Development of Edible Coatings Based on Pineapple Peel (Ananas Comosus L.) and Yam Starch (Dioscorea alata) for Application in Acerola (Malpighia emarginata DC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Yam Starch Extraction (YS)
2.2.2. Preparation of Pearl Pineapple Peel Flour (PPPF)
2.2.3. Characterization of PPPF
Physical Analysis and Technological Properties of PPPF
- Water Absorption Index (WAI)
- Water Solubility Index (WSI)
- Swelling volume (SV)
Physical–Chemical Analysis of PPPF
Determination of Total Phenolic Compound Content
Determination of Antioxidant Activity
2.2.4. Preparation of Edible Coating
2.2.5. Characterization of Edible Coating
Thickness
Moisture
Color and Opacity
Water Solubility
Mechanical Properties
Determination of Total Phenolic Compound Content and Antioxidant Activity
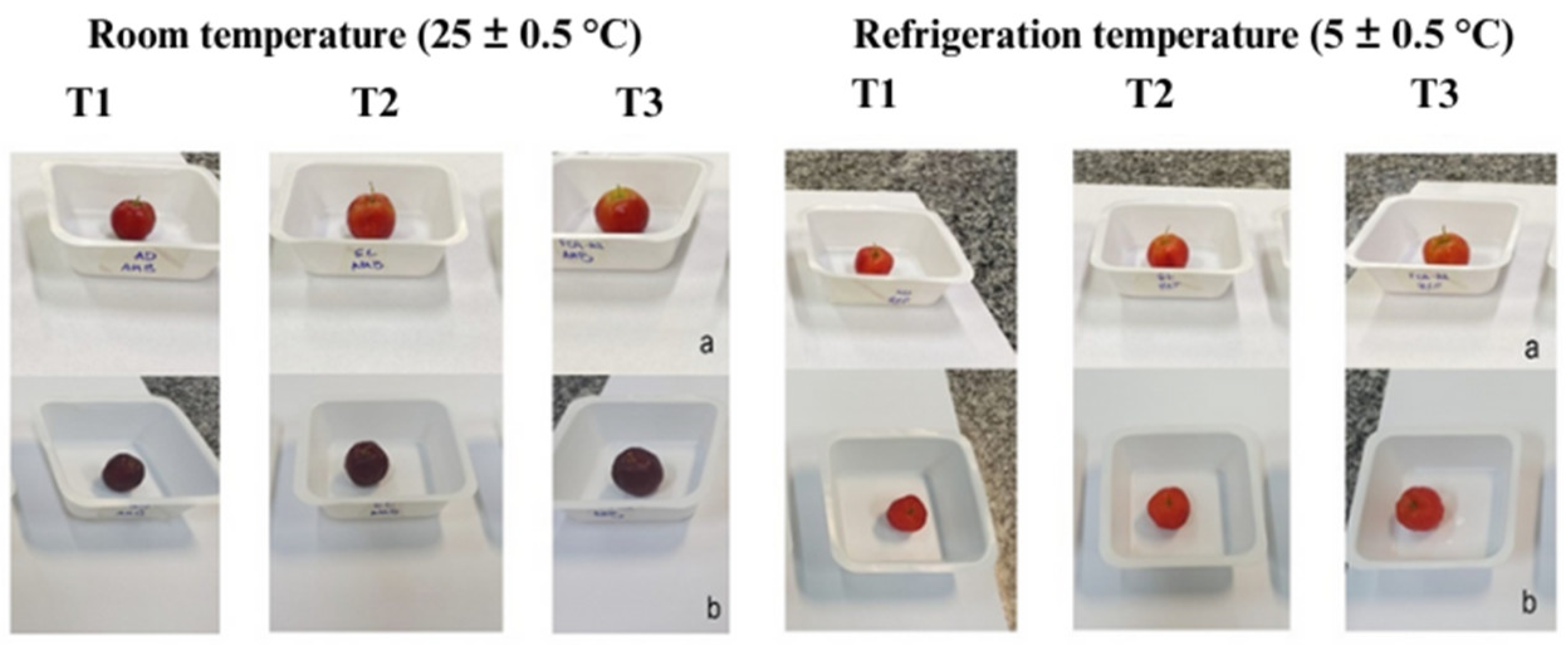
2.2.6. Evaluation of Quality Parameters of Coated Acerola Fruits during Storage at Room and Refrigerated Temperatures
Microbiological Analysis of Coated Acerolas
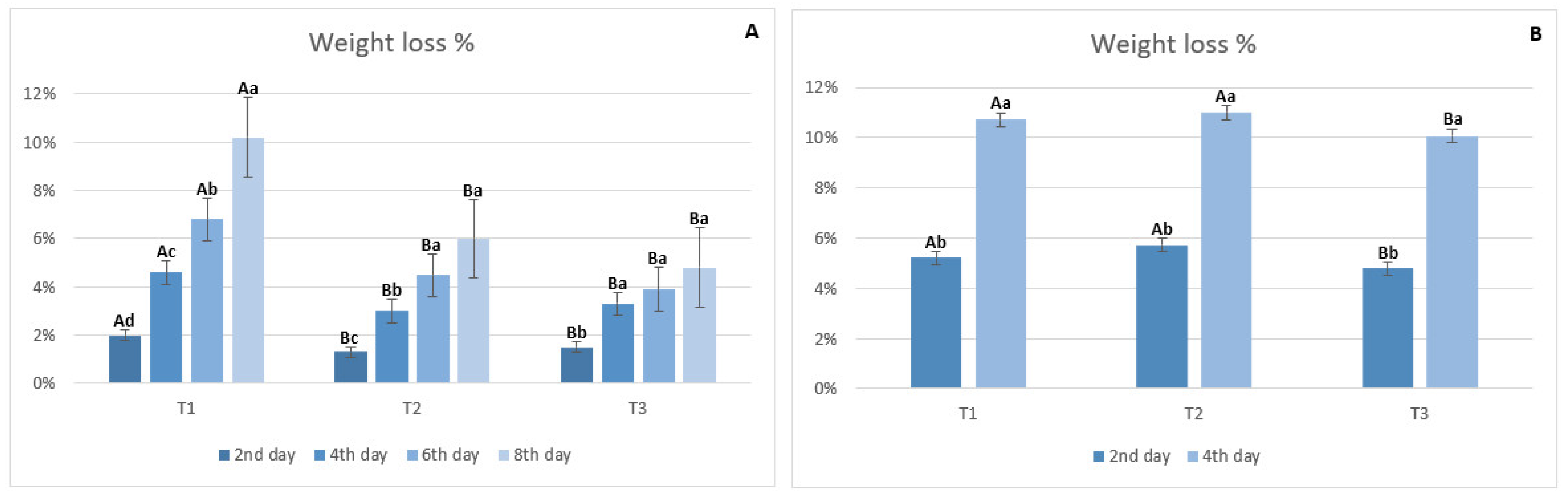
Weight Loss
Instrumental Color
Total Soluble Solids
pH
Total Titratable Acidity in Molar Acid
2.3. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Pearl Pineapple Peel Flour (PPPF)
3.1.1. Physical and Technological Properties of PPPF
3.1.2. Physical and Physicochemical Characterization of PPPF
3.1.3. Dosage of Total Phenolic Compounds
3.1.4. Antioxidant Activity of PPPF
3.2. Characterization of PPPF-YS Coating
3.2.1. Thickness
3.2.2. Moisture Content
3.2.3. Colorimetric Properties and Opacity
3.2.4. Water Solubility
3.2.5. Mechanical Properties
3.2.6. Total Phenolic Compound Content and Antioxidant Activity
3.3. Effect of Coating on Microbiological and Physicochemical Parameters of Acerola Stored at Room and Refrigerated Temperatures
3.3.1. Microbiological Evaluation
3.3.2. Weight Loss
3.3.3. Instrumental Color
3.3.4. Analysis of Total Soluble Solids, pH, and Total Titratable Acidity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros, R.; Nascimento, D.; Araújo, D.; Batista, F.; Lima, A.; Cruz-Filho, I.; Oliveira, M.; Melo, C. Phytochemical analysis, nutritional profile and immunostimulatory activity of aqueous extract from Malpighia emarginata DC leaves. Biocatal. Agric. Biotechnol. 2020, 23, 101442. [Google Scholar]
