Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Other Chemicals
2.2. Horned Melon Peel Extraction
2.2.1. Cloud Point Extraction of Horned Melon Peel
2.2.2. Conventional Water Extraction of Horned Melon Peel
2.3. Analytical Methods
2.3.1. Determination of Total Carotenoids Content (TCsp)
2.3.2. Determination of Total Phenols Content (TPsp and TPwp)
2.3.3. Determination of DPPH Radical Scavenging Activity (DPPHsp and DPPHwp)
2.3.4. Determination of Reducing Power (RPsp and RPwp)
2.3.5. Determination of ABTS Radical Scavenging Activity (ABTSsp and ABTSwp)
2.4. Experimental Design
2.5. Evaluation of Selected Food-Related Parameters
2.6. Statistical Analysis
2.6.1. Principal Component Analysis
2.6.2. Response Surface Methodology
2.6.3. Standard Scores
3. Results and Discussion
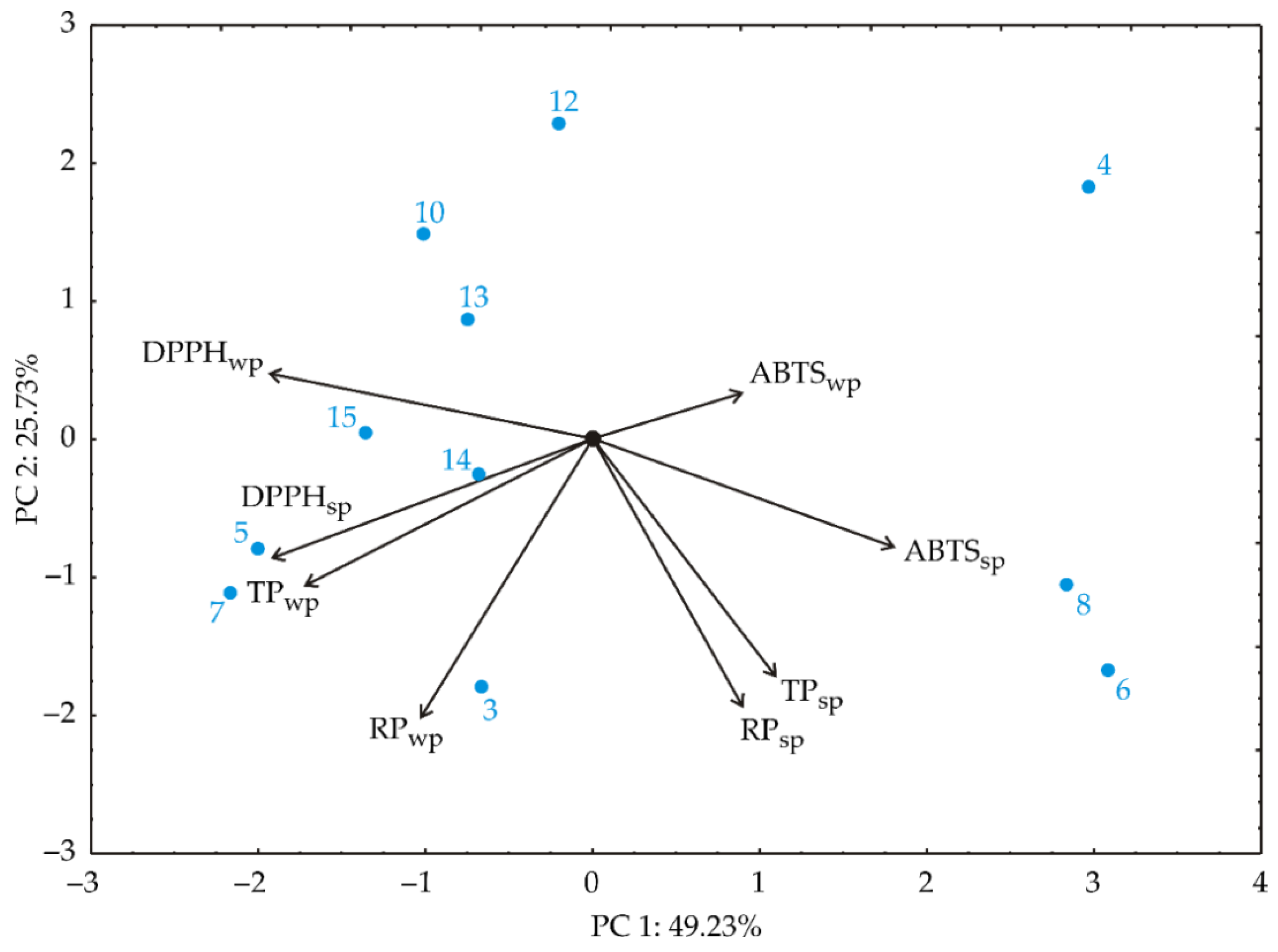
3.1. Principal Component Analysis
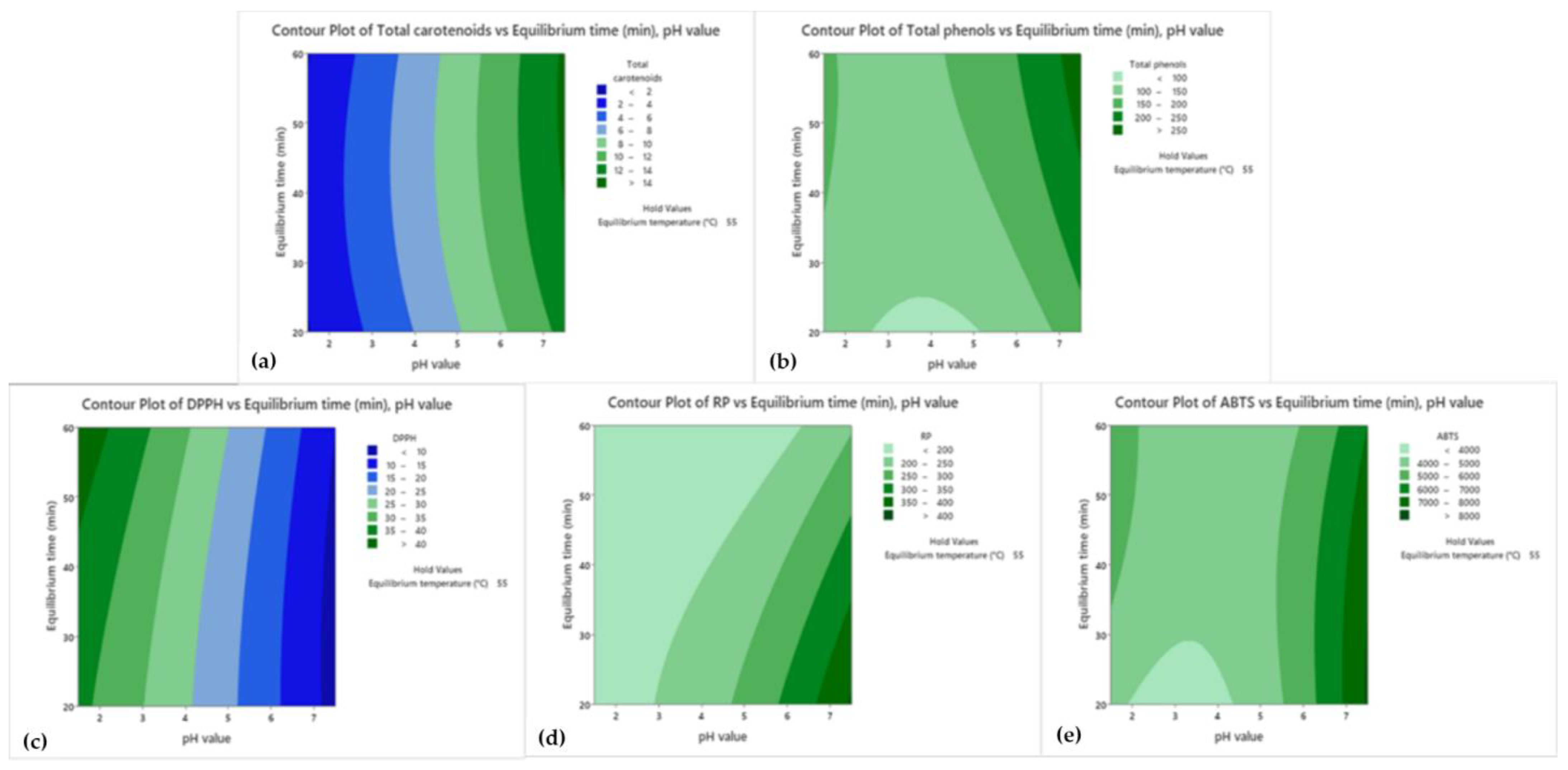
3.2. Response Surface Method
3.3. Standard Score
3.4. Verification of CPE Optimization Parameters and Assessment with Conventional Water Extraction
- Total carotenoids (TCsp): 13.80 mg β-carotene/100 g;
- Total phenols (TPsp): 236.14 mg GAE/100 g;
- DPPH (DPPHsp): 9.67 μmol TE/100 g;
- Reducing power (RPsp): 306.94 μmol TE/100 g;
- ABTS (ABTSsp): 7415.47 μmol TE/100 g.
