Optimizing the Extraction of Bioactive Compounds (Polyphenols, Lipids, and Alpha-Tocopherol) from Almond Okara to Unlock Its Potential as Functional Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation: Production of Almond Okara
2.3. Dry Matter Determination of Okara
2.4. Extraction Procedures and Experimental Design
Extraction Kinetics: Effect of Extraction Time on Total Polyphenols’ Yield
- Modeling of the kinetics of solid–liquid extraction: Peleg model
- -
- Effect of the okara-to-solvent ratio on the total polyphenol yield
- -
- Effect of ethanol–water mixtures at different percentages as well as extraction temperatures on the phenolic compounds content and antiradical activity of the extracts
- -
- Optimization of phenolic compound extraction from okara: experimental design
2.5. Determination of the Total Polyphenol and Tannin Yields in Aqueous and Ethanolic Extracts
- -
- Determination of the total polyphenol yield (YTP)
- -
- Determination of the tannin yield (YT) with hide powder method
2.6. Determination of the Total Flavonoid Yield (YTF) in Aqueous and Ethanolic Extracts
2.7. Antioxidant Activity
- -
- Evaluation of total antioxidant activity by the phosphomolybdenum method
- -
- DPPH free radical-scavenging capacity
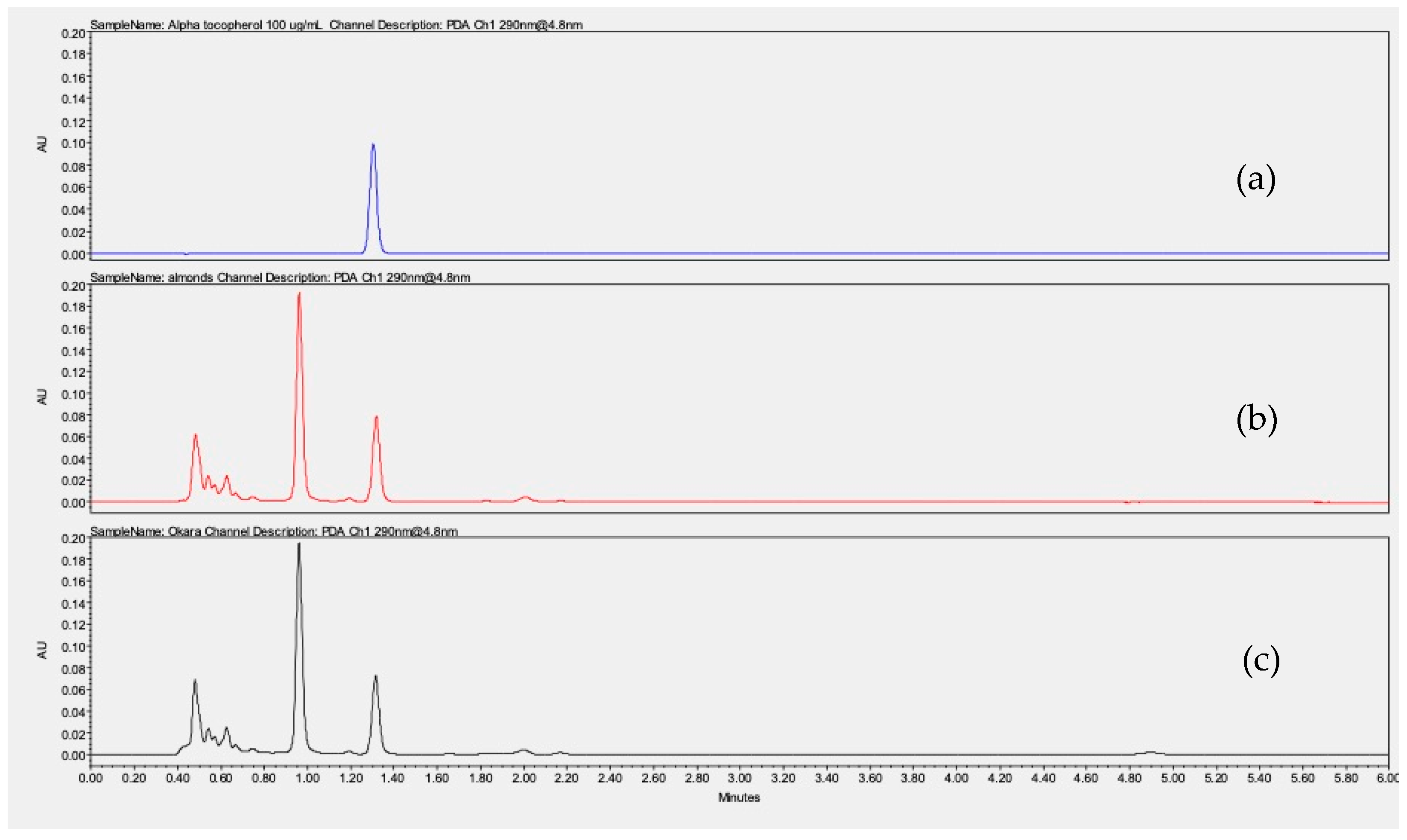
2.8. Alpha-Tocopherol Quantification
- -
- Preparation of the sample
- -
- Instrumentation and Chromatographic Conditions
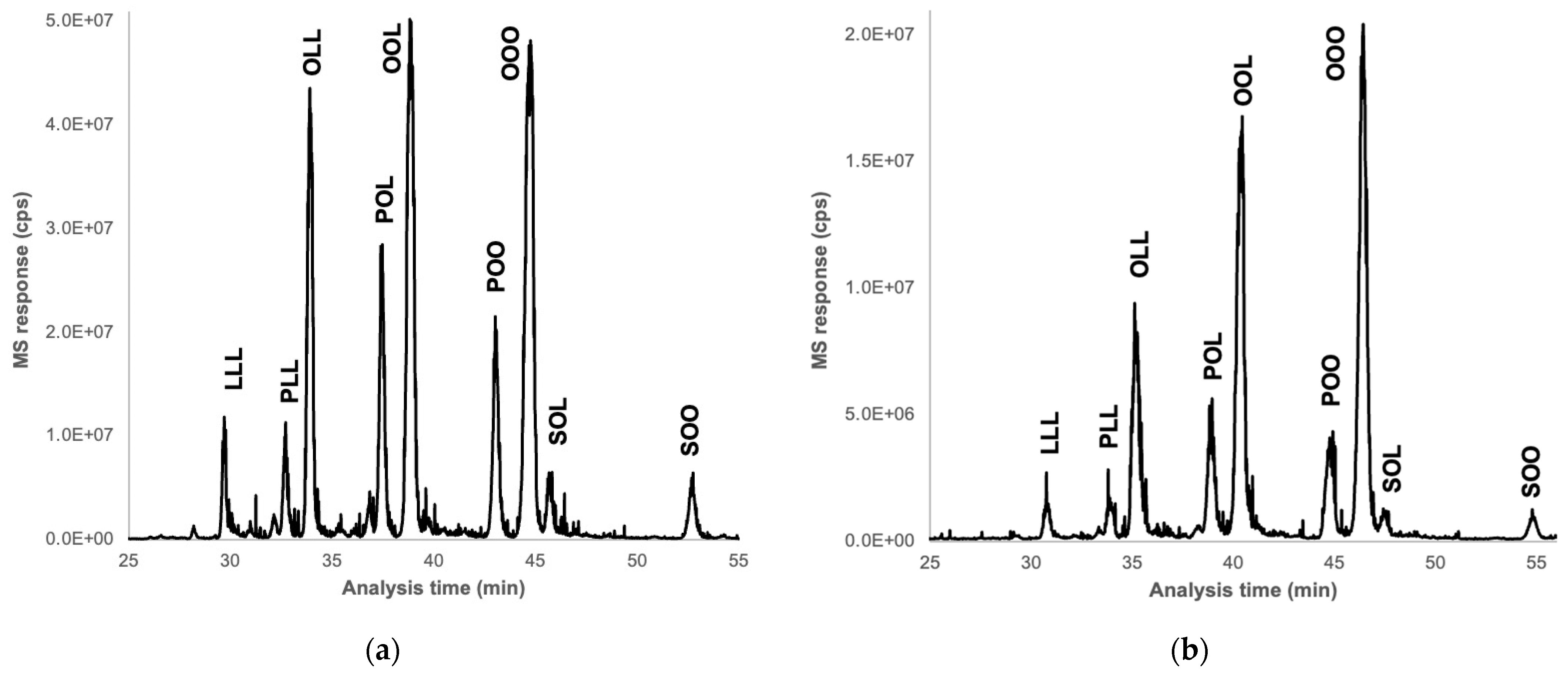
2.9. Analysis of Triglycerides
- -
- Preparation of the sample
- -
- Chromatographic system and mass spectrometry