- Ferreira, I.; Silva, V.; Vilvert, J.; Souza, F.; Freitas, S.; Lima, M. Brazilian varieties of acerola (Malpighia emarginata DC) produced under tropical semi-arid conditions: Bioactive phenolic compounds, sugars, organic acids, and antioxidant capacity. J. Food Biochem. 2021, 45, 13829. [Google Scholar]
- Silva, P.; Nogueira, G.; Duarte, C.; Barrozo, M. A New Rotary Dryer Assisted by Infrared Radiation for Drying of Acerola Residues. Waste Biomass Valorization 2021, 12, 3395–3406. [Google Scholar] [CrossRef]
- Santos, N.S.; Silva, J.C.S.; Araújo, C.A.; Lima, K.F.; Silva, F.G.A. Caracterização da conservação refrigerada da acerola (Malpighia emarginata) sob atmosfera modificada. Divers. J. 2020, 5, 12–19. [Google Scholar] [CrossRef]
- Souza, J.; Santana, E.; Silva, A.; Souza, A. Avaliação físico-química de acerola, Malpighia emarginata d.c., proveniente de macapá-amapá. J. Biol. Pharm. Agric. Manag. 2020, 16, 2. [Google Scholar]
- Inversen, L.; Rovina, K.; Vonnie, J.; Matanjun, P.; Erna, K.; Aqila, N.; Felícia, W.; Funk, A. The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review. Molecules 2020, 27, 5604. [Google Scholar] [CrossRef]
- Pinto, A.; Santos, K.; Santos, L.; Lubenow, L.; Coutinho, W.; Pereira, W.; Siqueira, A. Qualidade pós-colheita de frutos de figo submetidos ao efeito de coberturas comestíveis. Sci. Electron. Arch. 2020, 13, 1. [Google Scholar]
- Sousa, R.; Novais, T.; Batista, F.; Zuñiga, A. Análise sensorial de cookie desenvolvidos com farinha da casca de abacaxi (Ananas comosus (L.) Merril). Res. Soc. Dev. 2020, 9, 45942816. [Google Scholar] [CrossRef]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O. Characterization of starch–water interactions and their effects on two key functional properties: Starch gelatinization and retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Egharevba, H. Chemical Properties of Starch and Its Application in the Food Industry. In: Chemical properties of starch. IntechOpen 2019, 32, 137–144. [Google Scholar] [CrossRef]
- Andrade, L.; Barbosa, N.; Pereira, J. Rendimento e características dos amidos de inhame e de taro. In Avanços em Ciência e Tecnologia de Alimentos, 1st ed.; Oliveira, R.J., Ed.; Editora Científica Digital: São Paulo, Brasil, 2021; Volume 3, pp. 236–243. [Google Scholar]
- Menezes, A.; Santos, S.; Neto, J.; Noronha, M.; Costa, J. Qualidade pós-colheita de diferentes acessos de inhames coletados nos estados de Alagoas e Sergipe. Nativa 2022, 10, 170–176. [Google Scholar] [CrossRef]
- Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of Edible Coating Enriched with Natural Antioxidant Extract and Bergamot Essential Oil on the Shelf Life of Strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, M.; Di Stasio, M.; Sorrentino, A.; La Cara, F.; Volpe, M. Active Edible Polysaccharide-Based Coating for Preservation of Fresh Figs (Ficus carica L.). Foods 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.; Soares, B.; Diniz, K.; Leal, C.; Canto, D.; Flores, M.; Tavares-Filho, J.; Galembeck, A.; Stamford, T.; Stamford-Arnauld, T.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Dias, P.; Sajiwanie, J.; Rathnayaka, R. Chemical Composition, Physicochemical and Technological Properties of Selected Fruit Peels as a Potential Food Source. Int. J. Fruit Sci. 2020, 20, 240–251. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G. Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Y.; Wong, C.; Cheng, K.; Chen, F. Evaluation of two methods for the extraction of antioxidants from medicinal plants. Anal. Bioanal. Chem. Res. 2007, 388, 483–488. [Google Scholar] [CrossRef]
- Silva, F.; Queiroga, R.; Souza, E.; Voss, G.; Borges, G.; Lima, M.; Pintado, M.; Vasconcelos, M. Incorporation of phenolic-rich ingredients from integral valorization of Isabel grape improves the nutritional, functional and sensory characteristics of probiotic goat milk yogurt. Food Chem. 2022, 369, 130957. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying animproved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019; p. 3172. [Google Scholar]
- Tafa, K.; Satheesh, N.; Abera, W. Mechanical properties of tef starch based edible films: Development and process optimization. Heliyon 2023, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Instrução Normativa n° 161, de 01 de Julho de 2022. Estabelece os Padrões Microbiológicos dos Alimentos; ANVISA—Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2022.
- Gol, N.; Patel, P.; Rao, R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 4185–4195. [Google Scholar] [CrossRef]
- SIGMASTAT (Programa de Computador). Versão 3.5. Point Richmond (Califórnia): Comercial; 2006. SIGMASTAT. Version 3.5. Systat Software, Inc.: Point Richmond, CA, USA, 2006. Available online: https://www.systatsoftware.com (accessed on 8 September 2024).