3.5. Evaluation of Selected Food-Related Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishangulyyev, R.; Kim, S.; Lee, S. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Daniel, S.; Kanthapazham, R.; Vanaraj, R.; Thambidurai, A.; Peter, L.S. A Critical Review on Food Waste Management for the Production of Materials and Biofuel. J. Hazard. Mater. Adv. 2023, 10, 100266. [Google Scholar] [CrossRef]
- Šeregelj, V.; Šovljanski, O.; Tumbas Šaponjac, V.; Vulić, J.; Ćetković, G.; Markov, S.; Čanadanović-Brunet, J. Horned Melon (Cucumis metuliferus E. Meyer Ex. Naudin)—Current Knowledge on Its Phytochemicals, Biological Benefits, and Potential Applications. Processes 2022, 10, 94. [Google Scholar] [CrossRef]
- Ani, O.N.; Achikanu, C.E.; Asogwa, K.K. Comparative Study of Phytonutrient Content and Antioxidant Activity of the Fruit Juices of Watermelon (Citrullus lanatus) and Horned Melon (Cucumis metuliferus). Trop. J. Nat. Prod. Res. (TJNPR) 2023, 7, 3781–3786. [Google Scholar] [CrossRef]
- Šovljanski, O.; Šeregelj, V.; Pezo, L.; Tumbas Šaponjac, V.; Vulić, J.; Cvanić, T.; Markov, S.; Ćetković, G.; Čanadanović-Brunet, J. Horned Melon Pulp, Peel, and Seed: New Insight into Phytochemical and Biological Properties. Antioxidants 2022, 11, 825. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Nath, P.C.; Ojha, A.; Debnath, S.; Sharma, M.; Nayak, P.K.; Sridhar, K.; Inbaraj, B.S. Valorization of Food Waste as Animal Feed: A Step towards Sustainable Food Waste Management and Circular Bioeconomy. Animals 2023, 13, 1366. [Google Scholar] [CrossRef]
- Sarker, A.; Ahmmed, R.; Ahsan, S.M.; Rana, J.; Ghosh, M.K.; Nandi, R. A Comprehensive Review of Food Waste Valorization for the Sustainable Management of Global Food Waste. Sustain. Food Technol. 2023, 2, 48–69. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent Advances in Extraction of Antioxidants from Plant By-Products Processing Industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent Trends in Extraction of Plant Bioactives Using Green Technologies: A Review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical Analysis of Green Extraction Techniques Used for Botanicals: Trends, Priorities, and Optimization Strategies-A Review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Travičić, V.; Cvanić, T.; Šovljanski, O.; Erceg, T.; Perović, M.; Stupar, A.; Ćetković, G. Updating the Status quo on the Eco-Friendly Approach for Antioxidants Recovered from Plant Matrices Using Cloud Point Extraction. Antioxidants 2024, 13, 280. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Giovanoudis, I.; Lalas, S.I. Exploring the Feasibility of Cloud-Point Extraction for Bioactive Compound Recovery from Food Byproducts: A Review. Biomass 2023, 3, 306–322. [Google Scholar] [CrossRef]
- Chatzilazarou, A.; Katsoyannos, E.; Gortzi, O.; Lalas, S.; Paraskevopoulos, Y.; Dourtoglou, E.; Tsaknis, J. Removal of Polyphenols from Wine Sludge Using Cloud Point Extraction. J. Air Waste Manag. Assoc. 2010, 60, 454–459. [Google Scholar] [CrossRef]
