Analysis of Aroma Characteristics of ‘Binzi’ and ‘Xiangguo’ Apple—Ancient Cultivars in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Post-Harvest Storage Conditions
2.2. Preparation of Apple Fruit Samples before VOC Detection
2.3. VOC of Apple Fruits Using Solid-Phase Microextraction, Gas Chromatography and Mass Spectrometry (SPME-GC-MS)
2.4. Identification and Quantitation of VOCs
2.5. Calculation of Odor Activity Value (OAV)
2.6. Data Analysis
3. Results
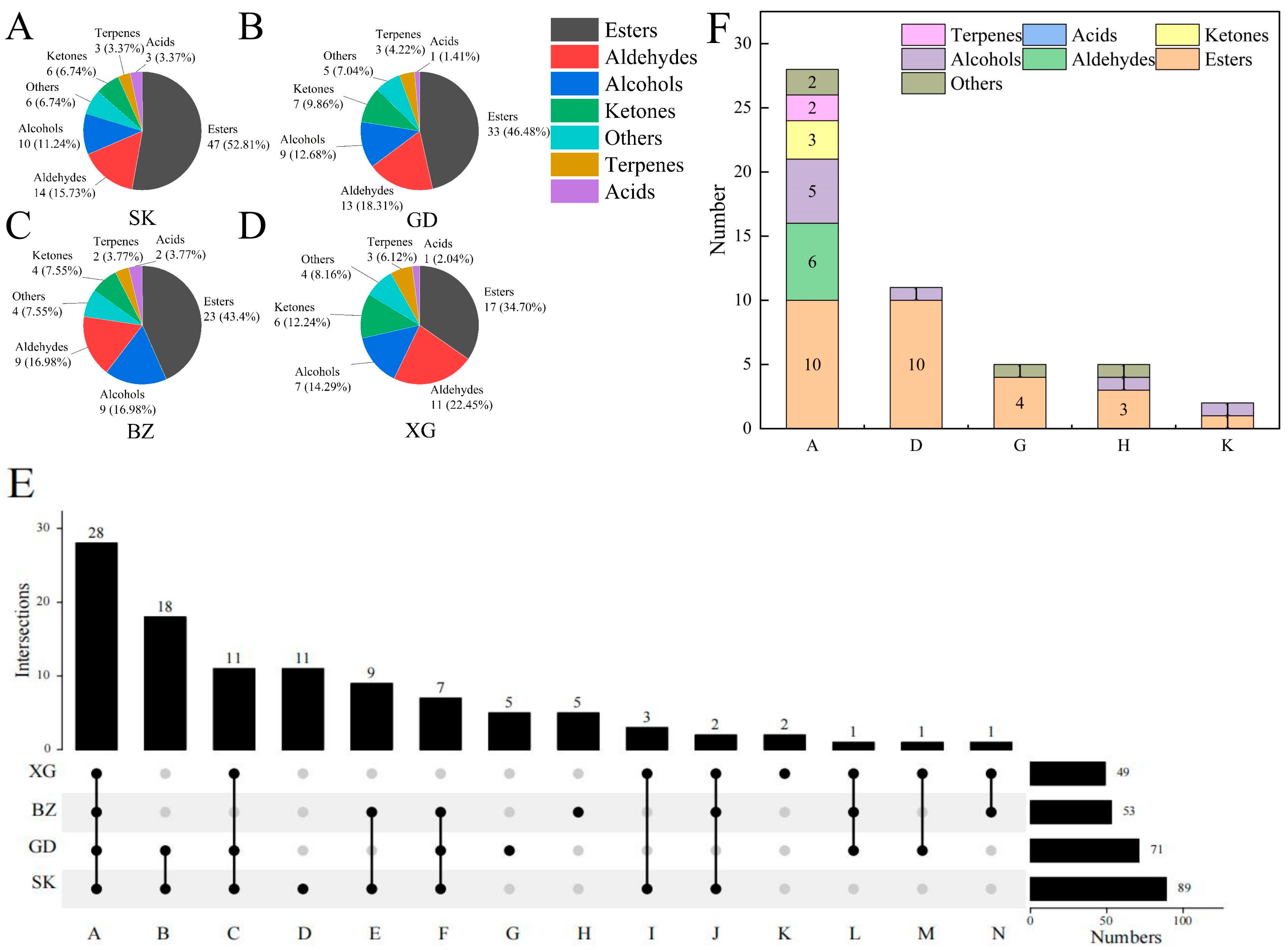
3.1. Identification and Determination of VOCs in Four Apple Cultivars
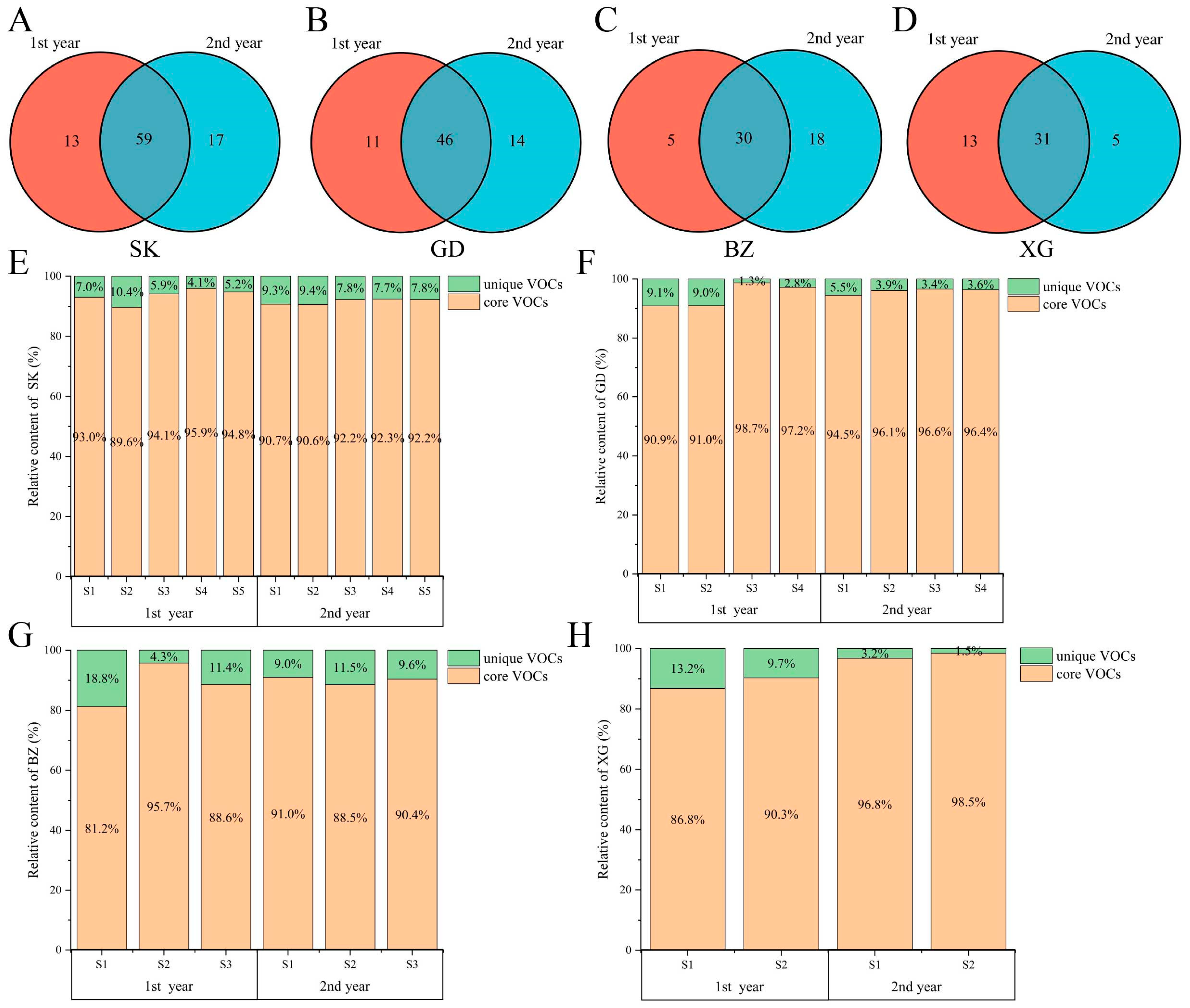
3.2. Core VOCs of Four Apple Cultivars in Two Years
3.3. Aroma Profile of Four Apple Cultivars during Storage
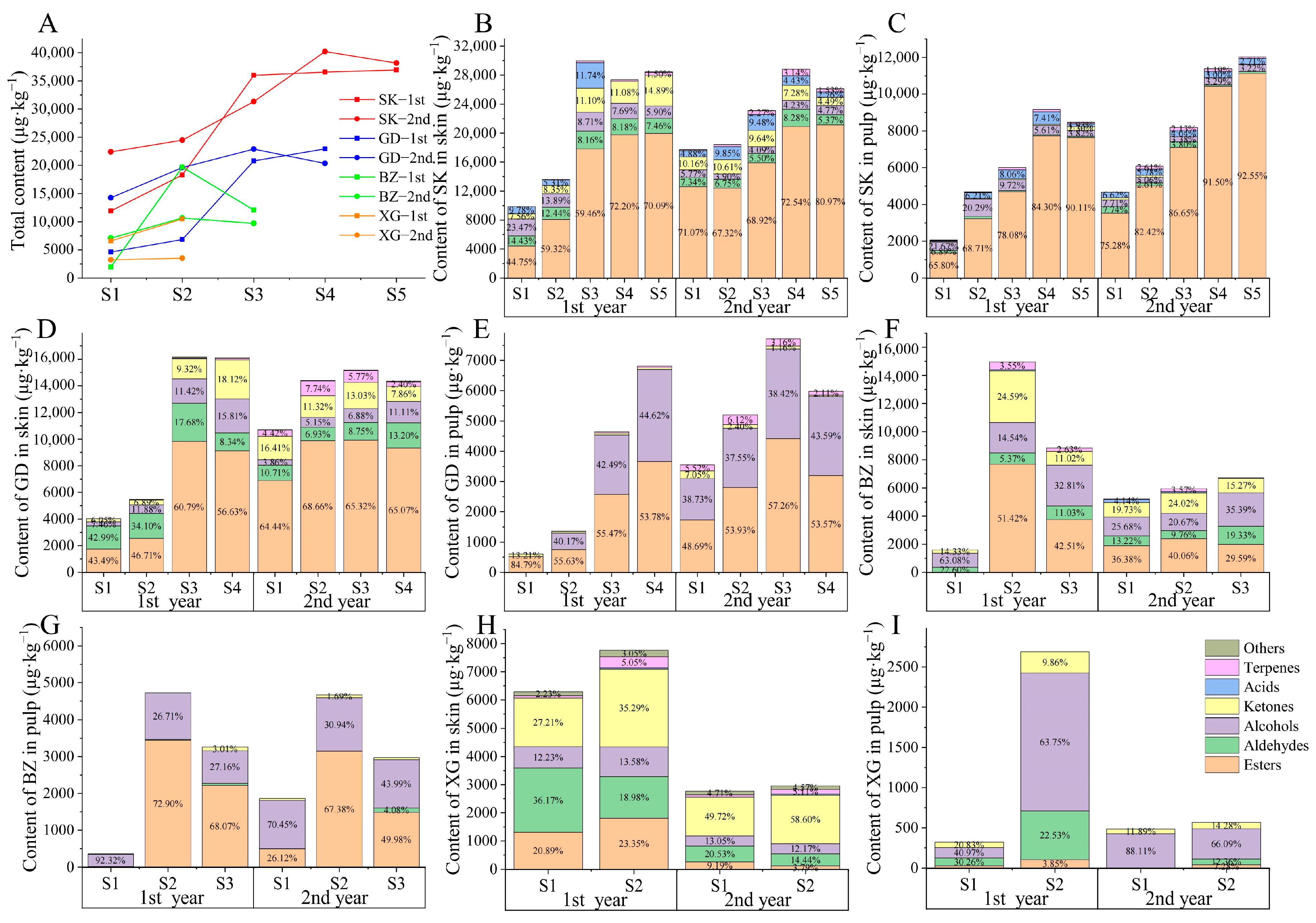
3.3.1. Changes in Total Aroma Content
3.3.2. Difference of Aroma Categories in Different Fruit Tissues
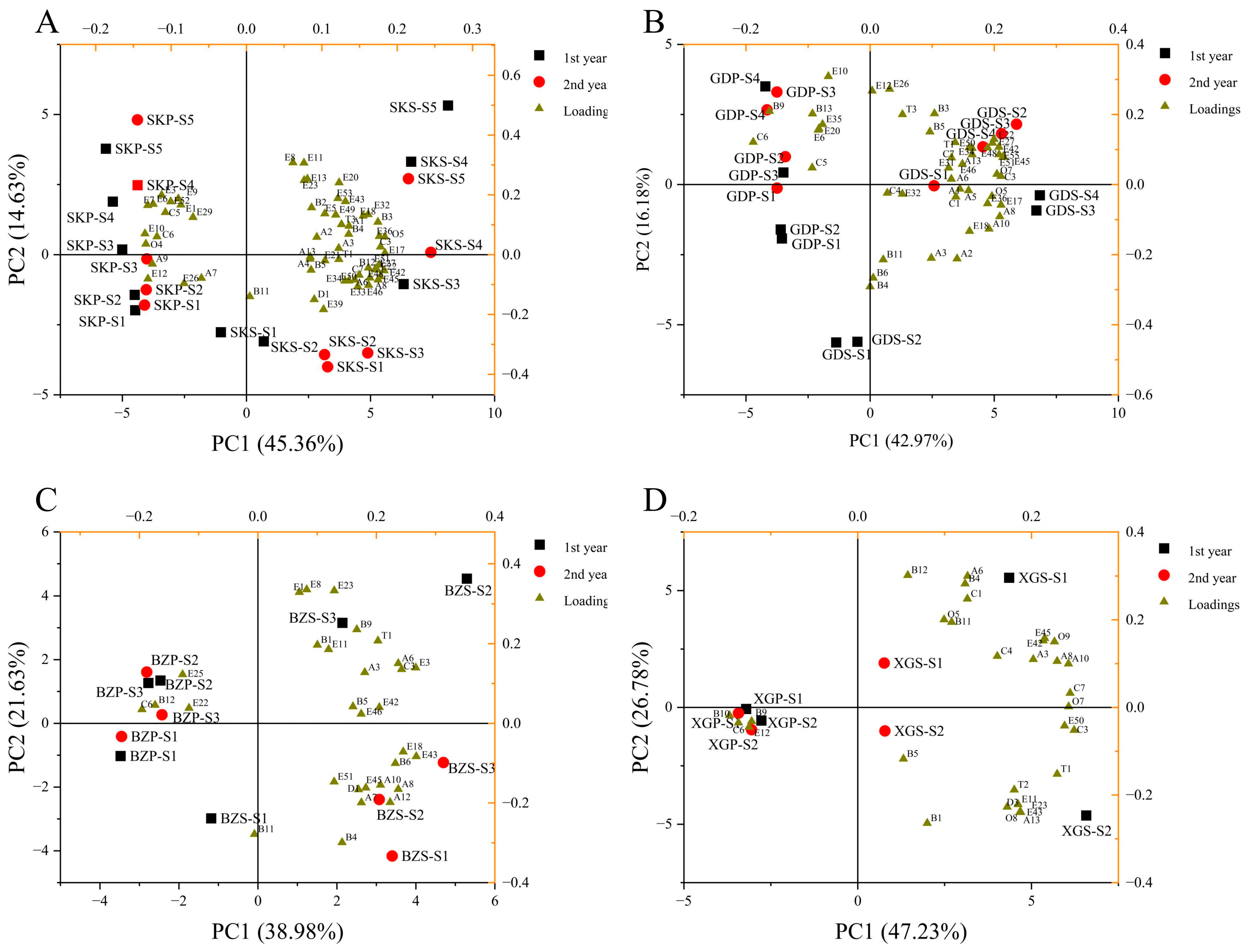
3.4. PCA of Four Apple Cultivars during Storage
3.5. OPLS-DA of Aroma Compounds during Storage
3.6. Identification of the Characteristic VOCs and Flavor Profiles
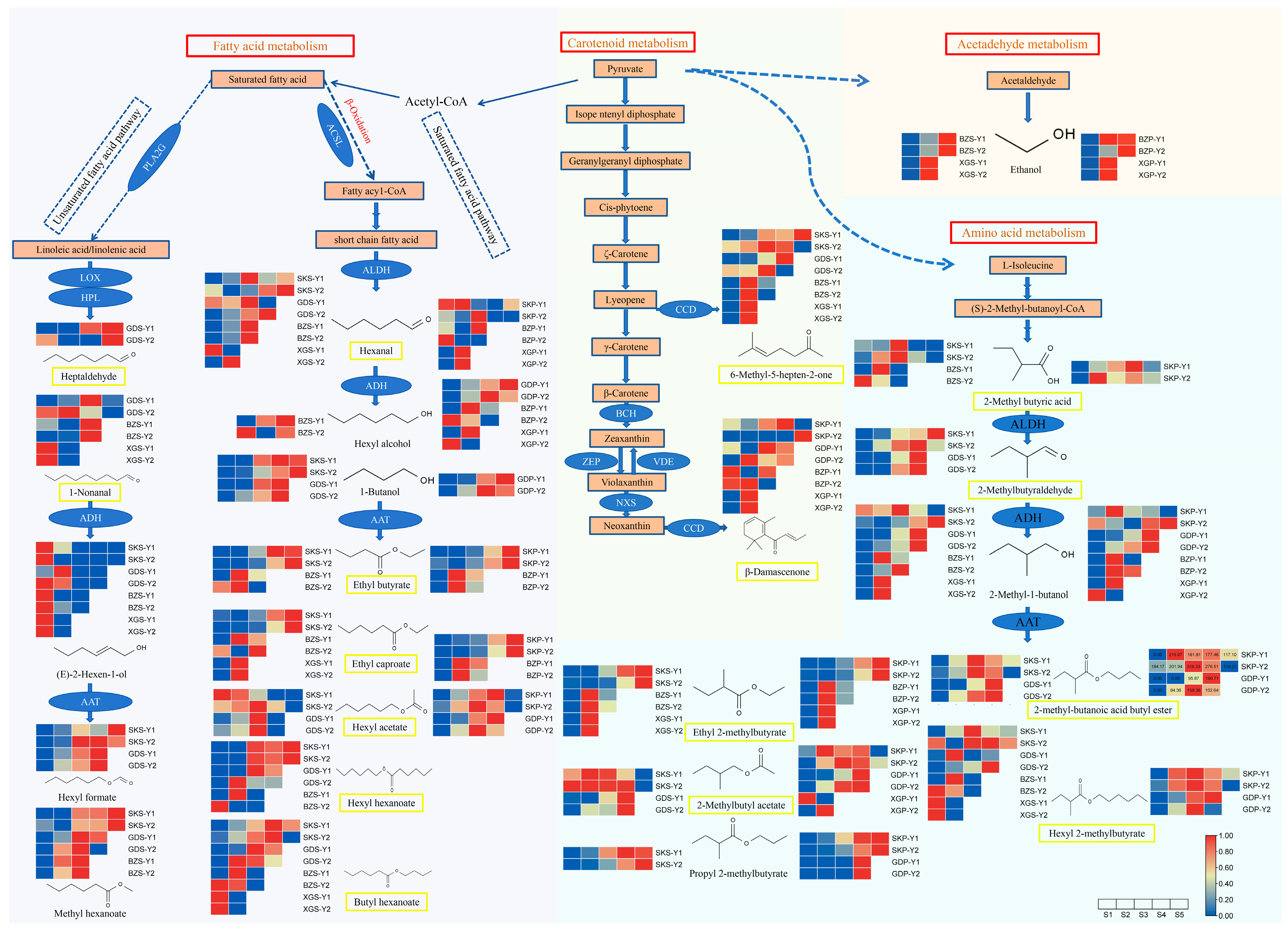
3.7. Differences in Metabolic Pathways of Main Aroma Compounds among Cultivars
4. Discussion
4.1. The VOCs of Apples
4.2. Dynamic Changes of VOCs during Storage Period
4.3. Characteristic VOCs and Flavor Profiles of Apples
4.4. Metabolism Pathway of VOCs in Apples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.X. Hebei Apple Records; China Agriculture Press: Beijing, China, 1985; pp. 121–200. [Google Scholar]
- Wang, D.J.; Wang, K.; Gao, Y.; Zhao, J.R.; Liu, L.J.; Gong, X.; Li, L.W. Preliminary investigation of modern distribution of Malus resources in China. J. Plant Genet. Resour. 2017, 18, 1116–1124. [Google Scholar] [CrossRef]
- Lu, Q.N.; Jia, D.X. The Fruit Tree Encyclopedia: Apple Volume; China Agricultural Science and Technology Press: Beijing, China; China Forestry Publishing House: Beijing, China, 1999; pp. 32–193. [Google Scholar]
- Chen, Y.Y.; Yin, H.; Wu, X.; Shi, X.J.; Qi, K.J.; Zhang, S.L. Comparative analysis of the volatile organic compounds in mature fruits of 12 Occidental pear (Pyrus communis L.) cultivars. Sci. Hortic. 2018, 240, 239–248. [Google Scholar] [CrossRef]
