Widely Targeted Metabolomic Analysis Reveals Dynamic Metabolic Changes in Yanbian Cattle during Dry-Aging Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Selection
2.2. Dry-Aged Beef Sample Collection
2.3. Metabolomic Profiling
2.3.1. Preparation and Extraction of Samples
2.3.2. T3 UPLC Conditions
2.3.3. ESI-QTRAP-MS/MS Conditions
2.3.4. Quantitative and Qualitative Metabolite Measurements
2.4. Statistical Analyses
3. Results
3.1. Dynamic Change in Metabolites and Multivariate Data Analysis
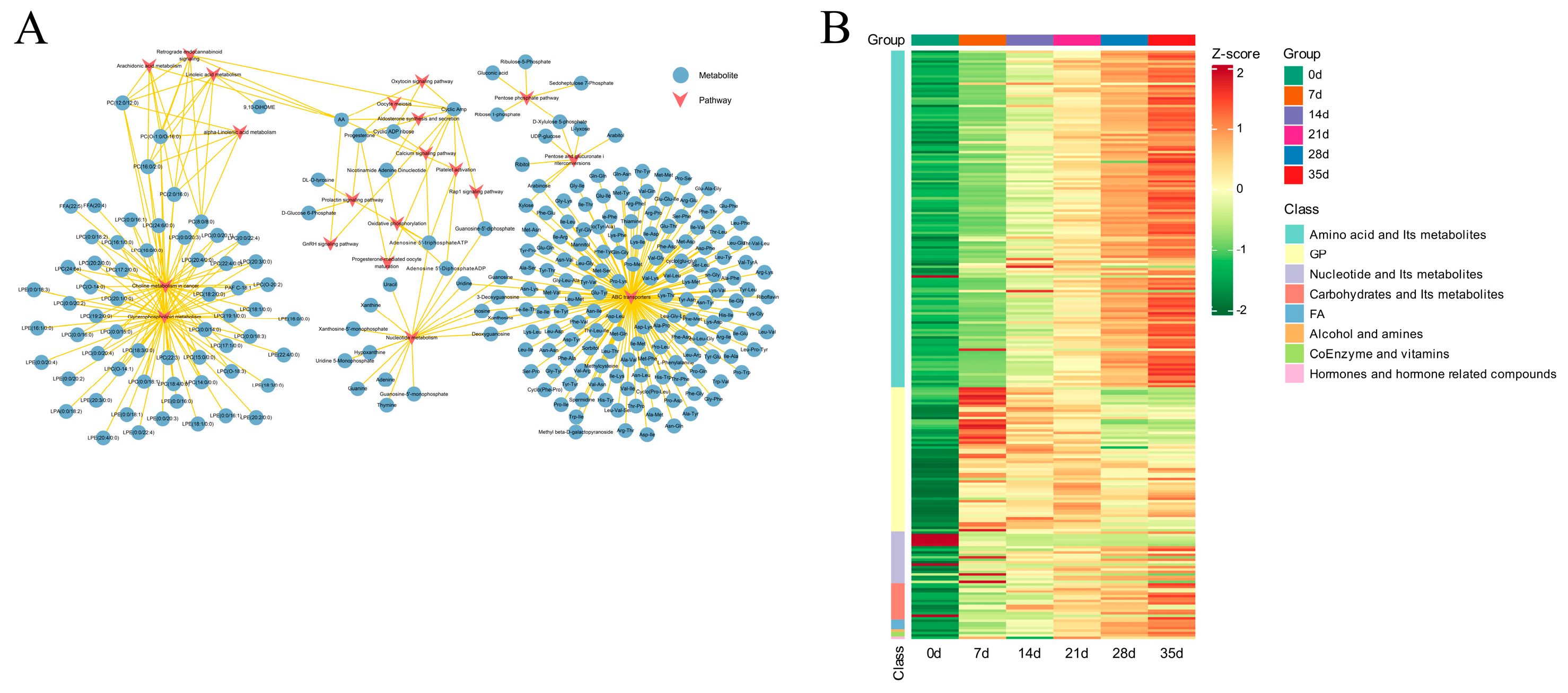
3.2. Identification of Characteristic Metabolites during the Dry-Aging Process in Yanbian Cattle
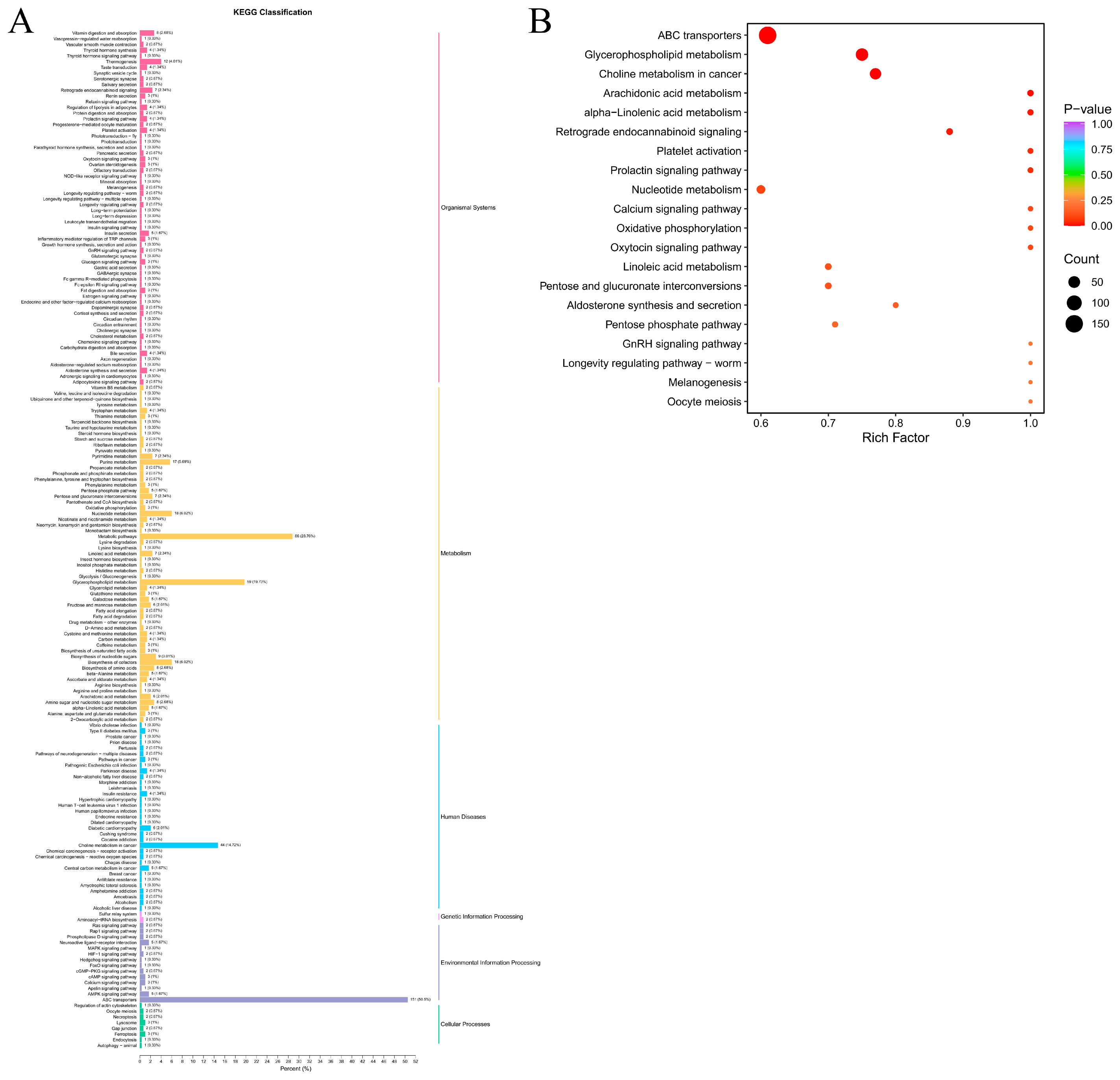
3.3. Metabolic Dry-Aging Process Pathways in Yanbian Cattle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resconi, V.C.; Bueno, M.; Escudero, A.; Magalhaes, D.; Ferreira, V.; Campo, M.M. Ageing and retail display time in raw beef odour according to the degree of lipid oxidation. Food Chem. 2018, 242, 288–300. [Google Scholar] [CrossRef]
- Lee, H.J.; Choe, J.; Kim, K.T.; Oh, M.; Lee, D.G.; Choi, Y.I.; Jo, C. Analysis of low-marbled Hanwoo cow meat aged with different dry-aging methods. Anim. Biosci. 2017, 30, 1733–1738. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Meyers, B.; Kim, H.W.; Liceaga, A.M.; Lemenager, R.P. Effects of stepwise dry/wet-aging and freezing on meat quality of beef loins. Meat Sci. 2016, 123, 57–63. [Google Scholar] [CrossRef]
- Stenström, H.; Li, X.; Hunt, M.C.; Lundström, K. Consumer preference and effect of correct or misleading information after ageing beef longissimus muscle using vacuum, dry ageing, or a dry ageing bag. Meat Sci. 2014, 96, 661–666. [Google Scholar] [CrossRef]
- Khan, M.I.; Jung, S.; Nam, K.C.; Jo, C. Postmortem aging of beef with a special reference to the dry aging. Korean J. Food Sci. Anim. Resour. 2016, 36, 159–169. [Google Scholar] [CrossRef]
- Ha, Y.; Hwang, I.; Ba, H.V.; Ryu, S.; Kim, Y.; Kang, S.M.; Kim, J.; Kim, Y.; Cho, S. Effects of dry- and wet-ageing on flavor compounds and eating quality of low fat Hanwoo beef muscles. Food Sci. Anim. Resour. 2019, 39, 655–667. [Google Scholar] [CrossRef]
