Volatile Organic Compounds Produced by Bacillus sp. Strain R2 Inhibit Aspergillus flavus Growth In Vitro and in Unhulled Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media
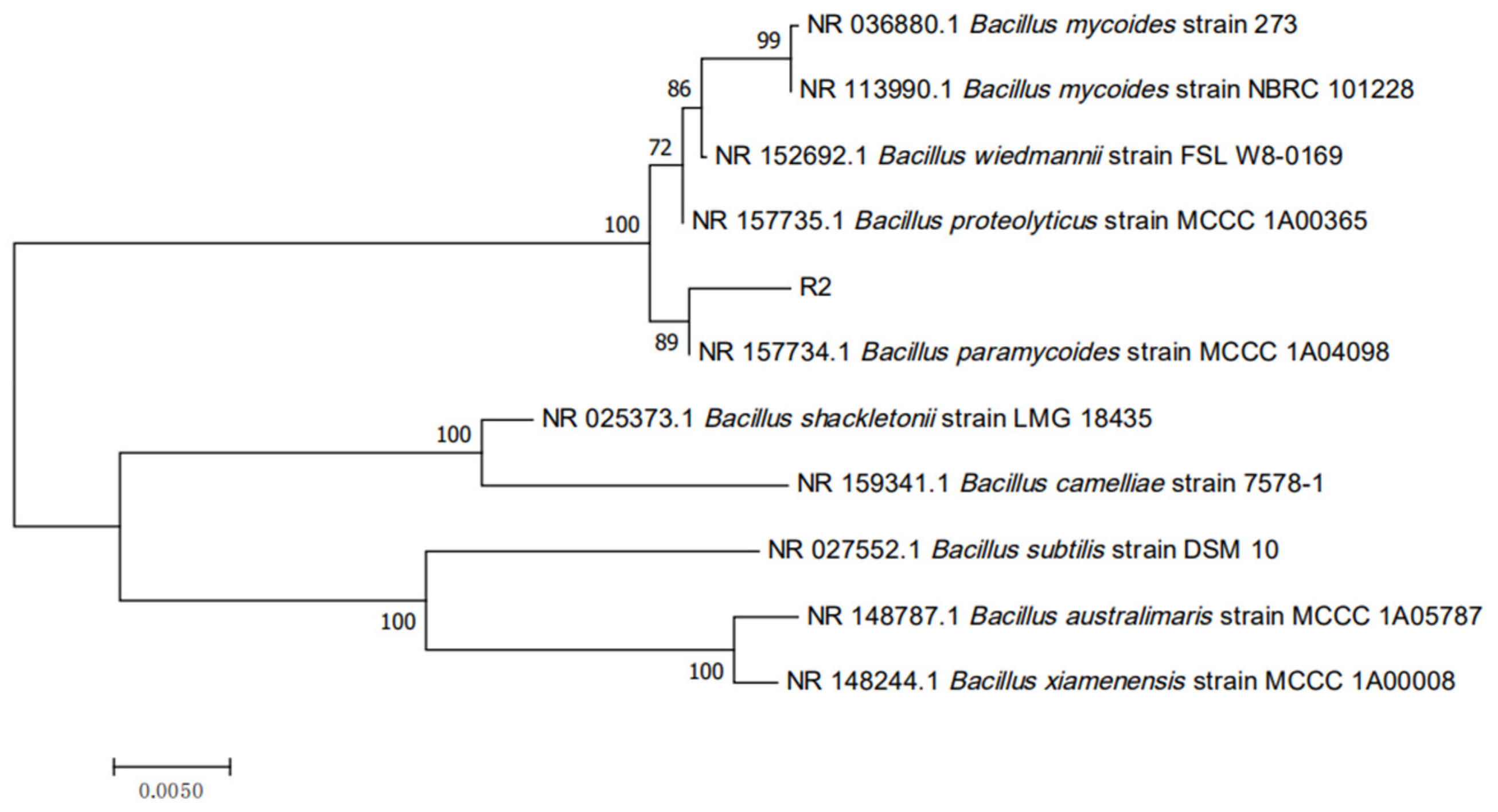
2.2. Identification of Strain R2
2.3. Effect of VOCs Produced by R2 on Spore Germination of A. flavus
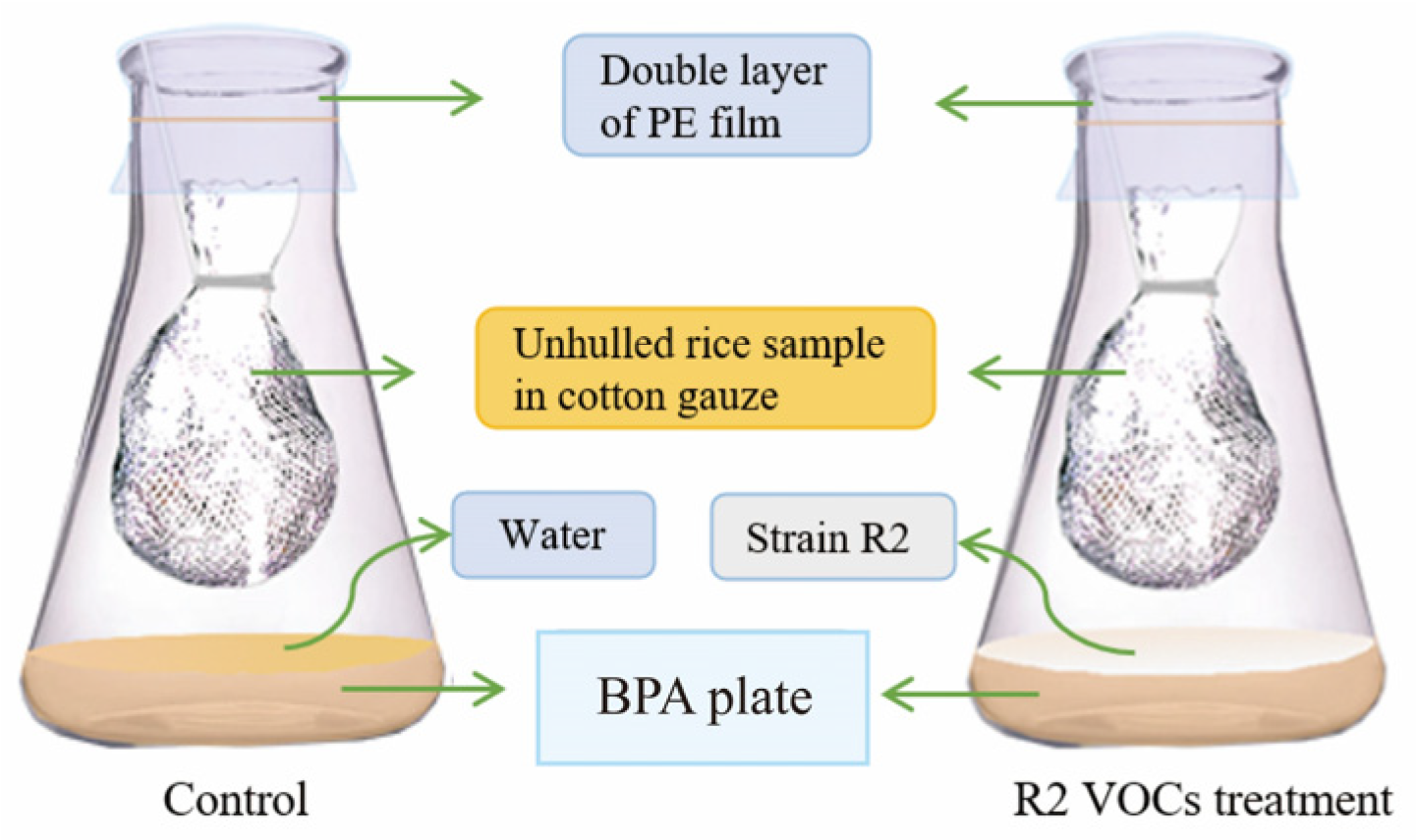
2.4. In Vivo Test of A. flavus Decay Control by R2 VOCs
2.5. Analysis of the VOCs Produced by Strain R2
2.6. Analysis of the Activity of Identified Individual VOCs against A. flavus
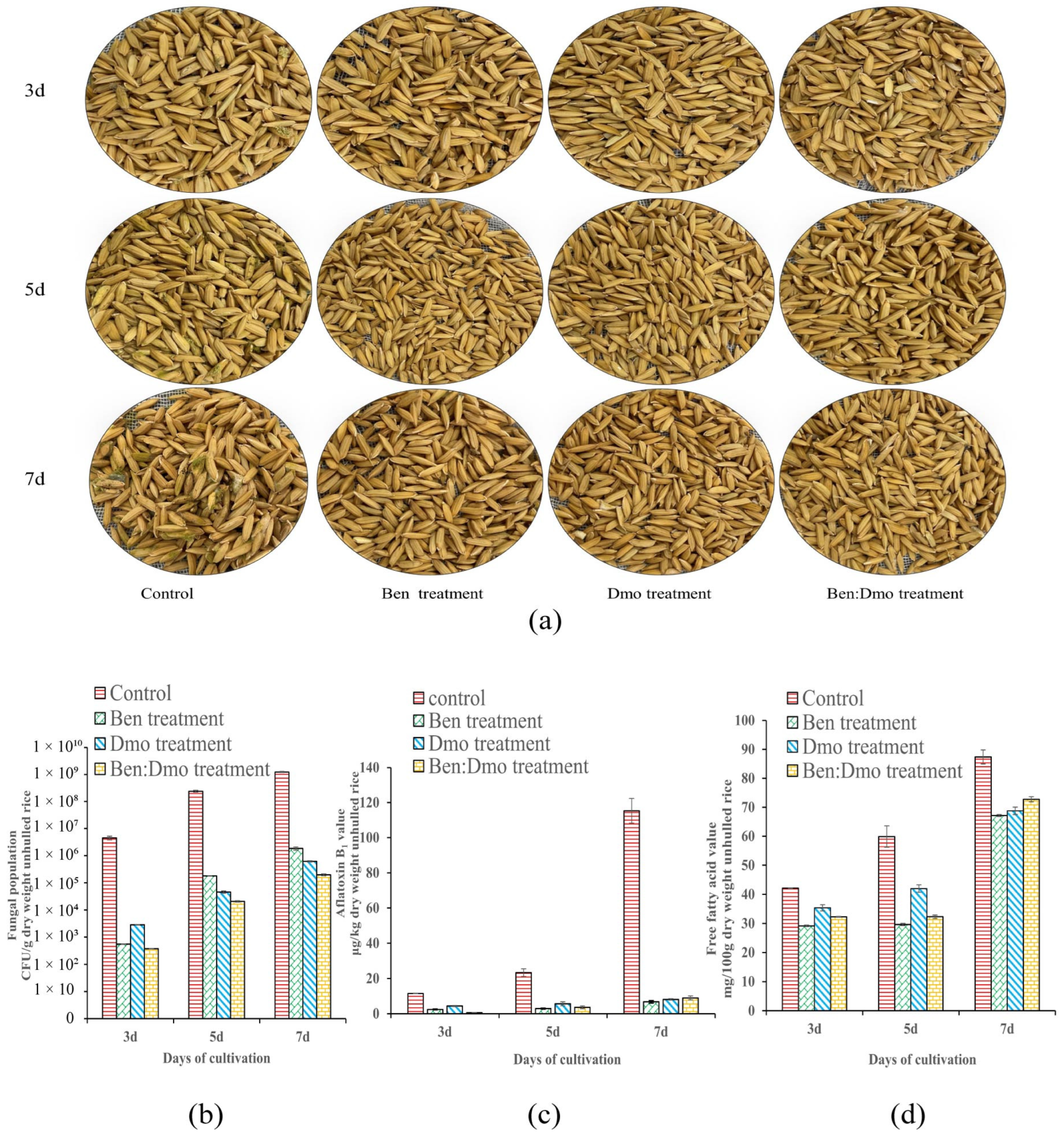
2.7. In Vivo Test of the Effect of Ben and Dmo on A. flavus Decay in Unhulled Rice
2.8. Statistical Analysis
3. Results
3.1. Bacillus Strain Isolation and Screening of Strains Producing High Antifungal Active VOCs
3.2. Physiological, Morphological, and Biochemical Properties of Bacillus Strain R2
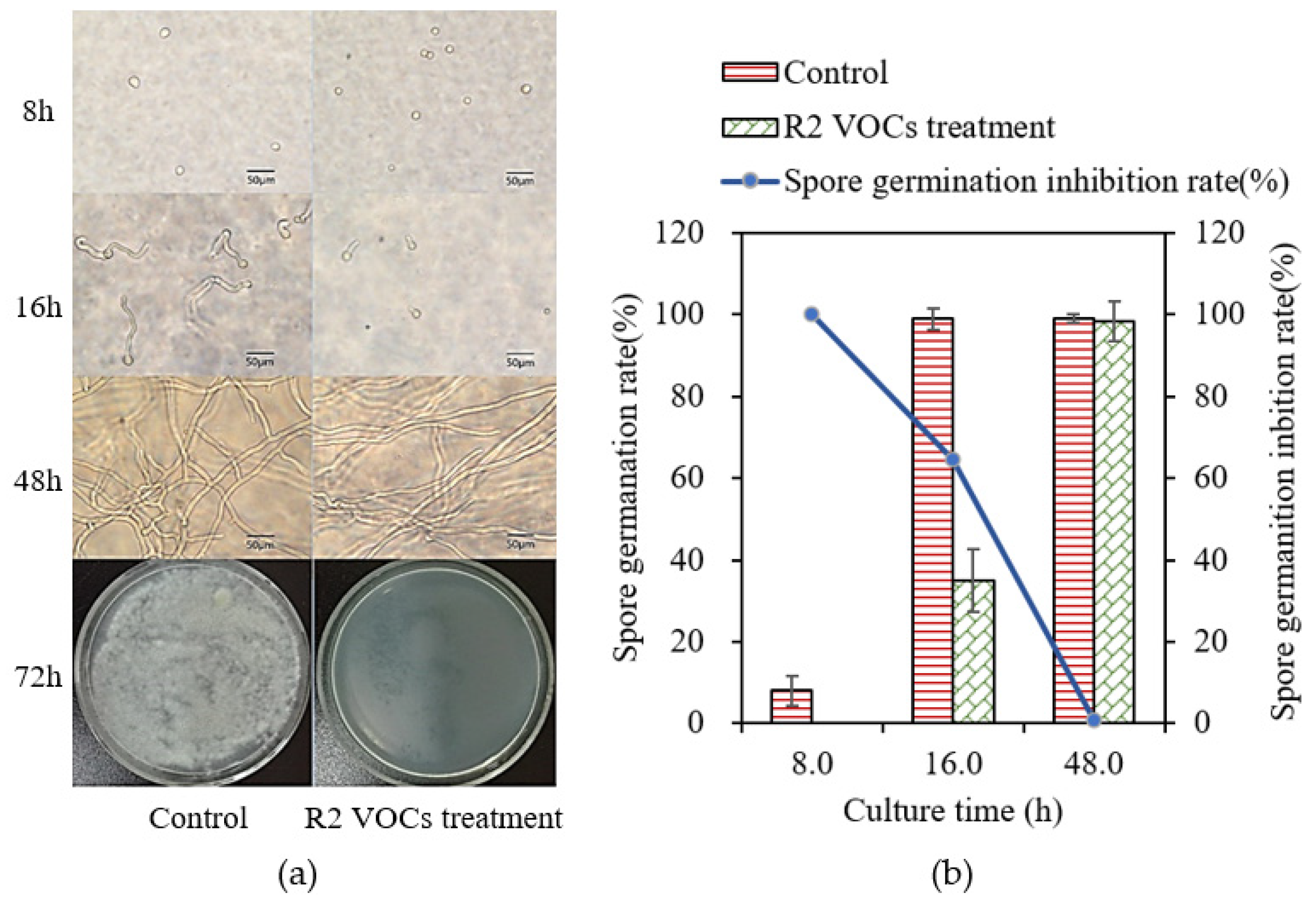
3.3. In Vitro R2 VOC Inhibition of Conidial Germination of A. flavus
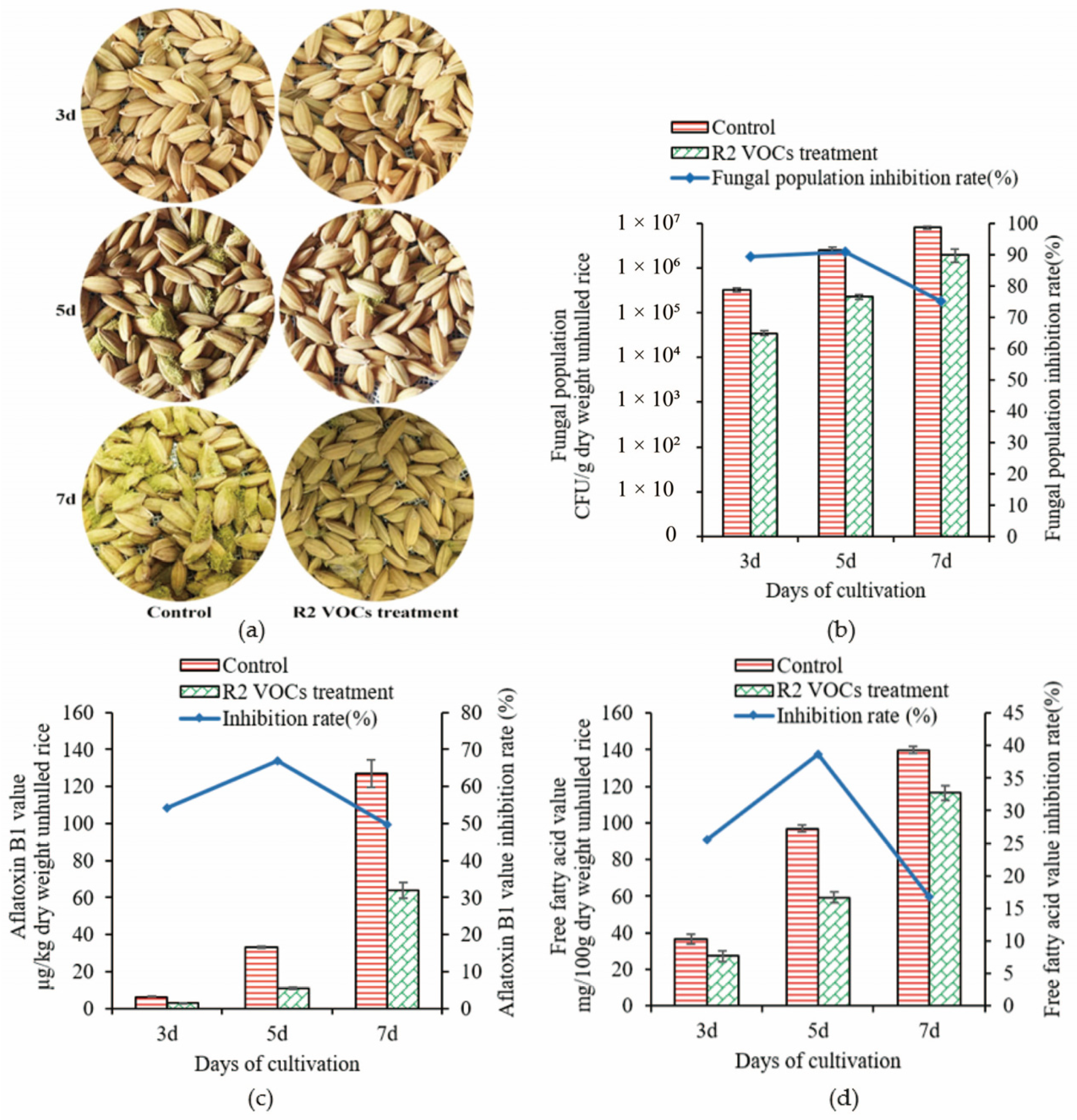
3.4. Effect of R2 VOCs on Rice Samples
3.5. Composition of VOCs Produced by Strain R2
3.6. Inhibitory Effect of the Individual Compounds against A. flavus
3.7. Antifungal Effect of Different Ben and Dmo Levels against A. flavus In Vitro
3.8. Inhibition Effect of Ben and Dmo against A. flavus in Unhulled Rice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, J.Y.; Jee, S.N.; Nam, Y.; Lee, H.; Ryoo, M., II.; Kim, K.D. Populations of Fungi and Bacteria Associated with Samples of Stored Rice in Korea. Mycobiology 2007, 35, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Oh, J.Y.; Kim, K.D. Biocontrol Activity of Volatile-Producing Bacillus Megaterium and Pseudomonas Protegens against Aspergillus flavus and Aflatoxin Production on Stored Rice Grains. Mycobiology 2017, 45, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Mwakinyali, S.E.; Ding, X.; Ming, Z.; Tong, W.; Zhang, Q.; Li, P. Recent Development of Aflatoxin Contamination Biocontrol in Agricultural Products. Biol. Control 2019, 128, 31–39. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Potential of Botanicals and Biocontrol Agents on Growth and Aflatoxin Production by Aspergillus flavus Infecting Rice Grains. Food Control 2009, 20, 173–178. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Microbe-Mediated Control of Mycotoxigenic Grain Fungi in Stored Rice with Focus on Aflatoxin Biodegradation and Biosynthesis Inhibition. Mycobiology 2016, 44, 67–78. [Google Scholar] [CrossRef]
- Je, W.P.; Choi, S.Y.; Hwang, H.J.; Kim, Y.B. Fungal Mycoflora and Mycotoxins in Korean Polished Rice Destined for Humans. Int. J. Food Microbiol. 2005, 103, 305–314. [Google Scholar]
- Klich, M.A. Environmental and Developmental Factors Influencing Aflatoxin Production by Aspergillus flavus and Aspergillus Parasiticus. Mycoscience 2007, 48, 71–80. [Google Scholar] [CrossRef]
- Agbetiameh, D.; Ortega-Beltran, A.; Awuah, R.T.; Atehnkeng, J.; Elzein, A.; Cotty, P.J.; Bandyopadhyay, R. Field Efficacy of Two Atoxigenic Biocontrol Products for Mitigation of Aflatoxin Contamination in Maize and Groundnut in Ghana. Biol. Control 2020, 150, 104351. [Google Scholar] [CrossRef]
- Kong, Q.; Chi, C.; Yu, J.; Shan, S.; Li, Q.; Li, Q.; Guan, B.; Nierman, W.C.; Bennett, J.W. The Inhibitory Effect of Bacillus Megaterium on Aflatoxin and Cyclopiazonic Acid Biosynthetic Pathway Gene Expression in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2014, 98, 5161–5172. [Google Scholar] [CrossRef]
- Al-Wadai, A.S.; Al-Othman, M.R.; Mahmoud, M.A.; El-Aziz, A.R.M.A. Molecular Characterization of Aspergillus flavus and Aflatoxin Contamination of Wheat Grains from Saudi Arabia. Genet. Mol. Res. 2013, 12, 3335–3352. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. International Agency for Research on Cancer Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans. Iarc Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556. [Google Scholar]