2.10. Statistical Analysis
3. Results
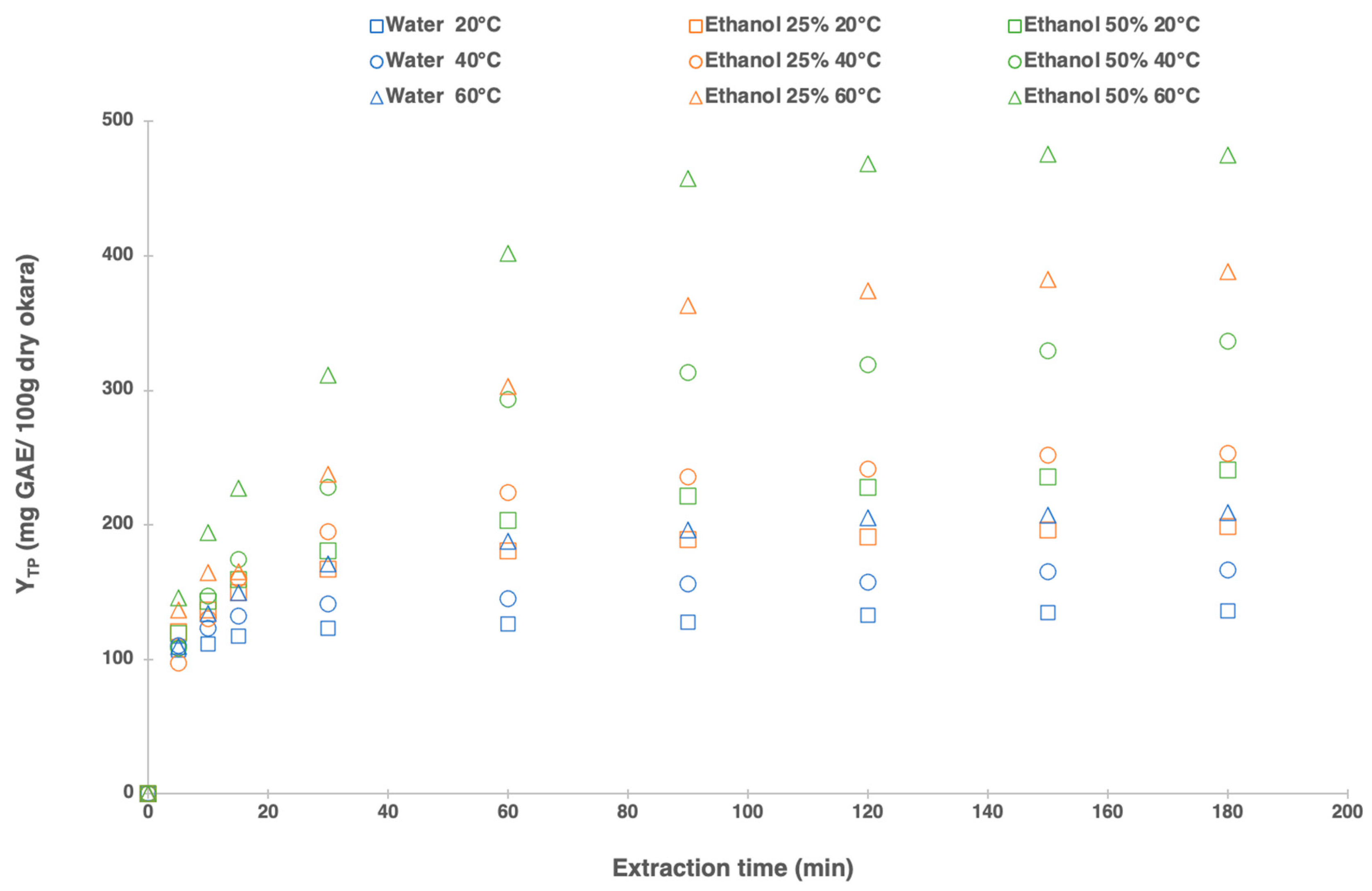
3.1. Extraction Kinetics: Effect of the Extraction Time on Total Polyphenol Yield
3.2. Modeling of Solid–Liquid Extraction Kinetic Parameters for TP Yield from Okara
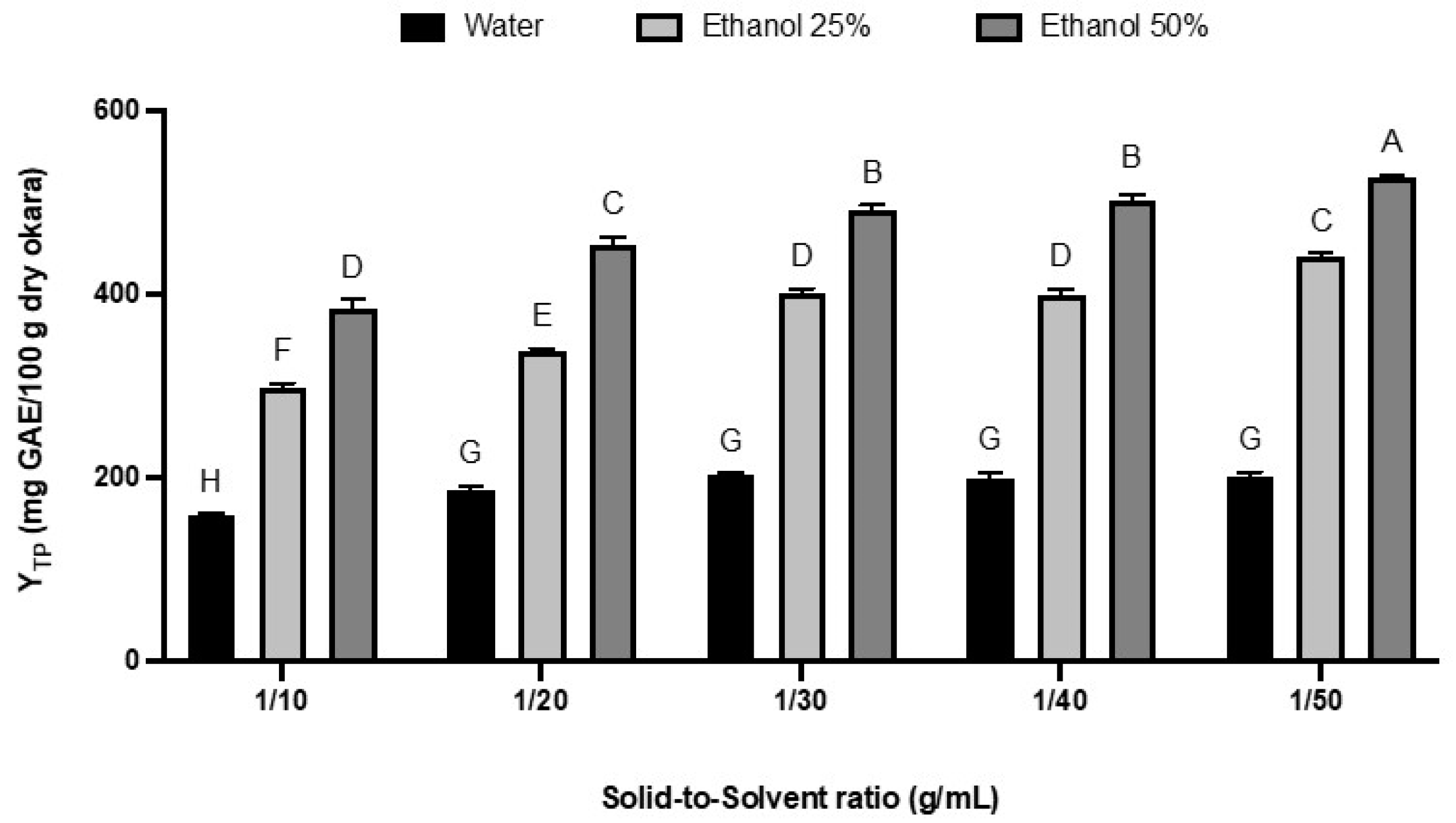
3.3. Solid-to-Solvent Ratio Effect on Total Polyphenol Yield
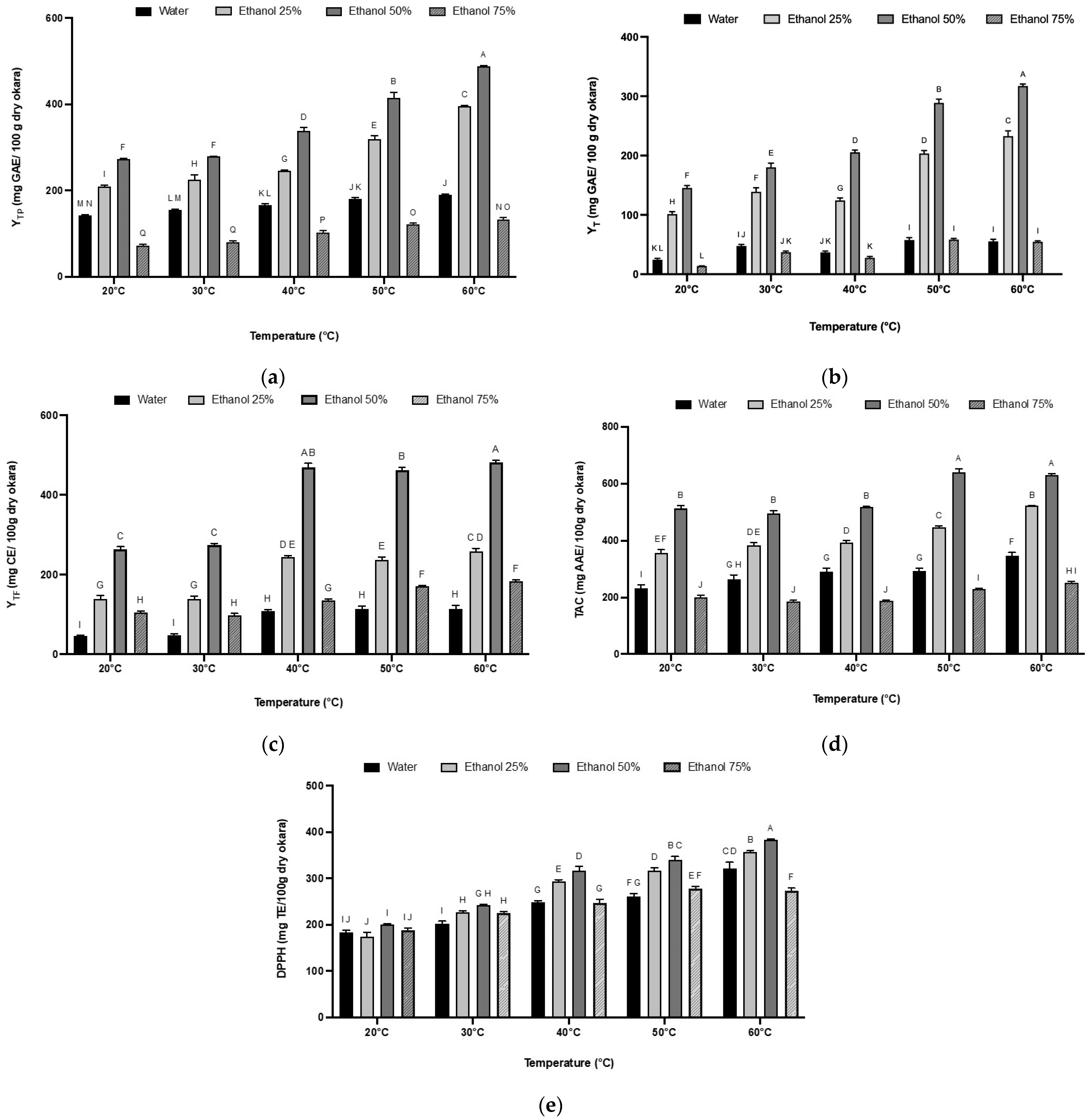
3.4. Polyphenol Recovery as a Function of Temperature and Ethanol Percentage
3.5. The Effect of Extraction Temperature and Solvent Mixture on Tannin and Flavonoid Contents