- Araguez, I.; Valpuesta, V. Metabolic engineering of aroma components in fruits. Biotechnol. J. 2013, 8, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Hewett, E. Factors affecting apple aroma/flavor volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Aprea, E.; Corollaro, M.L.; Betta, E.; Endrizzi, I.; Dematte, M.L.; Biasioli, F.; Gasperi, F. Sensory and instrumental profiling of 18 apple cultivars to investigate the relation between perceived quality and odour and flavour. Food Res. Int. 2012, 49, 677–686. [Google Scholar] [CrossRef]
- Espino-Diaz, M.; Sepulveda, D.R.; Gonzalez-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A review. Food Technol. Biotechnol. 2016, 54, 375–394. [Google Scholar] [CrossRef]
- Schiller, D.; Contreras, C.; Vogt, J.; Dunemann, F.; Defilippi, B.G.; Beaudry, R.; Schwab, W. A dual positional specific lipoxygenase functions in the generation of flavor compounds during climacteric ripening of apple. Hortic. Res. 2015, 2, 323–351. [Google Scholar] [CrossRef]
- Salas, N.A.; Gonzalez-Aguilar, G.A.; Jacobo-Cuellar, J.L.; Espino, M.; Sepulveda, D.; Guerrero, V.; Olivas, G.I. Volatile compounds in golden delicious apple fruit (Malus domestica) during cold storage. Rev. Fitotec. Mex. 2016, 39, 159–173. [Google Scholar] [CrossRef]
- Yang, S.B.; Hao, N.N.; Meng, Z.P.; Li, Y.J.; Zhao, Z.Y. Identification, comparison and classification of volatile compounds in peels of 40 apple cultivars by HS-SPME with GC-MS. Foods 2021, 10, 1051. [Google Scholar] [CrossRef]
- Yang, S.B.; Meng, Z.P.; Fan, J.; Yan, L.Y.; Yang, Y.Z.; Zhao, Z.Y. Evaluation of the volatile profiles in pulp of 85 apple cultivars (Malus domestica) by HS-SPME combined with GC-MS. J. Food Meas. Charact. 2021, 15, 4215–4225. [Google Scholar] [CrossRef]
- Villatoro, C.; Altisent, R.; Echeverria, G.; Graell, J.; Lopez, M.L.; Lara, I. Changes in biosynthesis of aroma volatile compounds during on-tree maturation of ‘Pink Lady’ apples. Postharvest Biol. Technol. 2008, 47, 286–295. [Google Scholar] [CrossRef]