- Groopman, J.D.; Kensler, T.W. Role of Metabolism and Viruses in Aflatoxin-Induced Liver Cancer. Toxicol. Appl. Pharmacol. 2005, 206, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hu, B.; Shao, L.; Tian, Y.; Jin, T.; Jin, Y.; Ji, S.; Fan, X. Integrated Analysis of Transcriptomics and Metabonomics Profiles in Aflatoxin B1-Induced Hepatotoxicity in Rat. Food Chem. Toxicol. 2013, 55, 444–455. [Google Scholar] [CrossRef]
- El Golli-Bennour, E.; Kouidhi, B.; Bouslimi, A.; Abid-Essefi, S.; Hassen, W.; Bacha, H. Cytotoxicity and Genotoxicity Induced by Aflatoxin B1, Ochratoxin A, and Their Combination in Cultured Vero Cells. J. Biochem. Mol. Toxicol. 2010, 24, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Meissonnier, G.M.; Pinton, P.; Laffitte, J.; Cossalter, A.M.; Gong, Y.Y.; Wild, C.P.; Bertin, G.; Galtier, P.; Oswald, I.P. Immunotoxicity of Aflatoxin B1: Impairment of the Cell-Mediated Response to Vaccine Antigen and Modulation of Cytokine Expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef]
- Robinson, H.J.; Phares, H.F.; Graessle, O.E. The Toxicological and Antifungal Properties of Thiabendazole. Ecotoxicol. Environ. Saf. 1978, 1, 471–476. [Google Scholar] [CrossRef]
- White, D.G.; Toman, J.; Burnette, D.C.; Jacobsen, B.J. The Effect of Postharvest Fungicide Application on Storage Fungi of Corn during Ambient Air Drying and Storage. Plant Dis. 1993, 77, 562–568. [Google Scholar] [CrossRef]
- White, D.G. Effects of postharvest oil and fungicide application on storage fungi in corn following high-temperature grain drying. Plant Dis. 1994, 78, 38–43. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The Science, Development, and Commercialization of Postharvest Biocontrol Products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical Treatments to Control Postharvest Diseases of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Kim, P., II.; Chung, K.C. Production of an Antifungal Protein for Control of Colletotrichum Lagenarium by Bacillus amyloliquefaciens MET0908. FEMS Microbiol. Lett. 2004, 234, 177–183. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Zhang, Y.; Shan, H.H.; Tong, Y.H.; Chen, X.J.; Liu, F.Q. Isolation and Identification of Antifungal Peptides from Bacillus amyloliquefaciens W10. Environ. Sci. Pollut. Res. 2017, 24, 25000–25009. [Google Scholar] [CrossRef] [PubMed]
