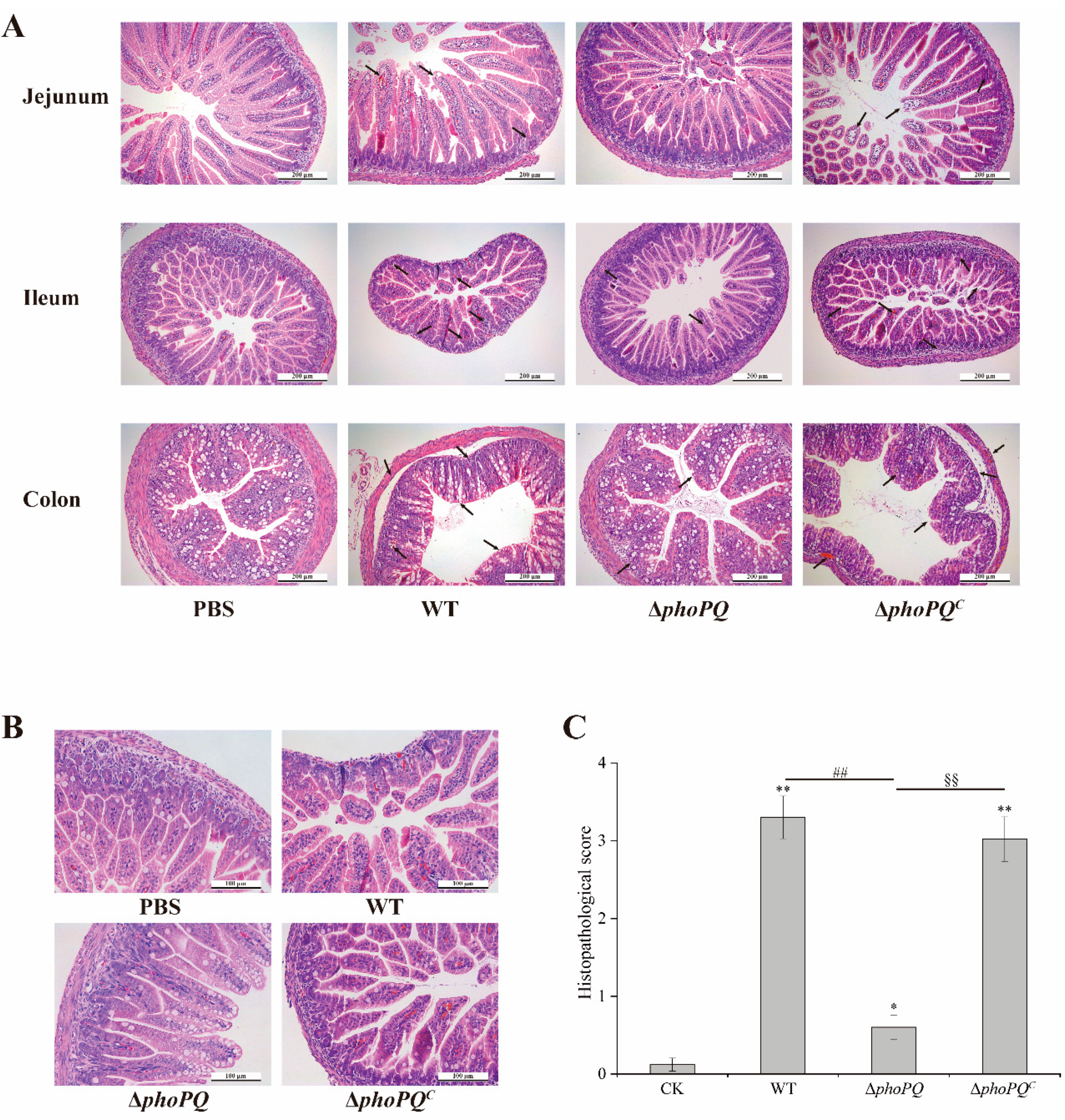
3.2. PhoP/PhoQ System Affected the Histopathological Damage
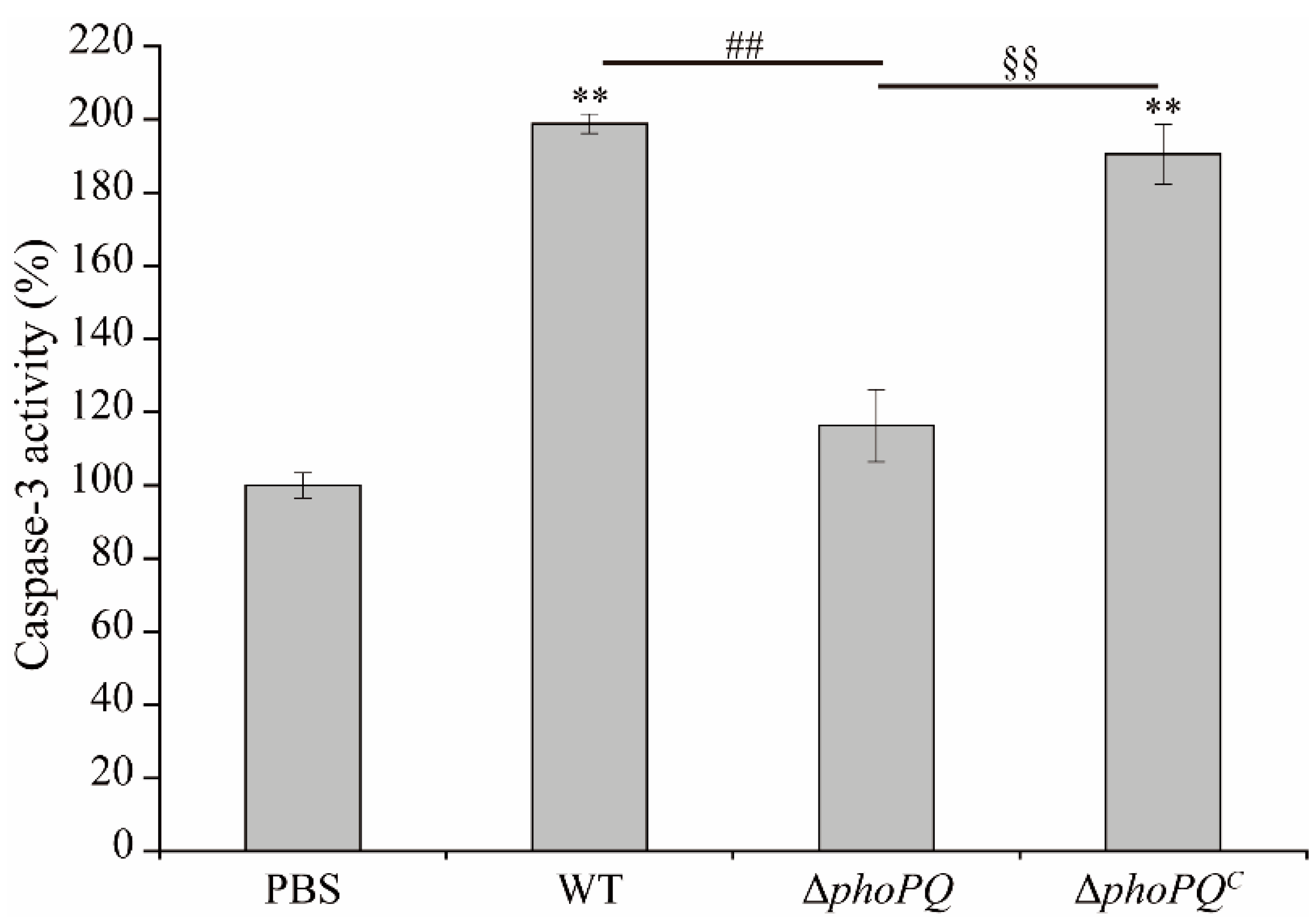
3.3. Deletion of phoPQ Gene in C. sakazakii Affected Caspase-3 Activity
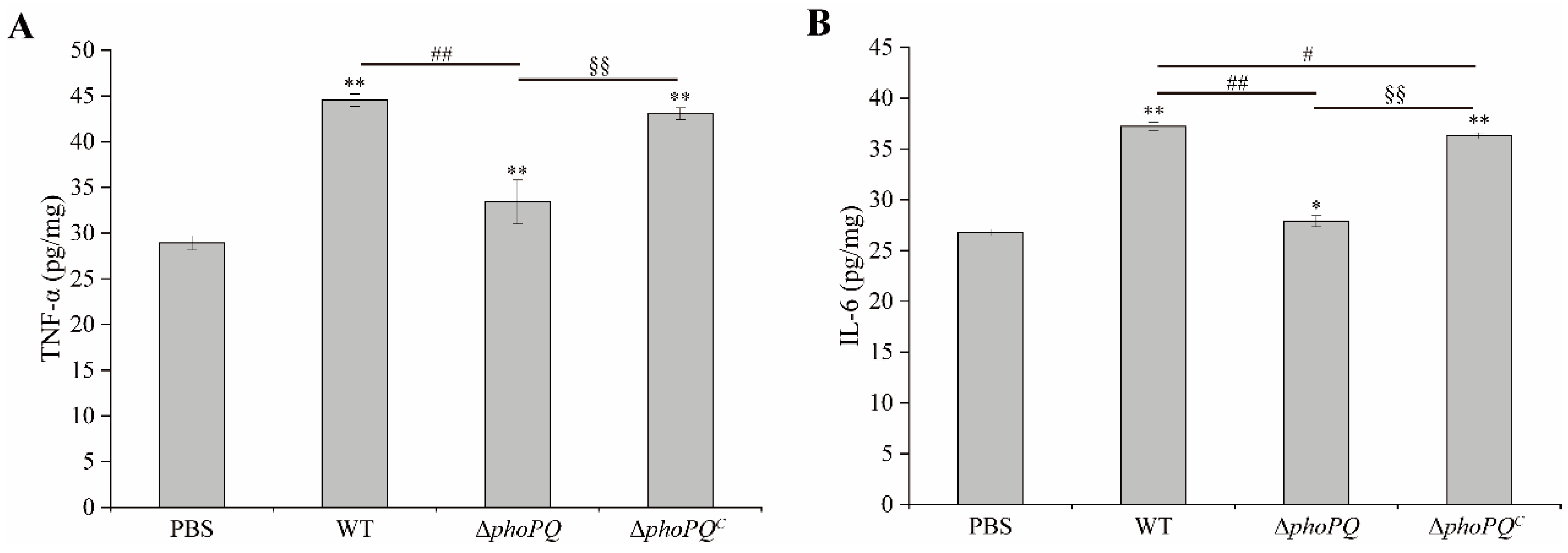
3.4. Deletion of phoPQ Gene in C. sakazakii Affected TNF-α and IL-6 Levels in Ileum
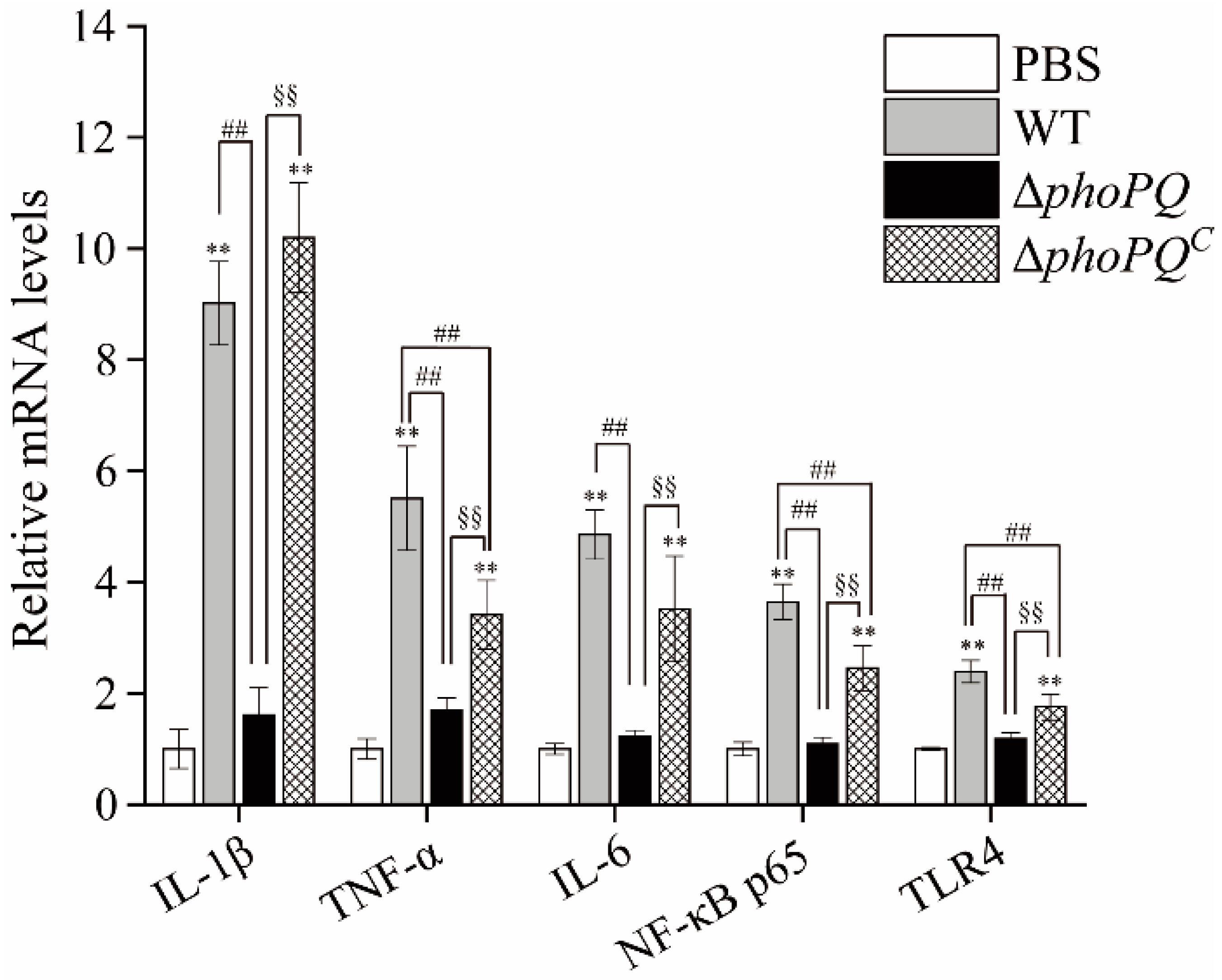
3.5. Deletion of phoPQ Gene Affected the Expression of Inflammation-Related Genes
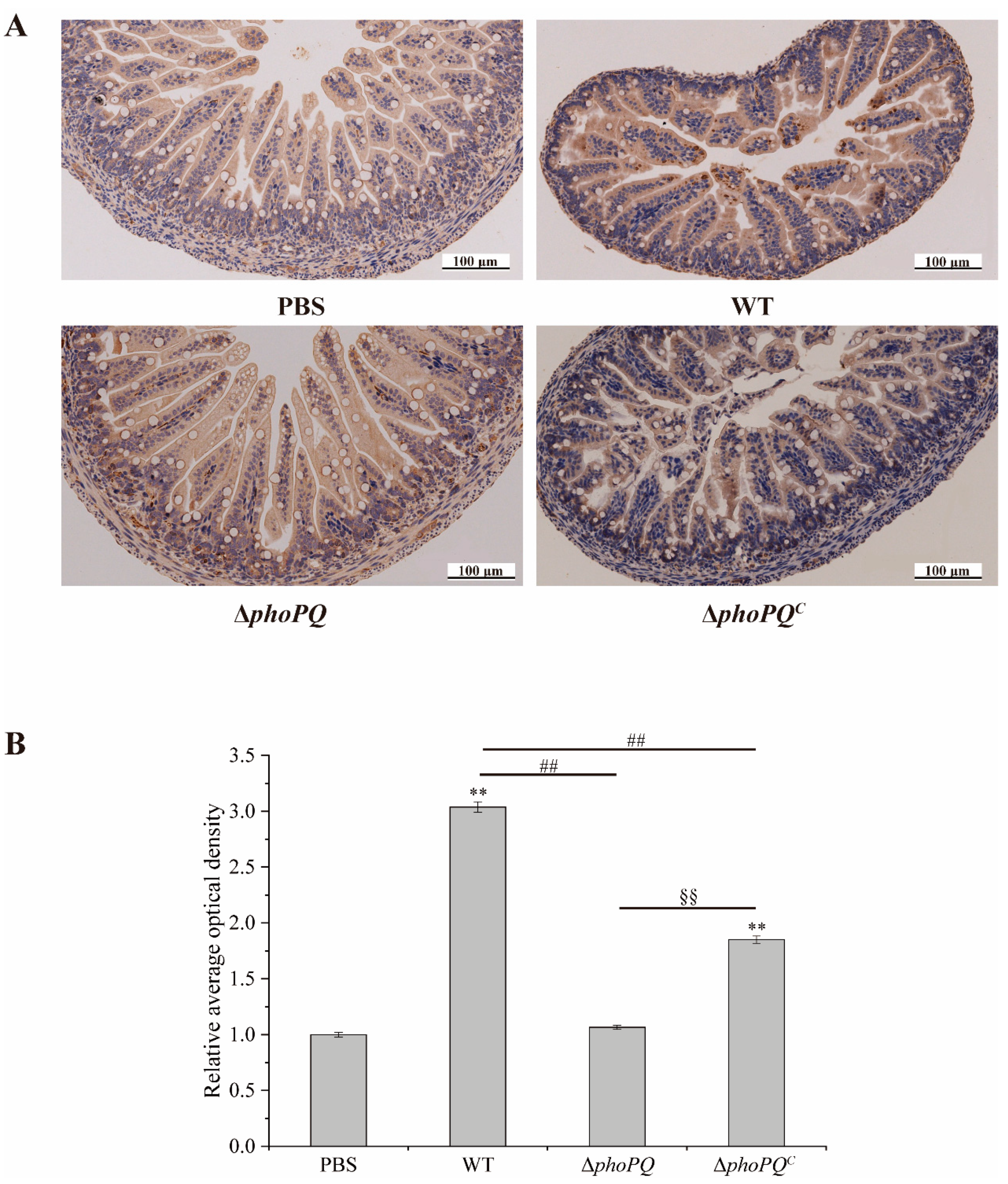
3.6. Deletion of phoPQ Gene Affected the Expression of NF-κB p65
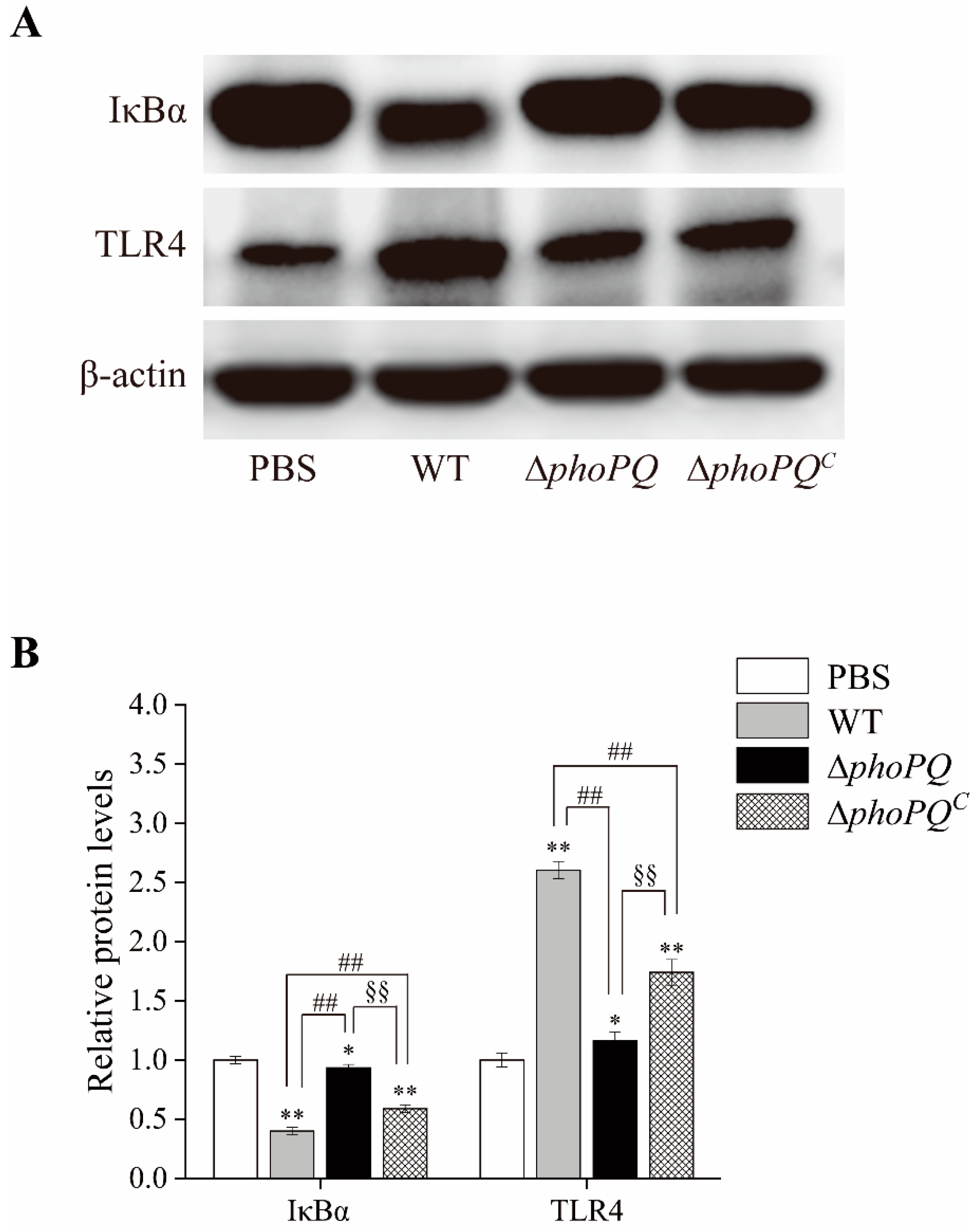
3.7. Deletion of phoPQ Gene in C. sakazakii Affected the Protein Expression of IκBα and TLR4 in the Ileum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhang, T.; Zhou, G.; Jiang, X.; Tao, M.; Zhang, J.; Zeng, X.; Wu, Z.; Pan, D.; Guo, Y. Prevention of necrotizing enterocolitis through milk polar lipids reducing intestinal epithelial apoptosis. J. Agric. Food Chem. 2020, 68, 7014–7023. [Google Scholar] [CrossRef]
- Hackam, D.J.; Sodhi, C.P. Bench to bedside—New insights into the pathogenesis of necrotizing enterocolitis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 468–479. [Google Scholar] [PubMed]
- Henry, M.; Moss, R. Necrotizing enterocolitis. Annu. Rev. Med. 2009, 60, 111–124. [Google Scholar] [CrossRef]
- Niño, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [PubMed]
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The potential role of phytonutrients flavonoids influencing gut microbiota in the prophylaxis and treatment of inflammatory bowel disease. Front. Nutr. 2021, 8, 798038. [Google Scholar] [CrossRef]
- Patel, R.M.; Denning, P.W. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr. Res. 2015, 78, 232–238. [Google Scholar]