- Yan, D.; Shi, J.R.; Ren, X.L.; Tao, Y.S.; Ma, F.W.; Li, R.; Liu, X.R.; Liu, C.H. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Li, D.M.; Li, Y.J.; Li, S.S.; Zhao, Z.Y. Brassinosteroids are involved in volatile compounds biosynthesis related to MdBZR1 in ‘Ruixue’ (Malus × domestica Borkh.) fruit. Postharvest Biol. Technol. 2022, 189, 111931. [Google Scholar] [CrossRef]
- Soomro, T.; Jordan, M.; Watts, S.; Migicovsky, Z.; Forney, C.F.; Song, J.; Myles, S. Genomic insights into apple aroma diversity. Fruit Res. 2023, 3, 27. [Google Scholar] [CrossRef]
- Dong, Z.D.; Song, S.W.; Song, C.H.; Zheng, X.B.; Jiao, L.; Wang, M.M.; Yan, Z.L.; Zhang, R.P.; Bai, T.H. Pedigree analysis and breeding inspiration of apple cultivars in China. Sci. Agric. Sin. 2020, 53, 4485–4496. [Google Scholar] [CrossRef]
- Lu, X.; Gao, Y.; Wang, K.; Sun, S.M.; Li, L.W.; Li, H.F.; Li, Q.S.; Feng, J.R.; Wang, D.J. Analysis of aroma characteristics in different cultivated apple strains. Sci. Agric. Sin. 2022, 55, 543–557. [Google Scholar] [CrossRef]
- Moreno-Peris, E.; Cortés-Olmos, C.; Díez-Díaz, M.; González-Mas, C.; de Luis-Margarit, A.; Fota, A.; Rodríguez-Burruezo, A. Hybridization in peppers (Capsicum spp.) to improve the volatile composition in fully ripe fruits: The effects of parent combinations and fruit tissues. Agronomy 2020, 10, 751. [Google Scholar] [CrossRef]
- Song, J.; Amyotte, B.; Yu, C.H.J.; Campbell-Palmer, L.; Vinqvist-Tymchuk, M.; Rupasinghe, H.P.V. Untargeted metabolomics analysis reveals the biochemical variations of polyphenols in a diverse apple population. Fruit Res. 2023, 3, 29. [Google Scholar] [CrossRef]
- Gao, G.W.; Liu, M.Y.; Kuang, L.X.; Li, J.; Li, H.F.; Xu, G.F. Gas chromatography-tandem mass spectrometry determination and risk assessment of 21 pesticides in jujubes. China Fruits 2023, 1, 95–99. [Google Scholar] [CrossRef]
- Reidel, R.V.B.; Melai, B.; Cioni, P.L.; Pistelli, L. Chemical composition of volatiles emitted by Ziziphus jujuba during different growth stages. Plant Biosyst. 2018, 152, 825–830. [Google Scholar] [CrossRef]
