Targeting Gut Microbiota with Probiotics and Phenolic Compounds in the Treatment of Atherosclerosis: A Comprehensive Review
Abstract
:1. Introduction
2. Atherosclerosis
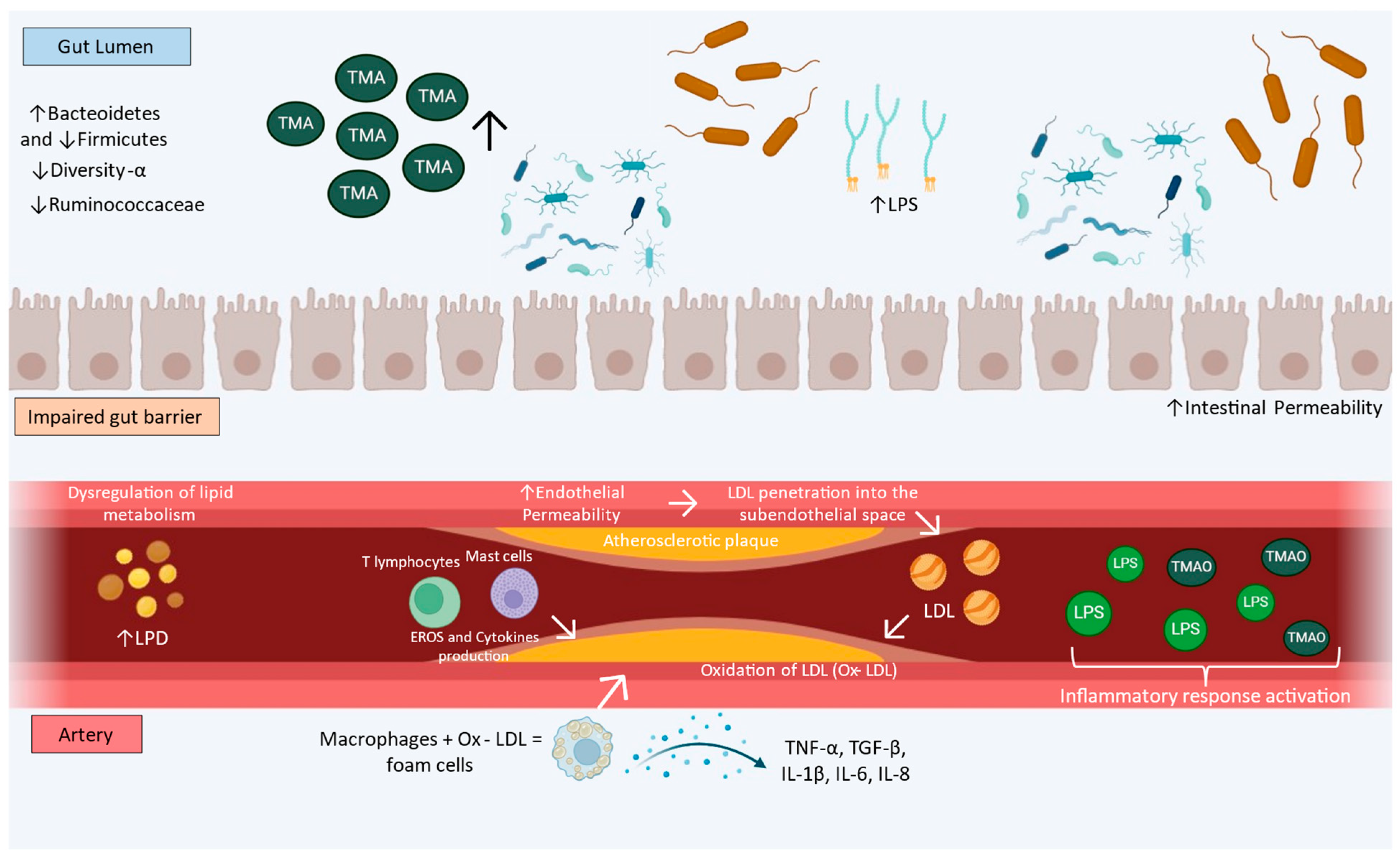
3. Gut Dysbiosis and Atherosclerosis
4. Probiotics and Atherosclerosis
4.1. Effects of Probiotics on Gut Microbiome in Atherosclerosis
4.2. Effects of Probiotics on Lipid Profile Aortic Plaque Deposition in Atherosclerosis
4.3. Effects of Probiotics on Endothelial Function in Atherosclerosis
4.4. Effects of Probiotics on Oxidative Stress and Inflammation in Atherosclerosis
5. Role of Quercetin on Atherosclerosis through Gut Microbiota
6. Role of Resveratrol in Atherosclerosis and Gut Microbiota
7. Role of Other Phenolic Compounds on Atherosclerosis and Gut Microbiota
7.1. Curcumin
7.2. Gallic Acid
7.3. Naringin
7.4. Procyanidin
7.5. Geraniin
7.6. Protocatechuic Acid
8. Combined Uses of Probiotics, Quercetin, and Resveratrol as Nutraceutical Candidates for Treating Atherosclerosis
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Parini, P.; Frikke-Schmidt, R.; Tselepis, A.D.; Moulin, P.; von Eckardstein, A.; Binder, C.J.; Catapano, A.L.; Ray, K.K.; Tokgozoglu, L. Taking action: European Atherosclerosis Society targets the United Nations Sustainable Development Goals 2030 agenda to fight atherosclerotic cardiovascular disease in Europe. Atherosclerosis 2021, 322, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Zuiderwijk, M.; Geerts, M.; van Rhijn, C.J.; van den Bogaerdt, A.; Hamming, J.F.; van Dijk, R.A.; Lindeman, J.H. Leukocyte Dynamics during the Evolution of Human Coronary Atherosclerosis: Conclusions from a Sevenfold, Chromogen-Based, Immunohistochemical Evaluation. Am. J. Pathol. 2018, 188, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Kritikou, E.; van der Heijden, T.; Swart, M.; van Duijn, J.; Slutter, B.; Wezel, A.; Smeets, H.J.; Maffia, P.; Kuiper, J.; Bot, I. Hypercholesterolemia Induces a Mast Cell-CD4(+) T Cell Interaction in Atherosclerosis. J. Immunol. 2019, 202, 1531–1539. [Google Scholar] [CrossRef]
- Vesnina, A.; Prosekov, A.; Atuchin, V.; Minina, V.; Ponasenko, A. Tackling Atherosclerosis via Selected Nutrition. Int. J. Mol. Sci. 2022, 23, 8233. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, C.; Lv, B.; He, S.; Zheng, Y.; Yang, W.; Wang, B.; Li, D.; Lin, J. Two-sample Mendelian randomization to study the causal association between gut microbiota and atherosclerosis. Front. Immunol. 2023, 14, 1282072. [Google Scholar] [CrossRef]
- Kumar, D.; Mukherjee, S.S.; Chakraborty, R.; Roy, R.R.; Pandey, A.; Patra, S.; Dey, S. The emerging role of gut microbiota in cardiovascular diseases. Indian Heart J. 2021, 73, 264–272. [Google Scholar] [CrossRef]
- Jonsson, A.L.; Backhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, X.; Hu, X.; Niu, H.; Tian, R.; Wang, H.; Pang, H.; Jiang, L.; Qiu, B.; Chen, X.; et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 2019, 7, 68. [Google Scholar] [CrossRef]
- Zhi, C.; Huang, J.; Wang, J.; Cao, H.; Bai, Y.; Guo, J.; Su, Z. Connection between gut microbiome and the development of obesity. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1987–1998. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.J.; van der Velden, S.; Rios-Morales, M.; van Faassen, M.J.R.; Loreti, M.G.; de Bruin, A.; et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Lepera, M.E.; Guaricci, A.I.; Forleo, C.; Cafiero, C.; Colella, M.; Palmirotta, R.; Santacroce, L. Might Gut Microbiota Be a Target for a Personalized Therapeutic Approach in Patients Affected by Atherosclerosis Disease? J. Pers. Med. 2023, 13, 1360. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Thompson, P.L.; Hamzah, J. Atherosclerosis: More Challenging and Complex Than We Thought. Clin. Ther. 2023, 45, 1017–1018. [Google Scholar] [CrossRef]
- Ziolkiewicz, A.; Kasprzak-Drozd, K.; Rusinek, R.; Markut-Miotla, E.; Oniszczuk, A. The Influence of Polyphenols on Atherosclerosis Development. Int. J. Mol. Sci. 2023, 24, 7146. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Reciprocal interactions between resveratrol and gut microbiota deepen our understanding of molecular mechanisms underlying its health benefits. Trends Food Sci. Technol. 2018, 81, 232–236. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Espirito-Santo, D.A.; Cordeiro, G.S.; Santos, L.S.; Silva, R.T.; Pereira, M.U.; Matos, R.J.B.; Boaventura, G.T.; Barreto-Medeiros, J.M. Cardioprotective effect of the quercetin on cardiovascular remodeling and atherosclerosis in rodents fed a high-fat diet: A systematic review. Chem. Biol. Interact. 2023, 384, 110700. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Li, D.; Ke, W.; Chen, F.; Hu, X. Targeting the gut microbiota with resveratrol: A demonstration of novel evidence for the management of hepatic steatosis. J. Nutr. Biochem. 2020, 81, 108363. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Wang, P.; Hu, X.; Zheng, L. Probiotics Bring New Hope for Atherosclerosis Prevention and Treatment. Oxidative Med. Cell. Longev. 2022, 2022, 3900835. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; de Souza, E.L.; Lacerda, D.C.; Cruz Neto, J.P.R.; Sales, L.C.S.; Silva Luis, C.C.; Pontes, P.B.; Cavalcanti Neto, M.P.; de Brito Alves, J.L. Evidence for Quercetin as a Dietary Supplement for the Treatment of Cardio-Metabolic Diseases in Pregnancy: A Review in Rodent Models. Foods 2022, 11, 2772. [Google Scholar] [CrossRef]
- Brito Sampaio, K.; Luiz de Brito Alves, J.; Mangueira do Nascimento, Y.; Fechine Tavares, J.; Sobral da Silva, M.; Dos Santos Nascimento, D.; Dos Santos Lima, M.; Priscila de Araujo Rodrigues, N.; Fernandes Garcia, E.; Leite de Souza, E. Nutraceutical formulations combining Limosilactobacillus fermentum, quercetin, and or resveratrol with beneficial impacts on the abundance of intestinal bacterial populations, metabolite production, and antioxidant capacity during colonic fermentation. Food Res. Int. 2022, 161, 111800. [Google Scholar] [CrossRef]
