Interactions of Saccharomyces cerevisiae and Lactiplantibacillus plantarum Isolated from Light-Flavor Jiupei at Various Fermentation Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Cultural Medium
2.2. Cultural Conditions and Growth Monitoring
2.3. Lactic Acid, Ethanol, and Reducing Sugar Measurement
2.4. Proteomic Analysis
2.5. Statistical Analysis
3. Results
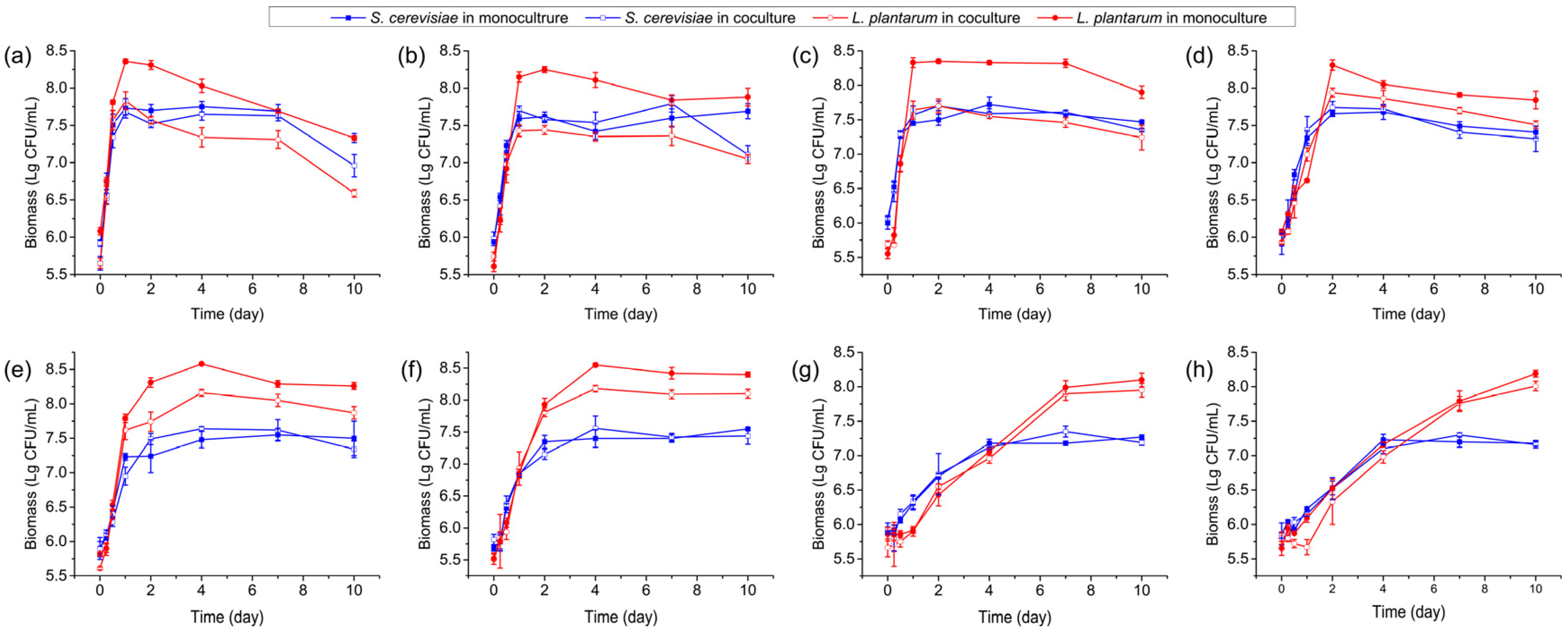
3.1. Microbial Biomass in Monoculture and Coculture Systems
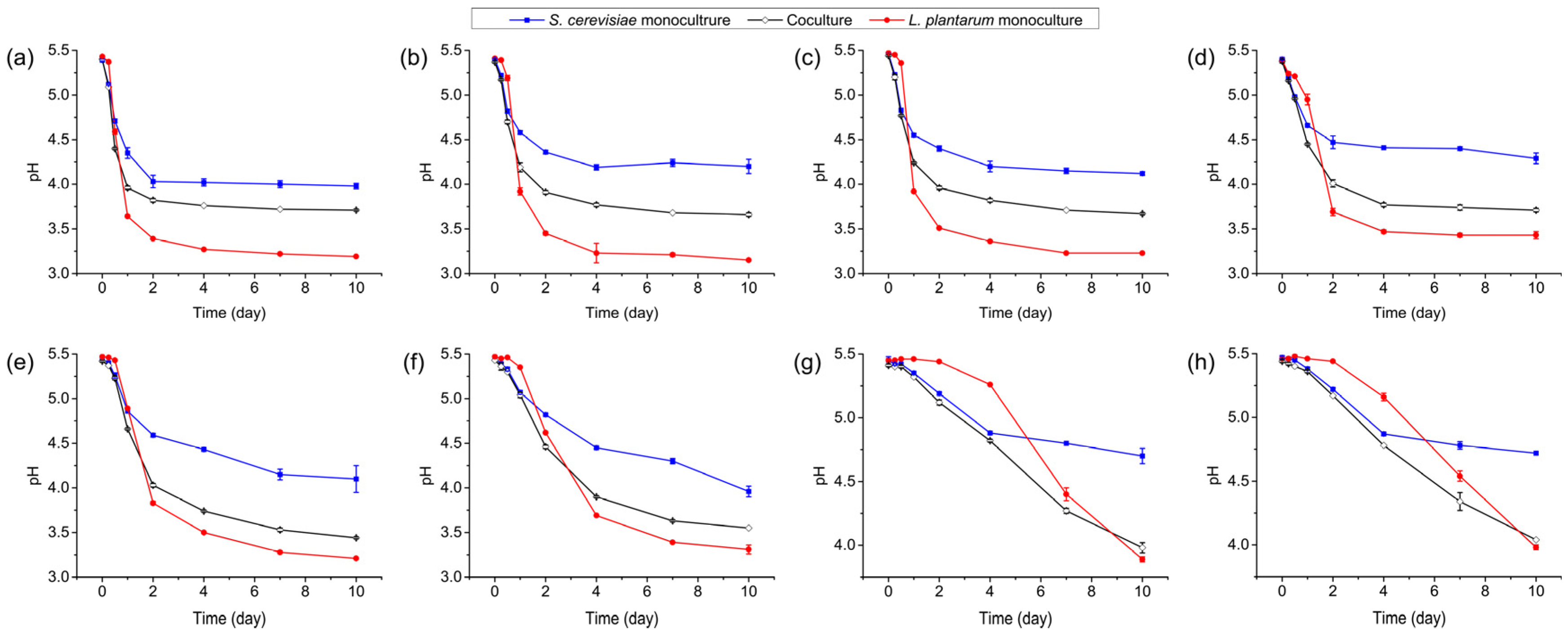
3.2. pH Changes in Monoculture and Coculture Systems
3.3. Lactic Acid Yield of L. plantarum R2 in Monoculture and Coculture Systems
3.4. Ethanol Production by S. cerevisiae Y28 in Monoculture and Coculture Systems
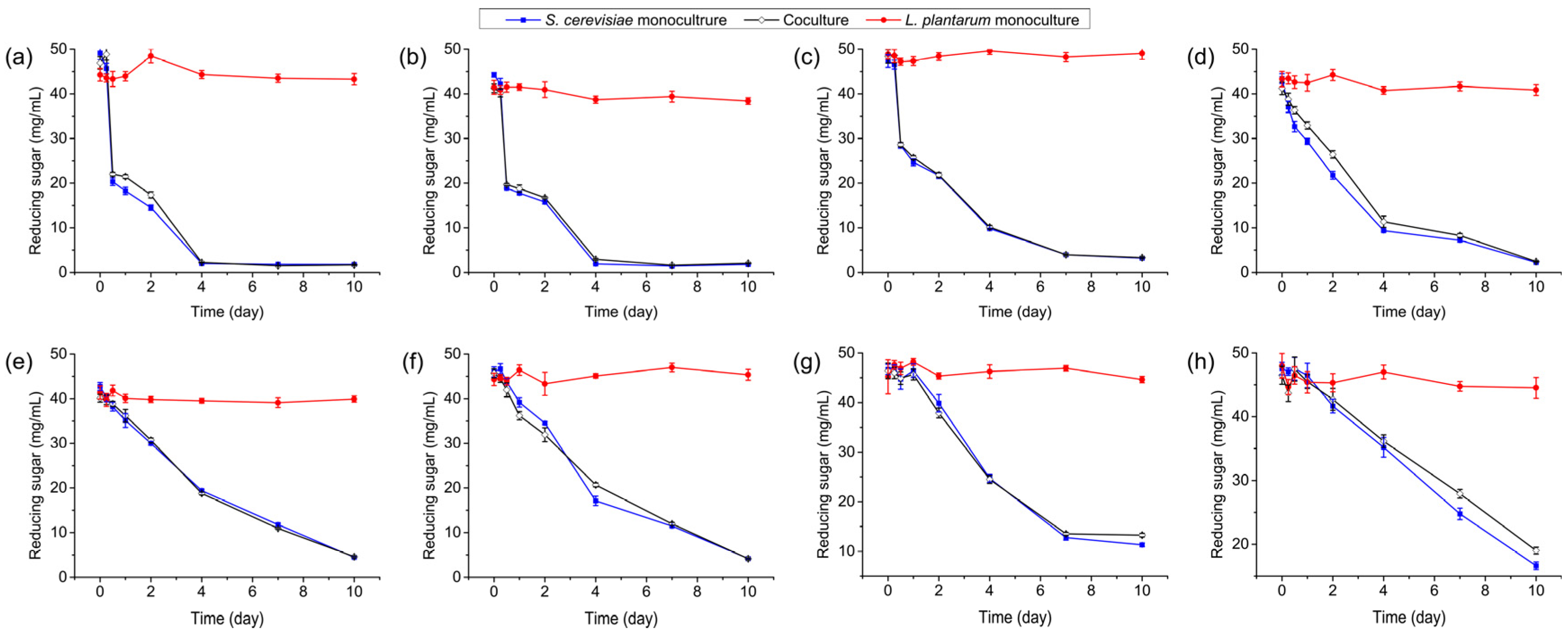
3.5. Consumption of Reducing Sugars
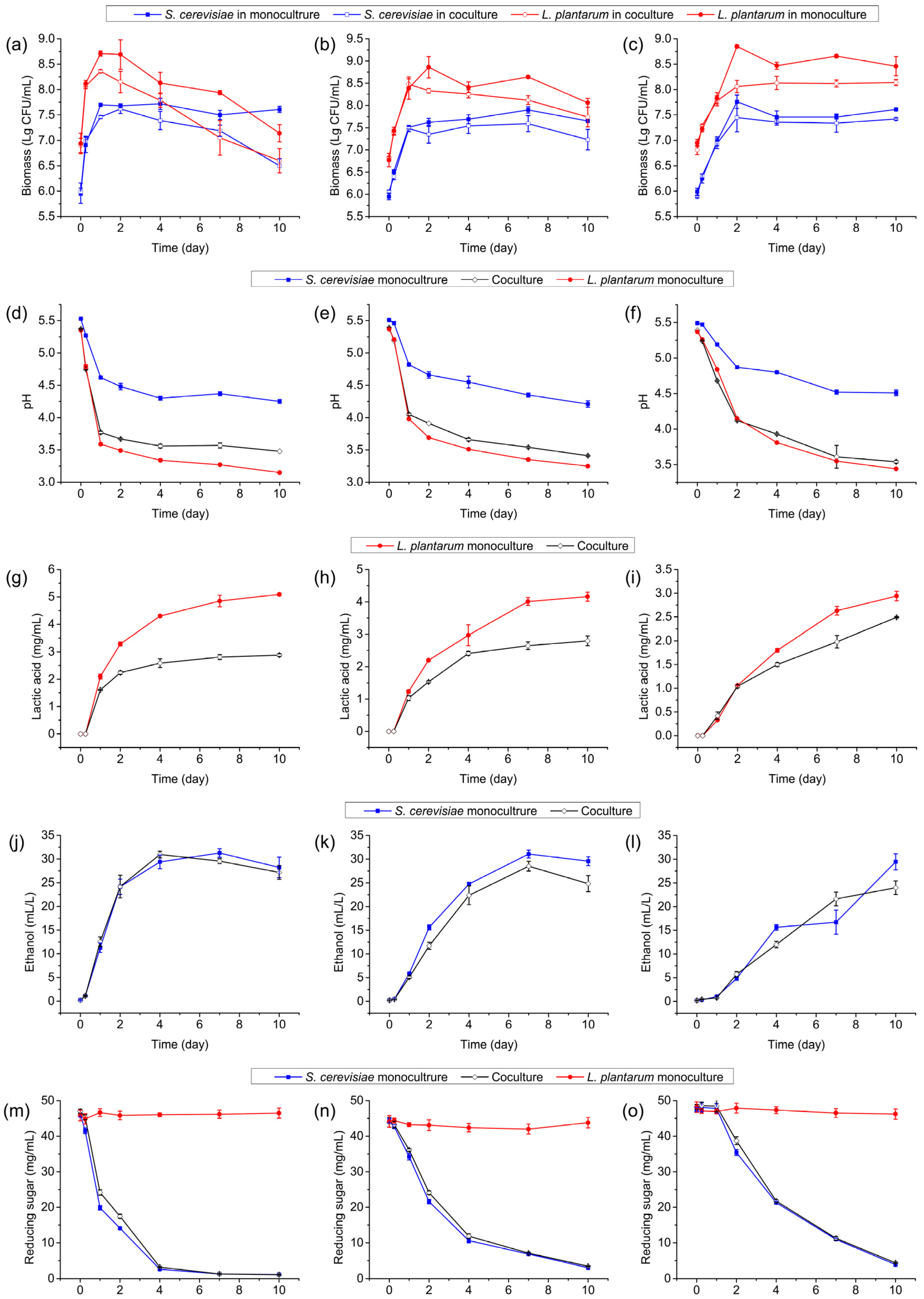
3.6. Effect of Elevated Initial Biomass of L. plantarum R2 on Interactions
3.7. Response of L. plantarum R2 to S. cerevisiae Y28 at Proteomic Level
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Biochem. Sci. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Tu, W.; Cao, X.; Cheng, J.; Li, L.; Zhang, T.; Wu, Q.; Xiang, P.; Shen, C.; Li, Q. Chinese Baijiu: The perfect works of microorganisms. Front. Microbiol. 2022, 13, 919044. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Sun, B. Chinese Baijiu and Whisky: Research reservoirs for flavor and functional food. Foods 2023, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Gao, X.; Shi, X.; Chen, S.; Xu, Y.; Tang, K. Comparison of the aroma-active compounds and sensory characteristics of different grades of light-flavor Baijiu. Foods 2023, 12, 1238. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, J.; Wang, R.; Zhang, N.; Zheng, F. A review on flavor of Baijiu and other world-renowned distilled liquors. Food Chem. X 2023, 20, 100870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Li, H.; Hui, M.; Pan, C. Strategies and challenges of microbiota regulation in Baijiu brewing. Foods 2024, 13, 1954. [Google Scholar] [CrossRef]
- Pang, X.; Han, B.; Huang, X.; Zhang, X.Z.; Hou, L.; Cao, M.; Gao, L.; Hu, G.; Chen, J. Effect of the environment microbiota on the flavour of light-flavour Baijiu during spontaneous fermentation. Sci. Rep. 2018, 8, 3396. [Google Scholar] [CrossRef]
- Wang, X.; Du, H.; Zhang, Y.; Xu, Y. Environmental microbiota drives microbial succession and metabolic profiles during Chinese liquor fermentation. Appl. Environ. Microbiol. 2018, 84, e02317–e02369. [Google Scholar] [CrossRef]
- Wei, J.; Du, H.; Xu, Y. Revealing the key microorganisms producing higher alcohols and their assembly processes during Jiang-flavor Baijiu fermentation. Food Biosci. 2024, 61, 104569. [Google Scholar] [CrossRef]
- Zha, M.; Sun, B.; Yin, S.; Mehmood, A.; Cheng, L.; Wang, C. Generation of 2-Furfurylthiol by carbon–sulfur lyase from the Baijiu yeast Saccharomyces cerevisiae G20. J. Agric. Food Chem. 2018, 66, 2114–2120. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, P.; Xu, Y.; Li, H.; Li, H.; Sun, J.; Sun, B. Isolation and characterization of yeast with benzenemethanethiol synthesis ability isolated from Baijiu Daqu. Foods 2023, 12, 2464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Du, G.; Chen, J.; Ren, T.; Wang, J.; Han, Y.; Zhen, P.; Zhao, X. The influence of seasons on the composition of microbial communities and the content of lactic acid during the fermentation of fen-flavor Baijiu. Fermentation 2022, 8, 740. [Google Scholar] [CrossRef]