- Cho, S.; Kang, S.M.; Kim, Y.S.; Kim, Y.C.; Ba, H.V.; Seo, H.W.; Lee, E.M.; Seong, P.N.; Kim, J.H. Comparison of drying yield, meat quality, oxidation stability and sensory properties of bone-in shell loin cut by different dry-aging conditions. Korean J. Food Sci. Anim. Resour. 2019, 38, 1131–1143. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, J.W.; Kim, M.; Oh, H.; Yoon, Y.; Jo, C. Changes in microbial composition on the crust by different air flow velocities and their effect on sensory properties of dry-aged beef. Meat Sci. 2019, 153, 152–158. [Google Scholar] [CrossRef]
- Setyabrata, D.; Cooper, B.R.; Sobreira, T.J.P.; Legako, J.F.; Martini, S.; Kim, Y.H.B. Elucidating mechanisms involved in flavor generation of dry-aged beef loins using metabolomics approach. Food Res. Int. 2020, 139, 109969. [Google Scholar] [CrossRef]
- Li, X.; Babol, J.; Wallby, A.; Lundström, K. Meat quality, microbiological status and consumer preference of beef gluteus medius aged in a dry ageing bag or vacuum. Meat Sci. 2013, 95, 229–234. [Google Scholar] [CrossRef]
- Kim, M.; Choe, J.; Lee, H.J.; Yoon, Y.; Yoon, S.; Jo, C. Effects of aging and aging method on physicochemical and sensory traits of different beef cuts. Food Sci. Anim. Resour. 2019, 39, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, D.; Tripathi, V.K.; Cho, S.; Kim, Y.; Hwang, I. Dry aging of beef; Review. J. Anim. Sci. Technol. 2016, 58, 20. [Google Scholar] [CrossRef]
- Ribeiro, F.A.; Lau, S.K.; Furbeck, R.A.; Herrera, N.J.; Henriott, M.L.; Bland, N.A.; Fernando, S.C.; Subbiah, J.; Sullivan, G.A.; Calkins, C.R. Ultimate pH effects on dry-aged beef quality. Meat Sci. 2020, 172, 108365. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, G.; Yin, X.; Ge, C.; Liao, G. Effects of different cooking methods on free fatty acid profile, water-soluble compounds and flavor compounds in Chinese Piao chicken meat. Food Res. Int. 2021, 149, 110696. [Google Scholar] [CrossRef]
- Mikami, N.; Toyotome, T.; Yamashiro, Y.; Sugo, K.; Yoshitomi, K.; Takaya, M.; Han, K.H.; Fukushima, M.; Shimada, K. Dry-aged beef manufactured in Japan: Microbiota identification and their effects on product characteristics. Food Res. Int. 2021, 140, 110020. [Google Scholar] [CrossRef] [PubMed]
- Terjung, N.; Witte, F.; Heinz, V. The dry aged beef paradox: Why dry aging is sometimes not better than wet aging. Meat Sci. 2021, 172, 108355. [Google Scholar] [CrossRef] [PubMed]