- Hassan, A.; Din, A.U.; Zhu, Y.; Zhang, K.; Li, T.; Wang, Y.; Xu, S.; Lei, H.; Yu, X.; Wang, G. Anti-atherosclerotic effects of Lactobacillus plantarum ATCC 14917 in ApoE−/− mice through modulation of proinflammatory cytokines and oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 6337–6350. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, H.Q.; Zhang, X.; Zhang, H.; Xia, J.; Ding, K.; Fang, Z.Y. Probiotic administration of lactobacillus rhamnosus GR-1 attenuates atherosclerotic plaque formation in ApoE−/− mice fed with a high-fat diet. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3533–3541. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Quan, G.; Wang, X.; Yang, L.; Zhong, L. Lactobacillus acidophilus ATCC 4356 prevents atherosclerosis via inhibition of intestinal cholesterol absorption in apolipoprotein E-knockout mice. Appl. Environ. Microbiol. 2014, 80, 7496–7504. [Google Scholar] [CrossRef]
- Khongrum, J.; Yingthongchai, P.; Boonyapranai, K.; Wongtanasarasin, W.; Aobchecy, P.; Tateing, S.; Prachansuwan, A.; Sitdhipol, J.; Niwasabutra, K.; Thaveethaptaikul, P.; et al. Safety and Effects of Lactobacillus paracasei TISTR 2593 Supplementation on Improving Cholesterol Metabolism and Atherosclerosis-Related Parameters in Subjects with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 661. [Google Scholar] [CrossRef]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Balzan, S.; Lubrano, V. LOX-1 receptor: A potential link in atherosclerosis and cancer. Life Sci. 2018, 198, 79–86. [Google Scholar] [CrossRef]
- Gioia, M.; Vindigni, G.; Testa, B.; Raniolo, S.; Fasciglione, G.F.; Coletta, M.; Biocca, S. Membrane Cholesterol Modulates LOX-1 Shedding in Endothelial Cells. PLoS ONE 2015, 10, e0141270. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.; Karathanasis, S.K.; Remaley, A.; Sposito, A.C. Role of LOX-1 (Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1) as a Cardiovascular Risk Predictor: Mechanistic Insight and Potential Clinical Use. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 153–166. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Chen, H.; Sawamura, T.; Ranganathan, S.; Mehta, J.L. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation 2003, 107, 612–617. [Google Scholar] [CrossRef]
- Markin, A.M.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Chakal, D.A.; Breshenkov, D.G.; Charchyan, E.R. The Role of Cytokines in Cholesterol Accumulation in Cells and Atherosclerosis Progression. Int. J. Mol. Sci. 2023, 24, 6426. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Chen, A.C.; Fang, T.J.; Ho, H.H.; Chen, J.F.; Kuo, Y.W.; Huang, Y.Y.; Tsai, S.Y.; Wu, S.F.; Lin, H.C.; Yeh, Y.T. A multi-strain probiotic blend reshaped obesity-related gut dysbiosis and improved lipid metabolism in obese children. Front. Nutr. 2022, 9, 922993. [Google Scholar] [CrossRef] [PubMed]
- Al Bataineh, M.T.; Kunstner, A.; Dash, N.R.; Alsafar, H.S.; Ragab, M.; Schmelter, F.; Sina, C.; Busch, H.; Ibrahim, S.M. Uncovering the relationship between gut microbial dysbiosis, metabolomics, and dietary intake in type 2 diabetes mellitus and in healthy volunteers: A multi-omics analysis. Sci. Rep. 2023, 13, 17943. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, M.; Chen, L.; Liu, L.; Tan, G.; Liu, J. Resveratrol promotes regression of renal carcinoma cells via a renin-angiotensin system suppression-dependent mechanism. Oncol. Lett. 2017, 13, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Dahal, R.H.; Kim, S.; Kim, Y.K.; Kim, E.S.; Kim, J. Insight into gut dysbiosis of patients with inflammatory bowel disease and ischemic colitis. Front. Microbiol. 2023, 14, 1174832. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Dong, L.; Jiang, Z.; Tan, L.; Luo, X.; Pei, G.; Qin, A.; Zhong, Z.; Liu, X.; Tang, Y.; et al. Probiotics ameliorate IgA nephropathy by improving gut dysbiosis and blunting NLRP3 signaling. J. Transl. Med. 2022, 20, 382. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M. Gut microbiota in overweight and obesity: Crosstalk with adipose tissue. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 164–183. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Dwivedi, G.; O’Gara, F.; Caparros-Martin, J.; Ward, N.C. The gut microbiome and cardiovascular disease: Current knowledge and clinical potential. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H923–H938. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Colangeli, L.; Escobar Marcillo, D.I.; Simonelli, V.; Iorio, E.; Rinaldi, T.; Sbraccia, P.; Fortini, P.; Guglielmi, V. The Crosstalk between Gut Microbiota and White Adipose Tissue Mitochondria in Obesity. Nutrients 2023, 15, 1723. [Google Scholar] [CrossRef]
- Andermann, T.; Antonelli, A.; Barrett, R.L.; Silvestro, D. Estimating Alpha, Beta, and Gamma Diversity Through Deep Learning. Front. Plant Sci. 2022, 13, 839407. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.E.; Martiny, J.B.H. Alpha-, beta-, and gamma-diversity of bacteria varies across habitats. PLoS ONE 2020, 15, e0233872. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2021, 12, 796025. [Google Scholar] [CrossRef]
- Prins, F.M.; Collij, V.; Groot, H.E.; Bjork, J.R.; Swarte, J.C.; Andreu-Sanchez, S.; Jansen, B.H.; Fu, J.; Harmsen, H.J.M.; Zhernakova, A.; et al. The gut microbiome across the cardiovascular risk spectrum. Eur. J. Prev. Cardiol. 2024, 31, 935–944. [Google Scholar] [CrossRef]