- Rodrigues, E.; Silva, F.; Barros, C.; Almeida, J.; Barros, N.; Nobre, E. Utilização de revestimentos comestíveis de óleo de girassol, pectina natural, gelatina incolor e fécula de mandioca na conservação pós colheita de acerola e goiaba. Braz. J. Dev. 2022, 8, 27542–27557. [Google Scholar] [CrossRef]
- Marcon, L.S. Elaboração e caracterização da farinha de cascas de poncã. In Trabalho de Conclusão de Curso (Tecnólogo em Alimentos); Universidade Tecnológica Federal do Paraná: Londrina, Brazil, 2021. [Google Scholar]
- Moraes, R.; Melo, K.; Oliveira, T.; Teles, J.; Peluzio, J.; Martins, G. Caracterização Química, física e tecnológica da farinha obtida a partir da casca de Buriti (Mauritia flexuosa L. f.). Braz. J. Dev. 2019, 5, 23307–23322. [Google Scholar] [CrossRef]
- Aquino, A. Farinha de Resíduos de Physalis Peruviana: Propriedades Tecnológicas e Sua Aplicação em Muffins; 6° Simpósio de Segurança Alimentar: Gramado, Brazil, 2018. [Google Scholar]
- Nolasco, M.V. Desenvolvimento e avaliação de filmes a partir de resíduo integral de cascas de batata (Solanum tuberosum L.). In Dissertação (Mestrado em Engenharia de Alimentos); Universidade Estadual de Campinas: Campinas, Brazil, 2023. [Google Scholar]
- Ferreira, S.; Mattos, E.; Almerindo, G.; Alves, F.; Bo, A. Obtenção e caracterização da farinha de bagaço de malte. In Anais do Congresso On-Line Brasileiro de Tecnologia de Cereais e Panificação. “Saudabilidade na Indústria de Cereais e Panificação”; Evento online; Universidade Federal de São João del-Rei: São João del-Rei, Brazil, 2020. [Google Scholar]
- Pereira, C.M. Caracterização físico-química e avaliação da inibição micelial da farinha de bacuri (Platonia insignis, Mart.). In Dissertação (Mestrado em Ciência e Tecnologia de Alimentos); Universidade Federal do Tocantins (UFT): Palmas, TO, Brazil, 2023. [Google Scholar]
- Robertson, J.; Monredon, F.; Dysseler, P.; Guillon, F.; Amado, R.; Hibault, J. Hydration properties of dietary fibre and resistant starch: A European collaborative study. Lebensm.-Wiss. Und Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Andrade-Neto, G. Produção e caracterização de filmes poliméricos para revestimento comestível em goiabas. In Trabalho de Conclusão de Curso (Bacharelado em Engenharia Química); Universidade Federal da Paraíba (UFPB): João Pessoa, Brazil, 2017. [Google Scholar]
- Silva, R.; Silva, S.; Wanderley, R.; Paiva, A.; Medeiros, A. Chemical and colorimetric characterization of orange peel, melon and pineapple flours. Res. Soc. Dev. 2020, 9, 7. [Google Scholar]
- Costa, M.J. Desenvolvimento de Mistura Para Bolo Com Adição de Farinha da Casca do Abacaxi (Ananas comuns L. Merril) E Farinha de Banana Verde (Musa spp.); Dissertação (Mestrado em Ciência e Tecnologia de Alimentos); Instituto Federal Goiano (IFGO): Rio Verde, Brazil, 2020. [Google Scholar]
- Nunes, J.; Lins, A.; Gomes, J.; Silva, W.; Silva, F. Influência da temperatura de secagem nas propriedades físico-química de resíduos abacaxi. Agropecuária Técnica 2017, 38, 41–46. [Google Scholar] [CrossRef]
- Silva, R.; Noma, C.; Santos, S.; Bellini, J.; Silva, V.; Silva, C.; Brum, F. Adição de valor ao resíduo de abacaxi: Caracterização e avaliação do potencial para meio de cultura de bactérias e leveduras. Concilium 2023, 23, 16. [Google Scholar]
- Oliveira, V.; Oliveira, I.; Mendes, F. Análises físico-químicas e composição nutricional da farinha de casca de abacaxi como aproveitamento de resíduos agroindustriais. In Ciência e Tecnologia de Alimentos: Pesquisa e Práticas Contemporâneas, 1st ed.; Oliveira, R.J., Ed.; Editora Científica Digital: São Paulo, Brasil, 2021; Volume 2, pp. 70–81. [Google Scholar]
- BRASIL. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. In Resolução n° 263, de 22 de Setembro de 2005. Regulamento Técnico para Produtos de Cereais, Amidos, Farinhas e Farelos; Diário Oficial da União: Brasília, Brazil, 2005. [Google Scholar]
- Fortes, R.; Brigagão, T.; Lourenço, C.; Carvalho, E.; Tavano, O.; Garcia, J. Caracterização física e química de farinha de arroz, farinhas de cascas de abacaxi e banana e farinha de sementes de abóbora. Res. Soc. Dev. 2020, 9, 436997293. [Google Scholar] [CrossRef]
- Duarte, S.; Almeida, F.; Valerio, G.; Dorini, L.; Gomes, V.; Costa, S.; Uliana, M. Biscoito Tipo Cookie com Adição de Farinha de Resíduos de Frutas; V Mostra de trabalhos de Engenharia Química: Santo Ângelo, Brazil, 2022. [Google Scholar]
- Souza, M.; Gomes, M.; Candeias, V.; Albuquerque-Júnior, N.; Januário, E.; Lima, D.; Vilar, S. Determination of the antioxidant capacity of pineapple peel powder extract by applying different extraction techniques. Res. Soc. Dev. 2021, 10, 10. [Google Scholar]
- Tavares, T.R.P.; Salomão, B.C.M. Avaliação da atividade antifúngica de extratos de resíduo de acerola (Malpighia emarginata) in natura e liofilizado. In Tecnologia de Alimentos: Tópicos Físicos, Químicos e Biológicos, 1st ed.; Oliveira, R.J., Ed.; Editora Científica Digital: São Paulo, Brasil, 2020; Volume 1, pp. 218–231. [Google Scholar]