3.6. The Effect of Extraction Temperature and Solvent Mixture on the Antioxidant Activity of Okara Extracts
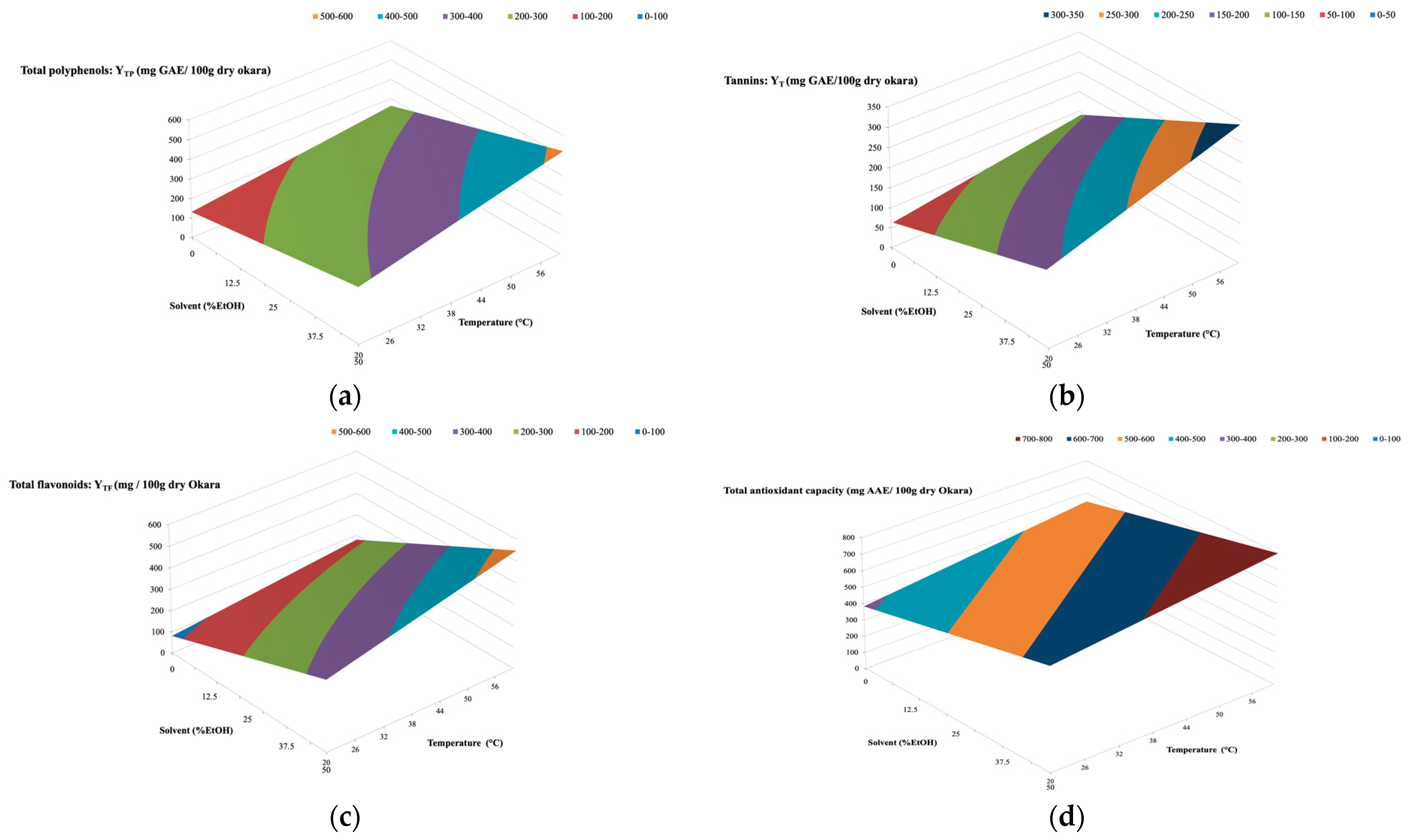
3.7. Experimental Design
3.8. Alpha-Tocopherol Quantification
3.9. Analysis of Triglycerides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Almond Milk Market Size & Share Analysis-Industry Research Report-Growth Trends. Available online: https://www.mordorintelligence.com/industry-reports/global-almond-milk-market (accessed on 30 March 2024).