- Traughber, C.A.; Iacano, A.J.; Neupane, K.; Khan, M.R.; Opoku, E.; Nunn, T.; Prince, A.; Sangwan, N.; Hazen, S.L.; Smith, J.D.; et al. Impavido attenuates inflammation, reduces atherosclerosis, and alters gut microbiota in hyperlipidemic mice. iScience 2023, 26, 106453. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Paradis, T.; Begue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- Alves, J.L.B.; Costa, P.; Sales, L.C.S.; Silva Luis, C.C.; Bezerra, T.P.T.; Souza, M.L.A.; Costa, B.A.; de Souza, E.L. Shedding light on the impacts of Spirulina platensis on gut microbiota and related health benefits. Crit. Rev. Food Sci. Nutr. 2024, 1–14. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Shen, X.; Li, L.; Sun, Z.; Zang, G.; Zhang, L.; Shao, C.; Wang, Z. Gut Microbiota and Atherosclerosis-Focusing on the Plaque Stability. Front. Cardiovasc. Med. 2021, 8, 668532. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Yoon, B.H.; Lee, S.M.; Choi, M.; Kim, E.H.; Lee, B.W.; Kim, S.Y.; Pack, C.G.; Sung, Y.H.; Baek, I.J.; et al. Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency. Exp. Mol. Med. 2022, 54, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.R.; Tong, Q.; Lin, Y.; Pan, L.B.; Fu, J.; Peng, R.; Zhang, X.F.; Zhao, Z.X.; Li, Y.; Yu, J.B.; et al. Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota. Signal Transduct. Target. Ther. 2022, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Panyod, S.; Wu, W.K.; Peng, S.Y.; Tseng, Y.J.; Hsieh, Y.C.; Chen, R.A.; Huang, H.S.; Chen, Y.H.; Chuang, H.L.; Hsu, C.C.; et al. Ginger essential oil and citral ameliorates atherosclerosis in ApoE−/− mice by modulating trimethylamine-N-oxide and gut microbiota. NPJ Sci. Food 2023, 7, 19. [Google Scholar] [CrossRef]
- Geng, J.; Yang, C.; Wang, B.; Zhang, X.; Hu, T.; Gu, Y.; Li, J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 2018, 97, 941–947. [Google Scholar] [CrossRef]
- Boini, K.M.; Hussain, T.; Li, P.L.; Koka, S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell. Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased Liver Localization of Lipopolysaccharides in Human and Experimental NAFLD. Hepatology 2020, 72, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chiou, J. Potential Benefits of Probiotics and Prebiotics for Coronary Heart Disease and Stroke. Nutrients 2021, 13, 2878. [Google Scholar] [CrossRef]
- O’Morain, V.L.; Ramji, D.P. The Potential of Probiotics in the Prevention and Treatment of Atherosclerosis. Mol. Nutr. Food Res. 2020, 64, e1900797. [Google Scholar] [CrossRef]
- Abdi, M.; Esmaeili Gouvarchin Ghaleh, H.; Ranjbar, R. Lactobacilli and Bifidobacterium as anti-atherosclerotic agents. Iran. J. Basic. Med. Sci. 2022, 25, 934–946. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, M.; Liu, Y.; Xu, M.; Shi, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Bifidobacterium breve and Bifidobacterium longum Attenuate Choline-Induced Plasma Trimethylamine N-Oxide Production by Modulating Gut Microbiota in Mice. Nutrients 2022, 14, 1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Y.; Li, X.; Zhang, T.; Liang, M.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota. Nutrients 2022, 14, 1272. [Google Scholar] [CrossRef]
- Hassan, A.; Luqman, A.; Zhang, K.; Ullah, M.; Din, A.U.; Xiaoling, L.; Wang, G. Impact of Probiotic Lactiplantibacillus plantarum ATCC 14917 on atherosclerotic plaque and its mechanism. World J. Microbiol. Biotechnol. 2024, 40, 198. [Google Scholar] [CrossRef]
- Chan, Y.K.; El-Nezami, H.; Chen, Y.; Kinnunen, K.; Kirjavainen, P.V. Probiotic mixture VSL#3 reduce high fat diet induced vascular inflammation and atherosclerosis in ApoE−/− mice. AMB Express 2016, 6, 61. [Google Scholar] [CrossRef]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, H.; Yang, X.; Li, Y.; Zhang, Z.; Chen, F.; Zhao, L.; Zhang, C. Lactobacillus Mucosae Strain Promoted by a High-Fiber Diet in Genetic Obese Child Alleviates Lipid Metabolism and Modifies Gut Microbiota in ApoE−/− Mice on a Western Diet. Microorganisms 2020, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Ren, W.; Wang, P.; Zheng, L. Lactobacillus rhamnosus GG protects against atherosclerosis by improving ketone body synthesis. Appl. Microbiol. Biotechnol. 2022, 106, 8233–8243. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.R.; Davies, T.S.; Moss, J.W.E.; Calvente, D.L.; Ramji, D.P.; Marchesi, J.R.; Pechlivanis, A.; Plummer, S.F.; Hughes, T.R. The anti-cholesterolaemic effect of a consortium of probiotics: An acute study in C57BL/6J mice. Sci. Rep. 2017, 7, 2883. [Google Scholar] [CrossRef]
- Bendali, F.; Kerdouche, K.; Hamma-Faradji, S.; Drider, D. In vitro and in vivo cholesterol lowering ability of Lactobacillus pentosus KF923750. Benef. Microbes 2017, 8, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zheng, J.; Zong, X.; Yang, X.; Zhang, Y.; Man, C.; Jiang, Y. Preventive Effect and Molecular Mechanism of Lactobacillus rhamnosus JL1 on Food-Borne Obesity in Mice. Nutrients 2021, 13, 3989. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Asemi, Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1594–1598. [Google Scholar] [CrossRef]
- Raygan, F.; Rezavandi, Z.; Bahmani, F.; Ostadmohammadi, V.; Mansournia, M.A.; Tajabadi-Ebrahimi, M.; Borzabadi, S.; Asemi, Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018, 10, 51. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, J.; Wang, R.; Wang, Y.; Shu, X.; Wang, P.; Chen, C.; Zhang, F. Limosilactobacillus fermentum TY-S11 ameliorates hypercholesterolemia via promoting cholesterol excretion and regulating gut microbiota in high-cholesterol diet-fed apolipoprotein E-deficient mice. Heliyon 2024, 10, e32059. [Google Scholar] [CrossRef]
- Yadav, R.; Khan, S.H.; Mada, S.B.; Meena, S.; Kapila, R.; Kapila, S. Consumption of Probiotic Lactobacillus fermentum MTCC: 5898-Fermented Milk Attenuates Dyslipidemia, Oxidative Stress, and Inflammation in Male Rats Fed on Cholesterol-Enriched Diet. Probiotics Antimicrob. Proteins 2019, 11, 509–518. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitacion, N.; Sanchez, M.; Romero, M.; Olivares, M.; Jimenez, R.; Duarte, J. The Probiotic Lactobacillus fermentum Prevents Dysbiosis and Vascular Oxidative Stress in Rats with Hypertension Induced by Chronic Nitric Oxide Blockade. Mol. Nutr. Food Res. 2018, 62, e1800298. [Google Scholar] [CrossRef]
- Palani Kumar, M.K.; Halami, P.M.; Serva Peddha, M. Effect of Lactobacillus fermentum MCC2760-Based Probiotic Curd on Hypercholesterolemic C57BL6 Mice. ACS Omega 2021, 6, 7701–7710. [Google Scholar] [CrossRef] [PubMed]