- Jin, T.; Zhan, X.; Pang, L.; Peng, B.; Zhang, X.; Zhu, W.; Yang, B.; Xia, X. CpxAR two-component system contributes to virulence properties of Cronobacter sakazakii. Food Microbiol. 2024, 117, 104393. [Google Scholar] [CrossRef]
- Blackwood, B.; Hunter, C. Cronobacter spp. Microbiol. Spectr. 2016, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Jiang, Y.; Feng, J.; Forsythe, S.J.; Li, R.; Zhou, Y.; Man, C. Antibiotic and desiccation resistance of Cronobacter sakazakii and C. malonaticus isolates from powdered infant formula and processing environments. Front. Microbiol. 2017, 8, 316. [Google Scholar] [CrossRef]
- Guo, X.; Yu, H.; Xiong, J.; Dai, Q.; Li, Y.; Zhang, W.; Liao, X.; He, X.; Zhou, H.; Zhang, K. Pseudomonas aeruginosa two-component system LadS/PA0034 regulates macrophage phagocytosis via fimbrial protein cupA1. mBio 2024, 15, e00616-24. [Google Scholar] [CrossRef]
- Monteagudo-Cascales, E.; Santero, E.; Canosa, I. The regulatory hierarchy following signal integration by the CbrAB two-component system: Diversity of responses and functions. Genes 2022, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, W.J.; Hancock, R.E.W. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 2009, 33, 279–294. [Google Scholar] [CrossRef]
- Zhu, Y.; Dou, Q.; Du, L.; Wang, Y. QseB/QseC: A two-component system globally regulating bacterial behaviors. Trends Microbiol. 2023, 31, 749–762. [Google Scholar] [PubMed]
- Singh, P.; Goar, H.; Paul, P.; Mehta, K.; Bamniya, B.; Vijjamarri, A.; Bansal, R.; Khan, H.; Karthikeyan, S.; Sarkar, D. Dual functioning by the PhoR sensor is a key determinant to Mycobacterium tuberculosis virulence. PLoS Genet. 2023, 19, e1011070. [Google Scholar] [CrossRef]