- Magalhães, M.; Gandra, K.; Cunha, L.; Lima, E. Obtenção da farinha do resíduo do processamento de acerola e avaliação de compostos bioativos e nutritivos. Res. Soc. Dev. 2021, 10, 14. [Google Scholar] [CrossRef]
- Arquelau, P.B.F. Desenvolvimento e Caracterização de Revestimentos Comestíveis a Partir de Farinha de Casca de Banana (Musa spp.) Prata (AAB); Dissertação (Mestrado em Nutrição); Universidade Federal de Minas Gerais: Belo Horizonte, Brazil, 2018. [Google Scholar]
- Silva, A.O. Desenvolvimento e Caracterização de Filmes Biopoliméricos à Base de Farinha de Bocaiuva (Acrocomia Aculeada) ou Polpa de pequi (Caryocar brasiliense); Tese (Doutorado em Biotecnologia e Biodiversidade); Universidade Federal da Grande Dourados: Dourados, Brazil, 2020. [Google Scholar]
- Mihai, M.; Legros, N.; Alemdar, A. Formulation-properties versatility of wood fiber biocomposites based on polylactide and polylactide/thermoplastic starch blends. Polym. Eng. Sci. 2014, 54, 1325–1340. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Souza, T.; Costa, M.; Nassur, R. Formulação e aplicação de revestimentos à base de farinha de casca de banana na conservação pós-colheita dos frutos. Res. Soc. Dev. 2021, 10, 391101018953. [Google Scholar] [CrossRef]
- González, A.; Barrera, G.; Galimberti, P.; Ribotta, P.; Igarzabal, C. Development of edible films prepared by soy protein and the galactomannan fraction extracted from Gleditsia triacanthos (Fabaceae) seed. Food Hydrocoll. 2019, 97, 105227. [Google Scholar] [CrossRef]
- Rodrigues, F.; Santos, S.; Lopes, M.; Guimarães, D.; Silva, E.; Souza-Filho, M.; Mattos, A.; Silva, L.; Azeredo, H.; Ricardo, N. Filmes antioxidantes e revestimentos à base de amido e fenólicos de Spondias purpurea L. Int. J. Biol. Macromol. 2021, 182, 354–365. [Google Scholar] [CrossRef]
- Narasgoudr, S.; Hegde, V.; Vanjeri, V.; Chougale, R.; Masti, S. Ethyl Vanillin Incorporated Chitosan/Poly(vinyl alcohol) Active Films for Food Packaging Applications. Carbohydr. Polym. 2020, 236, 116049. [Google Scholar] [CrossRef]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef] [PubMed]
- Iahnke, A.O.S. Filmes Biodegradáveis com Propriedades Funcionais Produzidos a Partir de Resíduos Industriais. Master’s Thesis, Instituto de Ciências e Tecnologia de Alimentos, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2015. [Google Scholar]
- Luz, R.; Ferreira, R.; Silva, C.; Miranda, B.; Piccoli, R.; Silva, M.; Paula, L.; Leles, M.; Fernandes, K.; Cruz, M.; et al. Development of a Halochromic, Antimicrobial, and Antioxidant Starch-Based Film Containing Phenolic Extract from Jaboticaba Peel. Foods 2023, 12, 653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, R.; Godoy, A.; Rosa, R.; Novak, R.; Olszewski, J. Caracterização fitoquímica e análise da atividade antimicrobiana e antioxidante dos extratos de Ilex paraguariensis, st hill.: Uma revisão de literatura. Braz. J. Dev. 2021, 7, 38806–38830. [Google Scholar] [CrossRef]
- Oliveira-Filho, J.G. Filmes Comestíveis Bioativos Baseados em Alginato, Polpa de Mangaba (Hancornia speciosa) e Saccharomyces boulardii. Master’s Thesis, Instituto Federal de Educação, Ciência e Tecnologia Goiano, Rio Verde, Brazil, 2022. [Google Scholar]
- Tomaz, P.; Vale, L.; Pereira-Filho, W.; Silva, F.; Dias, G.; Rosa Neto, N.; Silva, L.; Carvalho, R. Conservação de frutos de mamão na pós colheita com uso de biofilme à base de fécula de mandioca. Conservation of papaya fruits in the post-harvest with the use of biofilme cassava starch based coating. Braz. J. Dev. 2021, 7, 88564–88574. [Google Scholar] [CrossRef]
- Silva, A.G. Seleção de Genótipos de Acerola Para o Processamento de Suco com Alta Qualidade e vida Útil. Master’s Thesis, Universidade Federal do Vale do São Francisco, Petrolina, Brazil, 2023. [Google Scholar]
- Coelho, P.B. Aplicação de Revestimentos Biodegradáveis em Manga ‘Tommy Atkins’ Com Fins de Conservação. Master’s Thesis, Universidade Federal do Vale do São Francisco, Juazeiro, Brazil, 2021. [Google Scholar]
- Turquett, L.; Bastos, R.; Lima, J.; Valente, G. Avaliação da cobertura comestível elaborada a partir de quitosana, farelo de arroz e fécula de mandioca na conservação pós colheita de morangos. Braz. J. Dev. 2021, 7, 33153–33171. [Google Scholar] [CrossRef]
- Razali, Z.; Somasundram, C.; Nurulain, S.; Kunasekaran, W.; Alias, M. Postharvest Quality of Cherry Tomatoes Coated with Mucilage from Dragon Fruit and Irradiated with UV-C. Polymers 2021, 13, 2919. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Instrução Normativa nº 49, de 26 de Setembro de 2018. Estabelece os Parâmetros Analíticos de Suco e de Polpa de Frutas; MAPA—Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2018.