- O’Morain, V.L.; Chan, Y.H.; Williams, J.O.; Alotibi, R.; Alahmadi, A.; Rodrigues, N.P.; Plummer, S.F.; Hughes, T.R.; Michael, D.R.; Ramji, D.P. The Lab4P Consortium of Probiotics Attenuates Atherosclerosis in LDL Receptor Deficient Mice Fed a High Fat Diet and Causes Plaque Stabilization by Inhibiting Inflammation and Several Pro-Atherogenic Processes. Mol. Nutr. Food Res. 2021, 65, e2100214. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, W.; Li, Y.; Luo, S.; Liu, Q.; Zhong, Y.; Jian, Z.; Bao, M. Lactobacillus acidophilus ATCC 4356 attenuates the atherosclerotic progression through modulation of oxidative stress and inflammatory process. Int. Immunopharmacol. 2013, 17, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Friques, A.G.; Arpini, C.M.; Kalil, I.C.; Gava, A.L.; Leal, M.A.; Porto, M.L.; Nogueira, B.V.; Dias, A.T.; Andrade, T.U.; Pereira, T.M.; et al. Chronic administration of the probiotic kefir improves the endothelial function in spontaneously hypertensive rats. J. Transl. Med. 2015, 13, 390. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men with Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Salari, A.; Kheirkhah, J.; Ghorbani, Z. The Effects of Probiotics on Inflammation, Endothelial Dysfunction, and Atherosclerosis Progression: A Mechanistic Overview. Heart Lung Circ. 2022, 31, e45–e71. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Z.; Zhao, Y.; Gao, Y.; Zhao, L.; Li, S. Weizmannia coagulans JA845 improves atherosclerosis induced by vitamin D3 and high-fat diet in rats through modulating lipid metabolism, oxidative stress, and endothelial vascular injury. J. Appl. Microbiol. 2023, 134, lxad165. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kitada, Y.; Naito, Y. Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients 2019, 11, 1188. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Tan, F.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus fermentum CQPC07 attenuates obesity, inflammation and dyslipidemia by modulating the antioxidant capacity and lipid metabolism in high-fat diet induced obese mice. J. Inflamm. 2021, 18, 5. [Google Scholar] [CrossRef]
- Tung, M.C.; Lan, Y.W.; Li, H.H.; Chen, H.L.; Chen, S.Y.; Chen, Y.H.; Lin, C.C.; Tu, M.Y.; Chen, C.M. Kefir peptides alleviate high-fat diet-induced atherosclerosis by attenuating macrophage accumulation and oxidative stress in ApoE knockout mice. Sci. Rep. 2020, 10, 8802. [Google Scholar] [CrossRef]
- Qi, J.J.; Wang, J.S.; Wang, Y. Long-term lactobacillus ATCC4356 supplementation prevents formation of atherosclerosis via P65-mediated reducing of inflammation and oxidative stress in apoE~(-/-) mice. Int. J. Clin. Exp. Med. 2016, 9, 12862–12870. [Google Scholar]
- Paulino do Nascimento, L.C.; Lacerda, D.C.; Ferreira, D.J.S.; de Souza, E.L.; de Brito Alves, J.L. Limosilactobacillus fermentum, Current Evidence on the Antioxidant Properties and Opportunities to be Exploited as a Probiotic Microorganism. Probiotics Antimicrob. Proteins 2022, 14, 960–979. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Khedmatgozar, H.; Nachvak, S.M.; Abdollahzad, H.; Moradinazar, M.; Sadeghpour Tabaei, A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Vissenaekens, H.; Grootaert, C.; Raes, K.; De Munck, J.; Smagghe, G.; Boon, N.; Van Camp, J. Quercetin Mitigates Endothelial Activation in a Novel Intestinal-Endothelial-Monocyte/Macrophage Coculture Setup. Inflammation 2022, 45, 1600–1611. [Google Scholar] [CrossRef]
- Ramireddy, L.; Tsen, H.Y.; Chiang, Y.C.; Hung, C.Y.; Wu, S.R.; Young, S.L.; Lin, J.S.; Huang, C.H.; Chiu, S.H.; Chen, C.C.; et al. Molecular Identification and Selection of Probiotic Strains Able to Reduce the Serum TMAO Level in Mice Challenged with Choline. Foods 2021, 10, 2931. [Google Scholar] [CrossRef]
- de Luna Freire, M.O.; Cruz Neto, J.P.R.; de Albuquerque Lemos, D.E.; de Albuquerque, T.M.R.; Garcia, E.F.; de Souza, E.L.; de Brito Alves, J.L. Limosilactobacillus fermentum Strains as Novel Probiotic Candidates to Promote Host Health Benefits and Development of Biotherapeutics: A Comprehensive Review. Probiotics Antimicrob. Proteins 2024, 16, 1483–1498. [Google Scholar] [CrossRef]
- de Luna Freire, M.O.; do Nascimento, L.C.P.; de Oliveira, K.A.R.; de Oliveira, A.M.; Dos Santos Lima, M.; Napoleao, T.H.; da Costa Silva, J.H.; Lagranha, C.J.; de Souza, E.L.; de Brito Alves, J.L. Limosilactobacillus fermentum Strains with Claimed Probiotic Properties Exert Anti-oxidant and Anti-inflammatory Properties and Prevent Cardiometabolic Disorder in Female Rats Fed a High-Fat Diet. Probiotics Antimicrob. Proteins 2023, 15, 601–613. [Google Scholar] [CrossRef]
- de Luna Freire, M.O.; do Nascimento, L.C.P.; de Oliveira, K.A.R.; de Oliveira, A.M.; Napoleao, T.H.; Lima, M.D.S.; Lagranha, C.J.; de Souza, E.L.; de Brito Alves, J.L. Effects of a Mixed Limosilactobacillus fermentum Formulation with Claimed Probiotic Properties on Cardiometabolic Variables, Biomarkers of Inflammation and Oxidative Stress in Male Rats Fed a High-Fat Diet. Foods 2021, 10, 2202. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Albuquerque, T.M.R.; Dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Sampaio, K.B.; do Nascimento, Y.M.; Tavares, J.F.; Cavalcanti, M.T.; de Brito Alves, J.L.; Garcia, E.F.; de Souza, E.L. Development and in vitro evaluation of novel nutraceutical formulations composed of Limosilactobacillus fermentum, quercetin and/or resveratrol. Food Chem. 2021, 342, 128264. [Google Scholar] [CrossRef]
- Sampaio, K.B.; de Brito Alves, J.L.; do Nascimento, Y.M.; Tavares, J.F.; da Silva, M.S.; Dos Santos Nascimento, D.; de Araujo Rodrigues, N.P.; Monteiro, M.C.; Garcia, E.F.; de Souza, E.L. Effects of Simulated Gastrointestinal Conditions on Combined Potentially Probiotic Limosilactobacillus fermentum 296, Quercetin, and/or Resveratrol as Bioactive Components of Novel Nutraceuticals. Probiotics Antimicrob. Proteins 2024, 16, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, Z.; Granato, D. Polyphenols in foods: Classification, methods of identification, and nutritional aspects in human health. Adv. Food Nutr. Res. 2021, 98, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Mirsafaei, L.; Reiner, Z.; Shafabakhsh, R.; Asemi, Z. Molecular and Biological Functions of Quercetin as a Natural Solution for Cardiovascular Disease Prevention and Treatment. Plant Foods Hum. Nutr. 2020, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, Y.; Yan, F.; Dong, M.; Ren, Y. Research progress of quercetin in cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1203713. [Google Scholar] [CrossRef]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARgamma, LXRalpha and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhao, C.H.; Yao, X.L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jiang, L.Y.; Wang, Y.C.; Ma, D.F.; Li, X. Quercetin Attenuates Atherosclerosis via Modulating Oxidized LDL-Induced Endothelial Cellular Senescence. Front. Pharmacol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Zhou, Y.; Tan, Y.; Feng, W.; Peng, C. Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Feng, J.; Li, Z.; Ma, H.; Yue, Y.; Hao, K.; Li, J.; Xiang, Y.; Min, Y. Quercetin alleviates intestinal inflammation and improves intestinal functions via modulating gut microbiota composition in LPS-challenged laying hens. Poult. Sci. 2023, 102, 102433. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zeng, Y.; Li, G.; Chen, J.; Chen, X. Quercetin improves high-fat diet-induced obesity by modulating gut microbiota and metabolites in C57BL/6J mice. Phytother. Res. 2022, 36, 4558–4572. [Google Scholar] [CrossRef]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-kappaB signaling pathway. Cell. Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef]
- Wu, D.N.; Guan, L.; Jiang, Y.X.; Ma, S.H.; Sun, Y.N.; Lei, H.T.; Yang, W.F.; Wang, Q.F. Microbiome and metabonomics study of quercetin for the treatment of atherosclerosis. Cardiovasc. Diagn. Ther. 2019, 9, 545–560. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Cross, T.L.; Everhart, J.; Kay, C.; Bolling, B.W.; Backhed, F.; Rey, F.E. Gut microbiota and diet matrix modulate the effects of the flavonoid quercetin on atherosclerosis. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Froldi, G.; Ragazzi, E. Selected Plant-Derived Polyphenols as Potential Therapeutic Agents for Peripheral Artery Disease: Molecular Mechanisms, Efficacy and Safety. Molecules 2022, 27, 7110. [Google Scholar] [CrossRef] [PubMed]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application—A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Yao, M.; Fei, Y.; Zhang, S.; Qiu, B.; Zhu, L.; Li, F.; Berglund, B.; Xiao, H.; Li, L. Gut Microbiota Composition in Relation to the Metabolism of Oral Administrated Resveratrol. Nutrients 2022, 14, 1013. [Google Scholar] [CrossRef]
- Huminiecki, L.; Atanasov, A.G.; Horbanczuk, J. Etiology of atherosclerosis informs choice of animal models and tissues for initial functional genomic studies of resveratrol. Pharmacol. Res. 2020, 156, 104598. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Luo, J.Y.; Lau, C.W.; Chen, Z.Y.; Tian, X.Y.; Huang, Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020, 177, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.R.; Bozorgi, M.; Khazaei, M.; Aftabi, M.; Bozorgi, A. Resveratrol Nanoformulation Inhibits Invasive Breast Cancer Cell Growth through Autophagy Induction: An In Vitro Study. Cell J. 2024, 26, 112–120. [Google Scholar] [CrossRef]
- Ei, Z.Z.; Srithawirat, T.; Chunhacha, P.; Chaotham, C.; Arunmanee, W.; Phookphan, P.; Chanvorachote, P. Resveratrol Shows Potent Senescence Reversal in Experimental Cellular Models of Particular Matter 2.5-induced Cellular Senescence in Human Dermal Papilla Cells. In Vivo 2024, 38, 665–673. [Google Scholar] [CrossRef]
- Zeini, S.; Davoodian, N.; Kazemi, H.; Shareghi Brojeni, M.; Ghani, E.; Arab Firouzjaei, M.; Atashabparvar, A. Resveratrol prevents cognitive impairment and hippocampal inflammatory response induced by lipopolysaccharide in a mouse model of chronic neuroinflammation. Physiol. Behav. 2024, 278, 114508. [Google Scholar] [CrossRef]
- Bartra, C.; Yuan, Y.; Vuraic, K.; Valdes-Quiroz, H.; Garcia-Baucells, P.; Slevin, M.; Pastorello, Y.; Sunol, C.; Sanfeliu, C. Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer’s Disease Inflammation. Antioxidants 2024, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, X.; Lv, N.; Wang, L.; Gan, J.; Jiang, X.; Wang, Y. Therapeutic Role and Potential Mechanism of Resveratrol in Atherosclerosis: TLR4/NF-kappaB/HIF-1alpha. Mediat. Inflamm. 2023, 2023, 1097706. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, Y.; Cai, X.; Gu, M.; Sun, J.; Qi, C.; Goulette, T.; Song, M.; Li, Z.; Xiao, H. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. 2020, 11, 1063–1073. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, L. Resveratrol provides benefits in mice with type II diabetes-induced chronic renal failure through AMPK signaling pathway. Exp. Ther. Med. 2018, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Wang, S.; Zhang, C.; Zheng, L.; Jia, Y.; Xu, M.; Zhu, T.; Zhang, Y.; Rong, R. Resveratrol Alleviates Inflammatory Responses and Oxidative Stress in Rat Kidney Ischemia-Reperfusion Injury and H2O2-Induced NRK-52E Cells via the Nrf2/TLR4/NF-kappaB Pathway. Cell. Physiol. Biochem. 2018, 45, 1677–1689. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Choi, W.; Ju Son, D.; Sung Kim, Y.; Kim, M.K.; Yoon, B.E.; Pyee, J.; Tae Hong, J.; Go, Y.M.; et al. Antiatherogenic Effect of Resveratrol Attributed to Decreased Expression of ICAM-1 (Intercellular Adhesion Molecule-1). Arterioscler. Thromb. Vasc. Biol. 2019, 39, 675–684. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Xie, X. Resveratrol inhibiting TGF/ERK signaling pathway can improve atherosclerosis: Backgrounds, mechanisms and effects. Biomed. Pharmacother. 2022, 155, 113775. [Google Scholar] [CrossRef]
- Zhou, L.; Long, J.; Sun, Y.; Chen, W.; Qiu, R.; Yuan, D. Resveratrol ameliorates atherosclerosis induced by high-fat diet and LPS in ApoE−/− mice and inhibits the activation of CD4(+) T cells. Nutr. Metab. 2020, 17, 41. [Google Scholar] [CrossRef]
- Xu, L.; Wang, R.; Liu, H.; Wang, J.; Mang, J.; Xu, Z. Resveratrol Treatment Is Associated with Lipid Regulation and Inhibition of Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) in Rabbits Fed a High-Fat Diet. Evid.-Based Complement. Altern. Med. 2020, 2020, 9641582. [Google Scholar] [CrossRef]