- Li, C.B.; Xin, M.; Li, L.; He, X.M.; Yi, P.; Tang, Y.Y.; Li, J.M.; Zheng, F.J.; Liu, G.M.; Sheng, J.F.; et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HSSPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gao, Y.; Wang, K.; Sun, S.; Liu, Z.; Yan, P.; Feng, J.R.; Li, Q.S.; Li, L.W.; Wang, D.J. Dwarf interstocks improve aroma quality of ‘Huahong’ apple (Malus × domestica). Agriculture 2022, 12, 1710. [Google Scholar] [CrossRef]
- Song, X.Y.; Dai, F.; Yao, J.R.; Li, Z.P.; Huang, Z.; Liu, H.J.; Zhu, Z.Y. Characterization of the volatile profile of feijoa (Acca sellowiana) fruit at different ripening stages by HS-SPME-GC/MS. LWT 2023, 184, 115011. [Google Scholar] [CrossRef]
- Xi, B.N.; Zhang, J.J.; Xu, X.; Li, C.; Shu, Y.; Zhang, Y.; Shi, X.M.; Shen, Y.H. Characterization and metabolism pathway of volatile compounds in walnut oil obtained from various ripening stages via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2024, 435, 137547. [Google Scholar] [CrossRef]
- Guo, X.Y.; Schwab, W.; Ho, C.T.; Song, C.K.; Wan, X.C. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chem. 2021, 376, 131933. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Echeverría, G.; Graell, J.; López, M.L.; Lara, I. Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biol. Technol. 2004, 31, 217–227. [Google Scholar] [CrossRef]
- Lester, G. Consumer preference quality attributes of melon fruit. Acta Hortic. 2006, 712, 175–181. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; Leeuwen, C.V.; Tominaga, T.; Soyer, J.P.; Gaudillere, J.P.; Denis, D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L cv Sauvignon blanc in field conditions. J. Sci. Food Agric. 2005, 85, 73–85. [Google Scholar] [CrossRef]
- Liu, X.J.; Hao, N.N.; Feng, R.F.; Meng, Z.P.; Li, Y.A.; Zhao, Z.Y. Transcriptome and metabolite profiling analyses provide insight into volatile compounds of the apple cultivar ‘Ruixue’ and its parents during fruit development. BMC Plant Biol. 2021, 21, 23. [Google Scholar] [CrossRef]
