The Production of Water Kefir Drink with the Addition of Dried Figs in the Horizontal Rotating Tubular Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Kefir Grain Maintenance and Inoculum Preparation
2.2. Water Kefir Production in Jars with the Addition of Different Dried Fruits
2.3. Water Kefir Production in the Horizontal Rotating Tubular Bioreactor (HRTB)
2.4. Liquid Sample Analysis
2.5. Statistical Analysis of Experimental Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.H.; Tan, J.P.; Borner, R.A.; Zhang, S.J.; Priour, S.; Lima, A.; Ngom-Bru, C.; Cotter, P.D.; Duboux, S. A temporal view of the water kefir microbiota and flavour attributes. Innov. Food Sci. Emerg. Technol. 2022, 80, 103084. [Google Scholar] [CrossRef]
- Assumpcao Fiorda, F.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Kumar Rakshit, S.; Binder Pagnocelli, M.G.; Porto de Souza Vandenberghe, L.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation—A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Eckel, V.P.L.; Vogel, R.F. Genomic and physiological insights into the lifestyle of Bifdobacterium species from water kefir. Arch. Microbiol. 2020, 202, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Leech, J.; Cabrera-Rubio, R.; Walsh, A.M.; Macori, G.; Walsh, C.J.; Barton, W. Fermented-Food Metagenomics Reveals Substrate-Associated Differences in Taxonomy and Health-Associated and Antibiotic Resistance