- Janssen, A.; de Bakker, V.; Aprianto, R.; Trebosc, V.; Kemmer, C.; Pieren, M.; Veening, J.W. Klebsiella pneumoniae OmpR facilitates lung infection through transcriptional regulation of key virulence factors. Microbiol. Spectr. 2023, 12, e0396623. [Google Scholar] [CrossRef]
- Fernandez-Ciruelos, B.; Potmis, T.; Solomin, V.; Wells, J. Cross-talk between QseBC and PmrAB two-component systems is crucial for regulation of motility and colistin resistance in Enteropathogenic Escherichia coli. PLoS Pathog. 2023, 19, e1011345. [Google Scholar] [CrossRef] [PubMed]
- Groisman, E.A.; Duprey, A.; Choi, J. How the PhoP/PhoQ system controls virulence and Mg2+ homeostasis: Lessons in signal transduction, pathogenesis, physiology, and evolution. Microbiol. Mol. Biol. Rev. 2021, 85, e0017620. [Google Scholar]
- Han, J.; Gao, X.; Luo, X.; Zhu, L.; Zhang, Y.; Dong, P. The role of PhoP/PhoQ system in regulating stress adaptation response in Escherichia coli O157:H7. Food Microbiol. 2023, 112, 104244. [Google Scholar] [CrossRef]
- Disney-McKeethen, S.; Seo, S.; Mehta, H.; Ghosh, K.; Shamoo, Y. Experimental evolution of Pseudomonas aeruginosa to colistin in spatially confined microdroplets identifies evolutionary trajectories consistent with adaptation in microaerobic lung environments. mBio 2023, 14, e0150623. [Google Scholar] [CrossRef]
- Cabezudo, I.; Lobertti, C.; Véscovi, E.; Furlan, R. Effect-directed synthesis of PhoP/PhoQ inhibitors to regulate Salmonella virulence. J. Agric. Food Chem. 2022, 70, 6755–6763. [Google Scholar] [CrossRef]
- Richardson, A.; Lambert, S.; Smith, M. Neonatal mice as models for Cronobacter sakazakii infection in infants. J. Food Prot. 2009, 72, 2363–2367. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Chen, K.; Zhang, L.; Zhang, Y.; Wang, X.; Xia, X. The role of PhoP/PhoQ two component system in regulating stress adaptation in Cronobacter sakazakii. Food Microbiol. 2021, 100, 103851. [Google Scholar] [CrossRef]