- Iida, F.; Miyazaki, Y.; Tsuyuki, R.; Kato, K.; Egusa, A.; Ogoshi, H.; Nishimura, T. Changes in taste compounds, breaking properties, and sensory attributes during dry aging of beef from Japanese black cattle. Meat Sci. 2016, 112, 46–51. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, H.; Wu, F.; Zhu, M.; Zhang, Y.; Cheng, N.; Xue, X.; Wu, L.; Cao, W. Molecular mechanism of mature honey formation by GC-MS- and LC-MS-based metabolomics. J. Agric. Food Chem. 2021, 69, 3362–3370. [Google Scholar] [CrossRef]
- Wehrens, R.; Weingart, G.; Mattivi, F. metaMS: An open-source pipeline for GC-MS-based untargeted metabolomics. J. Chromatogr. B 2014, 966, 109–116. [Google Scholar] [CrossRef]
- Sugimoto, M.; Obiya, S.; Kaneko, M.; Enomoto, A.; Honma, M.; Wakayama, M.; Soga, T.; Tomita, M. Metabolomic Profiling as a Possible Reverse Engineering Tool for Estimating Processing Conditions of Dry-Cured Hams. J. Agric. Food Chem. 2017, 65, 402–410. [Google Scholar] [CrossRef]
- Beiglböck, H.; Wolf, P.; Pfleger, L.; Caliskan, B.; Fellinger, P.; Zettinig, G.; Kenner, L.; Trattnig, S.; Kautzky-Willer, A.; Krssak, M.; et al. Effects of thyroid function on phosphodiester concentrations in skeletal muscle and liver: An in vivo NMRS study. J. Clin. Endocrinol. Metab. 2020, 105, e4866–e4874. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kamiya, M.; Higuchi, M. Gas Chromatography-Mass Spectrometry-Based Metabolomic Analysis of Wagyu and Holstein Beef. Metabolites 2020, 10, 95. [Google Scholar] [CrossRef]
- Ijaz, M.; Zhang, D.Q.; Hou, C.L.; Mahmood, M.; Hussain, Z.; Zheng, X.C.; Li, X. Changes in postmortem metabolites profile of atypical and typical DFD beef. Meat Sci. 2022, 193, 108922. [Google Scholar] [CrossRef]
- Peng, Y.; Hong, J.; Raftery, D.; Xia, Q.; Du, D. Metabolomic-based clinical studies and murine models for acute pancreatitis disease: A review. BBA Mol. Basis Dis. 2021, 1867, 166123. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, S.; Zang, M.; Zhao, B.; Zhu, N.; Li, S.; Wu, Q.; Liu, B.; Zhao, Y.; Qiao, X.; et al. Non-targeted metabolomics analysis of metabolite changes in beef during dry aging. Food Sci. 2023, 44, 249–256. [Google Scholar] [CrossRef]
- Chen, X.; Mao, Y.; Liang, R.; Zhu, L.; Yang, X.; Hopkins, D.L.; Zhang, Y. LC-MS-based metabolomics reveals metabolite dynamic changes of beef after superchilling early post-mortem. Food Res. Int. 2024, 183, 114208. [Google Scholar] [CrossRef]