- Elkahoui, S.; Djébali, N.; Karkouch, I.; Ibrahim, A.H.; Kalai, L.; Bachkouel, S.; Tabbene, O.; Limam, F. Mass Spectrometry Identification of Antifungal Lipopeptides from Bacillus Sp. BCLRB2 against Rhizoctonia solani and Sclerotinia sclerotiorum. Appl. Biochem. Microbiol. 2014, 50, 161–165. [Google Scholar] [CrossRef]
- Yan, H.; Yun, J.; Ai, D.; Zhang, W.; Bai, J.; Guo, J. Two Novel Cationic Antifungal Peptides Isolated from Bacillus pumilus HN-10 and Their Inhibitory Activity against Trichothecium Roseum. World J. Microbiol. Biotechnol. 2018, 34, 21. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Wu, Y.; Ling, N.; Wei, Z.; Huang, Q.; Shen, Q. Effects of Volatile Organic Compounds Produced by Bacillus amyloliquefaciens on the Growth and Virulence Traits of Tomato Bacterial Wilt Pathogen Ralstonia Solanacearum. Appl. Microbiol. Biotechnol. 2016, 100, 7639–7650. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Biocontrol Activity of Volatile-Producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium Spp. Predominant in Stored Rice Grains: Study II. Mycobiology 2018, 46, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Shen, L.; Zhang, J.B.; Wu, A.B.; He, W.J.; Yuan, Q.S.; He, J.-D.; Liao, Y.C. The Shewanella algae Strain YM8 Produces Volatiles with Strong Inhibition Activity against Aspergillus pathogens and Aflatoxins. Front. Microbiol. 2015, 6, 1091. [Google Scholar] [CrossRef]
- Saleh, A.E.; Ul-Hassan, Z.; Zeidan, R.; Al-Shamary, N.; Al-Yafei, T.; Alnaimi, H.; Higazy, N.S.; Migheli, Q.; Jaoua, S. Biocontrol Activity of Bacillus megaterium BM344-1 against Toxigenic Fungi. ACS Omega 2021, 6, 10984–10990. [Google Scholar] [CrossRef]
- Di Francesco, A.; Zajc, J.; Gunde-Cimerman, N.; Aprea, E.; Gasperi, F.; Placì, N.; Caruso, F.; Baraldi, E. Bioactivity of Volatile Organic Compounds by Aureobasidium Species against Gray Mold of Tomato and Table Grape. World J. Microbiol. Biotechnol. 2020, 36, 1–11. [Google Scholar]