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, I.; et al. Almonds (Prunus dulcis Mill, D.A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S.J. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef]
- Berryman, C.E.; West, S.G.; Fleming, J.A.; Bordi, P.L.; Kris-Etherton, P.M. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: A randomized controlled trial. J. Am. Heart Assoc. 2015, 4, e000993. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Lapsley, K.; Ellis, P.R. A review of the impact of processing on nutrient bioaccessibility and digestion of almonds. Int. J. Food Sci. Technol. 2016, 51, 1937–1946. [Google Scholar] [CrossRef]
- Richardson, D.P.; Astrup, A.; Cocaul, A.; Ellis, P. The nutritional and health benefits of almonds: A healthy food choice. Food Sci. Technol. Bull. Funct. Foods 2009, 6, 41–50. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mattes, R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef]
- Zhu, Y.; Wilkinson, K.L.; Wirthensohn, M. Changes in fatty acid and tocopherol content during almond (Prunus dulcis, cv. Nonpareil) kernel development. Sci. Hortic. 2017, 225, 150–155. [Google Scholar] [CrossRef]
- Liu, Y.; Hwang, H.-J.; Kim, H.-S.; Park, H. Time and Intervention Effects of Daily Almond Intake on the Changes of Lipid Profile and Body Composition Among Free-Living Healthy Adults. J. Med. Food 2018, 21, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kodad, O.; Rafel Socias I Company; Alonso, J.M. Genotypic and Environmental Effects on Tocopherol Content in Almond. Antioxidants 2018, 7, 6. [Google Scholar] [CrossRef]
- López-Ortiz, C.M.; Prats-Moya, S.; Beltrán Sanahuja, A.; Maestre-Pérez, S.E.; Grané-Teruel, N.; Martín-Carratalá, M.L. Comparative study of tocopherol homologue content in four almond oil cultivars during two consecutive years. J. Food Compos. Anal. 2008, 21, 144–151. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayı, H.; Hanoglu, A.; Hanoglu, D.Y.; Yavuz, D.O.; Ozek, T.; Vatansever, S. Fatty acid composition and anticancer activity in colon carcinoma cell lines of Prunus dulcis seed oil. Pharm. Biol. 2017, 55, 1239–1248. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of Agri-Food By-Products in the Food Industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular Economy in the Agri-Food Sector. A Systematic Literature Review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- De Angelis, D.; Pasqualone, A.; Squeo, G.; Summo, C. Almond okara as a valuable ingredient in biscuit preparation. J. Sci. Food Agric. 2023, 103, 1676–1683. [Google Scholar] [CrossRef]
- Aiello, G.; Xu, R.; Pugliese, R.; Bartolomei, M.; Li, J.; Bollati, C.; Rueller, L.; Robert, J.; Arnoldi, A.; Lammi, C. Quality Assessment of the Protein Ingredients Recovered by Ultrasound-Assisted Extraction from the Press Cakes of Coconut and Almond Beverage Preparation. Foods 2022, 11, 3693. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Puchades, A.; Jiménez-Hernández, N.; Betoret, E.; Gosalbes, M.J.; Betoret, N. Almond (Prunus dulcis) Bagasse as a Source of Bioactive Compounds with Antioxidant Properties: An In Vitro Assessment. Antioxidants 2023, 12, 1229. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. 4.08-Extraction Techniques for the Determination of Phenolic Compounds in Food. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 159–180. [Google Scholar]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Alami, M.; Mashkour, M.; Shahraki, M.H. Optimization of Ultrasound-Assisted Stabilization and Formulation of Almond Milk. J. Food Process Preserv. 2016, 40, 828–839. [Google Scholar] [CrossRef]
- Galván D’Alessandro, L.; Dimitrov, K.; Vauchel, P.; Nikov, I. Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem. Eng. Res. Des. 2014, 92, 1818–1826. [Google Scholar] [CrossRef]
- Hadrich, B.; Dimitrov, K.; Kriaa, K. Modelling Investigation and Parameters Study of Polyphenols Extraction from Carob (Ceratonia siliqua L.) Using Experimental Factorial Design. J. Food Process Preserv. 2017, 41, e12769. [Google Scholar] [CrossRef]
- Wiesneth, S.; Jürgenliemk, G. Total phenolic and tannins determination: A modification of Ph. Eur. 2.8.14 for higher throughput. Pharm.-Int. J. Pharm. Sci. 2017, 72, 195–196. [Google Scholar]
- Liu, L.; Sun, Y.; Laura, T.; Liang, X.; Ye, H.; Zeng, X. Determination of polyphenolic content and antioxidant activity of kudingcha made from Ilex kudingcha C. J. Tseng. Food Chem. 2009, 112, 35–41. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Cicco, N.; Lattanzio, V. The Influence of Initial Carbonate Concentration on the Folin-Ciocalteu Micro-Method for the Determination of Phenolics with Low Concentration in the Presence of Methanol: A Comparative Study of Real-Time Monitored Reactions. Am. J. Anal. Chem. 2011, 2, 840–848. [Google Scholar] [CrossRef][Green Version]