- More, P.R.; Arya, S.S. A Novel, Green Cloud Point Extraction and Separation of Phenols and Flavonoids from Pomegranate Peel: An Optimization Study Using RCCD. J. Environ. Chem. Eng. 2019, 7, 103306. [Google Scholar] [CrossRef]
- Vichapong, J.; Santaladchaiyakit, Y.; Burakham, R.; Srijaranai, S. Cloud-Point Extraction and Reversed-Phase High Performance Liquid Chromatography for Analysis of Phenolic Compounds and Their Antioxidant Activity in Thai Local Wines. J. Food Sci. Technol. 2014, 51, 664–672. [Google Scholar] [CrossRef]
- de Araújo Padilha, C.E.; de Azevedo, J.C.S.; de Sousa, F.C.; de Oliveira, S.D.; de Souza, D.F.; de Oliveira, J.A.; de Macedo, G.R.; dos Santos, E.S. Recovery of Polyphenols from Camu-Camu (Myrciaria Dubia H.B.K. McVaugh) Depulping Residue by Cloud Point Extraction. Chin. J. Chem. Eng. 2018, 26, 2471–2476. [Google Scholar] [CrossRef]
- Motikar, P.D.; More, P.R.; Arya, S.S. A Novel, Green Environment-Friendly Cloud Point Extraction of Polyphenols from Pomegranate Peels: A Comparative Assessment with Ultrasound and Microwave-Assisted Extraction. Sep. Sci. Technol. 2021, 56, 1014–1025. [Google Scholar] [CrossRef]
- Zafar, Z.; Bhatti, I.A.; Hanif, M.A.; Zia, M.A. Enhanced Recovery of Phenolics from Acalypha Fruticosa by Micelle-Mediated Extraction, Antioxidant, Antimutagenic, Antimicrobial Evaluation, and Chemical Profiling. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Implementation of Cloud Point Extraction Using Surfactants in the Recovery of Polyphenols from Apricot Cannery Waste. Eng 2023, 4, 1225–1235. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Antioxidant Capacity in Two Different Cultivars of Ripe and Unripe Peaches Utilizing the Cloud-Point Extraction Method. AgriEngineering 2023, 5, 2139–2154. [Google Scholar] [CrossRef]
- Sazdanić, D.; Atanacković Krstonošić, M.; Ćirin, D.; Cvejić, J.; Alamri, A.; Galanakis, C.M.; Krstonošić, V. Non-Ionic Surfactants-Mediated Green Extraction of Polyphenols from Red Grape Pomace. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100439. [Google Scholar] [CrossRef]
- Vieira, F.A.; Ventura, S.P.M. Efficient Extraction of Carotenoids from Sargassum Muticum Using Aqueous Solutions of Tween 20. Mar. Drugs 2019, 17, 310. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Development of a Cloud Point Extraction Technique Based on Lecithin for the Recovery of Carotenoids from Liquid Tomato Wastewater. Waste 2023, 1, 105–114. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Travičić, V.; Šovljanski, O.; Tomić, A.; Perović, M.; Milošević, M.; Ćetković, N.; Antov, M. Augmenting Functional and Sensorial Quality Attributes of Kefir through Fortification with Encapsulated Blackberry Juice. Foods 2023, 12, 4163. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Cetković, G.; Moreno, D.A.; Canadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Krunić, M. Anthocyanin Profiles and Biological Properties of Caneberry (Rubus Spp.) Press Residues. J. Sci. Food Agric. 2014, 94, 2393–2400. [Google Scholar] [CrossRef]
