Dynamic Changes of Active Components and Volatile Organic Compounds in Rosa roxburghii Fruit during the Process of Maturity
Abstract
1. Introduction
2. Materials and Methods
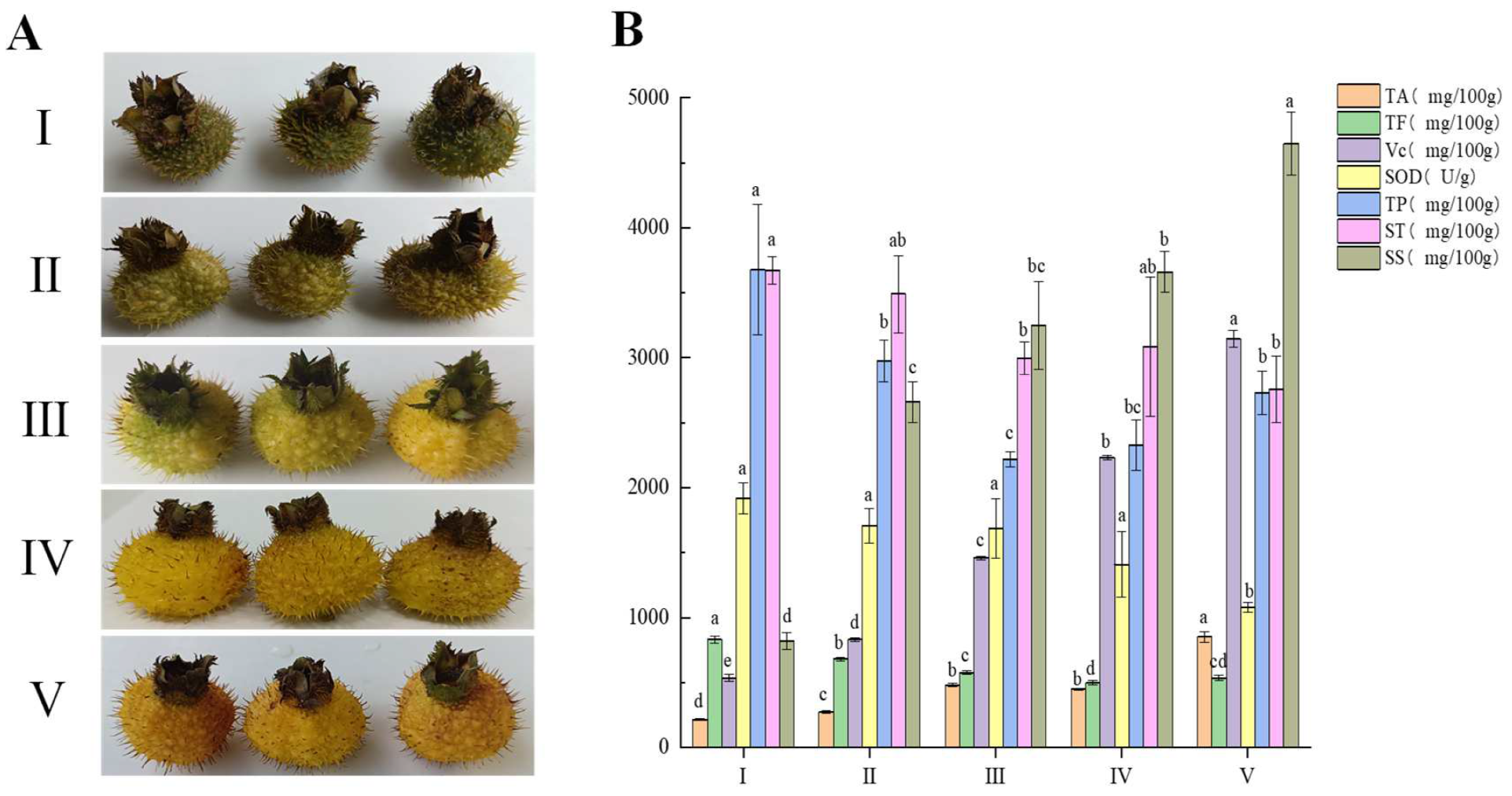
2.1. Plant Materials and Samples
2.2. Chemicals and Reagents
2.3. Instruments and Equipment
2.4. Determination of Active Ingredients in Rosa roxburghii Fruit
2.4.1. Measurement of Total Acid in Rosa roxburghii Fruit
2.4.2. Measurement of Total Flavonoid in Rosa roxburghii Fruit
2.4.3. Measurement of Vitamin C in Rosa roxburghii Fruit
2.4.4. Measurement of Superoxide Dismutase in Rosa roxburghii Fruit
2.4.5. Measurement of Total Phenolic in Rosa roxburghii Fruit
2.4.6. Measurement of Soluble Tannin in Rosa roxburghii Fruit
2.4.7. Measurement of Soluble Sugar in Rosa roxburghii Fruit
2.5. Determination of Relative Contents of Volatile Organic Compounds (VOCs) in Rosa roxburghii Fruit
2.6. Relative Odor Activity Value of VOCs
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes of Active Components of R. roxburghii Fruit at Different Stages of Maturity
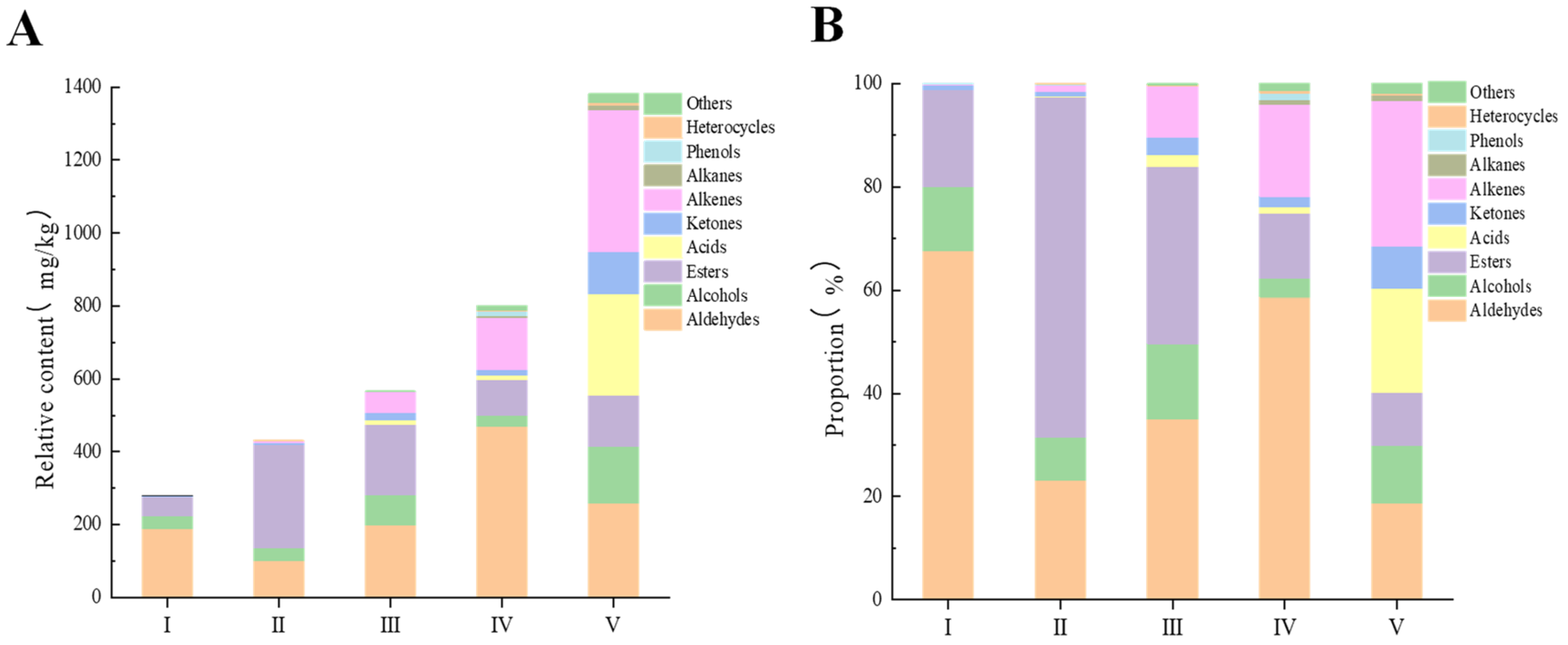
3.2. The Composition and Changes of VOCs in the Ripening Process of R. roxburghii Fruit
3.2.1. Dynamic Changes of Volatile Aldehydes during the Ripening Process of R. roxburghii
3.2.2. Dynamic Changes of Volatile Alcohols during the Ripening Process of R. roxburghii
3.2.3. Dynamic Changes of Volatile Esters during the Ripening Process of R. roxburghii
3.2.4. Dynamic Changes of Volatile Acids during the Ripening Process of R. roxburghii
3.2.5. Dynamic Changes of Volatile Ketones during the Ripening Process of R. roxburghii
3.2.6. Dynamic Changes of Volatile Alkenes during the Ripening Process of R. roxburghii
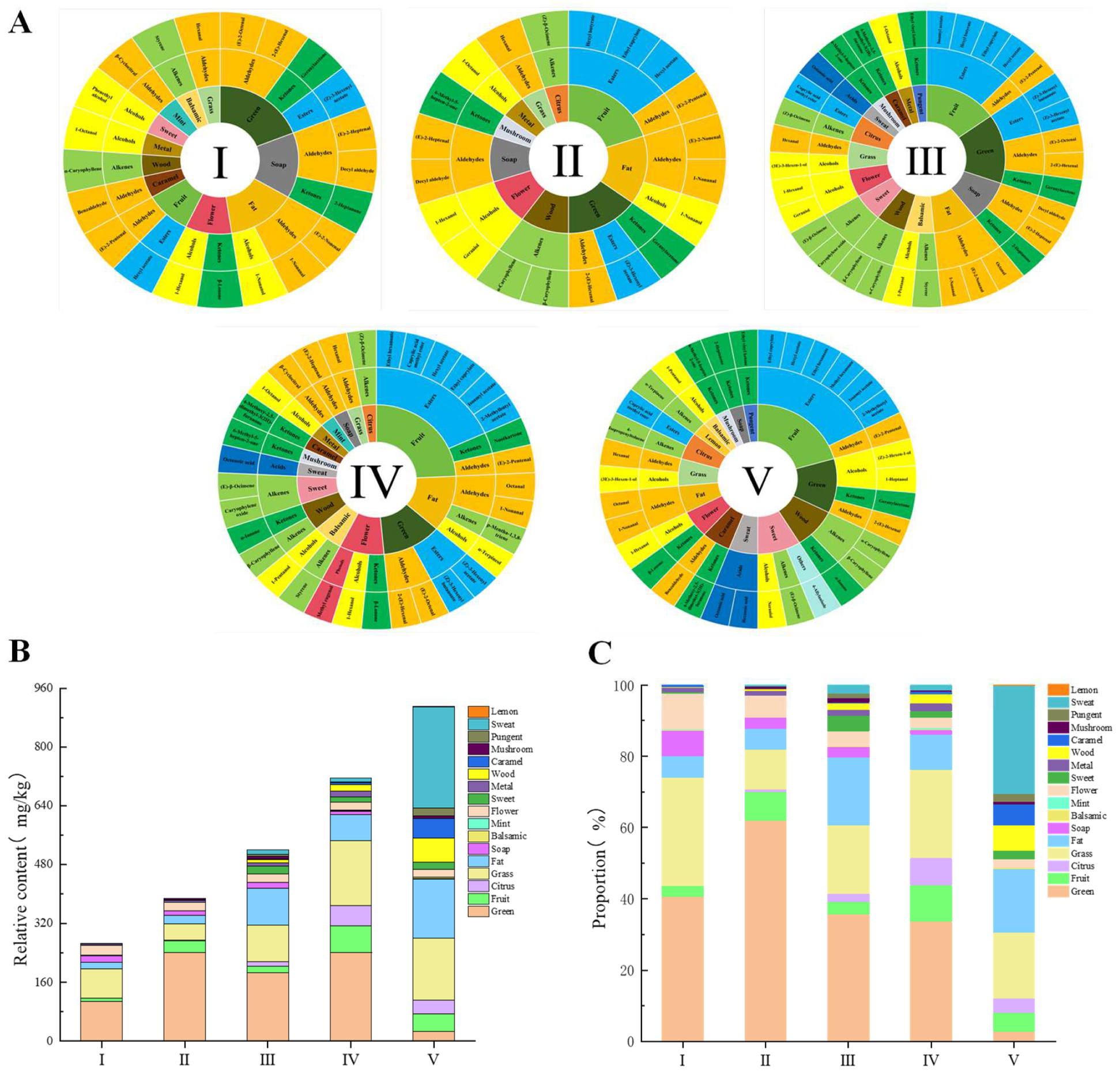
3.3. Analysis of ROAV Values of VOCs during the Ripening Process of R. roxburghii
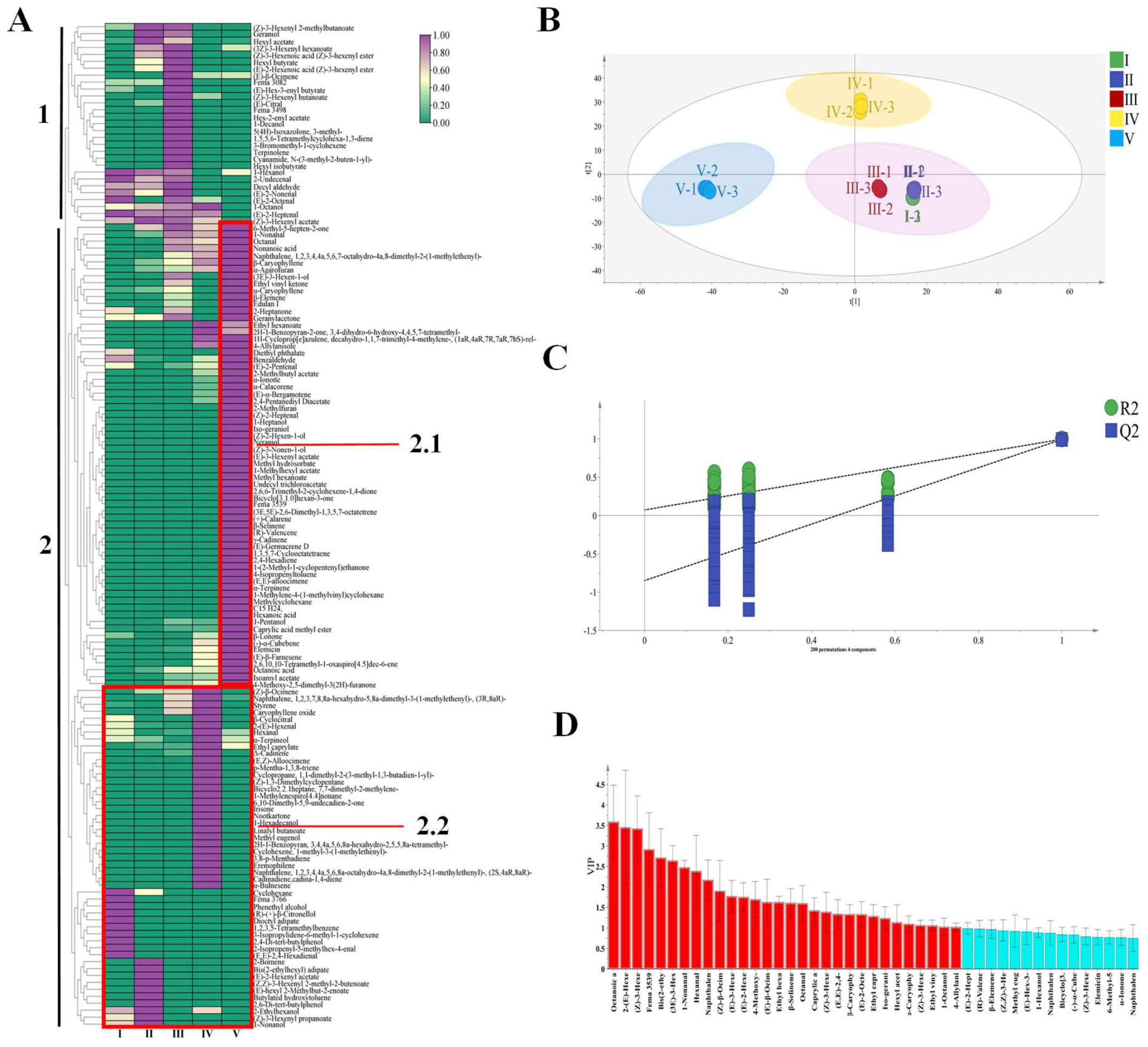
3.4. Differential Volatile Aroma Screening and OPLS-DA Analysis of VOCs during Ripening of R. roxburghii
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Fang, W.; Wang, Z.; Chen, Y. Physicochemical, biological properties, and flavour profile of Rosa roxburghii Tratt, Pyracantha fortuneana, and Rosa laevigata Michx fruits: A comprehensive review. Food Chem. 2022, 366, 130509. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, G.; Li, Q.; Yan, H. Transcriptome Analysis Reveals Regulatory Networks and Hub Genes in the Flavonoid Metabolism of Rosa roxburghii. Horticulturae 2023, 9, 233. [Google Scholar] [CrossRef]
- Wang, L.-T.; Zhang, S.; Fu, L.-N.; Chang, Y.-H.; Nie, S.-M.; Fu, Y.-J. Simultaneous quantification and quality control of flavor and functional phytochemicals in Rosa roxburghii fruit through multiple reaction monitoring mass spectrometry. J. Food Compos. Anal. 2023, 119, 105227. [Google Scholar] [CrossRef]
- Sheng, X.; Huang, M.; Li, T.; Li, X.; Cen, S.; Li, Q.; Huang, Q.; Tang, W. Characterization of aroma compounds in Rosa roxburghii Tratt using solvent-assisted flavor evaporation headspace-solid phase microextraction coupled with gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. X 2023, 18, 100632. [Google Scholar] [CrossRef]
- Su, L.; Wu, M.; Zhang, T.; Zhong, Y.; Cheng, Z. Identification of key genes regulating the synthesis of quercetin derivatives in Rosa roxburghii through integrated transcriptomics and metabolomics. J. Integr. Agric. 2024, 23, 876–887. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Zhang, H.; Sun, W.; Li, Z.; Zhang, F.; Zhang, H.; Chen, F.; Zhang, H.; An, J.; et al. Antimicrobial mechanism of strictinin isomers extracted from the root of Rosa roxburghii Tratt (Ci Li Gen). J. Ethnopharmacol. 2020, 250, 112498. [Google Scholar] [CrossRef]
- Xu, P.; Cai, X.; Zhang, W.; Li, Y.; Qiu, P.; Lu, D.; He, X. Flavonoids of Rosa roxburghii Tratt exhibit radioprotection and anti-apoptosis properties via the Bcl-2(Ca2+)/Caspase-3/PARP-1 pathway. Apoptosis 2016, 21, 1125–1143. [Google Scholar] [CrossRef]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, H.; Baldazzi, V.; van Leeuwen, C.; Bertin, N.; Gautier, H.; Wu, B.; Duchene, E.; Gomes, E.; Delrot, S.; et al. Inter-Species Comparative Analysis of Components of Soluble Sugar Concentration in Fleshy Fruits. Front. Plant Sci. 2016, 7, 649. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [CrossRef]