- Zhang, X. Metabolic Activity Assessment of Key Microorganisms in Jiupei of Light-Flavor Baijiu. Master’s Thesis, Shanxi University, Taiyuan, China, 2022. [Google Scholar]
- Xue, Y.A.; Tang, F.; Cai, W.; Zhao, X.; Song, W.; Zhong, J.A.; Liu, Z.; Guo, Z.; Shan, C. Bacterial diversity, organic acid, and flavor analysis of dacha and ercha fermented grains of fen flavor Baijiu. Front. Microbiol. 2022, 12, 769290. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Nie, Y.; Du, H.; Xu, Y. Reduced lactic acid strengthens microbial community stability and function during Jiang-flavour Baijiu fermentation. Food Biosci. 2024, 59, 103935. [Google Scholar] [CrossRef]
- Ding, Y.; Niu, Y.; Chen, Z.; Dong, S.; Li, H. Discovery of novel Lactobacillus plantarum co-existence-associated influencing factor(s) on Saccharomyces cerevisiae fermentation performance. LWT Food Sci. Technol. 2021, 135, 110268. [Google Scholar] [CrossRef]
- He, X.; Liu, B.; Xu, Y.; Chen, Z.; Li, H. Effects of Lactobacillus plantarum on the ethanol tolerance of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2021, 105, 2597–2611. [Google Scholar] [CrossRef]
- Liu, J.; Huang, T.; Liu, G.; Ye, Y.; Soteyome, T.; Seneviratne, G.; Xiao, G.; Xu, Z.; Kjellerup, B.V. Microbial interaction between Lactiplantibacillus plantarum and Saccharomyces cerevisiae: Transcriptome level mechanism of cell-cell antagonism. Microbiol. Spectr. 2022, 10, e01422–e01433. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Q.; Tan, X.; Zhang, S.; Zeng, L.; Tang, J.; Xiang, W. Characterization of γ-aminobutyric acid (GABA)-producing Saccharomyces cerevisiae and coculture with Lactobacillus plantarum for mulberry beverage brewing. J. Biosci. Bioeng. 2020, 129, 447–453. [Google Scholar] [CrossRef]