- Hou, C.Y.; Chen, Y.W.; Hazeena, S.H.; Tain, Y.L.; Hsieh, C.W.; Chen, D.Q.; Liu, R.Y.; Shih, M.K. Cardiovascular risk of dietary trimethylamine oxide precursors and the therapeutic potential of resveratrol and its derivatives. FEBS Open Bio 2024, 14, 358–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, E.; Ma, L.; Zhai, P. Dietary resveratrol increases the expression of hepatic 7alpha-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis. 2012, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef]
- Sudhir, K. Clinical review: Lipoprotein-associated phospholipase A2, a novel inflammatory biomarker and independent risk predictor for cardiovascular disease. J. Clin. Endocrinol. Metab. 2005, 90, 3100–3105. [Google Scholar] [CrossRef]
- Li, J.; Zhong, Z.; Yuan, J.; Chen, X.; Huang, Z.; Wu, Z. Resveratrol improves endothelial dysfunction and attenuates atherogenesis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2019, 67, 63–71. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Hu, M.M.; Xin, Y.F.; Gang, C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm. Res. 2015, 64, 321–332. [Google Scholar] [CrossRef]
- Raj, P.; Thandapilly, S.J.; Wigle, J.; Zieroth, S.; Netticadan, T. A Comprehensive Analysis of the Efficacy of Resveratrol in Atherosclerotic Cardiovascular Disease, Myocardial Infarction and Heart Failure. Molecules 2021, 26, 6600. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P.; et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 2013, 5, 205ra133. [Google Scholar] [CrossRef]
- Juan, M.E.; Maijo, M.; Planas, J.M. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J. Pharm. Biomed. Anal. 2010, 51, 391–398. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, Y.; Wang, D.; Zhao, W.; Hu, X.; Chen, F.; Zhao, X. Protective Effects of Dietary Resveratrol against Chronic Low-Grade Inflammation Mediated through the Gut Microbiota in High-Fat Diet Mice. Nutrients 2022, 14, 1994. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Sung, M.M.; Kim, T.T.; Denou, E.; Soltys, C.M.; Hamza, S.M.; Byrne, N.J.; Masson, G.; Park, H.; Wishart, D.S.; Madsen, K.L.; et al. Improved Glucose Homeostasis in Obese Mice Treated With Resveratrol Is Associated With Alterations in the Gut Microbiome. Diabetes 2017, 66, 418–425. [Google Scholar] [CrossRef]
- Zhang, J.; Ou, C.; Chen, M. Curcumin attenuates cadmium-induced atherosclerosis by regulating trimethylamine-N-oxide synthesis and macrophage polarization through remodeling the gut microbiota. Ecotoxicol. Environ. Saf. 2022, 244, 114057. [Google Scholar] [CrossRef]
- Clark, M.; Centner, A.M.; Ukhanov, V.; Nagpal, R.; Salazar, G. Gallic acid ameliorates atherosclerosis and vascular senescence and remodels the microbiome in a sex-dependent manner in ApoE−/− mice. J. Nutr. Biochem. 2022, 110, 109132. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, C.; Tian, G.; Wei, X.; Ma, Z.; Cui, J.; Wei, R.; Bao, Y.; Kong, W.; Zheng, J. Naringin Alleviates Atherosclerosis in ApoE−/− Mice by Regulating Cholesterol Metabolism Involved in Gut Microbiota Remodeling. J. Agric. Food Chem. 2020, 68, 12651–12660. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Li, W.; You, B.; Yu, J.; Huang, X.; Yang, R. Gut Microbiota Composition Affects Procyanidin A2-Attenuated Atherosclerosis in ApoE−/− Mice by Modulating the Bioavailability of Its Microbial Metabolites. J. Agric. Food Chem. 2021, 69, 6989–6999. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Wang, X.; Li, J.; Zhao, P.; Xi, X.; Feng, Y.; Yin, L.; Tian, J.; Li, H.; Liu, X.; et al. Anti-atherosclerotic effects of geraniin through the gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway in mice. Phytomedicine 2022, 101, 154104. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice—Role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Olszowy-Tomczyk, M. A Concise Profile of Gallic Acid-From Its Natural Sources through Biological Properties and Chemical Methods of Determination. Molecules 2023, 28, 1186. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, V.S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Dar, A.H.; Ayaz Mukarram, S.; Harsanyi, E.; Kovacs, B. Phytochemical Properties, Extraction, and Pharmacological Benefits of Naringin: A Review. Molecules 2023, 28, 5623. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.; Yang, M.; Zhang, L.; Wei, R.; Meng, K.; Bao, Y.; Zhang, L.; Zheng, J. Four Citrus Flavanones Exert Atherosclerosis Alleviation Effects in ApoE−/− Mice via Different Metabolic and Signaling Pathways. J. Agric. Food Chem. 2021, 69, 5226–5237. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Wang, L.; Fumoto, T.; Masumoto, S.; Shoji, T.; Miura, T.; Naraoka, M.; Matsuda, N.; Imaizumi, T.; Ohkuma, H. Regression of atherosclerosis with apple procyanidins by activating the ATP-binding cassette subfamily A member 1 in a rabbit model. Atherosclerosis 2017, 258, 56–64. [Google Scholar] [CrossRef]
- Okuda, T.; Mori, K.; Terayama, K.; Higuchi, K.; Hatano, T. [Isolation of geraniin from plants of Geranium and Euphorbiaceae (author’s transl)]. Yakugaku Zasshi 1979, 99, 543–545. [Google Scholar] [CrossRef]
- Ito, H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011, 77, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Boakye, Y.D.; Agyare, C.; Abotsi, W.K.; Ayande, P.G.; Ossei, P.P. Anti-inflammatory activity of aqueous leaf extract of Phyllanthus muellerianus (Kuntze) Exell. and its major constituent, geraniin. J. Ethnopharmacol. 2016, 187, 17–27. [Google Scholar] [CrossRef]
- Elendran, S.; Wang, L.W.; Prankerd, R.; Palanisamy, U.D. The physicochemical properties of geraniin, a potential antihyperglycemic agent. Pharm. Biol. 2015, 53, 1719–1726. [Google Scholar] [CrossRef]
- Shui, G.; Bao, Y.M.; Bo, J.; An, L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Tang, H.; Song, S.; Lu, L.; Zhang, P.; Li, X. Effects of protocatechuic acid on ameliorating lipid profiles and cardio-protection against coronary artery disease in high fat and fructose diet fed in rats. J. Vet. Med. Sci. 2020, 82, 1387–1394. [Google Scholar] [CrossRef]
- Ding, H.; Liu, J.; Chen, Z.; Huang, S.; Yan, C.; Kwek, E.; He, Z.; Zhu, H.; Chen, Z.Y. Protocatechuic acid alleviates TMAO-aggravated atherosclerosis via mitigating inflammation, regulating lipid metabolism, and reshaping gut microbiota. Food Funct. 2024, 15, 881–893. [Google Scholar] [CrossRef]