- Dong, B.; Tang, H.; Zhu, D.; Yao, Q.; Han, H.; He, K.; Ding, X. Benzothiazole Treatment Regulates the Reactive Oxygen Species Metabolism and Phenylpropanoid Pathway of Rosa roxburghii Fruit to Delay Senescence during Low Temperature Storage. Front. Plant Sci. 2021, 12, 753261. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Meng, X.; Song, H.; Gao, L.; Gao, Y.; Chen, W.; Huan, W.; Suo, J.; Yu, W.; Hu, Y.; et al. A comprehensive metabolomics analysis of Torreya grandis nuts with the effective de-astringent treatment during the postharvest ripening stage. Food Chem. 2023, 398, 133859. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, J.; Luo, C.; Rehman, L.; Li, X.; Xie, X. Advances in detecting fruit aroma compounds by combining chromatography and spectrometry. Sci. Food Agric. 2023, 103, 4755–4766. [Google Scholar] [CrossRef]
- Al Haj Ishak Al Ali, R.; Mondamert, L.; Halwani, J.; Jandry, J.; Nassif, N.; Shaban, A.; Berjeaud, J.M.; Labanowski, J. Temporal evolution of organochlorine and organophosphate pesticide residues in wells in the Akkar Region (Lebanon). Environ. Monit. Assess. 2022, 195, 121. [Google Scholar] [CrossRef]
- Leoni, C.; Majorani, C.; Cresti, R.; Marcello, I.; Berardi, E.; Fava, L.; Attias, L.; D’Ilio, S. Determination and risk assessment of phthalates in face masks. An Italian study. Hazard. Mater. 2023, 443 Pt A, 130176. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Xiao, Z.; Sun, X.; Wang, P.; Zhu, J.; Cao, X. Characterization of Volatile Compounds of Rosa roxburghii Tratt by Gas Chromatography-Olfactometry, Quantitative Measurements, Odor Activity Value, and Aroma Intensity. Molecules 2021, 26, 6202. [Google Scholar] [CrossRef]
- Huang, M.; Li, T.; Hardie, W.J.; Tang, W.; Li, X. Comparative characterization and sensory significance of volatile compounds in Rosa roxburghii Tratt fruit from five geographic locations in Guizhou, China. Flavour. Fragr. J. 2022, 37, 163–180. [Google Scholar] [CrossRef]
- Zhang, S.; Xiang, L.J.; Long, X.X.; Guo, L.J.; Wei, X.; Zhou, Y.Q.; Feng, T.T.; Zhou, Y.; Yin, X. Anti-Inflammatory and alpha-Glucosidase Inhibitory Triterpenoid with Diverse Carbon Skeletons from the Fruits of Rosa roxburghii. Agric. Food Chem. 2024, 72, 11503–11514. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, X.; He, Z.; Li, W.; Ren, X.; Lin, X. Dynamic changes of total acid and bacterial communities during the traditional fermentation of Hong Qu glutinous rice wine. Electron. J. Biotechnol. 2020, 43, 23–31. [Google Scholar] [CrossRef]
- Sammani, M.S.; Clavijo, S.; Cerdà, V. Recent, advanced sample pretreatments and analytical methods for flavonoids determination in different samples. TrAC Trends Anal. Chem. 2021, 138, 116220. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Savić, Z.; Brala, A.; Levaj, B.; Bursać Kovačević, D.; Biško, A. Evaluation of phenolic content and antioxidant capacity of blueberry cultivars (Vaccinium corymbosum L.) grown in the Northwest Croatia. Food Technol. Biotechnol. 2010, 48, 214–221. [Google Scholar]
- Sonia, S.; Singh, S. Tribulus terrestris L. fruits: An extensive Pharmacognostical and Phytochemical Quality Assesment using UV-spectrophotometer. Res. J. Pharm. Technol. 2022, 15, 5431–5435. [Google Scholar] [CrossRef]
- Yue, S.Y.; Zhou, R.R.; Nan, T.G.; Huang, L.Q.; Yuan, Y. [Comparison of major chemical components in Puerariae Thomsonii Radix and Puerariae Lobatae Radix]. Zhongguo Zhong Yao Za Zhi 2022, 47, 2689–2697. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Second Enlarged and Revised Edition; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; ISBN 978-7-03-058320-8. [Google Scholar]
- Van Gemert, L.J. Compilations of Flavour Threshold Values in Water and Other Media; Second Enlarged and Revised Edition; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; ISBN 978-7-03-045311-2. [Google Scholar]
- Xu, J.; Vidyarthi, S.K.; Bai, W.; Pan, Z. Nutritional constituents, health benefits and processing of Rosa Roxburghii: A review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Huang, M.; Xu, Q.; Deng, X.X. L-Ascorbic acid metabolism during fruit development in an ascorbate-rich fruit crop chestnut rose (Rosa roxburghii Tratt). Plant Physiol. 2014, 171, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xia, H.; Guo, Y.; Liu, X.; Lin, L.; Wang, J.; Xu, K.; Lv, X.; Hu, R.; Liang, D. Dynamic Changes in Ascorbic Acid Content during Fruit Development and Ripening of Actinidia latifolia (an Ascorbate-Rich Fruit Crop) and the Associated Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 5808. [Google Scholar] [CrossRef]