- Osaki, C.; Yamaguchi, K.; Urakawa, S.; Nakashima, Y.; Sugita, K.; Nagaishi, M.; Mitsuiki, S.; Kuraoka, T.; Ogawa, Y.; Sato, H. The Bacteriological Properties of Bacillus Strain TM-I-3 and Analysis of the Volatile Antifungal Compounds Emitted by This Bacteria. Biocontrol Sci. 2019, 24, 129–136. [Google Scholar] [CrossRef]
- You, W.; Ge, C.; Jiang, Z.; Chen, M.; Li, W.; Shao, Y. Screening of a Broad-Spectrum Antagonist-Bacillus Siamensis, and Its Possible Mechanisms to Control Postharvest Disease in Tropical Fruits. Biol. Control 2021, 157, 104584. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Teixidó, N.; Di Francesco, A.; Usall, J.; Ugolini, L.; Torres, R.; Mari, M. Antifungal Effect of Volatile Organic Compounds Produced by Bacillus amyloliquefaciens CPA-8 against Fruit Pathogen Decays of Cherry. Food Microbiol. 2017, 64, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Z.; Cai, M.Y. Handbook of Identification of Common Bacterial Systems; Science Press: Beijing, China, 2001; pp. 62–65. (In Chinese) [Google Scholar]
- Wang, Q.Y.; Lin, Q.L.; Peng, K.; Cao, J.Z.; Yang, C.; Xu, D. Surfactin Variants from Bacillus Subtilis Natto CSUF5 and Their Antifungal Properities against Aspergillus Niger. J. Biobased Mater. Bioenergy 2017, 11, 210–215. [Google Scholar] [CrossRef]
- Tian, P.P.; Lv, Y.Y.; Yuan, W.J.; Zhang, S.B.; Hu, Y. Sen. Effect of Artificial Aging on Wheat Quality Deterioration during Storage. J. Stored Prod. Res. 2019, 80, 50–56. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of Endophytic Bacillus velezensis ZSY-1 Strain and Antifungal Activity of Its Volatile Compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Park, C.E.; Kim, Y.S.; Park, K.J.; Kim, B.K. Changes in Physicochemical Characteristics of Rice during Storage at Different Temperatures. J. Stored Prod. Res. 2012, 48, 25–29. [Google Scholar] [CrossRef]
- Liu, C.; Yin, X.H.; Wang, Q.G. Antagonistic Activities of Volatiles Produced by Two Bacillus Strains against Monilinia fructicola in Peach Fruit. J. Organ. Behav. 2007, 28, 303–325. [Google Scholar] [CrossRef]