- Emami, C.; Mittal, R.; Wang, L.; Ford, H.; Prasadarao, N. Recruitment of dendritic cells is responsible for intestinal epithelial damage in the pathogenesis of necrotizing enterocolitis by Cronobacter sakazakii. J. Immunol. 2011, 186, 7067–7079. [Google Scholar] [CrossRef]
- Weng, M.Q.; Ganguli, K.; Zhu, W.S.; Shi, H.N.; Walker, W.A. Conditioned medium from Bifidobacteria infantis protects against Cronobacter sakazakii-induced intestinal inflammation in newborn mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G779–G787. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Hussein, H.; Wang, H.; Rabbi, M.F.; Bernstein, C.N.; Ghia, J.E.; Sly, L.M. Stability of reference genes for messenger RNA quantification by real-time PCR in mouse dextran sodium sulfate experimental colitis. PLoS ONE 2016, 11, e0156289. [Google Scholar] [CrossRef]
- He, Y.; Yao, X.; Taylor, N.; Bai, Y.; Lovenberg, T.; Bhattacharya, A. RNA sequencing analysis reveals quiescent microglia isolation methods from postnatal mouse brains and limitations of BV2 cells. J. Neuroinflammation 2018, 15, 153. [Google Scholar] [CrossRef]
- Zhang, N.; Huan, Y.; Huang, H.; Song, G.M.; Sun, S.J.; Shen, Z.F. Atorvastatin improves insulin sensitivity in mice with obesity induced by monosodium glutamate. Acta Pharmacol. Sin. 2010, 31, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Boytard, L.; Hadi, T.; Silvestro, M.; Qu, H.; Kumpfbeck, A.; Sleiman, R.; Fils, K.; Alebrahim, D.; Boccalatte, F.; Kugler, M.; et al. Lung-derived HMGB1 is detrimental for vascular remodeling of metabolically imbalanced arterial macrophages. Nat. Commun. 2020, 11, 4311. [Google Scholar] [CrossRef]