- Sanmartin, M.; Drogoudi, P.A.; Lyons, T.; Pateraki, I.; Barnes, J.; Kanellis, A.K. Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 2003, 216, 918–928. [Google Scholar] [CrossRef]
- Yang, Y. Cloning of APX Gene and Its Expression during AsA Accumulation in Rosa roxburghii; Guizhou University: Guiyang, China, 2008. [Google Scholar]
- Cheng, H.; Kong, W.; Tang, T.; Ren, K.; Zhang, K.; Wei, H.; Lin, T. Identification of Key Gene Networks Controlling Soluble Sugar and Organic Acid Metabolism During Oriental Melon Fruit Development by Integrated Analysis of Metabolic and Transcriptomic Analyses. Front. Plant Sci. 2022, 13, 830517. [Google Scholar] [CrossRef]
- Kohli, P.; Gupta, R. Alkaline pectinases: A review. Biocatal. Agric. Biotechnol. 2015, 4, 279–285. [Google Scholar] [CrossRef]
- Ramirez, E.; Brenes, M.; Garcia, P.; Medina, E.; Romero, C. Oleuropein hydrolysis in natural green olives: Importance of the endogenous enzymes. Food Chem. 2016, 206, 204–209. [Google Scholar] [CrossRef]
- Tian, S.; Qin, G.; Li, B. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. Int. 2015, 22, 4099–4121. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—Mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. Int. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhang, Q.; Zhang, Y.H.; Lu, X.Y.; Fu, W.M.; He, J.Y. Chemical Analysis of Dietary Constituents in Rosa roxburghii and Rosa sterilis Fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Xia, N.; Sun, Q.; Duan, C.; Pan, Q. Evolution of Seed-Soluble and Insoluble Tannins during Grape Berry Maturation. Molecules 2023, 28, 3050. [Google Scholar] [CrossRef]
- Magallhães, M.M.; Barros, R.S.; Finger, F.L. Changes in structural carbohydrates in developing fruit of Myrciaria jaboticaba. Sci. Hortic. 1996, 66, 17–22. [Google Scholar] [CrossRef]
- Salminen, J.P.; Roslin, T.; Karonen, M.; Sinkkonen, J.; Pihlaja, K.; Pulkkinen, P. Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. Chem. Ecol. 2004, 30, 1693–1711. [Google Scholar] [CrossRef]
- Zhang, B.; Xi, W.; Wei, W.; Shen, J.; Ferguson, I.; Chen, K. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Technol. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Garcia, C.V.; Stevenson, R.J.; Atkinson, R.G.; Winz, R.A.; Quek, S.Y. Changes in the bound aroma profiles of ‘Hayward’ and ‘Hort16A’ kiwifruit (Actinidia spp.) during ripening and GC-olfactometry analysis. Food Chem. 2013, 137, 45–54. [Google Scholar] [CrossRef]
- Buettner, A. (Ed.) Springer Handbook of Odor; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Li, G.; Jia, H.; Li, J.; Wang, Q.; Zhang, M.; Teng, Y. Emission of volatile esters and transcription of ethylene- and aroma-related genes during ripening of ‘Pingxiangli’ pear fruit (Pyrus ussuriensis Maxim). Sci. Hortic. 2014, 170, 17–23. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; López, M.L.; Echeverría, G.; Lara, I. Volatile ester-synthesising capacity in ‘Tardibelle’ peach fruit in response to controlled atmosphere and 1-MCP treatment. Food Chem. 2010, 123, 698–704. [Google Scholar] [CrossRef]
- Hernandezorte, P.; Cersosimo, M.; Loscos, N.; Cacho, J.; Garciamoruno, E.; Ferreira, V. The development of varietal aroma from non-floral grapes by yeasts of different genera. Food Chem. 2008, 107, 1064–1077. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Manriquez, D.; El-Sharkawy, I.; Flores, F.B.; El-Yahyaoui, F.; Regad, F.; Bouzayen, M.; Latche, A.; Pech, J.C. Two highly divergent alcohol dehydrogenases of melon exhibit fruit ripening-specific expression and distinct biochemical characteristics. Plant Mol. Biol. 2006, 61, 675–685. [Google Scholar] [CrossRef]
- Menager, I.; Jost, M.; Aubert, C. Changes in physicochemical characteristics and volatile constituents of strawberry (Cv. Cigaline) during maturation. Agric. Food Chem. 2004, 52, 1248–1254. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Manriquez, D.; Flores, F.B.; Regad, F.; Bouzayen, M.; Latche, A.; Pech, J.C. Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Mol. Biol. 2005, 59, 345–362. [Google Scholar] [CrossRef]
- Xi, W.; Zheng, H.; Zhang, Q.; Li, W. Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef]