- Luo, M.L.; Zhou, X.; Sun, H.J.; Zhou, Q.; Ge, W.Y.; Sun, Y.Y.; Yao, M.M.; Ji, S.J. Insights into profiling of volatile ester and LOX-pathway related gene families accompanying post-harvest ripening of ‘Nanguo’ pears. Food Chem. 2021, 335, 127665. [Google Scholar] [CrossRef]
- Saquet, A.A.; Almeida, D. Sensory and instrumental assessments during ripening of ‘Rocha’ pear: The role of temperature and the inhibition of ethylene action on fruit quality. Technol. Hortic. 2023, 3, 23. [Google Scholar] [CrossRef]
- Contreras, C.H.; Tjellström, H.; Beaudry, R.M. Relationships between free and esterified fatty acids and LOX-derived volatiles during ripening in apple. Postharvest Biol. Technol. 2015, 112, 105–113. [Google Scholar] [CrossRef]
- Zhang, A.D.; Zhang, Q.Y.; Li, J.Z.; Gong, H.S.; Fan, X.G.; Yang, Y.Q.; Liu, X.F.; Yin, X.R. Transcriptome co-expression network analysis identifies key genes and regulators of ripening kiwifruit ester biosynthesis. BMC Plant Biol. 2020, 20, 103. [Google Scholar] [CrossRef]
- Li, Z.Q.; Chen, C.Y.; Zou, D.F.; Li, J.W.; Huang, Y.Y.; Zheng, X.B.; Tan, B.; Cheng, J.; Wang, W.; Zhang, L.L.; et al. Ethylene accelerates grape ripening via increasing VvERF75-induced ethylene synthesis and chlorophyll degradation. Fruit Res. 2023, 3, 3. [Google Scholar] [CrossRef]
- Wang, Q.H.; Gao, F.; Chen, X.X.; Wu, W.J.; Wang, L.; Shi, J.L.; Huang, Y.; Shen, Y.Y.; Wu, G.L.; Guo, J.X. Characterization of key aroma compounds and regulation mechanism of aroma formation in local Binzi (Malus pumila × Malus asiatica) fruit. BMC Plant Biol. 2022, 22, 532. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Rega, P.; Migliozzi, T.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci. Hortic. 2013, 162, 341–350. [Google Scholar] [CrossRef]
- Cozzolino, R.; Giulio, B.D.; Petriccione, M.; Martignetti, A.; Pellicano, M.P. Comparative analysis of volatile metabolites, quality and sensory attributes of Actinidia chinensis fruit. Food Chem. 2020, 316, 126340. [Google Scholar] [CrossRef]
- Liu, H.C.; Yu, Y.S.; Zhou, H.L.; Zou, B.; Yu, Y.Y.; Yang, J.G.; Xu, Y.J.; Chen, X.W.; Yang, F. Evaluation of dynamic changes and regularity of volatile flavor compounds for different green plum (Prunus mume Sieb. et Zucc) varieties during the ripening process by HS-GCIMS with PLS-DA. Foods 2023, 12, 551. [Google Scholar] [CrossRef]