- Jin, C.J.; Liu, J.Y.; Jin, R.Y.; Yao, Y.P.; He, S.; Lei, M.; Peng, X.L. Linarin ameliorates dextran sulfate sodium-induced colitis in C57BL/6J mice via the improvement of intestinal barrier, suppression of inflammatory responses and modulation of gut microbiota. Food Funct. 2022, 13, 10574–10586. [Google Scholar] [CrossRef]
- Gao, J.; Han, Z.; Li, P.; Zhang, H.; Du, X.; Wang, S. Outer membrane protein F is involved in biofilm formation, virulence and antibiotic resistance in Cronobacter sakazakii. Microorganisms 2021, 9, 2338. [Google Scholar] [CrossRef]
- Shi, C.; Jin, T.; Guo, D.; Zhang, W.; Yang, B.; Su, D.; Xia, X. Citral attenuated intestinal inflammation induced by Cronobacter sakazakii in newborn mice. Foodborne Pathog. Dis. 2020, 17, 243–252. [Google Scholar] [CrossRef]
- Ufuk, C.; Cuneyt, T.; Utku, S.; Halil Ibrahim, Y.; Esra, C.; Ufuk, A.; Ismail, K.; Eyyup, K. Ginger (Zingiber officinale Roscoe) for the treatment and prevention of necrotizing enterocolitis. J. Ethnopharmacol. 2018, 225, 297–308. [Google Scholar]
- Janina, M.; Lilith, R.; Christoph, H.; Mats Ingmar, F.; Kirstin, F.; Delfina, M.; Jürgen, H.; Michael, Z.; Mercedes, G.d.A.; Katja, M.; et al. Antimicrobial peptides (AMPs) and the microbiome in preterm infants: Consequences and opportunities for future therapeutics. Int. J. Mol. Sci. 2024, 25, 6684. [Google Scholar] [CrossRef] [PubMed]
- Manabu, K.; Sally, K. Caspases and kinases in a death grip. Cell 2009, 138, 838–854. [Google Scholar]
- Yang, G.; Jin, T.; Yin, S.; Guo, D.; Zhang, C.; Xia, X.; Shi, C. trans-Cinnamaldehyde mitigated intestinal inflammation induced by Cronobacter sakazakii in newborn mice. Food Funct. 2019, 10, 2986–2996. [Google Scholar] [CrossRef]