- Singha, P.; Muthukumarappan, K. Effects of Processing Conditions on the System Parameters during Single Screw Extrusion of Blend Containing Apple Pomace. J. Food Process Eng. 2017, 40, e12513. [Google Scholar] [CrossRef]
- Brlek, T.; Pezo, L.; Voća, N.; Krička, T.; Vukmirović, Đ.; Čolović, R.; Bodroža-Solarov, M. Chemometric Approach for Assessing the Quality of Olive Cake Pellets. Fuel Process. Technol. 2013, 116, 250–256. [Google Scholar] [CrossRef]
- Takla, S.S.; Shawky, E.; Hammoda, H.M.; Darwish, F.A. Green Techniques in Comparison to Conventional Ones in the Extraction of Amaryllidaceae Alkaloids: Best Solvents Selection and Parameters Optimization. J. Chromatogr. A 2018, 1567, 99–110. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; International Life Sciences Institute: Washington, DC, USA, 2001; 71p. [Google Scholar]
- Britton, G. Structure and Properties of Carotenoids in Relation to Function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Kiai, H.; Raiti, J.; El-Abbassi, A.; Hafidi, A. Recovery of Phenolic Compounds from Table Olive Processing Wastewaters Using Cloud Point Extraction Method. J. Environ. Chem. Eng. 2018, 6, 1569–1575. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A. Response Surface Modeling and Optimization of Polyphenols Extraction from Apple Pomace Based on Nonionic Emulsifiers. Agronomy 2020, 10, 92. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Approach to Optimization of FRAP Methodology for Studies Based on Selected Monoterpenes. Molecules 2020, 25, 5267. [Google Scholar] [CrossRef]
- Chat, O.A.; Najar, M.H.; Mir, M.A.; Rather, G.M.; Dar, A.A. Effects of Surfactant Micelles on Solubilization and DPPH Radical Scavenging Activity of Rutin. J. Colloid Interface Sci. 2011, 355, 140–149. [Google Scholar] [CrossRef]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, P.; Cañigueral, S. Antioxidant Activity of Tween-20 and Tween-80 Evaluated through Different in-Vitro Tests. J. Pharm. Pharmacol. 2015, 67, 666–672. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Voulgaris, A.; Katsoulis, K.; Lalas, S.I.; Roussis, I.G.; Gortzi, O. Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater. Foods 2023, 12, 497. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Isolation of Polyphenols from Two Waste Streams of Clingstone Peach Canneries Utilizing the Cloud Point Extraction Method. Biomass 2023, 3, 291–305. [Google Scholar] [CrossRef]