- Xie, S.; Liu, J.; Gu, S.; Chen, X.; Jiang, H.; Ding, T. Antifungal Activity of Volatile Compounds Produced by Endophytic Bacillus subtilis DZSY21 against Curvularia Lunata. Ann. Microbiol. 2020, 70, 1–10. [Google Scholar] [CrossRef]
- He, C.N.; Ye, W.Q.; Zhu, Y.Y.; Zhou, W.W. Antifungal Activity of Volatile Organic Compounds Produced by Bacillus Methylotrophicus and Bacillus thuringiensis against Five Common Spoilage Fungi on Loquats. Molecules 2020, 25, 3360. [Google Scholar] [CrossRef]
- Arrebola, E.; Sivakumar, D.; Korsten, L. Effect of Volatile Compounds Produced by Bacillus Strains on Postharvest Decay in Citrus. Biol. Control 2010, 53, 122–128. [Google Scholar] [CrossRef]
- Elkahoui, S.; Djébali, N.; Yaich, N.; Azaiez, S.; Hammami, M.; Essid, R.; Limam, F. Antifungal Activity of Volatile Compounds-Producing Pseudomonas P2 Strain against Rhizoctonia solani. World J. Microbiol. Biotechnol. 2015, 31, 175–185. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Lai, Q.; Zeng, R.; Ye, D.; Xu, J.; Shao, Z. Proposal of Nine Novel Species of the Bacillus Cereus Group. Int. J. Syst. Evol. Microbiol. 2017, 67, 2499–2508. [Google Scholar] [CrossRef]
- El-Sersawy, M.M.; Hassan, S.E.D.; El-Ghamry, A.A.; El-Gwad, A.M.A.; Fouda, A. Implication of Plant Growth-Promoting Rhizobacteria of Bacillus Spp. as Biocontrol Agents against Wilt Disease Caused by Fusarium oxysporum Schlecht. in Vicia faba L. Biomol. Concepts 2021, 12, 197–214. [Google Scholar] [CrossRef]
- Shastri, B.; Kumar, R.; Lal, R.J. Isolation, Characterization and Identification of Indigenous Endophytic Bacteria Exhibiting PGP and Antifungal Traits from the Internal Tissue of Sugarcane Crop. Sugar Tech. 2020, 22, 563–573. [Google Scholar] [CrossRef]
- Xu, X.; Wu, X.; Wu, T.; Zeng, M. Growth-Promoting and Adverse-Resistant Characteristics of JYZ-SD5, a Tree Rhizobacterium and Its Species Identification. Biotechnol. Bull. 2019, 35, 31–38. [Google Scholar]