- Hejna, M.; Kovanda, L.; Rossi, L.; Liu, Y. Mint oils: In vitro ability to perform anti-inflammatory, antioxidant, and antimicrobial activities and to enhance intestinal barrier integrity. Antioxidants 2021, 10, 1004. [Google Scholar] [CrossRef]
- Bu, C.; Hu, M.; Su, Y.; Yuan, F.; Zhang, Y.; Xia, J.; Jia, Z.; Zhang, L. Cell-permeable JNK-inhibitory peptide regulates intestinal barrier function and inflammation to ameliorate necrotizing enterocolitis. J. Cell Mol. Med. 2024, 28, e18534. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, Z.; Lin, R.; Liu, Y.; Zhi, F. Bacteroides fragilis strain ZY-312 defense against Cronobacter sakazakii-induced necrotizing enterocolitis in vitro and in a neonatal rat model. mSystems 2019, 4, e00305-19. [Google Scholar] [CrossRef]
- Rajendra, K.; Bhesh Raj, S.; Shraddha, T.; Evan Peter, W.; Lillian, Z.; Parimal, S.; Min, Z.; Balamurugan, S.; Balaji, B.; Subbarao Malireddi, R.K.; et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: Past, present and future prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef]
- Kaplina, A.; Kononova, S.; Zaikova, E.; Pervunina, T.; Petrova, N.; Sitkin, S. Necrotizing enterocolitis: The role of hypoxia, gut microbiome, and microbial metabolites. Int. J. Mol. Sci. 2023, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Y.; Jiang, K.; Yang, Y.; Zhao, G.; Guo, S.; Guo, G. MicroRNA-106a provides negative feedback regulation in lipopolysaccharide-induced inflammation by targeting TLR4. Int. J. Biol. Sci. 2019, 15, 2308–2319. [Google Scholar] [CrossRef]
- Yang, R.; Hu, X.; Xie, X.; Chen, H.; Fang, H.; Zhu, L.; Li, Z. Propionic acid targets the TLR4/NF-κB signaling pathway and inhibits LPS-induced intestinal barrier dysfunction: In vitro and in vivo studies. Front. Pharmacol. 2020, 11, 573475. [Google Scholar] [CrossRef]