- Matsusaka, Y.; Kawabata, J. Evaluation of Antioxidant Capacity of Non-Edible Parts of Some Selected Tropical Fruits. Food Sci. Technol. Res. 2010, 16, 467–472. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Salem, R.D.; Mortazavian, A.M.; Rocha, R.S.; Cruz, A.G. Effects of herbal extracts on quality traits of yogurts, cheeses, fermented milks, and ice creams: A technological perspective. Curr. Opin. Food Sci. 2018, 19, 52. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Wellness 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Almeida, H.H.; Barros, L.; Barreira, J.C.; Calhelha, R.C.; Heleno, S.A.; Sayer, C.; Miranda, C.G.; Leimann, F.V.; Barreiro, M.F.; Ferreira, I.C. Bioactive evaluation and application of different formulations of the natural colorant curcumin (E100) in a hydro-philic matrix (yogurt). Food Chem. 2018, 261, 224–232. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, C.; Sun, R.; Ni, S.; Xia, Q. Effect of emulsification process on multiple lipid particles encapsulating both coenzyme Q10 and tea polyphenols. J. Food Process Eng. 2015, 38, 144–154. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Mohammadinejad, R.; Ashrafizadeh, M. Drug delivery systems for resveratrol, a non-flavonoid polyphenol: Emerging evidence in last decades. J. Drug Deliv. Sci. Technol. 2019, 51, 591–604. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]




| Code | Variables | Coded levels | ||||
| −1 | 0 | 1 | ||||
| Actual levels | ||||||
| X1 | pH value | 1.5 | 4.5 | 7.5 | ||
| X2 | Equilibrium temperature (°C) | 35 | 55 | 75 | ||
| X3 | Equilibrium time (min) | 20 | 40 | 60 | ||
| Experimental design | ||||||
| Run | X1 | X2 | X3 | |||
| 1 | −1 | −1 | 0 | |||
| 2 | 1 | −1 | 0 | |||
| 3 | −1 | 1 | 0 | |||
| 4 | 1 | 1 | 0 | |||
| 5 | −1 | 0 | −1 | |||
| 6 | 1 | 0 | −1 | |||
| 7 | −1 | 0 | 1 | |||
| 8 | 1 | 0 | 1 | |||
| 9 | 0 | −1 | −1 | |||
| 10 | 0 | 1 | −1 | |||
| 11 | 0 | −1 | 1 | |||
| 12 | 0 | 1 | 1 | |||
| 13 | 0 | 0 | 0 | |||
| 14 | 0 | 0 | 0 | |||
| 15 | 0 | 0 | 0 | |||
| Run | Surfactant-Rich Phase (sp) | ||||
|---|---|---|---|---|---|
| TCsp | TPsp | DPPHsp | RPsp | ABTSsp | |
| (mg β-Carotene/100 g) | (mg GAE/100 g) | (μmol TE/100 g) | |||
| 1 | no phase separation occurred | ||||
| 2 | no phase separation occurred | ||||
| 3 | 1.26 ± 0.50 a | 175.41 ± 3.33 f | 32.18 ± 2.42 f | 224.50 ± 20.41 de | 6360.79 ± 133.51 f |
| 4 | 14.65 ± 0.70 d | 129.91 ± 7.37 c | 4.90 ± 2.11 a | 137.07 ± 5.83 b | 7728.79 ± 164.56 h |
| 5 | 1.87 ± 0.44 a | 127.90 ± 7.35 c | 36.22 ± 1.15 g | 188.31 ± 23.23 d | 4253.30 ± 163.29 a |
| 6 | 12.55 ± 0.93 d | 180.02 ± 6.92 f | 8.30 ± 3.85 b | 406.26 ± 2.95 f | 8100.58 ± 116.45 g |
| 7 | 1.87 ± 0.71 a | 155.62 ± 3.48 e | 43.44 ± 3.38 h | 187.33 ± 11.65 d | 5659.71 ± 219.51 e |
| 8 | 14.29 ± 0.36 d | 277.34 ± 19.20 g | 10.16 ± 2.23 c | 244.08 ± 3.98 e | 6976.56 ± 450.90 fg |
| 9 | no phase separation occurred | ||||
| 10 | 9.02 ± 0.58 c | 62.33 ± 0.56 a | 21.68 ± 1.41 d | 144.01 ± 5.41 c | 4199.67 ± 56.38 a |
| 11 | no phase separation occurred | ||||
| 12 | 5.02 ± 0.72 b | 96.10 ± 3.34 b | 19.29 ± 3.23 d | 100.22 ± 8.57 a | 5125.92 ± 8.92 d |
| 13 | 6.90 ± 0.71 b | 127.30 ± 2.45 c | 21.30 ± 1.80 d | 176.25 ± 14.20 d | 4072.85 ± 84.77 a |
| 14 | 8.34 ± 0.43 c | 135.96 ± 2.78 d | 25.75 ± 2.72 e | 242.55 ± 9.84 e | 4770.06 ± 281.08 c |