- Schieberle, P. Odour-active compounds in moderately roasted sesame. Food Chem. 1996, 55, 145–152. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.X.; Chen, Y.L.; Xiao, L.K.; Zhang, X.L.; Yang, C.H.W.; Li, Z.J.; Zhu, M.Z.; Liu, Z.H.; Wang, Y.L. Discrimination and characterization of the volatile profiles of five Fu brick teas from different manufacturing regions by using HS-SPME/GC-MS and HS-GC-IMS. Curr. Res. Food Sci. 2022, 5, 1788–1807. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.Y.; Qin, Y.Y.; Jiang, L.W.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Wyllie, S.G.; Leach, D.N.; Nonhebel, H.N.; Lusunzi, I. Biochemical pathways for the formation of esters in ripening fruit. In Flavor Science; Woodhead Publishing: Cambridge, UK, 1996. [Google Scholar]
- Hadi, M.A.M.E.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Knee, M.; Hatfield, S. The metabolism of alcohols by apple fruit tissue. J. Sci. Food Agric. 1981, 32, 593–600. [Google Scholar] [CrossRef]
- Paillard, N.M.M. Biosynthesis of apple volatiles—Alcohol and ester formations beginning with fatty-acids. Phytochemistry 1979, 18, 1165–1171. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K.S. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- Olias, J.M.; Sanz, C.; Rios, J.J.; Pérez, A.G. Substrate specificity of alcohol acyltransferase from strawberry and banana fruits. Fruit Flavors 1995, 596, 134–141. [Google Scholar]
- Peng, B.; Hao, X.S.; Zhang, Q.; Cai, W.C.; Zhao, X.X.; Tang, F.X.; Shan, C.H. Effects of low-temperature storage on the aroma composition and quality of Golden Empress Hami melons: A transcriptomic and metabolomic analysis. Food Biosci. 2024, 57, 103422. [Google Scholar] [CrossRef]
- Goepfert, S.; Poirier, Y. β-oxidation in fatty acid degradation and beyond. Curr. Opin. Plant Biol. 2007, 10, 245–251. [Google Scholar] [CrossRef]