- Chhinder, P.S.; Matthew, D.N.; Richard, S.; Shonan, S.; Congrong, M.; Maria, F.B.; Thomas, P., Jr.; Anthony, M.R.; Amin, A.; Misty, G.; et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012, 143, 708–718.e5. [Google Scholar]
- Yu, R.; Jiang, S.; Tao, Y.; Li, P.; Yin, J.; Zhou, Q. Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-κB signaling pathways. J. Cell Physiol. 2019, 234, 13431–13438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; He, Y.; Zhu, X.; Ai, Q.; Shi, Y. β-glucan protects against necrotizing enterocolitis in mice by inhibiting intestinal inflammation, improving the gut barrier, and modulating gut microbiota. J. Transl. Med. 2023, 21, 14. [Google Scholar] [CrossRef]
- Sun, Q.; Ji, Y.C.; Wang, Z.L.; She, X.; He, Y.; Ai, Q.; Li, L.Q. Sodium butyrate alleviates intestinal inflammation in mice with necrotizing enterocolitis. Mediat. Inflamm. 2021, 2021, 6259381. [Google Scholar] [CrossRef]
- De Plaen, I.G.; Liu, S.X.L.; Tian, R.; Neequaye, I.; May, M.J.; Han, X.-B.; Hsueh, W.; Jilling, T.; Lu, J.; Caplan, M.S. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr. Res. 2007, 61, 716–721. [Google Scholar] [CrossRef]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G608–G617. [Google Scholar] [CrossRef] [PubMed]
- Carabajal, M.A.; Asquith, C.R.M.; Laitinen, T.; Tizzard, G.J.; Yim, L.; Rial, A.; Chabalgoity, J.A.; Zuercher, W.J.; Véscovi, E.G. Quinazoline-based antivirulence compounds selectively target Salmonella PhoP/PhoQ signal transduction system. Antimicrob. Agents Chemother. 2019, 64, e01744-19. [Google Scholar] [CrossRef] [PubMed]
- Carlos, A.L.; Ignacio, C.; Fernán, O.G.; Víctor, B.; Christian, M.; Ricard, L.E.F.; Eleonora, G.V. An allosteric inhibitor of the PhoQ histidine kinase with therapeutic potential against Salmonella infection. J. Antimicrob. Chemother. 2024, 79, 1820–1830. [Google Scholar]