| 15 | 8.80 ± 0.41 c | 135.18 ± 1.08 d | 27.61 ± 1.95 e | 200.66 ± 8.68 de | 4477.45 ± 41.27 b |
| Run | Water phase (wp) | ||||
| TPwp | DPPHwp | RPwp | ABTSwp | ||
| (mg GAE/100 g) | (μmol TE/100 g) | ||||
| 1 | no phase separation occurred | ||||
| 2 | no phase separation occurred | ||||
| 3 | 428.25 ± 33.90 e | 512.60 ± 8.53 e | 934.46 ± 73.75 g | 8752.68 ± 280.63 c | |
| 4 | 198.61 ± 5.15 a | 120.39 ± 15.81 b | 179.23 ± 9.08 a | 8771.61 ± 439.02 c | |
| 5 | 422.16 ± 17.24 e | 478.79 ± 14.91 d | 783.90 ± 17.19 f | 3521.47 ± 474.82 a | |
| 6 | 313.49 ± 8.49 d | 85.19 ± 2.54 a | 519.16 ± 65.93 e | 8900.48 ± 847.70 cd | |
| 7 | 461.62 ± 6.10 f | 770.68 ± 11.18 i | 737.75 ± 55.72 f | 6238.70 ± 342.31 b | |
| 8 | 298.08 ± 4.27 c | 145.22 ± 7.93 c | 465.96 ± 21.82 d | 10,080.85 ± 208.80 d | |
| 9 | no phase separation occurred | ||||
| 10 | 395.27 ± 7.96 e | 636.58 ± 39.80 gh | 519.75 ± 32.39 e | 11,359.71 ± 388.24 d | |
| 11 | no phase separation occurred | ||||
| 12 | 272.07 ± 4.45 b | 658.28 ± 3.36 gh | 240.21 ± 27.70 b | 6907.59 ± 736.16 b | |
| 13 | 437.80 ± 11.59 e | 548.64 ± 1.80 f | 342.82 ± 25.42 c | 8572.07 ± 159.05 c | |
| 14 | 452.62 ± 6.38 f | 600.20 ± 9.76 g | 546.86 ± 33.65 e | 10,654.54 ± 335.51 d | |
| 15 | 428.25 ± 33.90 e | 512.60 ± 8.53 e | 934.46 ± 73.75 g | 8752.68 ± 280.63 c | |
| Responses | TPsp | TPwp | DPPHsp | DPPHwp | RPsp | RPwp | ABTSsp | ABTSwp |
|---|---|---|---|---|---|---|---|---|
| TCsp | 0.300 | −0.633 * | −0.896 *** | −0.723 * | 0.276 | −0.662 * | 0.526 | 0.587 |
| TPsp | −0.146 | −0.216 | −0.557 | 0.552 | 0.202 | 0.583 | 0.100 | |
| TPwp | 0.818 ** | 0.704 * | 0.078 | 0.662 * | −0.670 * | −0.153 | ||
| DPPHsp | 0.790 ** | −0.211 | 0.732 * | −0.591 | −0.524 | |||
| DPPHwp | −0.506 | 0.278 | −0.808 ** | −0.227 | ||||
| RPsp | 0.307 | 0.510 | 0.184 | |||||
| RPwp | −0.126 | −0.269 | ||||||
| ABTSsp | 0.205 |
| Terms | df | TCsp | TPsp | TPwp | DPPHsp | DPPHwp | RPsp | RPwp | ABTSsp | ABTSwp |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 2.1 × 102 ** | 5.7 × 102 * | 4.5 × 104 ** | 1.1 × 103 ** | 2.7 × 105 * | 8.3 × 102 | 3.5 × 105 * | 5.2 × 106 * | 7.1 × 106 |
| Temp | 1 | 0.3 | 4.7 × 103 ** | 1.9 × 104 ** | 6.8 | 1.0 × 102 | 1.6 × 104 | 1.5 × 104 | 6.6 × 105 | 2.1 × 106 |
| t | 1 | 3.3 | 3.1 × 103 ** | 4.1 × 103 * | 1.5 | 1.3 × 104 | 5.2 × 103 | 3.6 × 104 | 3.8 × 105 | 2.1 × 106 |
| pH2 | 1 | 0.2 | 1.0 × 104 ** | 5.7 × 103 * | 3.3 | 2.0 × 105 * | 7.5 × 103 | 7.4 × 104 | 1.1 × 107 * | 2.7 × 106 |
| t2 | 1 | 1.1 | 2.8 × 102 | 1.9 × 103 * | 1.6 | 5.6 × 103 | 4.7 × 101 | 9.6 × 101 | 2.1 × 105 | 1.1 × 106 |
| pH × Temp | 1 | 1.1 | 5.8 × 103 ** | 2.9 × 103 * | 3.7 | 4.6 × 103 | 1.7 × 104 | 7.9 × 104 | 4.9 × 105 | 7.0 × 106 |
| pH × t | 1 | 0.8 | 1.2 × 103 * | 7.5 × 102 | 7.2 | 1.3 × 104 | 6.5 × 103 | 1.2 × 101 | 1.6 × 106 | 5.9 × 105 |
| Temp × t | 1 | 7.9 | 2.8 × 102 | 6.1 × 103 * | 1.6 × 101 | 7.9 × 103 | 4.8 × 102 | 1.8 × 104 | 2.1 × 105 | 1.4 × 107 |
| Error | 2 | 2.0 × 10 | 4.6 × 101 | 1.8 × 102 | 2.1 × 101 | 7.6 × 103 | 2.2 × 103 | 2.3 × 104 | 2.5 × 105 | 5.4 × 106 |