- Gómez, J.F.M.; Cônsolo, N.R.B.; Antonelo, D.S.; Beline, M.; Gagaoua, M.; Higuera-Padilla, A.; Colnago, L.A.; Gerrard, D.E.; Silva, S.L. Impact of cattle feeding strategy on the beef metabolome. Metabolites 2022, 12, 640. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, X. Metabolite profile differences among different storage time in beef preserved at low temperature. J. Food Sci. 2019, 84, 3163–3171. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Zamudio, G.D.; Silva, L.; Vieira, N.M.; Vilela, R.; Assis, D.; Assis, G.; Esteada, M.M.; Rodrigues, R.T.; Duarte, M.S.; Chizzotti, M.L. Effect of short-term dietary protein restriction before slaughter on meat quality and skeletal muscle metabolomic profile in culled ewes. Livest. Sci. 2022, 261, 104956. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, L.; Su, R.; Corazzin, M.; Yang, Z.; Dou, L.; Hu, G.; Zhang, Y.; Liu, T.; Guo, Y.; et al. Widely targeted metabolomic analysis reveals the dynamic changes of metabolites during postmortem chilled aging in Mongolian sheep. Food Chem. 2024, 431, 137035. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, D.; Wang, Y.; Tian, J.; Mu, B.; Cui, M.; Li, G. Study on changes in tenderness of Yanbian cattle during dry and wet-aging. Food Mach. 2024, 40, 17–22. [Google Scholar] [CrossRef]
- Álvarez, S.; Mullen, A.M.; Hamill, R.; O’Neill, E.; Alvarez, C. Dry-aging of beef as a tool to improve meat quality. Impact of processing conditions on the technical and organoleptic meat properties. Adv. Food Nutr. Res. 2021, 95, 97–130. [Google Scholar] [CrossRef]
- Sun, D.; Peng, A.; Tan, J.; Piao, C.; Lü, A.; Mu, B.; Li, G. Effect of dry-aging process on the quality of rump beef from Yanbian cattle. Food Mach. 2023, 39, 159–165+172. [Google Scholar] [CrossRef]
- Sha, H.; Li, S.; Li, J.; Zhao, J.; Su, D. Widely targeted metabolomics and network pharmacology reveal the nutritional potential of Yellowhorn (Xanthoceras sorbifolium Bunge) leaves and flowers. Foods 2024, 13, 1274. [Google Scholar] [CrossRef]
- Da Silva Bernardo, A.P.; Ferreira, F.M.S.; Silva, A.C.M.; Prestes, F.S.; Francisco, V.C.; Nassu, R.T.; Nascimento, M.S.; Pflanzer, S.B. Dry-aged and wet-aged beef: Effects of aging time and temperature on microbiological profile, physicochemical characteristics, volatile compound profile and weight loss of meat from Nellore cattle (Bos indicus). Anim. Prod. Sci. 2021, 61, 497–1509. [Google Scholar] [CrossRef]