Determinants. mSystems 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, C.; Gottstein, W.; Blasche, S.; Kim, Y.; Abel-Kistrup, M.; Swiegers, H.; Saerens, S.; Edwards, N.; Patil, K.R.; Teusink, B.; et al. Finding Functional Differences between Species in a Microbial Community: Case Studies in Wine Fermentation and Kefir Culture. Front. Microbiol. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Verce, M.; De Vuyst, L.; Weckx, S. Shotgun Metagenomics of a Water Kefir Fermentation Ecosystem Reveals a Novel Oenococcus Species. Front. Microbiol. 2019, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Laureys, D.; De Vuyst, L. Microbial Species Diversity, Community Dynamics, and Metabolite Kinetics of Water Kefir Fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef]
- Laureys, D.; Aerts, M.; Van Damme, P.; De Vuyst, L. Oxygen and diverse nutrients influence the water kefir fermentation process. Food Microbiol. 2018, 73, 351–361. [Google Scholar] [CrossRef]
- Lynch, M.K.; Wilkinson, S.; Daenen, L.; Arendt, K.E. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- Laureys, D.; Aerts, M.; Vandamme, P.; De Vuyst, L. The Buffer Capacity and Calcium Concentration of Water Influence the Microbial Species Diversity, Grain Growth, and Metabolite Production During Water Kefir Fermentation. Front. Microbiol. 2019, 10, 2876. [Google Scholar] [CrossRef]