| R2 | 0.992 | 0.998 | 0.998 | 0.985 | 0.987 | 0.965 | 0.956 | 0.989 | 0.889 | |
| adj R2 | 0.958 | 0.992 | 0.989 | 0.927 | 0.936 | 0.826 | 0.782 | 0.943 | 0.444 |
| CPE Optimization | |||
|---|---|---|---|
| CPE phase: | Surfactant-rich phase | ||
| Goal for CPE outcomes values: | Maximizing | ||
| Weight coefficient for CPE outcomes: | equal for all (1) | ||
| Importance of CPE outcomes: | equal for all (1) | ||
| Optimized CPE parameters | |||
| pH value (/) | 7.32 | ||
| Equilibrium temperature (°C) | 55 | ||
| Equilibrium time (min) | 43.03 | ||
| CPE outcomes for optimized CPE parameters | |||
| Predicted CPE outcomes | Experimentally obtained CPE outcomes | ||
| TC (mg β carotene/100 g) | 13.80 | TC (mg β-carotene/100 g) | 13.11 |
| TP (mg GAE/100 g) | 236.14 | TP (mg GAE/100 g) | 239.71 |
| DPPH (μmol TE/100 g) | 9.67 | DPPH (μmol TE/100 g) | 9.05 |
| RP (μmol TE/100 g) | 306.94 | RP (μmol TE/100 g) | 308.63 |
| ABTS (μmol TE/100 g) | 7415.47 | ABTS (μmol TE/100 g) | 7398.58 |
| Comparison with conventional water extraction | |||
| TC (mg β carotene/100 g) | nd * | ||
| TP (mg GAE/100 g) | 165.21 ± 0.21 | ||
| DPPH (μmol TE/100 g) | 117.78 ± 0.36 | ||
| RP (μmol TE/100 g) | 156.28 ± 1.47 | ||
| ABTS (μmol TE/100 g) | 3274.02 ± 5.16 | ||
| Microbiological Parameter (log CFU/mL or g) | Raw Materials | Obtained Extracts | ||||
|---|---|---|---|---|---|---|
| Water | NaCl | Tween 80 | Lyophilized Horned Melon Peel | Water-Based Extract | CPE Extract | |
| Total viable count | 3 | 2.5 | 2.8 | 3.5 | 3.5 | 2 |
| Escherichia coli | <1 | <1 | <1 | <1 | <1 | <1 |
| Salmonella spp. | nd | nd | nd | nd | nd | nd |
| Staphylococcus aureus | <1 | <1 | <1 | <1 | <1 | <1 |
| Listeria monocytogenes | nd | nd | nd | nd | nd | nd |
| Yeast and mold | 0.3 | 0.8 | 1.1 | 1.4 | 2.5 | 1 |
| Bacillus cereus | <1 | 1.8 | <1 | 0.4 | 1.5 | 0.5 |
| Enterobacteriaceae | <1 | <1 | <1 | <1 | <1 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travičić, V.; Cvanić, T.; Vidović, S.; Pezo, L.; Hidalgo, A.; Šovljanski, O.; Ćetković, G. Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction. Foods 2024, 13, 2863. https://doi.org/10.3390/foods13182863
Travičić V, Cvanić T, Vidović S, Pezo L, Hidalgo A, Šovljanski O, Ćetković G. Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction. Foods. 2024; 13(18):2863. https://doi.org/10.3390/foods13182863
Chicago/Turabian StyleTravičić, Vanja, Teodora Cvanić, Senka Vidović, Lato Pezo, Alyssa Hidalgo, Olja Šovljanski, and Gordana Ćetković. 2024. "Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction" Foods 13, no. 18: 2863. https://doi.org/10.3390/foods13182863
APA StyleTravičić, V., Cvanić, T., Vidović, S., Pezo, L., Hidalgo, A., Šovljanski, O., & Ćetković, G. (2024). Sustainable Recovery of Polyphenols and Carotenoids from Horned Melon Peel via Cloud Point Extraction. Foods, 13(18), 2863. https://doi.org/10.3390/foods13182863