- Zhang, R.; Ross, A.; Yoo, M. Metabolic fingerprinting of in-bag dry- and wet-aged lamb with rapid evaporative ionisation mass spectroscopy. Food Chem. 2021, 347, 128999. [Google Scholar] [CrossRef]
- Sentandreu, M.; Coulis, G.; Ouali, A. Role of muscle endopeptidases and their inhibitors in meat tenderness. Trends Food Sci. Technol. 2002, 13, 400–421. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Yu, Q.; Han, L.; Ma, Z. Effects of Lysosomal-Mitochondrial Apoptotic Pathway on Tenderness in Postmortem Bovine Longissimus Muscle. J. Agric. Food Chem. 2019, 67, 4578–4587. [Google Scholar] [CrossRef]
- Muroya, S.; Ueda, S.; Komatsu, T.; Miyakawa, T.; Ertbjerg, P. MEATabolomics: Muscle and meat metabolomics in domestic animals. Metabolites 2020, 10, 188. [Google Scholar] [CrossRef]
- Ryu, S.; Park, M.R.; Maburutse, B.E.; Lee, W.J.; Park, D.; Cho, S.; Hwang, I.; Oh, Y.; Kim, Y. Diversity and characteristics of the meat microbiological community on dry aged beef. J. Microbiol. Biotechnol. 2017, 28, 105–108. [Google Scholar] [CrossRef]
- Hulánková, R.; Kameník, J.; Saláková, A.; Závodský, D.; Borilova, G. The effect of dry aging on instrumental, chemical and microbiological parameters of organic beef loin muscle. LWT Food Sci. Techonol. 2018, 89, 559–565. [Google Scholar] [CrossRef]
- Setyabrata, D.; Vierck, K.; Sheets, T.R.; Legako, J.F.; Cooper, B.R.; Johnson, T.A.; Kim, Y.H.B. Characterizing the flavor precursors and liberation mechanisms of various dry-aging methods in cull beef loins using metabolomics and microbiome approaches. Metabolites 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, D.; Peng, A.; Li, T.; Li, H.; Mu, B.; Wang, J.; Cui, M.; Piao, C.; Li, G. Hydrolysis of beef sarcoplasmic protein by dry-aged beef-isolated Penicillium oxalicum and its associated metabolic pathways. Foods 2024, 13, 1038. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.; Witte, F.; Sieksmeyer, T.; Januschweski, E.; Terjung, N.; Hertel, C.; Heinz, V.; Juadjur, A.; Gibis, M. Metabolic and microbial analyses of the surface and inner part of wet-aged and dry-aged beef. J. Food Sci. 2023, 88, 4357–4387. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Q.; Liu, Z. Genome-Wide Identification, Characterization and Phylogenetic Analysis of 50 Catfish ATP-Binding Cassette (ABC) Transporter Genes. PLoS ONE 2013, 8, e63895. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.W.K.; Chung, H.H.; Gan, H.M. Genome-wide identification, characterization and phylogenetic analysis of 52 striped catfish (Pangasianodon hypophthalmus) ATP-binding cassette (ABC) transporter genes. Trop. Life Sci. Res. 2022, 33, 257–293. [Google Scholar] [CrossRef]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and flavor chemistry characteristics of Australian beef: Influence of intramuscular fat, feed, and breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino acids production focusing on fermentation technologies—A review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef]
- Bao, Y.; Zhu, Y.; Ren, X.; Zhang, Y.; Peng, Z.; Zhou, G. Formation and Inhibition of Lipid Alkyl Radicals in Roasted Meat. Foods 2020, 9, 572. [Google Scholar] [CrossRef]
- Utama, D.T.; Kim, Y.J.; Jeong, H.S.; Kim, J.; Barido, F.H.; Lee, S.K. Comparison of meat quality, fatty acid composition and aroma volatiles of dry-aged beef from Hanwoo cows slaughtered at 60 or 80 months old. Asian-Austral. J. Anim. Sci. 2020, 33, 157. [Google Scholar] [CrossRef]
- Ali, M.; Nam, K. Physicochemical attributes, oxidative stability, and microbial profile of boneless sirloin and bone-in T-bone steaks from Hanwoo steer with reference to dry-aging. J. Anim. Sci. Techonol. 2021, 63, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, H.; Yoon, J.; Kim, M.; Jo, C. Effect of different aging methods on the formation of aroma volatiles in beef strip loins. Foods 2021, 10, 146. [Google Scholar] [CrossRef]
- Lee, D.H.; Kulkarni, K.P.; Kim, B.O.; Seok, Y.M.; Song, J.T.; Lee, J.D. Comparative assessment of quality characteristics of Chungkookjang made from soybean seeds differing in oleic acid concentration. J. Funct. Foods 2019, 52, 529–536. [Google Scholar] [CrossRef]
- Ba, H.V.; Park, K.; Dashmaa, D.; Hwang, I. Effect of muscle type and vacuum chiller ageing period on the chemical compositions, meat quality, sensory attributes and volatile compounds of Korean native cattle beef. Anim. Sci. J. 2013, 85, 164–173. [Google Scholar] [CrossRef]