- Bamba, M.; Bordage, S.; Sahuc, M.-E.; Moureu, S.; Samaillie, J.; Roumy, V.; Vauchel, P.; Dimitrov, K.; Rouillé, Y.; Dubuisson, J.; et al. Anti-HCV Tannins from Plants Traditionally Used in West Africa and Extracted With Green Solvents. Front. Pharmacol. 2022, 12, 789688. [Google Scholar] [CrossRef]
- Moreno Gracia, B.; Laya Reig, D.; Rubio-Cabetas, M.J.; Sanz García, M.Á. Study of Phenolic Compounds and Antioxidant Capacity of Spanish Almonds. Foods 2021, 10, 2334. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Jamei, P. Properties of biological activity of ten wild almond (Prunus amygdalus L.) species. Turk. J. Biol. 2012, 36, 201–209. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Yang, S.; Chen, Y.; Zhao, M.; Ashraf, M.; Jiang, Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem. 2009, 116, 1–7. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Sinakos, Z.; Papageorgiou, V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother. Res. PTR 2005, 19, 997–1000. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Słowik-Borowiec, M.; Głąb, N.; Stach, S.; Szpyrka, E. A Miniaturized Sample Preparation Method for the Determination of Vitamins A and E in Food Products. Molecules 2023, 28, 3449. [Google Scholar] [CrossRef]
- Gros, Q.; Wolniaczyk, M.; Duval, J.; West, C.; Horie, S.; Toyota, Y.; Funada, Y.; Lesellier, E. Comparison of the triglyceride composition of vegetable samples with ultra-high efficiency/low-pressure supercritical fluid chromatography—Mass spectrometry. J. Food Compos. Anal. 2023, 115, 104960. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Xiao, L.; Wu, M.; Apaliya, M.T. Impact of extraction parameters and their optimization on the nutraceuticals and antioxidant properties of aqueous extract mulberry leaf. Int. J. Food Prop. 2018, 21, 717–732. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef]
- Tan, P.W.; Tan, C.; Chun Wai, H. Antioxidant properties: Effects of solid-to-solvent ratio on antioxidant compounds and capacities of Pegaga (Centella asiatica). Int. Food Res. J. 2011, 18, 557–562. [Google Scholar]
- Dent, M.; Verica, D.-U.; Penić, M.; Brncic, M.; Bosiljkov, T.; Levaj, B. The Effect of Extraction Solvents, Temperature and Time on the Composition and Mass Fraction of Polyphenols in Dalmatian Wild Sage (Salvia officinalis L.) Extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Galvan d’Alessandro, L.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mandal, N.; Dey, A.; Mondal, B. An approach towards optimization of the extraction of polyphenolic antioxidants from ginger (Zingiber officinale). J. Food Sci. Technol. 2014, 51, 3301–3308. [Google Scholar] [CrossRef]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of bioactive compounds from mango (Mangifera indica L. var. Carabao) seed kernel with ethanol–water binary solvent systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.-L.; Vorobiev, E. Valorization of oilseed residues: Extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind. Crops Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Li, F.; Ning, S.; Li, Y.; Yu, Y.; Shen, C.; Duan, G. Optimisation of Infrared-assisted Extraction of Rutin from Crude Flos Sophorae Immaturus Using Response Surface Methodology and HPLC Analysis. Phytochem. Anal. 2012, 23, 292–298. [Google Scholar] [CrossRef]
- Mou, Q.; He, J.; Yin, R.; Yang, B.; Fu, M.; Fang, J.; Li, H. Response Surface Optimized Infrared-Assisted Extraction and UHPLC Determination of Flavonoid Types from Flos Sophorae. Molecules 2017, 22, 1000. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, B.; Wulandari, S.; Amalia Nurfitriani, R.; Awaludin, A. The potential solvent for tannin extraction as a feed additive made of coffee husk (Coffea canephora) using Soxhlet Method. IOP Conf. Ser. Earth Environ. Sci. 2022, 980, 012024. [Google Scholar] [CrossRef]
- Tomsone, L.; Kruma, Z.; Galoburda, R. Comparison of different solvents and extraction methods for isolation of phenolic compounds from horseradish roots (Armoracia rusticana). World Acad. Sci. Eng. Technol. 2012, 64, 903–908. [Google Scholar]
- Downey, M.O.; Hanlin, R.L. Comparison of Ethanol and Acetone Mixtures for Extraction of Condensed Tannin from Grape Skin. J. Enol. Vitic. 2010, 31, 31. [Google Scholar] [CrossRef][Green Version]
- Chanda, S.V.; Kaneria, M.J. Optimization of Conditions for the Extraction of Antioxidants from Leaves of Syzygium cumini L. Using Different Solvents. Food Anal. Methods 2012, 5, 332–338. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, e6792069. [Google Scholar] [CrossRef]