- Cai, X.; Zhang, J.; Chen, M.; Wu, Y.; Wang, X.; Chen, J.; Zhang, J.; Shen, X.; Qu, D.; Jiang, H. The effect of the potential PhoQ histidine kinase inhibitors on Shigella flexneri virulence. PLoS ONE 2011, 6, e23100. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence (5′-3′) | References |
|---|---|---|
| IL-1β | F: GCAACTGTTCCTGAACTCAACT R: ATCTTTTGGGGTCCGTCAACT | [25] |
| TNF-α | F: CCCTCACACTCAGATCATCTTCT R: GCTACGACGTGGGCTACAG | [26] |
| IL-6 | F: TAGTCCTTCCTACCCCAATTTCC R: TTGGTCCTTAGCCACTCCTTC | [26] |
| NF-κB p65 | F: AGGCTTCTGGGCCTTATGTG R: TGCTTCTCTCGCCAGGAATAC | [27] |
| TLR4 | F: ATGGCATGGCTTACACCACC R: GAGGCCAATTTTGTCTCCACA | [28] |
| GAPDH | F: AGGTCGGTGTGAACGGATTTG R: TGTAGACCATGTAGTTGAGGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, Y.; Wang, Y.; Qiao, Z.; Liu, Y.; Xia, X. PhoP/PhoQ Two-Component System Contributes to Intestinal Inflammation Induced by Cronobacter sakazakii in Neonatal Mice. Foods 2024, 13, 2808. https://doi.org/10.3390/foods13172808
Ma Y, Zhang Y, Wang Y, Qiao Z, Liu Y, Xia X. PhoP/PhoQ Two-Component System Contributes to Intestinal Inflammation Induced by Cronobacter sakazakii in Neonatal Mice. Foods. 2024; 13(17):2808. https://doi.org/10.3390/foods13172808
Chicago/Turabian StyleMa, Yan, Yingying Zhang, Yuting Wang, Zhu Qiao, Yingying Liu, and Xiaodong Xia. 2024. "PhoP/PhoQ Two-Component System Contributes to Intestinal Inflammation Induced by Cronobacter sakazakii in Neonatal Mice" Foods 13, no. 17: 2808. https://doi.org/10.3390/foods13172808
APA StyleMa, Y., Zhang, Y., Wang, Y., Qiao, Z., Liu, Y., & Xia, X. (2024). PhoP/PhoQ Two-Component System Contributes to Intestinal Inflammation Induced by Cronobacter sakazakii in Neonatal Mice. Foods, 13(17), 2808. https://doi.org/10.3390/foods13172808