- Hoti, G.; Matencio, A.; Rubin Pedrazzo, A.; Cecone, C.; Appleton, S.L.; Khazaei Monfared, Y.; Caldera, F.; Trotta, F. Nutraceutical Concepts and Dextrin-Based Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4102. [Google Scholar] [CrossRef]
- Dama, A.; Shpati, K.; Daliu, P.; Dumur, S.; Gorica, E.; Santini, A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients 2024, 16, 507. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jung, S.K. Nutraceuticals for prevention of atherosclerosis: Targeting monocyte infiltration to the vascular endothelium. J. Food Biochem. 2020, 44, e13200. [Google Scholar] [CrossRef]
- Aquila, G.; Marracino, L.; Martino, V.; Calabria, D.; Campo, G.; Caliceti, C.; Rizzo, P. The Use of Nutraceuticals to Counteract Atherosclerosis: The Role of the Notch Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 5470470. [Google Scholar] [CrossRef]
- Moss, J.W.; Ramji, D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.E.; Williams, J.O.; Ramji, D.P. Nutraceuticals as therapeutic agents for atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Guo, J.; Dai, R.; Zhu, C.; Zhang, Z. Targeting gut microbiota and immune crosstalk: Potential mechanisms of natural products in the treatment of atherosclerosis. Front. Pharmacol. 2023, 14, 1252907. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Gil-Sanchez, I.; Ayuda-Duran, B.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Bartolome, B.; Moreno-Arribas, M.V. An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuno, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- de Llano, D.G.; Gil-Sánchez, I.; Esteban-Fernández, A.; Ramos, A.M.; Fernández-Díaz, M.; Cueva, C.; Moreno-Arribas, M.V.; Bartolomé, B. Reciprocal beneficial effects between wine polyphenols and probiotics: An exploratory study. Eur. Food Res. Technol. 2017, 243, 531–538. [Google Scholar] [CrossRef]
- Succi, M.; Tremonte, P.; Pannella, G.; Tipaldi, L.; Cozzolino, A.; Coppola, R.; Sorrentino, E. Survival of commercial probiotic strains in dark chocolate with high cocoa and phenols content during the storage and in a static in vitro digestion model. J. Funct. Foods 2017, 35, 60–67. [Google Scholar] [CrossRef]
- Dos Santos, A.S.; de Albuquerque, T.M.R.; de Brito Alves, J.L.; de Souza, E.L. Effects of Quercetin and Resveratrol on in vitro Properties Related to the Functionality of Potentially Probiotic Lactobacillus Strains. Front. Microbiol. 2019, 10, 2229. [Google Scholar] [CrossRef]
- Dos Santos Nascimento, D.; Sampaio, K.B.; do Nascimento, Y.M.; de Souza, T.A.; de Souza, F.S.; Junior, J.V.C.; Tavares, J.F.; da Silva, M.S.; de Brito Alves, J.L.; de Souza, E.L. Evaluating the Stability of a Novel Nutraceutical Formulation Combining Probiotic Limosilactobacillus fermentum 296, Quercetin, and Resveratrol Under Different Storage Conditions. Probiotics Antimicrob. Proteins 2024, 16, 13–25. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, G.; Zhao, X.; Zhang, H.; Ren, M.; Song, X.; Chang, H.; Jing, Z. Probiotics combined with atorvastatin administration in the treatment of hyperlipidemia: A randomized, double-blind, placebo-controlled clinical trial. Medicine 2024, 103, e37883. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ma, T.; Li, Y.; Yang, N.; Li, B.; Zhou, X.; Guo, S.; Zhang, S.; Kwok, L.Y.; Sun, Z.; et al. Bifidobacterium lactis Probio-M8 Adjuvant Treatment Confers Added Benefits to Patients with Coronary Artery Disease via Target Modulation of the Gut-Heart/-Brain Axes. mSystems 2022, 7, e0010022. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Macchi, C.; Botta, M.; Dall’Orto, D.; Del Puppo, M.; Bertolotti, M.; Bosisio, R.; Mombelli, G.; et al. Nutraceutical approach for the management of cardiovascular risk—A combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: Results from a randomized, double-blind, placebo-controlled study. Nutr. J. 2019, 18, 13. [Google Scholar] [CrossRef]
- Guerrero-Bonmatty, R.; Gil-Fernandez, G.; Rodriguez-Velasco, F.J.; Espadaler-Mazo, J. A Combination of Lactoplantibacillus plantarum Strains CECT7527, CECT7528, and CECT7529 Plus Monacolin K Reduces Blood Cholesterol: Results from a Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 1206. [Google Scholar] [CrossRef]


| Treatment | Dose | Model | Time Intervention | Outcomes in Gut Microbiota | References |
|---|---|---|---|---|---|
| Lactiplantibacillus plantarum ATCC 14917 | 109 CFU | ApoE−/− mice fed a high-fat diet | 12 weeks | ↑ intestinal integrity ↑ mRNA expression ZO-1, occluding, claudin-3, MUC-3 ↓ LPS in mesenteric adipose tissue. | Hassan et al., 2024 [78] |
| Probiotic Mixture (B. breve, B. longum, B. infantis, L. acidophilus, L. platarum, L. paracasei, L. bulgaricus, S. thermophilus) | 2.78 × 1011 CFU/day | ApoE−/− female mice fed a high-fat diet | 12 weeks | ↔ Distinct compositional profile of the gut microbiota in the ileum, and colon. | Chan et al., 2016 [79] |
| L. plantarum ZDY04 | 1 × 109 CFU | Female BALB/c mice fed a chow diet with 1.3% choline chloride | 4 weeks | ↓ serum TMAO ↓ cecal TMA ~ cecal microbiota richness and diversity ↑ Lachnospiraceae, Erysipelotrichaceae, and Bacteroidaceae ↓ Aerococcaceae (families) ↑ Enterorhabdus, Succinivibrionaceae UGG-002, Lachnospiraceae UGG-006, Lachnospiraceae NK4A136, Ruminiclostridium 9, Lachnospiraceae XPB1014, Ruminococcaceae UCG-014, Ruminococcaceae NK4A214, Christensenellaceae R-7, and Rikenellaceae RC9 (genus) ↔ Distinct microbiota structure | Qiu et al., 2018 [80] |
| Lactobacillus mucosae A1 | 1 × 109 CFU | ApoE−/− mice fed a high-fat high-cholesterol | 13 weeks | ~ decreases serum TMA, TMAO, and LBP ~ riches and diversity ↔ Distinct microbiota structure ↓ Oscillibacter, Ruminiclostridium, Harryflintia, Enterorhabdus, Anaerovorax, Eubacterium, Turicibacter, Enterococcus, unclassified Ruminococcaceae, unclassified Clostridiales, unclassified Lachnospiraceae | Jiang et al., 2020 [81] |