- Lu, X.G.; Meng, G.L.; Jin, W.G.; Gao, H. Effects of 1-MCP in combination with Ca application on aroma volatiles production and softening of ‘Fuji’ apple fruit. Sci. Hortic. 2018, 229, 91–98. [Google Scholar] [CrossRef]
- Winterhalter, P.; Rouseff, R.L. Carotenoid-derived aroma compounds: An introduction. In Carotenoid-Derived Aroma Compounds; Winterhalter, P., Rouseff, R.L., Eds.; American Chemical Society: Washington, DC, USA, 2002; pp. 1–17. [Google Scholar]
- Jia, L.T.; Wang, L.; Xia, Q.; Luo, W.Q.; Baldwin, E.A.; Zhang, X.; Jiang, L.; Li, J.; Zhao, Y.D.; Qiao, X.; et al. Expression patterns of volatile compounds during ‘FL 47’ tomato ripening and their response to exogenous methyl salicylate (MeSA) fumigation. Postharvest Biol. Technol. 2023, 203, 112414. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Which impact for beta-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]

| Compound | Aroma Descripition | Odor Threshold (µg/kg) | Fruit Harvest Day (S1) | Key Aroma Transition Period | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SK | GD | BZ | XG | SK | GD | BZ | XG | |||
| Methyl 2-methylbutyrate | Apple, Fruit, Green Apple, Strawberry | 0.25 | 1388.35 | – | – | – | 1679.36 | – | – | – |
| Ethyl butyrate | Pineapple, fruity, apple | 1 | 36.09 | – | 0.00 | – | 378.47 | – | 3439.60 | – |
| Ethyl 2-methylbutyrate | Apple, Ester, Green Apple, Kiwi, Strawberry | 0.1 | 332.13 | – | 0.00 | 608.27 | 9268.73 | – | 15,469.63 | 8740.38 |
| 2-Methylbutyl acetate | Apple, Banana, Pear | 5 | 154.18 | 17.36 | – | 0.00 | 204.46 | 74.90 | – | 0.00 |
| Ethyl caproate | Apple peel, fruit | 1 | 153.26 | – | 0.00 | 0.00 | 249.09 | – | 1167.97 | 83.08 |
| Hexyl acetate | Apple, Banana, Grass | 2 | 227.07 | 164.92 | – | – | 92.73 | 532.83 | – | – |
| Hexyl 2-methylbutyrate | Fruity, green, apple | 6 | 103.91 | 12.97 | 0.00 | 74.47 | 527.09 | 183.89 | 19.27 | 41.42 |
| Hexanal | Grass, green, leaves, vinous | 4 | 282.97 | 320.70 | 23.32 | 392.64 | 402.54 | 407.84 | 42.80 | 257.22 |
| 1-Nonanal | Aldehyde, citrus, fatty, floral, green | 1 | – | 166.74 | 26.49 | 151.52 | – | 335.04 | 0.00 | 116.24 |
| 2-Methyl butyric acid | Pungent, cheese, fruity | 5.8 | 166.19 | – | 0.00 | – | 606.44 | – | 13.58 | – |