- Sheng, Z.-L.; Wan, P.-F.; Dong, C.-L.; Li, Y.-H. Optimization of total flavonoids content extracted from Flos populi using response surface methodology. Ind. Crops Prod. 2013, 43, 778–786. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of Total Phenolic Compounds, Flavonoids, Anthocyanins and Tannins from Grape Byproducts by Response Surface Methodology. Influence of Solid-Liquid Ratio, Particle Size, Time, Temperature and Solvent Mixtures on the Optimization Process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef]
- Chaalal, M.; Ouchemoukh, S.; Mehenni, C.; Salhi, N.; Soufi, O.; Ydjedd, S.; Louaileche, H. Phenolic contents and in vitro antioxidant activity of four commonly consumed nuts in algeria. Acta Aliment. 2019, 48, 125–131. [Google Scholar] [CrossRef]
- Murakami, M.; Yamaguchi, T.; Takamura, H.; Matoba, T. Effects of Ascorbic Acid and α-Tocopherol on Antioxidant Activity of Polyphenolic Compounds. J. Food Sci. 2006, 68, 1622–1625. [Google Scholar] [CrossRef]
- Cherif, A.; Sebei, K.; Boukhchina, S.; Kallel, H.; Belkacemi, K.; Arul, J. Kernel fatty acid and triacylglycerol composition for three almond cultivars during maturation. J. Am. Oil Chem. Soc. 2004, 81, 901–905. [Google Scholar] [CrossRef]
- Martín-Carratalá, M.L.; Llorens-Jordá, C.; Berenguer-Navarro, V.; Grané-Teruel, N. Comparative study on the triglyceride composition of almond kernel oil. A new basis for cultivar chemometric characterization. J. Agric. Food Chem. 1999, 47, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
| 20 °C | 40 °C | 60 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | EtOH 25% | EtOH 50% | Water | EtOH 25% | EtOH 50% | Water | EtOH 25% | EtOH 50% | |
| K1 | 90.97 | 50.15 | 36.49 | 58.33 | 27.25 | 24.32 | 37.79 | 22.70 | 29.00 |
| K2 | 132.29 | 196.73 | 237.92 | 161.02 | 263.06 | 361.30 | 212.05 | 424.29 | 534.94 |
| NMRSD% | 2.5 | 2.8 | 3.9 | 3.5 | 1.5 | 5.6 | 2.8 | 2.4 | 2.7 |
| Run | YTP (exp) | YTP (Model) | YT (exp) | YT (Model) | YTF (exp) | YTF (Model) | TAC (exp) | TAC (Model) |
|---|---|---|---|---|---|---|---|---|
| 1 | 108.9 | 105.5 | 66.9 | 43.7 | 66.5 | 68.2 | 163.3 | 150.8 |
| 2 | 172.9 | 168.7 | 128.9 | 121.7 | 115.7 | 117.1 | 230.6 | 237.5 |
| 3 | 205.6 | 192.4 | 166.9 | 166.2 | 272.6 | 271.6 | 246.4 | 263.6 |
| 4 | 356.7 | 362 | 311.2 | 318.2 | 340.3 | 338.9 | 304.6 | 350.3 |
| 5 | 138.7 | 131.4 | 54.9 | 65.8 | 81.9 | 80.7 | 396.4 | 383.3 |
| 6 | 251.7 | 262.8 | 131.5 | 143.8 | 180.1 | 178.6 | 540.6 | 546.9 |
| 7 | 283.2 | 285.3 | 189.6 | 188.3 | 335 | 331.1 | 618.6 | 635.1 |
| 8 | 521.7 | 523 | 331.9 | 340.3 | 552.7 | 548.5 | 789.4 | 789.7 |
| 9 | 255.6 | 253.9 | 178.7 | 173.5 | 262.2 | 241.8 | 431.4 | 420.8 |
| 10 | 253.7 | 253.9 | 172.6 | 173.5 | 262.6 | 241.8 | 436.2 | 420.8 |
| 11 | 255.1 | 253.9 | 180 | 173.5 | 255.3 | 241.8 | 430.8 | 420.8 |
| 12 | 177.5 | 178.7 | 111.4 | 116 | 169.3 | 187.9 | 341.3 | 358.2 |
| 13 | 363.1 | 329.1 | 260.9 | 231 | 275.8 | 295.8 | 490.7 | 483.4 |
| 14 | 152.9 | 167.1 | 58.6 | 93.7 | 122.7 | 111.2 | 323.2 | 329.6 |
| 15 | 325.9 | 340.7 | 238.9 | 253.3 | 373.1 | 372.5 | 570.7 | 511.9 |
| 16 | 188.4 | 207.2 | 144.9 | 162.4 | 185.4 | 198.9 | 232.4 | 250.6 |
| 17 | 304.6 | 300.6 | 221.5 | 184.6 | 259.9 | 248.7 | 570.5 | 591 |
| NMRSD % | 0.029 | 0.062 | 0.026 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, M.; Dimitrov, K.; Samaillie, J.; Caux, B.; Sahpaz, S.; Blanchemain, N.; West, C.; Rivière, C. Optimizing the Extraction of Bioactive Compounds (Polyphenols, Lipids, and Alpha-Tocopherol) from Almond Okara to Unlock Its Potential as Functional Food. Foods 2024, 13, 2828. https://doi.org/10.3390/foods13172828
Taha M, Dimitrov K, Samaillie J, Caux B, Sahpaz S, Blanchemain N, West C, Rivière C. Optimizing the Extraction of Bioactive Compounds (Polyphenols, Lipids, and Alpha-Tocopherol) from Almond Okara to Unlock Its Potential as Functional Food. Foods. 2024; 13(17):2828. https://doi.org/10.3390/foods13172828
Chicago/Turabian StyleTaha, Mariam, Krasimir Dimitrov, Jennifer Samaillie, Benjamin Caux, Sevser Sahpaz, Nicolas Blanchemain, Caroline West, and Céline Rivière. 2024. "Optimizing the Extraction of Bioactive Compounds (Polyphenols, Lipids, and Alpha-Tocopherol) from Almond Okara to Unlock Its Potential as Functional Food" Foods 13, no. 17: 2828. https://doi.org/10.3390/foods13172828
APA StyleTaha, M., Dimitrov, K., Samaillie, J., Caux, B., Sahpaz, S., Blanchemain, N., West, C., & Rivière, C. (2024). Optimizing the Extraction of Bioactive Compounds (Polyphenols, Lipids, and Alpha-Tocopherol) from Almond Okara to Unlock Its Potential as Functional Food. Foods, 13(17), 2828. https://doi.org/10.3390/foods13172828