- Gao, H.; Li, P.; Xu, X.; Zeng, Q.; Guan, W. Research on Volatile Organic Compounds from Bacillus Subtilis CF-3: Biocontrol Effects on Fruit Fungal Pathogens and Dynamic Changes during Fermentation. Front. Microbiol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Kim, J.H.; Chan, K.L.; Mahoney, N.; Campbell, B.C. Antifungal Activity of Redox-Active Benzaldehydes That Target Cellular Antioxidation. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 23. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; Wen, S.Y. Exploring the Caste-Specific Multi-Layer Defense Mechanism of Formosan Subterranean Termites, Coptotermes formosanus Shiraki. Int. J. Mol. Sci. 2017, 18, 2694. [Google Scholar] [CrossRef]
- Mannaa, M.; Oh, J.Y.; Kim, K.D. Microbe-Mediated Control of Aspergillus flavus in Stored Rice Grains with a Focus on Aflatoxin Inhibition and Biodegradation. Ann. Appl. Biol. 2017, 171, 376–392. [Google Scholar] [CrossRef]
- Rajer, F.U.; Wu, H.; Xie, Y.; Xie, S.; Raza, W.; Tahir, H.A.S.; Gao, X. Volatile Organic Compounds Produced by a Soil-Isolate, Bacillus subtilis FA26 Induce Adverse Ultra-Structural Changes to the Cells of Clavibacter michiganensis ssp. Sepedonicus, the Causal Agent of Bacterial Ring Rot of Potato. Microbiology 2017, 163, 523–530. [Google Scholar] [CrossRef]
- Li, S.F.; Zhang, S.B.; Lv, Y.Y.; Zhai, H.C.; Hu, Y.; Cai, J.P. Heptanal Inhibits the Growth of Aspergillus flavus through Disturbance of Plasma Membrane Integrity, Mitochondrial Function and Antioxidant Enzyme Activity. LWT 2022, 154, 112655. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, L.; Wang, C.; Mu, W.; Liu, F. Bioactive Evaluation and Application of Antifungal Volatiles Generated by Soil Bacteria. J. Plant Prot. 2009, 36, 97–105. [Google Scholar]
- Xia, K.; Zhang, C.; Zhang, X.; Cao, J.; He, L.; Liu, C. Control of Grey Mould by Sodium Diacetate Treatments and Its Effects on Postharvest Quality of ‘Red Globe’ Grapes. Physiol. Mol. Plant Pathol. 2023, 125, 102014. [Google Scholar] [CrossRef]