| Compound | Aroma Descripition | Odor Threshold (µg/kg) | Fruit Harvest Day (S1) | Key Aroma Transition Period | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SK | GD | BZ | XG | SK | GD | BZ | XG | |||
| Ethyl butyrate | Pineapple, fruity, apple | 1 | 38.55 | – | 0.00 | – | 112.99 | – | 1993.95 | – |
| Ethyl 2-methylbutyrate | Apple, Ester, Green Apple, Kiwi, Strawberry | 0.1 | 171.95 | – | 0.00 | 151.44 | 2717.36 | – | 9328.17 | 1036.50 |
| 2-Methylbutyl acetate | Apple, Banana, Pear | 5 | 148.21 | 18.16 | – | 2.06 | 247.94 | 83.06 | – | 0.00 |
| Ethyl caproate | Apple peel, fruit | 1 | 0.00 | – | 0.00 | – | 78.16 | – | 325.01 | – |
| Hexyl acetate | Apple, Banana, Grass | 2 | 85.07 | 96.45 | – | – | 317.65 | 417.48 | – | – |
| Hexanal | Grass, green, leaves, vinous | 4 | 10.64 | – | 3.87 | 12.05 | 4.54 | – | 0.00 | 136.53 |
| β-Damascenone | Apple, rose, honey | 0.05 | 691.43 | 795.87 | 241.95 | 775.81 | 769.58 | 921.84 | 0.00 | 1574.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Liu, Z.; Gao, Y.; Wang, K.; Sun, S.; Guo, H.; Tian, W.; Wang, L.; Li, Z.; Li, L.; et al. Analysis of Aroma Characteristics of ‘Binzi’ and ‘Xiangguo’ Apple—Ancient Cultivars in China. Foods 2024, 13, 2869. https://doi.org/10.3390/foods13182869
Lu X, Liu Z, Gao Y, Wang K, Sun S, Guo H, Tian W, Wang L, Li Z, Li L, et al. Analysis of Aroma Characteristics of ‘Binzi’ and ‘Xiangguo’ Apple—Ancient Cultivars in China. Foods. 2024; 13(18):2869. https://doi.org/10.3390/foods13182869
Chicago/Turabian StyleLu, Xiang, Zhao Liu, Yuan Gao, Kun Wang, Simiao Sun, Hanxin Guo, Wen Tian, Lin Wang, Zichen Li, Lianwen Li, and et al. 2024. "Analysis of Aroma Characteristics of ‘Binzi’ and ‘Xiangguo’ Apple—Ancient Cultivars in China" Foods 13, no. 18: 2869. https://doi.org/10.3390/foods13182869
APA StyleLu, X., Liu, Z., Gao, Y., Wang, K., Sun, S., Guo, H., Tian, W., Wang, L., Li, Z., Li, L., Feng, J., & Wang, D. (2024). Analysis of Aroma Characteristics of ‘Binzi’ and ‘Xiangguo’ Apple—Ancient Cultivars in China. Foods, 13(18), 2869. https://doi.org/10.3390/foods13182869