- Aguiar, M.C.S.; Silvério, F.O.; de Pinho, G.P.; Lopes, P.S.N.; Fidêncio, P.H.; Ventura, S.J. Volatile compounds from fruits of Butia capitata at different stages of maturity and storage. Food Res. Int. 2014, 62, 1095–1099. [Google Scholar] [CrossRef]
- Zapata, J.; Mateo-Vivaracho, L.; Cacho, J.; Ferreira, V. Comparison of extraction techniques and mass spectrometric ionization modes in the analysis of wine volatile carbonyls. Anal. Chim. Acta 2010, 660, 197–205. [Google Scholar] [CrossRef]
- Lavid, N.; Schwab, W.; Kafkas, E.; Koch-Dean, M.; Bar, E.; Larkov, O.; Ravid, U.; Lewinsohn, E. Aroma biosynthesis in strawberry: S-adenosylmethionine:furaneol o-methyltransferase activity in ripening fruits. Agric. Food Chem. 2002, 50, 4025–4030. [Google Scholar] [CrossRef]
- Schiefner, A.; Sinz, Q.; Neumaier, I.; Schwab, W.; Skerra, A. Structural basis for the enzymatic formation of the key strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone. Biol. Chem. 2013, 288, 16815–16826. [Google Scholar] [CrossRef] [PubMed]
- Wein, M.; Lewinsohn, E.; Schwab, W. Metabolic fate of isotopes during the biological transformation of carbohydrates to 2,5-dimethyl-4-hydroxy-3(2h)-furanone in strawberry fruits. Agric. Food Chem. 2001, 49, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC x GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.G.; Sanz, C.; Olias, R.; Olias, J.M. Lipoxygenase and hydroperoxide lyase activities in ripening strawberry fruits. Agric. Food Chem. 1999, 47, 249–253. [Google Scholar] [CrossRef]
- Contreras, C.; Tjellström, H.; Beaudry, R.M. Relationships between free and esterified fatty acids and LOX-derived volatiles during ripening in apple. Postharvest Biol. Technol. 2016, 112, 105–113. [Google Scholar] [CrossRef]
- Shi, F.; Zhou, X.; Zhou, Q.; Tan, Z.; Yao, M.M.; Wei, B.D.; Ji, S.J. Membrane lipid metabolism changes and aroma ester loss in low-temperature stored Nanguo pears. Food Chem. 2018, 245, 446–453. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Haruna, S.A.; Zhou, C.; Wang, Y.; Ouyang, Q.; Chen, Q. Identification of characteristic volatiles and metabolomic pathway during pork storage using HS-SPME-GC/MS coupled with multivariate analysis. Food Chem. 2022, 373 Pt A, 131431. [Google Scholar] [CrossRef]
- Mano, J.; Torii, Y.; Hayashi, S.; Takimoto, K.; Matsui, K.; Nakamura, K.; Inze, D.; Babiychuk, E.; Kushnir, S.; Asada, K. The NADPH:quinone oxidoreductase P1-zeta-crystallin in Arabidopsis catalyzes the alpha,beta-hydrogenation of 2-alkenals: Detoxication of the lipid peroxide-derived reactive aldehydes. Plant Cell Physiol. 2002, 43, 1445–1455. [Google Scholar] [CrossRef]
- Cabrita, M.J.; Freitas, A.M.C.; Laureano, O.; Stefano, R.D. Glycosidic aroma compounds of some Portuguese grape cultivars. J. Sci. Food Agric. 2006, 86, 922–931. [Google Scholar] [CrossRef]
- Plotto, A.; Margaría, C.A.; Goodner, K.L.; Goodrich, R.; Baldwin, E.A. Odour and flavour thresholds for key aroma components in an orange juice matrix: Terpenes and aldehydes. Flavour. Fragr. J. 2004, 19, 491–498. [Google Scholar] [CrossRef]
- Winterhalter, P.; Rouseff, R. Carotenoid-Derived Aroma Compounds: An Introduction. In Carotenoid-Derived Aroma Compounds; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2001; pp. 1–17. [Google Scholar]
- Choi, Y.J.; Lee, S.Y. Microbial production of short-chain alkanes. Nature 2013, 502, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Cui, C.; Zhang, S.; Zhu, J.; Peng, C.; Cai, H.; Yang, X.; Hou, R. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Cai, D.; Zhang, R.; Zhu, Y.; Zhang, D.; Qiao, L.; Liu, Y. Mass spectrometry-based metabolomics approach to reveal differential compounds in pufferfish soups: Flavor, nutrition, and safety. Food Chem. 2019, 301, 125261. [Google Scholar] [CrossRef] [PubMed]
| No. | Compounds | RT | RI | LRI | Relative Content (mg/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | |||||
| 1 | (E)-2-Pentenal | 9.582 | 744 | ND | 4.34 | 2.20 | 2.80 | 4.02 | 8.32 |
| 2 | Hexanal | 10.962 | 801 | 806.1 | 81.13 | 43.26 | 49.14 | 177.11 | 66.54 |
| 3 | 2- (E)-Hexenal | 12.733 | 847 | 861.7 | 55.46 | 21.12 | 19.45 | 209.15 | 18.37 |
| 4 | (E,E)-2,4-Hexadienal | 14.399 | 931 | 921.4 | 4.58 | ND | ND | ND | ND |
| 5 | (Z)-2-Heptenal | 15.389 | ND | 960.5 | ND | ND | ND | ND | 1.95 |
| 7 | (E)-2-Heptenal | 15.57 | 978 | 967.6 | 16.54 | 9.86 | 11.28 | 9.46 | ND |
| 6 | Benzaldehyde | 15.751 | 961 | 968.4 | 1.48 | 0.09 | ND | 0.60 | 2.26 |