- Rocha, A.; Costa, S.; Lima, T.; Silva, A.; Barão, C.; Pimental, T.; Ushirobira, T.; Marcolino, V. Aplicação do biopolímero de amido de cassava e amido de milho na conservação pós-colheita de guava. Braz. J. Dev. 2020, 6, 6658–6680. [Google Scholar] [CrossRef]
- Repolho, R.; Oliveira, W.; Carvalho, A.; Sanches, A.; Sousa, J. Application of edible coatings in conservation of acerola. Appl. Res. Agrotechnol. 2019, 12, 59–69. [Google Scholar]
- Sapelli, K.; Faria, C.; Botelho, R. Postharvest conservation of peaches with the use of edible coatings added with yerba mate extract. Braz. J. Food Technol. 2020, 23, 2019044. [Google Scholar] [CrossRef]
| Granulometry | ||||
|---|---|---|---|---|
| Mesh | Size (mm) | Initial Weight (g) | Final Weight (g) % | Retention |
| 16 | 1.19 | 456.62 ± 0.32 | 519.61 * ± 0.40 | 62.99 ± 0.08 |
| 18 | 1.00 | 458.35 ± 0.10 | 464.09 * ± 0.19 | 5.74 ± 0.09 |
| 20 | 0.84 | 513.07 ± 0.14 | 524.34 * ± 0.24 | 11.32 ± 0.10 |
| 35 | 0.50 | 379.33 ± 0.23 | 385.85 * ± 0.65 | 6.52 ± 0.43 |
| Base | <0.07 | 387.07 ± 0.17 | 402.58 * ± 0.28 | 15.51 ± 0.11 |
| Parameters | PPPF |
|---|---|
| WAI (g absorbed/g dry weight) | 2.85 ± 0.14 |
| Water solubility (%) | 27.55 ± 2.95 |
| SV (mL/g dry weight) | 10.5 ± 0.00 |
| Parameters | PPPF |
|---|---|
| Water activity | 0.29 ± 0.00 |
| Titratable acidity (%) | 1.87 ± 0.04 |
| pH | 4.18 ± 0.16 |
| Moisture (%) | 4.69 ± 0.31 |
| Ash (%) | 4.10 ± 0.00 |
| Lipids (%) | 1.26 ± 0.23 |
| Proteins (%) | 3.07 ± 0.00 |
| Carbohydrates (%) | 78.59 ± 0.03 |
| Total fiber (%) | 10.12 ± 0.00 |
| Parameters | White Background | Black Background |
|---|---|---|
| Luminosity (L*) | 83.68 ± 0.78 | 37.92 ± 0.36 |
| a* | −1.71 ± 0.11 | −1.06 ± 0.16 |
| b* | 21.13 ± 2.39 | 6.88 ± 0.43 |
| Coating | DPPH (%) | ABTS (%) | PC (mg GAE/g) |
|---|---|---|---|
| PPPF-YS | 28.85 ± 0.27 | <LOD | 278.68 ± 0.45 |
| FC | <LOD | <LOD | 85.60 ± 0.56 |
| Microbial Parameter | Treatments | Time (Days) | Microbiological Standards * | ||
|---|---|---|---|---|---|
| 0 | 2 | 4 | |||
| E. coli | T1 | Abs | Abs | Abs | 5 × 103 UFC/g |
| T2 | Abs | Abs | Abs | ||
| T3 | Abs | Abs | Abs | ||
| Salmonella spp. | T1 | Abs | Abs | Abs | Abs |
| T2 | Abs | Abs | Abs | ||
| T3 | Abs | Abs | Abs | ||
| Microbial Parameter | Treatments | Time (Days) | Microbiological Standards * | ||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |||
| E. coli | T1 | Abs | Abs | Abs | Abs | Abs | 5 × 103 UFC/g |
| T2 | Abs | Abs | Abs | Abs | Abs | ||