- Gerardi, C.; Tristezza, M.; Giordano, L.; Rampino, P.; Perrotta, C.; Baruzzi, F.; Capozzi, V.; Mita, G.; Grieco, F. Exploitation of Prunus mahaleb fruit by fermentation with selected strains of Lactobacillus plantarum and Saccharomyces cerevisiae. Food Microbiol. 2019, 84, 103262. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Wang, H.; Yang, F.; Chen, L.; Hao, F.; Lv, X.; Du, H.; Xu, Y. Effects of initial temperature on microbial community succession rate and volatile flavors during Baijiu fermentation process. Food Res. Int. 2021, 141, 109887. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, Y.; Xie, Y.; Zhang, H.; Jiang, K.; Pang, X.; Huang, M. Metabolic characteristics of lactic acid bacteria and interaction with yeast isolated from light-flavor Baijiu fermentation. Food Biosci. 2022, 50, 102102. [Google Scholar] [CrossRef]
- Zhuang, X.; Wu, Q.; Xu, Y. Physiological characteristics of Zygosaccharomyces bailii and its interaction with Bacillus licheniformis in Chinese maotai-flavor liquor making. Microbiol. China 2017, 44, 251–262. [Google Scholar]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; Andersen, K.R.; Kilstrup, M.; Martinussen, J.; Switzer, R.L.; Willemoës, M. Phosphoribosyl diphosphate (PRPP): Biosynthesis, enzymology, utilization, and metabolic significance. Microbiol. Mol. Biol. Rev. 2016, 81, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Bergkessel, M. Regulation of protein biosynthetic activity during growth arrest. Curr. Opin. Microbiol. 2020, 57, 62–69. [Google Scholar] [CrossRef]
- Piir, K.; Paier, A.; Liiv, A.; Tenson, T.; Maiväli, Ü. Ribosome degradation in growing bacteria. EMBO Rep. 2011, 12, 458–462. [Google Scholar] [CrossRef]