- Rutenberg, R.; Bernstein, S.; Fallik, E.; Paster, N.; Poverenov, E. The Improvement of Propionic Acid Safety and Use during the Preservation of Stored Grains. Crop Prot. 2018, 110, 191–197. [Google Scholar] [CrossRef]
- Wang, L.M.; Xing, F.G.; Lyu, C.; Liu, Y. Study on the Anti-Mould and Anti-Mycotoxins Effects of Combined Essential Oils in Maize. J. Nucl. Agric. Sci. 2018, 35, 732–739. [Google Scholar]


| Compound | Chemical Formula | Retention Time (min) | Peak Area (%) |
|---|---|---|---|
| 2,4-Dimethylheptane | C9H20 | 4.51 | 0.716 |
| 2,4-Dimethyl-1-heptene | C9H18 | 5.34 | 0.256 |
| 2,6-Dimethyl-nonane | C11H24 | 8.58 | 3.227 |
| 3,7-Dimethyl-1-octanol | C10H22O | 10.12 | 0.211 |
| Benzaldehyde | C7H6O | 10.64 | 2.023 |
| 4-Carbonitrile-pyrazole | C4H3N3 | 10.98 | 0.485 |
| 3-Ethyl-3-methyl heptane | C10H22 | 12.71 | 0.306 |
| n-Pentadecane | C15H32 | 13.1 | 0.584 |
| n-Nonadecane | C19H40 | 13.63 | 1.027 |
| 2-Butyl-1-octanol | C12H26O | 13.75 | 0.196 |
| n-Hexadecane | C16H34 | 13.98 | 0.431 |
| 3,3-Dimethyl-1-butanol | C6H14O | 14.25 | 0.535 |
| 2,3-Butanediol | C4H10O2 | 14.74 | 0.199 |
| 2-Methyl-2-oxazoline | C4H7NO | 15.46 | 0.579 |
| 3-Butyn-1-ol | C4H6O | 16.85 | 0.212 |
| 2-Methylglutaronitrile | C6H8N2 | 17.24 | 1.858 |
| Tert-butyl nonaneperoxoate | C13H26O3 | 17.55 | 0.122 |
| Ethyl propiolate | C5H6O2 | 20.15 | 0.476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, K.; Yu, L.; Lin, Q.; Zhou, W. Volatile Organic Compounds Produced by Bacillus sp. Strain R2 Inhibit Aspergillus flavus Growth In Vitro and in Unhulled Rice. Foods 2024, 13, 2898. https://doi.org/10.3390/foods13182898
Wang Q, Zhang K, Yu L, Lin Q, Zhou W. Volatile Organic Compounds Produced by Bacillus sp. Strain R2 Inhibit Aspergillus flavus Growth In Vitro and in Unhulled Rice. Foods. 2024; 13(18):2898. https://doi.org/10.3390/foods13182898
Chicago/Turabian StyleWang, Qingyun, Kaige Zhang, Lu Yu, Qinlu Lin, and Wenhua Zhou. 2024. "Volatile Organic Compounds Produced by Bacillus sp. Strain R2 Inhibit Aspergillus flavus Growth In Vitro and in Unhulled Rice" Foods 13, no. 18: 2898. https://doi.org/10.3390/foods13182898