| Lactobacillus rhamnosus GG | 1 × 107 CFU once a week | ApoE−/− mice were fed a high-fat diet with an additional Lactobacillus rhamnosus GG suspension | 12 weeks | ~ Chao 1 and Ace indices ↑ Simpson and Shannon indices ↓ Proteobacteria ↑ Firmicutes ↑ Lactobacillus ↓ Desulfovibrionaceae (genus) ↔ Distinct microbiota diversity | Zhai et al., 2022 [82] |
| L. plantarum, L. reuteri, L. casei, B. breve, and B. adolescentis | 1 × 109 CFU | Female C57BL/6 mice fed a Paigen atherogenic diet | 16 weeks | ~ α-diversity ↔ Distinct microbiota diversity with an overlap among probiotic intervention groups. | Wang et al., 2022 [77] |
| Treatment | Dose | Model | Time Intervention | Outcomes in Gut Microbiota | Reference |
|---|---|---|---|---|---|
| Quercetin | 100 µg/day | LDLR−/− fed a high-fat diet | 12 weeks | ↑ α-diversity ↑ Akkermansia, Bacteroides, Parabacteroides, and Ruminococcus (phylum) | Nie et al., 2019 [128] |
| Quercetin | 100 mg/kg/day | ApoE−/− mice fed a high-cholesterol diet | 12 weeks | ↑ Streptophyta, Enterobacter, Mobilitalea, Clostridium, Phascolarctobacterium, Candidatus Stoquefichus, Faecalimonas, Faecalibaculum, Anaerovibrio, Deltaproteobacteria Unclassified, and Acutalibacter (genus) | Wu et al., 2019 [129] |
| Quercetin | 0.1% w/w added to diet | ApoE−/− mice fed a high-MAC diet supplemented with quercetin | 16 weeks | ↑ gut microbiota richness Atherosclerotic plaque areas were negatively associated with the Eggerthellaceae and Erysipelotrichaceae families and positively associated with the Lactobacillaceae family. | Kasahara et al., 2023 [130] |
| Resveratrol | 0.4% added to diet | ApoE−/− mice fed a choline-rich diet supplemented with resveratrol | 4 months | ↑ Bacteroides, Akkermansia, Lactobacillus, and Bifidobacterium (genus). ↑ Fecal BA loss. | Chen et al. 2016 [165] |
| Curcumin | 100 mg/kg b.w. by gavage | ApoE−/− mice fed a high-fat diet | 2 months | ~ α diversity ↑ Firmicute/Bacteroidetes ratio ↑ Abundance of Verrucomicrobia Downregulation Lactobacillaceae family ↑ Unspecifield_S24_7 and Akkermansia abundance ↓ Lactobacillus abundance ↓ TMAO plasma levels | Zhang; Ou; Chen., 2022 [168] |
| Curcumin | dosage of 100 mg/kg | LDLr−/− mice fed a high-fat, high-cholesterol Western-type diet | 16 weeks | ↓ Intestinal permeability ↓ LPS plasma levels ↓ Plasma appearance of FITIC- dextran | Gosh et al., 2014 [174] |
| Gallic Acid | 0.2% gallic acid in drinking water | ApoE−/− mice fed a Paigen atherogenic purified diet | 2 weeks | α diversity similar ↓ Ratio of obligate anaerobic/anaerobic bacteria in females ↓ Ratio of gram-positive/negative bacteria in males ↓ Atherosclerotic plaque formation in males | Clark et al., 2022 [169] |
| Naringin | dosage of 100 mg/kg/day | Female ApoE−/− fed a high-fat diet | 16 weeks | ↑ Fecal excretion total lipid, total bile acids, and coprostanol ↑ Firmicutes and ↓Bacteroidetes and Verrucomicrobia phylum abundance ↓ Bacteroides, Bifidobacterium, Lactococcus e Clostridium sensu_stricto_1 ↓ Streptococcus, Desulfovibrio, Parasutterella, and Bacteroides | Wang et al., 2020 [170] |
| Naringin, hesperidin, naringenin, and hesperidin | 100 mg/kg/day of each polyphenol | Female ApoE−/− mice fed a high-fat diet | 16 weeks | ↑ Lactobacillus, Eubacterium coprostanoligenes, and Eubacterium brachy ↓ Bacteroides, Lactococcus, and Clostridium_sensu_stricto_1 | Wang et al., 2021 [177] |
| Procyanidin A2 | 110 mg/kg body weight/day | Male ApoE−/− fed a high-fat diet | 12 weeks | ↑ α diversity ↓ Firmicutes/Bacteroidetes ratio ↑ Verrucomicrobia relative abundance ↑ Relative abundance of Akkermansia, Prevotellaceae, and Coriobacteriaceae_UGG-002 | Yang et al., 2021 [171] |
| Geraniin | 80 mg/kg of body weight/day dissolved in drinking water | ApoE−/− mice fed a high-choline diet (0.08% choline diet and 1% choline diet) | 12 weeks | ↓ TMAO plasma levels ↑ Bacteroides, Alistipes, Flavonifractor, Clostridium XIVb, Butyricicoccus, Anaeroplasma, Clostridium XI, Enterorhabdus, Erysipeiotrichaceae_incertae_sedis, Anaerotruncus, Intestinimonas, Vampirovibrio, and Butyricimonas ↓ Phenomena’s, Rhizobium, Stenotrophomonas, Burkholdeira, Serratia, Ruminococcus, Blautia, Parasutterella, Pseudomonas, Roseburia, and Streptophyta | Lin et al., 2022 [172] |
| Protocatechuic acid | Western diet supplemented with protocatechuic acid (0.5% or 1.0%) | Male ApoE−/− mice fed a Western diet supplemented with TMAO | 12 weeks | ↓ TNF-α, MCP-1, IL-1β, and IL-6 plasma levels ↑ α diversity | Ding et al., 2024 [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Neto, J.P.R.; de Luna Freire, M.O.; de Albuquerque Lemos, D.E.; Ribeiro Alves, R.M.F.; de Farias Cardoso, E.F.; de Moura Balarini, C.; Duman, H.; Karav, S.; de Souza, E.L.; de Brito Alves, J.L. Targeting Gut Microbiota with Probiotics and Phenolic Compounds in the Treatment of Atherosclerosis: A Comprehensive Review. Foods 2024, 13, 2886. https://doi.org/10.3390/foods13182886
Cruz Neto JPR, de Luna Freire MO, de Albuquerque Lemos DE, Ribeiro Alves RMF, de Farias Cardoso EF, de Moura Balarini C, Duman H, Karav S, de Souza EL, de Brito Alves JL. Targeting Gut Microbiota with Probiotics and Phenolic Compounds in the Treatment of Atherosclerosis: A Comprehensive Review. Foods. 2024; 13(18):2886. https://doi.org/10.3390/foods13182886
Chicago/Turabian StyleCruz Neto, José Patrocínio Ribeiro, Micaelle Oliveira de Luna Freire, Deborah Emanuelle de Albuquerque Lemos, Rayanne Maira Felix Ribeiro Alves, Emmily Ferreira de Farias Cardoso, Camille de Moura Balarini, Hatice Duman, Sercan Karav, Evandro Leite de Souza, and José Luiz de Brito Alves. 2024. "Targeting Gut Microbiota with Probiotics and Phenolic Compounds in the Treatment of Atherosclerosis: A Comprehensive Review" Foods 13, no. 18: 2886. https://doi.org/10.3390/foods13182886
APA StyleCruz Neto, J. P. R., de Luna Freire, M. O., de Albuquerque Lemos, D. E., Ribeiro Alves, R. M. F., de Farias Cardoso, E. F., de Moura Balarini, C., Duman, H., Karav, S., de Souza, E. L., & de Brito Alves, J. L. (2024). Targeting Gut Microbiota with Probiotics and Phenolic Compounds in the Treatment of Atherosclerosis: A Comprehensive Review. Foods, 13(18), 2886. https://doi.org/10.3390/foods13182886