- Dai, X.; Zhu, M. Coupling of ribosome synthesis and translational capacity with cell growth. Trends Biochem. Sci. 2020, 45, 681–692. [Google Scholar] [CrossRef]
- Scott, M.; Gunderson, C.W.; Mateescu, E.M.; Zhang, Z.; Hwa, T. Interdependence of cell growth and gene expression: Origins and consequences. Science 2010, 330, 1099–1102. [Google Scholar] [CrossRef]
- Hausmann, C.D.; Ibba, M. Aminoacyl-tRNA synthetase complexes: Molecular multitasking revealed. FEMS Microbiol. Rev. 2008, 32, 705–721. [Google Scholar] [CrossRef]
- Ferro, I.; Liebeton, K.; Ignatova, Z. Growth-rate dependent regulation of tRNA level and charging in Bacillus licheniformis. J. Mol. Biol. 2017, 429, 3102–3112. [Google Scholar] [CrossRef]
- Ho, J.M.; Bakkalbasi, E.; Soll, D.; Miller, C.A. Drugging tRNA aminoacylation. RNA Biol. 2018, 15, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Xiao, Y.; Li, R.; Li, H.; Wang, P.; Yang, X.; Zhang, Y. Transcriptome and metabolome profiling to elucidate the mechanism underlying the poor growth of Streptococcus suis serotype 2 after orphan response regulator CovR deletion. Front. Vet. Sci. 2023, 10, 1280161. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Du, H.; Xu, Y. Cooperative response of Pichia kudriavzevii and Saccharomyces cerevisiae to lactic acid stress in Baijiu fermentation. J. Agric. Food. Chem. 2020, 68, 4903–4911. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.P.; Jin, B.; Lant, P. Direct fermentation of potato starch wastewater to lactic acid by Rhizopus oryzae and Rhizopus arrhizus. Bioprocess. Biosyst. Eng. 2005, 27, 229–238. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Qian, H.; Yang, Y.; Zhu, X.; Wu, Q.; Mu, Y.; Huang, Z. Analysis of saccharification products of high-concentration glutinous rice fermentation by Rhizopus nigricans Q3 and alcoholic fermentation of Saccharomyces cerevisiae GY-1. ACS Omega 2021, 6, 8038–8044. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, C.; Guo, Z.; Li, S.; Zhu, Z.; Grimi, N.; Xiao, J. Fermentation of Betaphycus gelatinum using Lactobacillus brevis: Growth of probiotics, total polyphenol content, polyphenol profile, and antioxidant capacity. Foods 2023, 12, 3334. [Google Scholar] [CrossRef]
- Bailey, J.M.; Whelan, W.J. Physical properties of starch: I. relationship between Iodine stain and chain length. J. Biol. Chem. 1961, 236, 969–973. [Google Scholar] [CrossRef]
- Dworkin, J.; Harwood, C.S. Metabolic reprogramming and longevity in quiescence. Annu. Rev. Microbiol. 2022, 76, 91–111. [Google Scholar] [CrossRef]
- Breeden, L.L.; Tsukiyama, T. Quiescence in Saccharomyces cerevisiae. Annu. Rev. Genet. 2022, 56, 253–278. [Google Scholar] [CrossRef]
- den Ridder, M.; van den Brandeler, W.; Altiner, M.; Daran-Lapujade, P.; Pabst, M. Proteome dynamics during transition from exponential to stationary phase under aerobic and anaerobic conditions in yeast. Mol. Cell. Proteom. 2023, 22, 100552. [Google Scholar] [CrossRef]
- Marco, M.L.; Kleerebezem, M. Assessment of real-time RT-PCR for quantification of Lactobacillus plantarum gene expression during stationary phase and nutrient starvation. J. Appl. Microbiol. 2008, 104, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Multiparametric approach to interactions between Saccharomyces cerevisiae and Lachancea thermotolerans during fermentation. Fermentation 2022, 8, 286. [Google Scholar] [CrossRef]