- Teixeira Magalhães, K.; Pereira, G.V.D.M.; Ribeiro Dias, D.; Freitas Schwan, R. Microbial communities and chemical changes during fermentation of sugary Brazilian kefir. World J. Microbiol. Biotechnol. 2010, 26, 1241–1250. [Google Scholar] [CrossRef]
- Laureys, D.; Van Jean, A.; Dumont, J.; De Vuyst, L. Investigation of the instability and low water kefir grain growth during an industrial water kefir fermentation process. Appl. Microbiol. Biotechnol. 2016, 101, 2811–2819. [Google Scholar] [CrossRef]
- Kohler, S.; Schmacht, M.; Troubounis, A.H.L.; Ludszuweit, M.; Rettberg, N.; Senz, M. Tradition as a Stepping Stone for a Microbial Defined Water Kefir Fermentation Process: Insights in Cell Growth, Bioflavoring, and Sensory Perception. Front. Microbiol. 2021, 12, 732019. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.; Scapini, T.; Frumi Camargo, A.; Bonatto, C.; Spitza Stefanski, F.; Pompeu de Jesus, E.; Techi Diniz, L.G.; Canhadas Bertan, L.; Resende Maldonado, R.; Treichel, H. Development of fermented beverage with water kefir in water-soluble coconut extract (Cocos nucifera L.) with inulin addition. Food Sci. Technol. 2021, 145, 111364. [Google Scholar] [CrossRef]
- Satir, G. The effects of fermentation with water kefir grains on two varieties of tigernut (Cyperus esculentus L.) milk. LWT 2022, 171, 114164. [Google Scholar] [CrossRef]
- Lins Mendes, R.M.; Cavalcanti de Andrade, R.H.; De Fatima Fonseca Marques, M.; Ribeiro de Andrade, E. Potential use of the passion fruit from caatinga in kefir. Food Biosci. 2021, 39, 100809. [Google Scholar] [CrossRef]