| 8 | Octanal | 16.494 | 1002 | 1005.0 | ND | ND | 20.81 | 9.97 | 57.75 |
| 9 | Cyanamide, N- (3-methyl-2-buten-1-yl)- | 17.76 | ND | 1066.3 | ND | ND | 4.82 | ND | ND |
| 10 | (E)-2-Octenal | 17.846 | 1064 | 1070.5 | 5.59 | ND | 4.72 | 0.93 | ND |
| 11 | 2-Isopropenyl-5-methylhex-4-enal | 18.255 | ND | 1090.3 | 0.33 | ND | ND | ND | ND |
| 12 | 1-Nonanal | 18.598 | 1105 | 1115.6 | 13.18 | 19.31 | 77.19 | 56.64 | 102.94 |
| 13 | Fema 3766 | 19.712 | 1162 | 1168.7 | 0.50 | ND | ND | ND | ND |
| 14 | (E)-2-Nonenal | 19.816 | 1164 | 1174.4 | 0.74 | 0.39 | 0.91 | ND | ND |
| 15 | Decyl aldehyde | 20.645 | 1204 | 1218.8 | 2.33 | 2.26 | 4.04 | ND | ND |
| 16 | β-Cyclocitral | 21.178 | 1222 | 1244.7 | 0.66 | ND | ND | 1.74 | ND |
| 17 | (E)-Citral | 21.911 | 1269 | 1285.0 | ND | 0.12 | 0.57 | ND | ND |
| 18 | 2-Undecenal | 23.853 | 1359 | 1378.2 | 1.90 | 1.10 | 1.63 | ND | ND |
| 19 | Fema 3082 | 23.91 | ND | 1378.2 | 0.27 | 0.30 | 1.11 | ND | ND |
| Aldehydes (19) | 189.01 | 100.00 | 198.47 | 469.63 | 258.13 | ||||
| 20 | 1-Pentanol | 9.648 | 763 | ND | ND | ND | 0.36 | 0.21 | 3.20 |
| 21 | (3E)-3-Hexen-1-ol | 12.571 | 852 | 864.1 | ND | ND | 50.71 | ND | 102.41 |
| 22 | (Z)-2-Hexen-1-ol | 12.857 | 865 | 867.6 | ND | ND | ND | ND | 0.84 |
| 23 | 1-Hexanol | 12.914 | 867 | 869.4 | 26.36 | 23.38 | 21.98 | 7.97 | 14.41 |
| 24 | 1-Heptanol | 15.665 | 968 | 971.4 | ND | ND | ND | ND | 2.11 |
| 25 | 2-Ethylhexanol | 17.179 | 1031 | 1040.0 | 1.75 | 3.14 | ND | 1.34 | ND |
| 26 | 1-Octanol | 18.036 | 1064 | 1079.7 | 3.33 | 5.61 | 8.91 | 15.68 | ND |
| 27 | Phenethyl alcohol | 19.064 | 1110 | 1133.3 | 1.03 | ND | ND | ND | ND |
| 28 | (Z)-3-Nonen-1-ol | 19.502 | 1126 | 1157.2 | ND | ND | ND | ND | 0.75 |
| 29 | 1-Nonanol | 19.969 | 1171 | 1182.2 | 1.31 | 2.86 | ND | ND | ND |
| 30 | α-Terpineol | 20.349 | 1192 | 1211.9 | 0.81 | 0.38 | ND | 2.99 | 0.72 |
| 31 | Neraniol | 20.921 | 1232 | 1233.3 | ND | ND | ND | ND | 1.30 |
| 32 | (R)- (+)-β-Citronellol | 21.054 | ND | 1240.2 | 0.17 | ND | ND | ND | ND |
| 33 | Iso-geraniol | 21.187 | 1273 | 1247.2 | ND | ND | ND | ND | 30.38 |
| 34 | Geraniol | 21.587 | 1260 | 1265.6 | ND | 0.46 | 0.40 | ND | ND |
| 35 | 1-Decanol | 21.835 | 1279 | 1281.0 | ND | ND | 0.30 | ND | ND |
| 36 | 1-Hexadecanol | 22.406 | 1876 | 1309.5 | ND | ND | ND | 1.36 | ND |
| Alcohols (17) | 34.76 | 35.82 | 82.65 | 29.54 | 156.11 | ||||
| 37 | Isoamyl acetate | 13.181 | 876 | 878.1 | ND | ND | 0.46 | 0.72 | 5.03 |
| 38 | 2-Methylbutyl acetate | 13.257 | 879 | 880.5 | ND | ND | ND | 0.66 | 3.70 |
| 39 | Methyl hexanoate | 14.523 | 923.2 | 926.3 | ND | ND | ND | ND | 8.77 |
| 40 | Methyl hydrosorbate | 14.732 | ND | 934.5 | ND | ND | ND | ND | 0.38 |
| 41 | Ethyl hexanoate | 16.37 | 1000 | 999.2 | ND | ND | ND | 32.04 | 15.35 |
| 42 | (E)-3-Hexenyl acetate | 16.551 | 1004 | 1007.8 | ND | ND | ND | ND | 56.61 |
| 43 | (Z)-3-Hexenyl acetate | 16.694 | 1007 | 1016.1 | 46.07 | 218.88 | 142.66 | 29.54 | ND |
| 44 | Hexyl acetate | 16.818 | 1011 | 1020.7 | 3.45 | 28.04 | 13.71 | 2.82 | 3.21 |
| 45 | Hex-2-enyl acetate | 16.875 | ND | 1023.5 | ND | ND | 2.20 | ND | ND |
| 46 | (E)-2-Hexenyl acetate | 16.912 | 1017 | 1025.3 | ND | 7.47 | ND | ND | ND |
| 47 | 1-Methylhexyl acetate | 17.284 | 1043 | 1043.3 | ND | ND | ND | ND | 1.35 |
| 48 | (Z)-3-Hexenyl propanoate | 18.626 | 1100 | 1109.8 | 0.88 | 2.07 | ND | ND | ND |
| 49 | Caprylic acid methyl ester | 18.912 | 1126 | 1124.9 | ND | ND | 0.46 | 0.60 | 35.41 |
| 50 | (E)-Hex-3-enyl butyrate | 20.207 | ND | 1195.8 | 0.75 | ND | 6.91 | ND | ND |
| 51 | (Z)-3-Hexenyl butanoate | 20.207 | 1187 | 1195.8 | ND | ND | 15.54 | 1.26 | ND |
| 52 | Hexyl isobutyrate | 20.292 | 1150 | 1200.4 | ND | ND | 0.40 | ND | ND |
| 53 | Hexyl butyrate | 20.321 | 1191 | 1201.9 | ND | 0.51 | 1.23 | ND | ND |
| 54 | Ethyl caprylate | 20.378 | 1195 | 1204.9 | ND | 0.39 | 0.41 | 31.58 | 4.32 |
| 55 | Linalyl butanoate | 21.064 | 1422 | 1240.8 | ND | ND | ND | 0.73 | ND |
| 56 | (Z)-3-Hexenyl 2-methylbutanoate | 21.083 | 1233.5 | 1241.7 | 0.27 | 1.37 | 1.30 | ND | ND |
| 57 | Fema 3498 | 22.063 | 1240 | 1292.9 | ND | ND | 0.92 | ND | ND |
| 58 | Undecyl trichloroacetate | 22.244 | ND | 1302.1 | ND | ND | ND | ND | 3.81 |
| 59 | (Z,Z)-3-Hexenyl 2-methyl-2-butenoate | 22.996 | 1282.2 | 1336.5 | ND | 2.86 | ND | ND | ND |
| 60 | (E)-hexyl 2-Methylbut-2-enoate | 23.091 | 1331 | 1340.8 | ND | 0.39 | ND | ND | ND |