- Bagheri, B.; Bauer, F.F.; Cardinali, G.; Setati, M.E. Ecological interactions are a primary driver of population dynamics in wine yeast microbiota during fermentation. Sci. Rep. 2020, 10, 4911. [Google Scholar] [CrossRef]
- Balsa-Canto, E.; Alonso-del-Real, J.; Querol, A. Temperature shapes ecological dynamics in mixed culture fermentations driven by two species of the Saccharomyces genus. Front. Bioeng. Biotechnol. 2020, 8, 915. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Xi, B.; Han, Y.; Li, J.; Luo, L.; Qu, C.; Li, J.; Liu, S.; Kang, L.; Bai, B.; et al. Interactions of Saccharomyces cerevisiae and Lactiplantibacillus plantarum Isolated from Light-Flavor Jiupei at Various Fermentation Temperatures. Foods 2024, 13, 2884. https://doi.org/10.3390/foods13182884
Yang P, Xi B, Han Y, Li J, Luo L, Qu C, Li J, Liu S, Kang L, Bai B, et al. Interactions of Saccharomyces cerevisiae and Lactiplantibacillus plantarum Isolated from Light-Flavor Jiupei at Various Fermentation Temperatures. Foods. 2024; 13(18):2884. https://doi.org/10.3390/foods13182884
Chicago/Turabian StyleYang, Pu, Bo Xi, Ying Han, Jiayang Li, Lujun Luo, Chaofan Qu, Junfang Li, Shuai Liu, Le Kang, Baoqing Bai, and et al. 2024. "Interactions of Saccharomyces cerevisiae and Lactiplantibacillus plantarum Isolated from Light-Flavor Jiupei at Various Fermentation Temperatures" Foods 13, no. 18: 2884. https://doi.org/10.3390/foods13182884
APA StyleYang, P., Xi, B., Han, Y., Li, J., Luo, L., Qu, C., Li, J., Liu, S., Kang, L., Bai, B., Zhang, B., Zhao, S., Zhen, P., & Zhang, L. (2024). Interactions of Saccharomyces cerevisiae and Lactiplantibacillus plantarum Isolated from Light-Flavor Jiupei at Various Fermentation Temperatures. Foods, 13(18), 2884. https://doi.org/10.3390/foods13182884