- Resende Maldonado, R.; Rocha Mendes Pedreira, A.J.; Buzaneli Cristianini, L.; Fernanda Guidi, M.; Oliveira Capato, M.; Avila, P.F.; Goldbeck, R.; Setsuko Kamimura, E. Application of soluble fibres in the osmotic dehydration of pineapples and reuse of effluent in a beverage fermented by water kefir. Food Sci. Technol. 2020, 132, 109819. [Google Scholar] [CrossRef]
- Atalar, I. Functional kefir production from high pressure homogenized hazelnut milk. Food Sci. Technol. 2019, 107, 256–263. [Google Scholar] [CrossRef]
- Bueno, R.S.; Ressutte, J.B.; Hata, N.N.Y.; Henrique-Bana, F.C.; Guergoletto, K.B.; De Oliveira, A.G.; Spinosa, W.A. Quality and shelf life assessment of a new beverage produced from water kefir grains and red pitaya. LWT 2021, 140, 110770. [Google Scholar] [CrossRef]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol production by lactic acid bacteria: A review. Int. Dairy J. 2002, I2, 2762–2795. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid bacteria: Physiology and carbon sources oxidation. Indian J. Microbiol. 2013, 53, 377. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sounderrajan, A.; Serventi, L. Water kefir: A review of its microbiological profile, antioxidant potential and sensory quality. Acta Sci. Nutr. Health 2020, 4, 10–17. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 10, e04974. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Lynch, K.M.; Nyhan, L.; Sahin, A.W.; O’ Riordan, P.; Luk, D.; Arendt, E.K. Influence of Substrate on the Fermentation Characteristics and Culture-Dependent Microbial Composition of Water Kefir. Fermentation 2023, 9, 28. [Google Scholar] [CrossRef]
- Oliveira, R.; Lages, F.; Silva-Graça, M.; Lucas, C. Fps1p channel is the mediator of the major part of glycerol passive diffusion in Saccharomyces cerevisiae: Artefacts and re-definitions. Biochim. Biophys. Acta 2003, 1613, 57. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Vandamme, E.J. Lactic Acid Bacteria and Bacteriocins: Their Practical Importance. In Bacteriocins of Lactic Acid Bacteria; De Vuyst, L., Vandamme, E.J., Eds.; Springer: Boston, MA, USA, 1994; pp. 1–11. [Google Scholar] [CrossRef]
- Condon, S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Lett. 1987, 46, 269–280. [Google Scholar] [CrossRef]
- Bozkir, E.; Yilmaz, B.; Sharma, H.; Esatbeyoglu, T.; Ozogul, F. Challenges in water kefir production and limitations in human consumption: A comprehensive review of current knowledge. Heliyon 2024, 10, e33501. [Google Scholar] [CrossRef] [PubMed]
- Cufaoglu, G.; Nur Erdinc, A. An alternative source of probiotics: Water kefir. Food Front. 2023, 21, 21–31. [Google Scholar] [CrossRef]
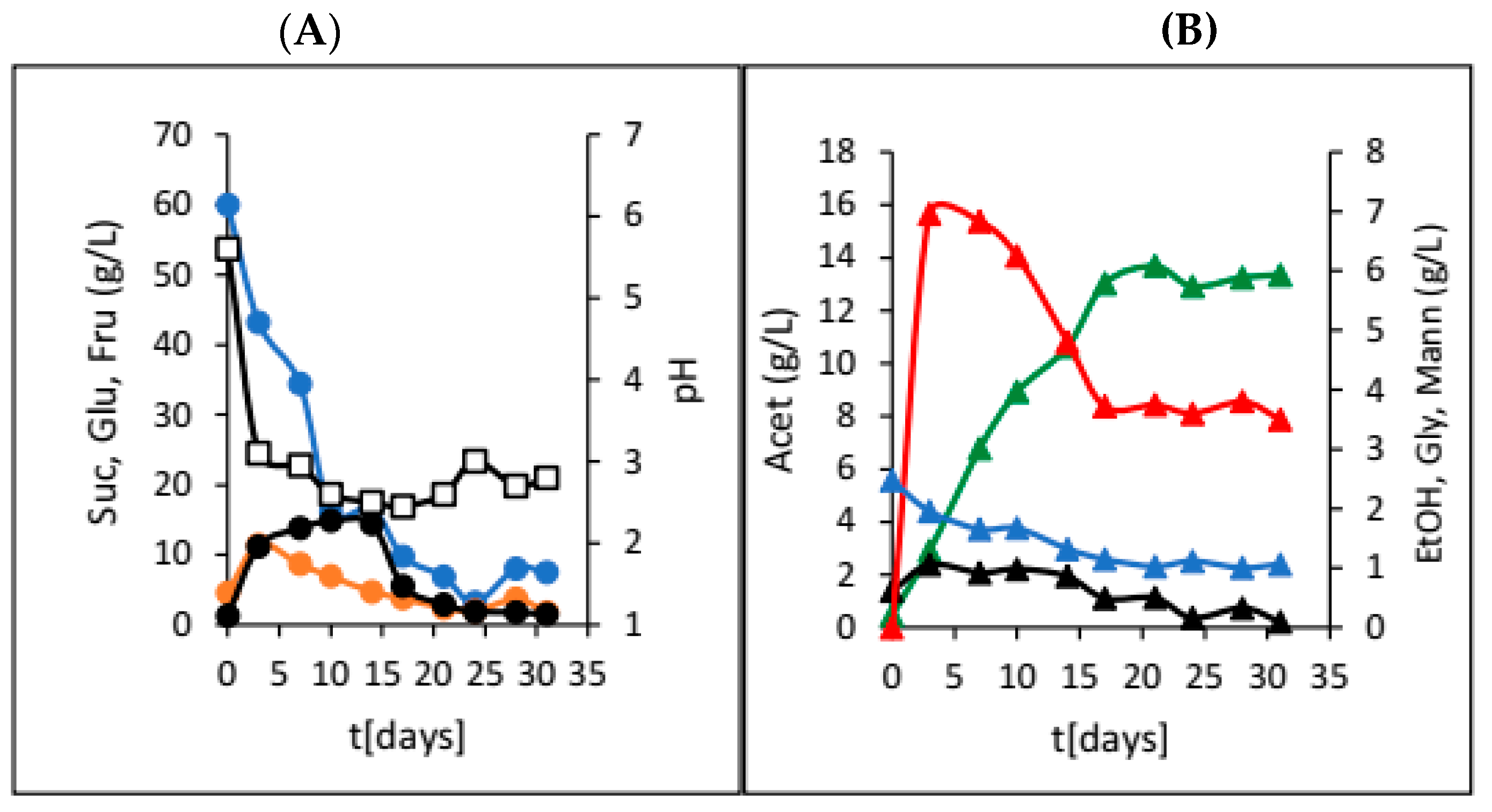
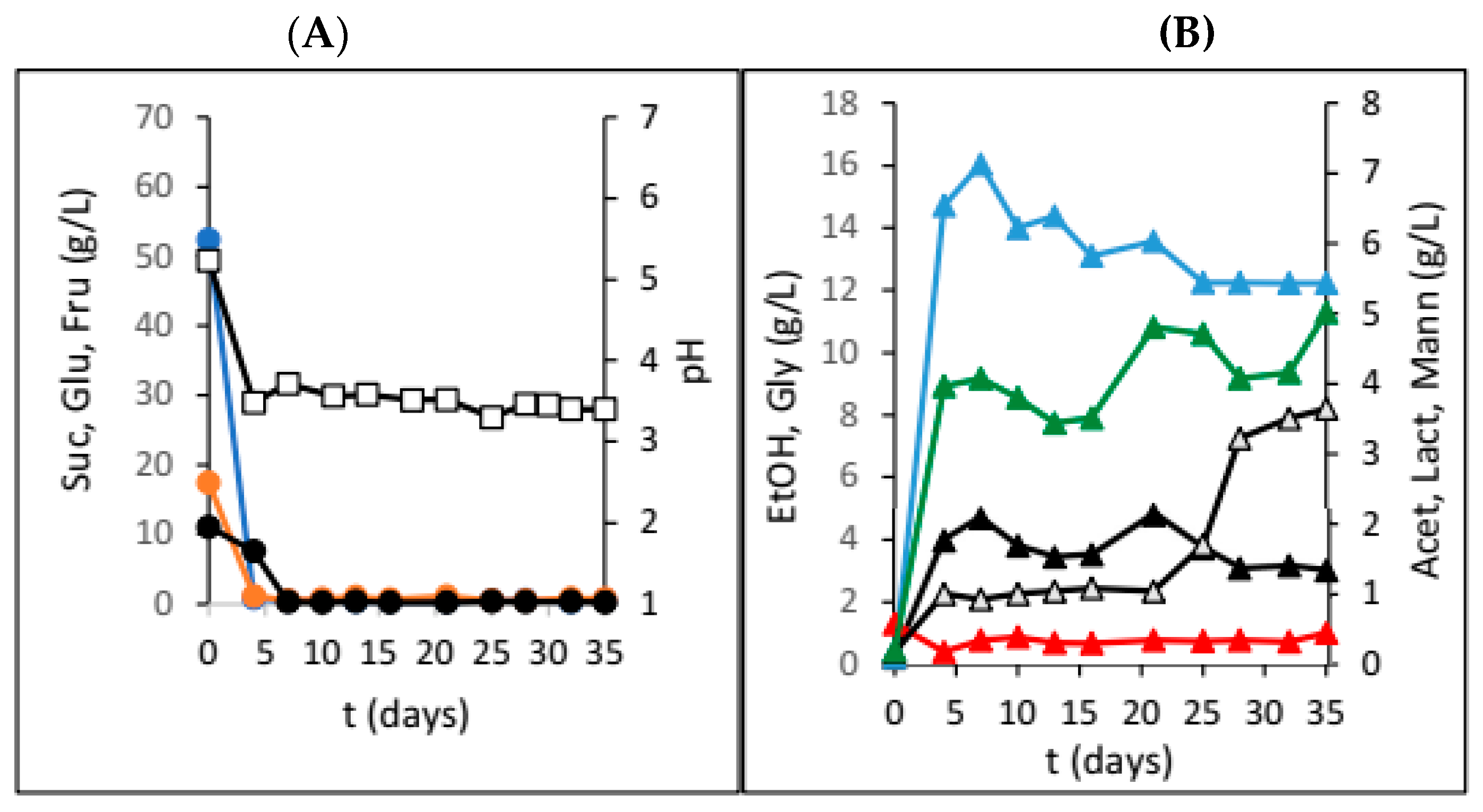