| 61 | (3Z)-3-Hexenyl hexanoate | 23.996 | 1380 | 1389.5 | ND | 1.01 | 1.51 | ND | 0.45 |
| 62 | (Z)-3-Hexenoic acid (Z)-3-hexenyl ester | 24.329 | ND | 1397.4 | ND | 2.79 | 5.84 | ND | ND |
| 63 | (E)-2-Hexenoic acid (Z)-3-hexenyl ester | 25.5 | ND | 1440.8 | ND | 0.35 | 0.80 | ND | ND |
| 64 | Diethyl phthalate | 29.622 | 1602.3 | 1606.5 | 1.08 | ND | ND | ND | 2.36 |
| 65 | Bis (2-ethylhexyl) adipate | 31.498 | ND | 1698.5 | ND | 17.86 | ND | ND | ND |
| 66 | Dioctyl adipate | 31.517 | ND | 1699.5 | 0.18 | ND | ND | ND | ND |
| Esters (30) | 52.69 | 283.97 | 194.35 | 99.94 | 140.75 | ||||
| 67 | Hexanoic acid | 15.999 | 981 | 984.6 | ND | ND | ND | ND | 7.93 |
| 68 | Octanoic acid | 19.94 | 1178 | 1178.1 | ND | 0.96 | 12.23 | 9.85 | 268.93 |
| 69 | Nonanoic acid | 21.454 | 1280 | 1269.6 | ND | ND | 1.00 | 0.82 | 1.33 |
| Acids (3) | ND | 0.96 | 13.22 | 10.67 | 278.19 | ||||
| 70 | Ethyl vinyl ketone | 7.392 | 680 | ND | ND | ND | 6.46 | ND | 20.51 |
| 71 | 2-Heptanone | 13.599 | 893 | 891.6 | 0.37 | ND | 0.45 | ND | 0.71 |
| 72 | Bicyclo [3.1.0]hexan-3-one | 14.209 | ND | 913.9 | ND | ND | ND | ND | 13.48 |
| 73 | 5 (4H)-Isoxazolone, 3-methyl- | 16.17 | ND | 991.3 | ND | ND | 0.81 | ND | ND |
| 74 | 6-Methyl-5-hepten-2-one | 16.256 | 987 | 994.7 | ND | 2.89 | 6.51 | 2.71 | 7.09 |
| 75 | 4-Methoxy-2,5-dimethyl-3 (2H)-furanone | 17.893 | 1065 | 1072.3 | ND | ND | 1.66 | 4.96 | 50.37 |
| 76 | 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | 19.426 | 1152 | 1153.1 | ND | ND | ND | ND | 0.42 |
| 77 | Irisone | 23.025 | ND | 1337.8 | ND | ND | ND | 1.92 | ND |
| 78 | α-Ionone | 25.633 | 1429 | 1441.9 | ND | ND | ND | 0.50 | 9.27 |
| 79 | Geranylacetone | 25.862 | 1453 | 1456.9 | 1.20 | 1.30 | 3.24 | ND | 4.14 |
| 80 | 6,10-Dimethyl-5,9-undecadien-2-one | 26.014 | 1456 | 1462.8 | ND | ND | ND | 1.83 | ND |
| 81 | β-Lonone | 27.214 | 1488 | 1503.3 | 0.60 | ND | ND | 1.28 | 7.67 |
| 82 | 2H-1-Benzopyran-2-one, 3,4-dihydro-6-hydroxy-4,4,5,7-tetramethyl- | 29.013 | ND | 1575.7 | ND | ND | ND | 1.64 | 1.07 |
| 83 | Nootkartone | 33.516 | 1800 | 1814.5 | ND | ND | ND | 0.88 | ND |
| Ketones (14) | 2.17 | 4.19 | 19.13 | 15.73 | 114.73 | ||||
| 84 | 1,3,5,7-Cyclooctatetraene | 13.695 | ND | 894.7 | ND | ND | ND | ND | 0.49 |
| 85 | Styrene | 13.885 | 894 | 900.0 | 0.05 | ND | 0.52 | 0.96 | ND |
| 86 | 2,4-Hexadiene | 14.428 | ND | 922.5 | ND | ND | ND | ND | 0.56 |
| 87 | 1- (2-Methyl-1-cyclopentenyl)ethanone | 16.855 | ND | 1022.5 | ND | ND | ND | ND | 1.40 |
| 88 | 3-Bromomethyl-1-cyclohexene | 17.008 | ND | 1029.9 | ND | ND | 0.57 | ND | ND |
| 89 | (E)-β-Ocimene | 17.389 | 1051 | 1048.4 | ND | ND | 21.88 | 2.03 | 2.51 |
| 90 | Fema 3539 | 17.484 | 1050 | 1053.0 | ND | ND | ND | ND | 99.74 |
| 91 | (Z)-β-Ocimene | 17.627 | 1040 | 1059.9 | ND | 2.90 | 11.09 | 53.77 | ND |
| 92 | 4-Isopropenyltoluene | 18.417 | 1090 | 1098.1 | ND | ND | ND | ND | 0.60 |
| 93 | (E,E)-alloocimene | 19.055 | ND | 1132.8 | ND | ND | ND | ND | 4.26 |
| 94 | (3E,5E)-2,6-Dimethyl-1,3,5,7-octatetrene | 19.131 | 1134 | 1136.9 | ND | ND | ND | ND | 4.72 |
| 95 | 1,2,3,5-Tetramethtylbenzene | 19.207 | 1127.9 | 1141.1 | 0.16 | ND | ND | ND | ND |
| 96 | 1,5,5,6-Tetramethylcyclohexa-1,3-diene | 19.207 | ND | 1141.1 | ND | ND | 0.24 | ND | ND |
| 97 | (E,Z)-Alloocimene | 19.207 | 1131 | 1141.1 | ND | ND | ND | 2.50 | ND |
| 98 | p-Mentha-1,3,8-triene | 19.283 | ND | 1145.2 | ND | ND | ND | 1.42 | ND |
| 99 | 2-Bornene | 21.33 | ND | 1254.6 | ND | 0.07 | ND | ND | ND |
| 100 | 3-Isopropylidene-6-methyl-1-cyclohexene | 21.34 | 1088 | 1255.2 | 0.05 | ND | ND | ND | ND |
| 101 | 2,6,10,10-Tetramethyl-1-oxaspiro[4.5]dec-6-ene | 22.672 | 1298 | 1336.5 | ND | ND | ND | 0.63 | 1.64 |
| 102 | (-)-α-Cubebene | 23.663 | 1351 | 1366.9 | ND | ND | ND | 2.91 | 9.60 |
| 103 | α-Terpinene | 23.939 | 1020 | 1379.5 | ND | ND | ND | ND | 1.06 |
| 104 | 3,8-p-Menthadiene | 24.005 | 1071 | 1382.5 | ND | ND | ND | 0.25 | ND |
| 105 | Terpinolene | 24.101 | 1090 | 1386.9 | ND | ND | 0.29 | ND | ND |
| 106 | Cyclohexene, 1-methyl-3- (1-methylethenyl)- | 24.101 | ND | 1386.9 | ND | ND | ND | 2.15 | ND |
| 107 | β-Elemene | 24.662 | 1392 | 1410.6 | ND | ND | 2.14 | ND | 19.12 |
| 108 | β-Caryophyllene | 25.586 | 1419 | 1446.3 | ND | 1.18 | 5.23 | 17.41 | 34.36 |
| 109 | (E)-α-Bergamotene | 25.69 | 1450 | 1450.3 | ND | ND | ND | 0.24 | 1.96 |