| T3 | Abs | Abs | Abs | Abs | Abs | ||
| Salmonella spp. | T1 | Abs | Abs | Abs | Abs | Abs | Abs |
| T2 | Abs | Abs | Abs | Abs | Abs | ||
| T3 | Abs | Abs | Abs | Abs | Abs | ||
| Treatments | Storage Days (25 ± 0.5 °C). | ||
|---|---|---|---|
| 0 | 2 | 4 | |
| L* | |||
| T1 | 26.63 ± 4.72 Aa | 24.22 ± 2.29 Aa | 18.21 ± 1.33 Bb |
| T2 | 31.87 ± 2.08 Aa | 23.15 ± 4.04 Ba | 17.53 ± 1.98 Bb |
| T3 | 30.59 ± 3.60 Aa | 27.48 ± 0.75 Aa | 27.76 ± 2.96 Aa |
| a* | |||
| T1 | 34.60 ± 2.81 Aa | 30.05 ± 3.02 Aa | 21.36 ± 1.72 Ba |
| T2 | 36.79 ± 0.97 Aa | 27.64 ± 3.82 Ba | 21.72 ± 0.76 Ca |
| T3 | 36.91 ± 1.47 Aa | 25.93 ± 1.55 Ba | 19.39 ± 1.62 Ca |
| b* | |||
| T1 | 11.95 ± 1.56 Ab | 8.87 ± 0.72 Ba | 5.06 ± 0.16 Ca |
| T2 | 14.79 ± 1.33 Aab | 8.46 ± 1.43 Ba | 5.86 ± 0.65 Ba |
| T3 | 15.48 ± 0.86 Aa | 8.03 ± 0.49 Ba | 5.57 ± 0.34 Ca |
| Treatments | Storage Days (5 ± 0.5 °C) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |
| L* | |||||
| T1 | 31.04 ± 3.68 Aa | 27.04 ± 3.49 ABa | 21.56 ± 5.45 ABa | 25.25 ± 2.91 ABa | 17.79 ± 0.04 Bb |
| T2 | 30.26 ± 6.22 Aa | 27.19 ± 3.88 ABa | 19.70 ± 2.48 ABa | 22.15 ± 2.12 ABa | 19.23 ± 0.08 Bb |
| T3 | 32.03 ± 1.80 Aa | 33.00 ± 3.48 Aa | 25.89 ± 3.27 ABa | 25.97 ± 3.04 ABa | 22.10 ± 0.08 Ba |
| a* | |||||
| T1 | 34.71 ± 2.08 Aa | 30.56 ± 2.99 ABa | 23.43 ± 5.87 Ba | 26.81 ± 3.26 ABa | 18.25 ± 0.04 Bb |
| T2 | 34.34 ± 3.61 Aa | 30.33 ± 1.52 ABa | 25.18 ± 0.67 Ba | 27.40 ± 2.68 Ba | 18.53 ± 0.04 Cb |
| T3 | 29.27 ± 4.62 Aa | 31.23 ± 2.53 Aa | 30.23 ± 1.05 Aa | 28.45 ± 2.06 ABa | 21.21 ± 0.01 Ba |
| b* | |||||
| T1 | 13.90 ± 1.49 Aa | 12.78 ± 0.32 ABa | 9.96 ± 3.74 ABa | 11.69 ± 1.69 ABa | 7.72 ± 0.05 Bb |
| T2 | 13.40 ± 2.64 Aa | 11.95 ± 2.03 ABa | 10.20 ± 1.09 ABa | 11.55 ± 1.53 ABa | 7.73 ± 0.04 Bb |
| T3 | 14.91 ± 0.56 Aa | 1511 ± 1.02 Aa | 13.57 ± 0.91 ABa | 12.13 ± 0.90 BCa | 10.34 ± 0.03 Ca |
| Treatments | Storage Days (25 ± 0.5 °C) | ||
|---|---|---|---|
| 0 | 2 | 4 | |
| Total soluble solids (°Brix) | |||
| T1 | 11.95 ± 0.92 Aa | 9.65 ± 0.21 Ba | 9.20 ± 0.00 Ba |
| T2 | 9.45 ± 1.06 Aa | 9.65 ± 0.21 Aa | 8.00 ± 0.28 Ab |
| T3 | 10.15 ± 0.50 Aa | 9.35 ± 0.21 Aa | 8.85 ± 0.07 Aa |
| pH | |||
| T1 | 3.44 ± 0.01 ABa | 3.35 ± 0.04 Ba | 3.56 ± 0.06 Aa |
| T2 | 3.43 ± 0.04 Aa | 3.37 ± 0.02 Aa | 3.47 ± 0.11 Aa |
| T3 | 3.44 ± 0.01 Aa | 3.27 ± 0.02 Aa | 3.42 ± 0.16 Aa |
| Total titratable acidity (% citric acid) | |||
| T1 | 1.48 ± 0.04 Ba | 1.62 ± 0.13 Ba | 2.01 ± 0.04 Aa |
| T2 | 1.40 ± 0.28 Aa | 1.55 ± 0.23 Aa | 1.68 ± 0.21 Aa |