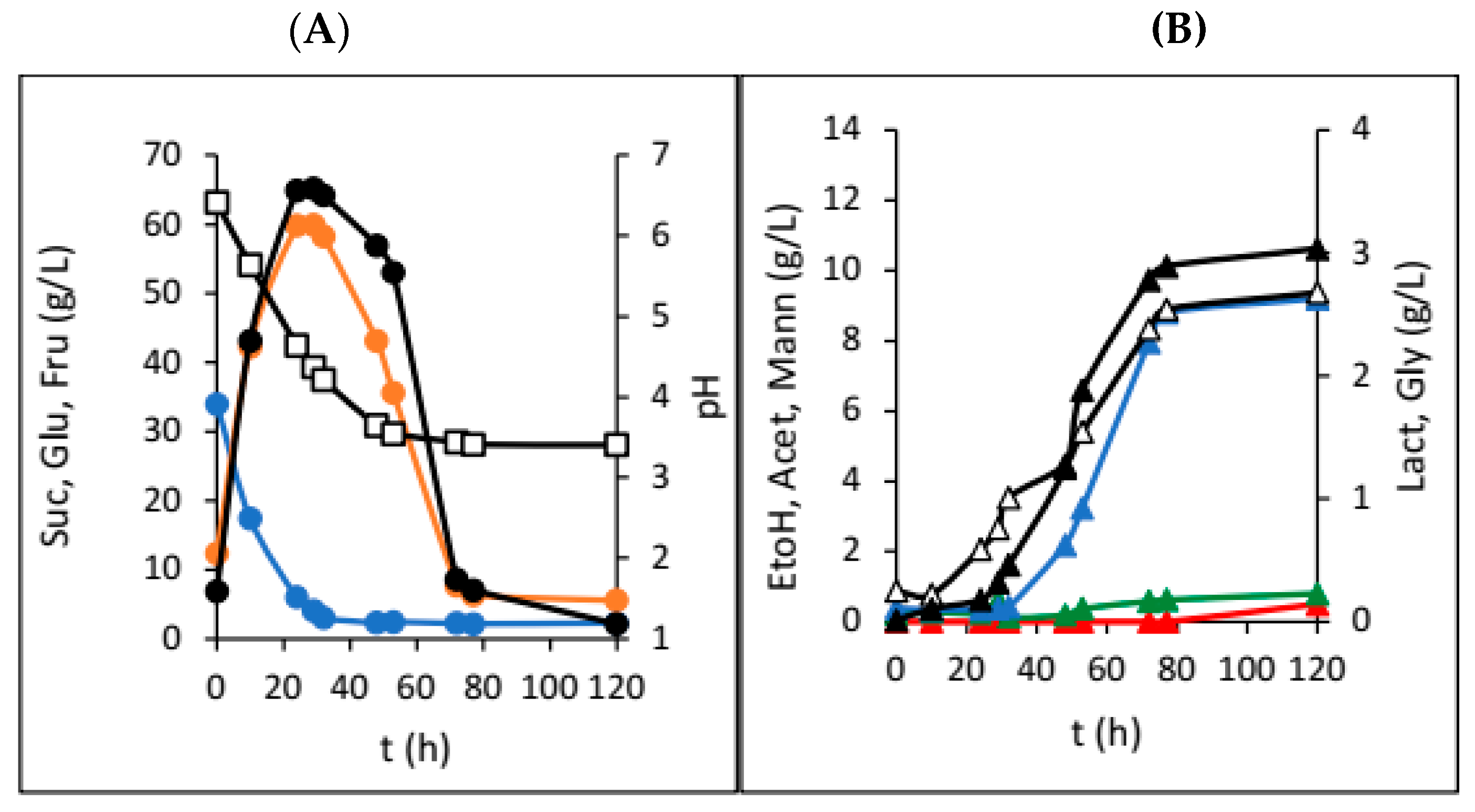
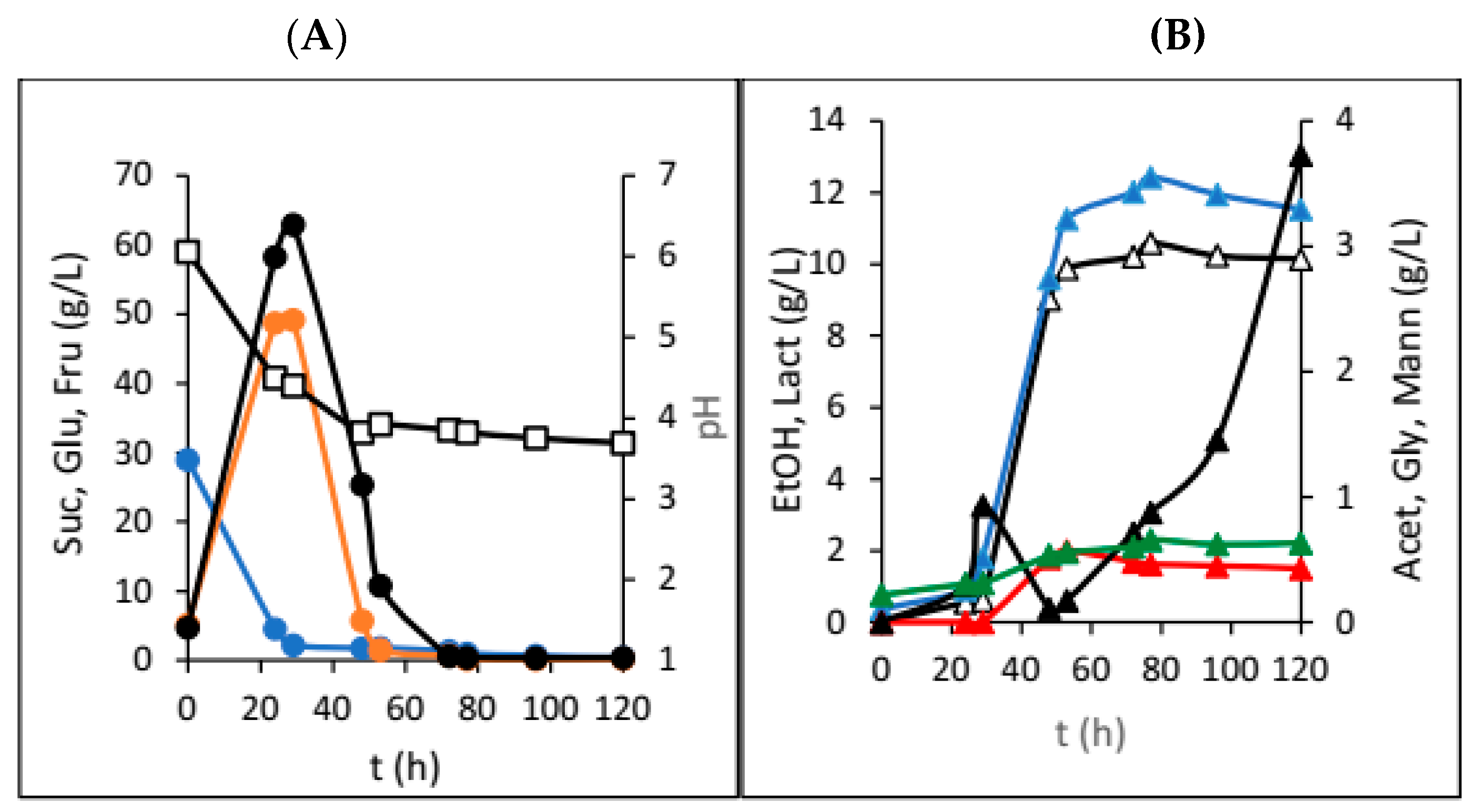
| Dried Fruit | γSUC (g/L) | γGLU (g/L) | γFRU (g/L) | γEtOH (g/L) | γGLY (g/L) | γACET (g/L) | γLAC (g/L) | γMANN (g/L) | pH |
|---|---|---|---|---|---|---|---|---|---|
| Plums | 10.0 | 0.0 | 3.0 | 1.0 | 0.5 | 7.0 | 0.0 | 4.0 | 2.9 |
| Raisins | 9.0 | 10.0 | 30.0 | 0.1 | 0.2 | 0.2 | 0.0 | 0.0 | 2.1 |
| Dates | 10.0 | 10.0 | 40.0 | 1.0 | 0.1 | 12.0 | 0.0 | 0.0 | 2.0 |
| Papaya | 20.0 | 21.0 | 50.0 | 1.3 | 0.5 | 6.0 | 0.0 | 0.0 | 2.4 |
| Cranberries | 2.0 | 30.0 | 52.0 | 1.0 | 0.3 | 6.5 | 0.0 | 1.5 | 2.2 |
| Apricots | 0.0 | 10.0 | 25.0 | 0.1 | 3.0 | 8.0 | 0.0 | 10.0 | 3.0 |
| 10 g of dried figs | 0.0 | 0.0 | 0.0 | 0.2 | 1.0 | 45.0 | 6.0 | 5.0 | 2.7 |
| 20 g of dried figs | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 55.0 | 12.0 | 0.0 | 2.8 |
| 30 g of dried figs | 0.0 | 0.0 | 0.0 | 12.2 | 3.0 | 5.0 | 3.6 | 0.4 | 3.5 |
|
Constant
Rotation Mode |
γSUC (g/L) |
γGLU (g/L) |
γFRU (g/L) |
γEtOH (g/L) |
γGLY (g/L) |
γACET (g/L) |
γLAC (g/L) |
γMANN (g/L) | pH |
|---|---|---|---|---|---|---|---|---|---|
| 3 rpm | 2.20 | 5.5 | 2.10 | 9.19 | 3.65 | 3.83 | 2.17 | 0.56 | 3.45 |
| 5 rpm | 0.77 | 0.26 | 0.04 | 11.72 | 3.66 | 3.87 | 2.16 | 0.48 | 3.7 |
| 7 rpm | 1.04 | 0.22 | 0.23 | 11.30 | 1.93 | 4.16 | 0.29 | 0.48 | 3.3 |
| Interval rotation mode | |||||||||
| 3, 57 min at 3 rpm | 0.39 | 0.26 | 0.38 | 11.52 | 3.72 | 0.63 | 10,14 | 0.43 | 3.85 |