| 110 | (E)-β-Farnesene | 25.957 | 1459 | 1460.6 | ND | ND | ND | 1.46 | 5.45 |
| 111 | (E)-Germacrene D | 26.328 | 1481 | 1474.9 | ND | ND | ND | ND | 0.81 |
| 112 | α-Caryophyllene | 26.49 | 1454 | 1481.2 | 0.30 | 0.86 | 3.59 | ND | 22.24 |
| 113 | Naphthalene, 1,2,3,4,4a,5,6,7-octahydro-4a,8-dimethyl-2- (1-methylethenyl)- | 27.271 | 1491.8 | 1511.7 | ND | ND | 6.77 | 27.01 | 96.67 |
| 114 | β-Selinene | 27.404 | 1475 | 1517.0 | ND | ND | ND | ND | 44.37 |
| 115 | (R)-Valencene | 27.509 | 1494 | 1521.2 | ND | ND | ND | ND | 17.73 |
| 116 | Naphthalene, 1,2,3,7,8,8a-hexahydro-5,8a-dimethyl-3- (1-methylethenyl)-, (3R,8aR)- | 27.557 | ND | 1523.1 | ND | ND | 3.24 | 9.83 | 0.20 |
| 117 | γ-Cadinene | 27.604 | 1502 | 1525.0 | ND | ND | ND | ND | 4.24 |
| 118 | Eremophilene | 27.661 | 1503 | 1527.2 | ND | ND | ND | 2.14 | ND |
| 119 | 1H-Cycloprop[e]azulene, decahydro-1,1,7-trimethyl-4-methylene-, (1aR,4aR,7R,7aR,7bS)-rel- | 27.757 | ND | 1531.0 | ND | ND | ND | 1.26 | 1.37 |
| 120 | (+)-Calarene | 27.994 | 1432 | 1540.5 | ND | ND | ND | ND | 1.31 |
| 121 | Δ-Cadinene | 28.271 | 1525 | 1551.5 | ND | ND | 0.25 | 4.82 | ND |
| 122 | Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-2- (1-methylethenyl)-, (2S,4aR,8aR)- | 28.366 | 1518 | 1555.3 | ND | ND | ND | 7.51 | ND |
| 123 | Cadinadiene,cadina-1,4-diene | 28.566 | 1542 | 1563.2 | ND | ND | ND | 0.22 | ND |
| 124 | Elemicin | 28.709 | 1554 | 1568.9 | ND | ND | 0.18 | 2.64 | 11.61 |
| 125 | α-Calacorene | 28.87 | 1540 | 1570.0 | ND | ND | ND | 0.15 | 1.34 |
| 126 | Caryophyllene oxide | 29.937 | 1617 | 1621.9 | ND | ND | 0.55 | 1.01 | ND |
| 127 | α-Bulnesene | 31.403 | 1526 | 1693.9 | ND | ND | ND | 0.71 | ND |
| Alkenes (44) | 0.56 | 5.00 | 56.54 | 143.01 | 389.34 | ||||
| 128 | Cyclohexane | 7.183 | ND | ND | 0.26 | 0.12 | ND | ND | ND |
| 129 | Methylcyclohexane | 8.411 | 736 | ND | ND | ND | ND | ND | 0.70 |
| 130 | (Z)-1,3-Dimethylcyclopentane | 15.818 | ND | 977.4 | ND | ND | ND | 0.46 | ND |
| 131 | Bicyclo2.2.1heptane, 7,7-dimethyl-2-methylene- | 18.312 | 951 | 1093.0 | ND | ND | ND | 0.16 | ND |
| 132 | 2,4-Pentanediyl Diacetate | 18.922 | ND | 1117.7 | ND | ND | ND | 0.68 | 8.62 |
| 133 | 1-Methylene-4- (1-methylvinyl)cyclohexane | 22.12 | 1007 | 1295.9 | ND | ND | ND | ND | 2.38 |
| 134 | Cyclopropane, 1,1-dimethyl-2- (3-methyl-1,3-butadien-1-yl)- | 22.273 | ND | 1303.4 | ND | ND | ND | 1.80 | ND |
| 135 | 1-Methylenespiro[4.4]nonane | 24.377 | ND | 1399.5 | ND | ND | ND | 2.79 | ND |
| 136 | C15 H24, | 26.947 | 1477 | 1498.8 | ND | ND | ND | ND | 1.96 |
| Alkanes (9) | 0.26 | 0.12 | ND | 5.89 | 13.66 | ||||
| 137 | Methyl eugenol | 24.805 | 1404 | 1416.1 | ND | ND | ND | 11.47 | ND |
| 138 | 2,4-Di-tert-butylphenol | 27.69 | 1519 | 1528.4 | 0.13 | ND | ND | ND | ND |
| 139 | 2,6-Di-tert-butylphenol | 27.69 | ND | 1528.4 | ND | 0.09 | ND | ND | ND |
| 140 | Butylated hydroxytoluene | 27.899 | 1514 | 1536.7 | ND | 0.66 | ND | ND | ND |
| Phenols (4) | 0.13 | 0.75 | ND | 11.47 | ND | ||||
| 141 | 2-Methylfuran | 9.239 | 600 | ND | ND | ND | ND | ND | 0.19 |
| 142 | α-Agarofuran | 28.994 | 1543 | 1580.3 | ND | 0.52 | 1.10 | 2.80 | 6.10 |
| Heterocycles (2) | ND | 0.52 | 1.10 | 2.80 | 6.29 | ||||
| 143 | 4-Allylanisole | 20.607 | 1199 | 1208.9 | ND | ND | ND | 11.00 | 16.35 |
| 144 | 2H-1-Benzopyran, 3,4,4a,5,6,8a-hexahydro-2,5,5,8a-tetramethyl- | 22.596 | ND | 1318.2 | ND | ND | ND | 0.61 | ND |
| 145 | Edulan I | 22.863 | 1315 | 1330.4 | ND | ND | 1.29 | ND | 8.59 |
| Others (5) | ND | ND | 1.29 | 11.61 | 24.94 | ||||
| Odor Descriptions | Class | Compounds | Odor Threshold (mg/kg) | ROAV | ||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||||
| Green | Aldehydes | 2- (E)-Hexenal | 0.0887 | 625.22 | 238.06 | 219.73 | 2363.27 | 207.05 |
| (E)-2-Octenal | 0.003 | 1862.51 | ND | 1572.29 | 310.21 | ND | ||
| Alcohols | 1-Heptanol | 0.0054 | ND | ND | ND | ND | 390.45 | |
| (Z)-2-Hexen-1-ol | 0.3593 | ND | ND | ND | ND | 2.33 | ||
| Esters | (Z)-3-Hexenyl acetate | 0.013 | 3544.18 | 16,836.82 | 10,973.98 | 2272.36 | ND | |
| (Z)-3-Hexenyl butanoate | 0.5 | ND | ND | 31.07 | 2.52 | ND | ||
| Ketones | Geranylacetone | 0.06 | 19.92 | 21.61 | 54.07 | ND | 68.93 | |
| Fruit | Aldehydes | (E)-2-Pentenal | 0.98 | 4.43 | 2.24 | 2.86 | 4.11 | 8.49 |
| Esters | Isoamyl acetate | 0.00015 | ND | ND | 3077.42 | 4794.89 | 33,556.71 | |
| Hexyl acetate | 0.115 | 29.99 | 243.78 | 119.23 | 24.50 | 27.90 | ||