- Yancey, E.J.; Dikeman, M.E.; Hachmeister, K.A.; Chambers, E.; Milliken, G.A. Flavor characterization of top-blade, top-sirloin, and tenderloin steaks as affected by pH, maturity, and marbling1,2,3. J. Anim. Sci. 2005, 83, 2618–2623. [Google Scholar] [CrossRef]
- Ali, A.; Karin, S. Flavor retention and release from beverages: A kinetic and thermodynamic perspective (review). J. Agric. Food Chem. 2018, 66, 9869–9881. [Google Scholar] [CrossRef]
- Muroya, S.; Oe, M.; Ojima, K.; Watanabe, A. Metabolomic approach to key metabolites characterizing postmortem aged loin muscle of Japanese Black (Wagyu) cattle. Anim. Biosci. 2019, 32, 1172–1185. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Critical Role During Fetal Development and Dietary Requirements in Adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef]
- You, L.; Luo, R. Effect of chilled ageing conditioning at 4 °C in lamb longissimus dorsi muscles on water-soluble flavour precursors as revealed by a metabolomic approach. J. Food Qual. 2019, 2019, 4529830. [Google Scholar] [CrossRef]
- Liu, J.; You, M.; Zhu, X.; Shi, W. Characterization of aroma characteristics of silver carp mince glycated with different reducing sugars. Food Chem. X 2024, 22, 101335. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Mu, B.; Liu, Y.; Zhao, C.; Li, H.; Wang, J.; Li, T.; Li, G.; Piao, C. Widely Targeted Metabolomic Analysis Reveals Dynamic Metabolic Changes in Yanbian Cattle during Dry-Aging Process. Foods 2024, 13, 2879. https://doi.org/10.3390/foods13182879
Sun D, Mu B, Liu Y, Zhao C, Li H, Wang J, Li T, Li G, Piao C. Widely Targeted Metabolomic Analysis Reveals Dynamic Metabolic Changes in Yanbian Cattle during Dry-Aging Process. Foods. 2024; 13(18):2879. https://doi.org/10.3390/foods13182879
Chicago/Turabian StyleSun, Depeng, Baide Mu, Yujia Liu, Changcheng Zhao, Hongmei Li, Juan Wang, Tingyu Li, Guanhao Li, and Chunxiang Piao. 2024. "Widely Targeted Metabolomic Analysis Reveals Dynamic Metabolic Changes in Yanbian Cattle during Dry-Aging Process" Foods 13, no. 18: 2879. https://doi.org/10.3390/foods13182879
APA StyleSun, D., Mu, B., Liu, Y., Zhao, C., Li, H., Wang, J., Li, T., Li, G., & Piao, C. (2024). Widely Targeted Metabolomic Analysis Reveals Dynamic Metabolic Changes in Yanbian Cattle during Dry-Aging Process. Foods, 13(18), 2879. https://doi.org/10.3390/foods13182879