| T3 | 1.37 ± 0.06 Aa | 1.40 ± 0.18 Aa | 1.76 ± 0.04 Aa |
| Treatments | Storage Days (5 ± 0.5 °C) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |
| Total soluble solids (°Brix) | |||||
| T1 | 11.94 ± 0.92 Aa | 9.35 ± 0.35 Ba | 8.10 ± 0.28 Ba | 9.00 ± 0.14 Ba | 9.95 ± 0.07 Ba |
| T2 | 9.44 ± 1.06 Aa | 9.55 ± 0.35 Aa | 9.50 ± 0.56 Aa | 9.15 ± 0.35 Aa | 9.70 ± 0.28 Aa |
| T3 | 10.14 ± 0.49 Aa | 9.05 ± 0.07 ABa | 9.05 ± 0.50 ABa | 8.55 ± 0.21 Ba | 9.30 ± 0.14 ABa |
| pH | |||||
| T1 | 3.43 ± 0.01 Aa | 3.51 ± 0.01 Aa | 3.38 ± 0.11 Aa | 3.49 ± 0.04 Aa | 3.41 ± 0.08 Aa |
| T2 | 3.42 ± 0.04 Ba | 3.43 ± 0.01 Ba | 3.50 ± 0.07 ABa | 3.43 ± 0.01 Ba | 3.61 ± 0.02 Aa |
| T3 | 3.43 ± 0.01 Aa | 3.49 ± 0.07 Aa | 3.47 ± 0.05 Aa | 3.52 ± 0.06 Aa | 3.56 ± 0.09 Aa |
| Total titratable acidity (% citric acid) | |||||
| T1 | 1.47 ± 0.04 Aa | 1.82 ± 0.02 Aa | 1.56 ± 0.12 Aa | 1.65 ± 0.00 Aa | 1.56 ± 0.21 Aa |
| T2 | 1.39 ± 0.27 Aa | 1.27 ± 0.09 Ab | 1.72 ± 0.09 Aa | 1.74 ± 0.04 Aa | 1.53 ± 0.09 Aa |
| T3 | 1.36 ± 0.05 CDa | 2.04 ± 0.10 Aa | 1.78 ± 0.08 ABa | 1.68 ± 0.04 BCa | 1.33 ± 0.09 Da |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvão, M.B.F.; Stamford, T.C.M.; de Melo, F.A.B.R.; de Lima, G.S.; de Oliveira, C.E.V.; de Oliveira, I.L.N.; Bidô, R.d.C.d.A.; Pintado, M.M.E.; de Oliveira, M.E.G.; Stamford, T.L.M. Development of Edible Coatings Based on Pineapple Peel (Ananas Comosus L.) and Yam Starch (Dioscorea alata) for Application in Acerola (Malpighia emarginata DC). Foods 2024, 13, 2873. https://doi.org/10.3390/foods13182873
Galvão MBF, Stamford TCM, de Melo FABR, de Lima GS, de Oliveira CEV, de Oliveira ILN, Bidô RdCdA, Pintado MME, de Oliveira MEG, Stamford TLM. Development of Edible Coatings Based on Pineapple Peel (Ananas Comosus L.) and Yam Starch (Dioscorea alata) for Application in Acerola (Malpighia emarginata DC). Foods. 2024; 13(18):2873. https://doi.org/10.3390/foods13182873
Chicago/Turabian StyleGalvão, Maria Brígida Fonseca, Thayza Christina Montenegro Stamford, Flávia Alexsandra Belarmino Rolim de Melo, Gerlane Souza de Lima, Carlos Eduardo Vasconcelos de Oliveira, Ingrid Luana Nicácio de Oliveira, Rita de Cássia de Araújo Bidô, Maria Manuela Estevez Pintado, Maria Elieidy Gomes de Oliveira, and Tania Lucia Montenegro Stamford. 2024. "Development of Edible Coatings Based on Pineapple Peel (Ananas Comosus L.) and Yam Starch (Dioscorea alata) for Application in Acerola (Malpighia emarginata DC)" Foods 13, no. 18: 2873. https://doi.org/10.3390/foods13182873