| Ethyl caprylate | 0.0193 | ND | 20.01 | 21.35 | 1636.14 | 223.99 | ||
| Hexyl butyrate | 0.203 | ND | 2.49 | 6.06 | ND | ND | ||
| 2-Methylbutyl acetate | 0.005 | ND | ND | ND | 132.12 | 336.56 | ||
| Ethyl Hexanoate | 0.0022 | ND | ND | ND | 14,562.77 | 6978.21 | ||
| Methyl hexanoate | 0.07 | ND | ND | ND | ND | 125.28 | ||
| Ketones | Nootkartone | 0.28 | ND | ND | ND | 4.73 | ND | |
| Citrus | Esters | Caprylic acid methyl ester | 0.2 | ND | ND | 2.32 | 2.98 | 177.04 |
| Alkenes | (Z)-β-Ocimene | 0.034 | ND | 85.38 | 326.18 | 1581.58 | ND | |
| 4-Isopropenyltoluene | 0.085 | ND | ND | ND | ND | 7.00 | ||
| Grass | Aldehydes | Hexanal | 0.005 | 16,225.60 | 8652.63 | 9827.86 | 35,421.65 | 13,307.02 |
| Alcohols | (3E)-3-Hexen-1-ol | 0.11 | ND | ND | 461.03 | ND | 930.99 | |
| Fat | Aldehydes | Octanal | 0.000587 | ND | ND | 35,446.06 | 16,981.53 | 98,386.72 |
| 1-Nonanal | 0.0011 | 11,982.85 | 17,551.64 | 70,171.81 | 51,494.71 | 93,581.44 | ||
| (E)-2-Nonenal | 0.00019 | 3919.81 | 2052.53 | 4809.00 | ND | ND | ||
| Alcohols | 1-Nonanol | 0.002 | 653.29 | 1427.64 | ND | ND | ND | |
| α-Terpineol | 1.2 | ND | ND | ND | 2.49 | ND | ||
| Alkenes | p-Mentha-1,3,8-triene | 0.015 | ND | ND | ND | 94.69 | ND | |
| Soap | Aldehydes | Decyl aldehyde | 0.003 | 778.21 | 754.34 | 1348.23 | ND | ND |
| (E)-2-Heptenal | 0.04 | 413.38 | 246.49 | 282.08 | 236.53 | ND | ||
| Ketones | 2-Heptanone | 0.14 | 2.65 | ND | 3.20 | ND | 5.10 | |
| Balsamic | Alcohols | 1-Pentanol | 0.1502 | ND | ND | 2.37 | 1.41 | 21.28 |
| Alkenes | Styrene | 0.0036 | 12.91 | ND | 143.33 | 265.82 | ND | |
| Mint | Aldehydes | β-Cyclocitral | 0.003 | 218.73 | ND | ND | 579.84 | ND |
| Flower | Alcohols | 1-Hexanol | 0.0056 | 4706.37 | 4174.89 | 3924.12 | 1422.84 | 2572.79 |
| Geraniol | 0.0011 | ND | 417.14 | 361.15 | ND | ND | ||
| Phenols | Methyl eugenol | 0.775 | ND | ND | ND | 14.80 | ND | |
| Ketones | β-Lonone | 0.000007 | 85,552.07 | ND | ND | 183,215.46 | 1,095,920.27 | |
| Sweet | Alcohols | Phenethyl alcohol | 0.56423 | 1.83 | ND | ND | ND | ND |
| Neraniol | 0.68 | ND | ND | ND | ND | 1.91 | ||
| Alkenes | (E)-β-Ocimene | 0.034 | ND | ND | 643.42 | 59.67 | 73.85 | |
| Caryophylene oxide | 0.41 | ND | ND | 1.35 | 2.45 | ND | ||
| Others | 4-Allylanisole | 0.0075 | ND | ND | ND | ND | 2180.27 | |
| Metal | Alcohols | 1-Octanol | 0.1258 | 26.45 | 44.60 | 70.81 | 124.62 | ND |
| Wood | Ketones | α-Ionone | 0.00378 | ND | ND | ND | 131.65 | 2452.49 |
| Alkenes | α-Caryophyllene | 0.16 | 1.87 | 5.36 | 22.47 | ND | 139.00 | |
| β-Caryophyllene | 0.064 | ND | 18.40 | 81.77 | 272.01 | 536.81 | ||
| Caramel | Aldehydes | Benzaldehyde | 0.75089 | 1.97 | ND | ND | ND | 3.01 |
| Ketones | 4-Methoxy-2,5-dimethyl-3 (2H)-furanone | 0.16 | ND | ND | 10.36 | 31.03 | 314.81 | |
| Mushroom | Ketones | 6-Methyl-5-hepten-2-one | 0.068 | ND | 42.48 | 95.68 | 39.79 | 104.31 |
| Pungent | Ketones | Ethyl vinyl ketone | 0.023 | ND | ND | 280.88 | ND | 891.90 |
| Sweat | Acids | Octanoic acid | 3 | ND | ND | 4.08 | 3.28 | 89.64 |
| Hexanoic acid | 5 | ND | ND | ND | ND | 1.59 | ||
| Lemon | Alkenes | α-Terpinene | 0.04 | ND | ND | ND | ND | 13.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Deng, J.; Wu, S.; Fei, Q.; Lin, D.; Chen, H.; Tao, G.; Meng, L.; Hu, Y.; Ma, F. Dynamic Changes of Active Components and Volatile Organic Compounds in Rosa roxburghii Fruit during the Process of Maturity. Foods 2024, 13, 2893. https://doi.org/10.3390/foods13182893
Xu S, Deng J, Wu S, Fei Q, Lin D, Chen H, Tao G, Meng L, Hu Y, Ma F. Dynamic Changes of Active Components and Volatile Organic Compounds in Rosa roxburghii Fruit during the Process of Maturity. Foods. 2024; 13(18):2893. https://doi.org/10.3390/foods13182893
Chicago/Turabian StyleXu, Su, Junyi Deng, Siyao Wu, Qiang Fei, Dong Lin, Haijiang Chen, Guangcan Tao, Lingshuai Meng, Yan Hu, and Fengwei Ma. 2024. "Dynamic Changes of Active Components and Volatile Organic Compounds in Rosa roxburghii Fruit during the Process of Maturity" Foods 13, no. 18: 2893. https://doi.org/10.3390/foods13182893
APA StyleXu, S., Deng, J., Wu, S., Fei, Q., Lin, D., Chen, H., Tao, G., Meng, L., Hu, Y., & Ma, F. (2024). Dynamic Changes of Active Components and Volatile Organic Compounds in Rosa roxburghii Fruit during the Process of Maturity. Foods, 13(18), 2893. https://doi.org/10.3390/foods13182893