| 6, 54 min at 3 rpm | 0.11 | 1.17 | 0.31 | 11.08 | 5.39 | 8.46 | 8.34 | 0.38 | 3.7 |
| 9, 51 min at 3 rpm | 0.56 | 0.84 | 0.30 | 10.23 | 4.35 | 5.90 | 3.25 | 0.42 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlečić, M.; Novak, M.; Trontel, A.; Marđetko, N.; Tominac, V.P.; Dobrinčić, A.; Kralj, M.; Šantek, B. The Production of Water Kefir Drink with the Addition of Dried Figs in the Horizontal Rotating Tubular Bioreactor. Foods 2024, 13, 2834. https://doi.org/10.3390/foods13172834
Pavlečić M, Novak M, Trontel A, Marđetko N, Tominac VP, Dobrinčić A, Kralj M, Šantek B. The Production of Water Kefir Drink with the Addition of Dried Figs in the Horizontal Rotating Tubular Bioreactor. Foods. 2024; 13(17):2834. https://doi.org/10.3390/foods13172834
Chicago/Turabian StylePavlečić, Mladen, Mario Novak, Antonija Trontel, Nenad Marđetko, Vlatka Petravić Tominac, Ana Dobrinčić, Monika Kralj, and Božidar Šantek. 2024. "The Production of Water Kefir Drink with the Addition of Dried Figs in the Horizontal Rotating Tubular Bioreactor" Foods 13, no. 17: 2834. https://doi.org/10.3390/foods13172834
APA StylePavlečić, M., Novak, M., Trontel, A., Marđetko, N., Tominac, V. P., Dobrinčić, A., Kralj, M., & Šantek, B. (2024). The Production of Water Kefir Drink with the Addition of Dried Figs in the Horizontal Rotating Tubular Bioreactor. Foods, 13(17), 2834. https://doi.org/10.3390/foods13172834








