Microbial and Quality Attributes of Beef Steaks under High-CO2 Packaging: Emitter Pads versus Gas Flushing
Abstract
:1. Introduction
2. Materials and Methods
2.1. CO2 Emitter Pads and Meat Samples: Selection and Suppliers
2.2. CO2 Emitter Pads, Gas Flushing, and Meat Sample Preparation
2.3. CO2 Emitter Pads and Meat Sample Analyses
2.4. Statistical Analysis
3. Results and Discussion
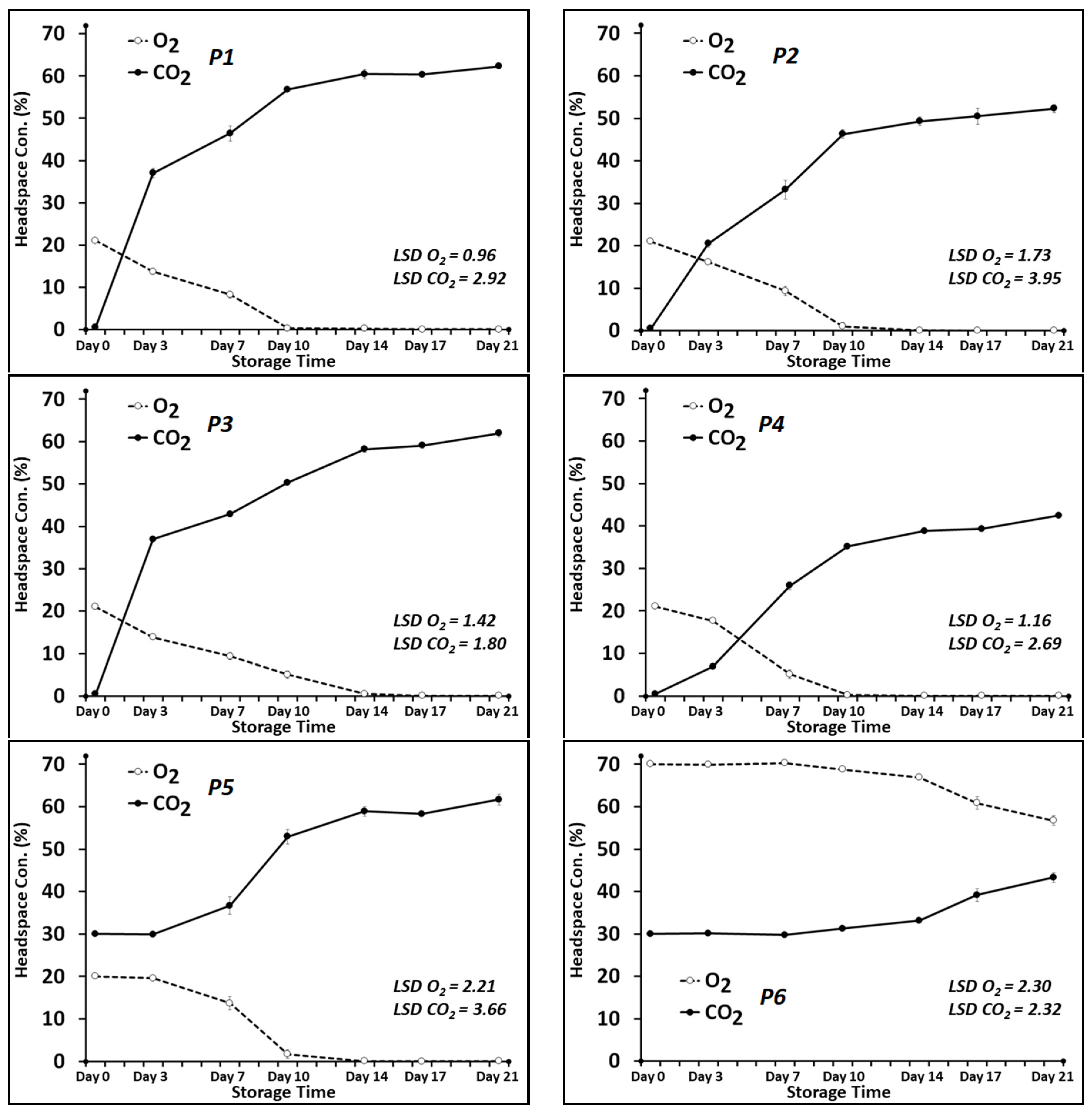
3.1. Headspace CO2 and O2 Levels
3.2. Color
3.3. pH
3.4. Microbiological Examination
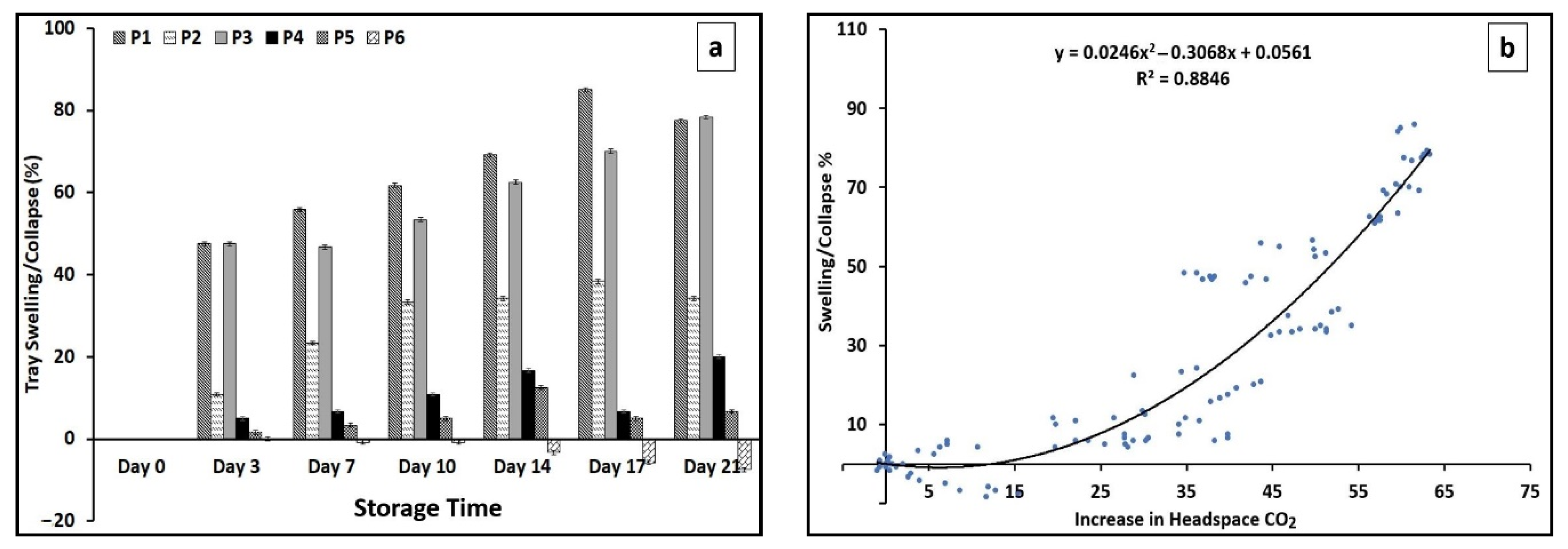
3.5. Tray Swelling/Collapse
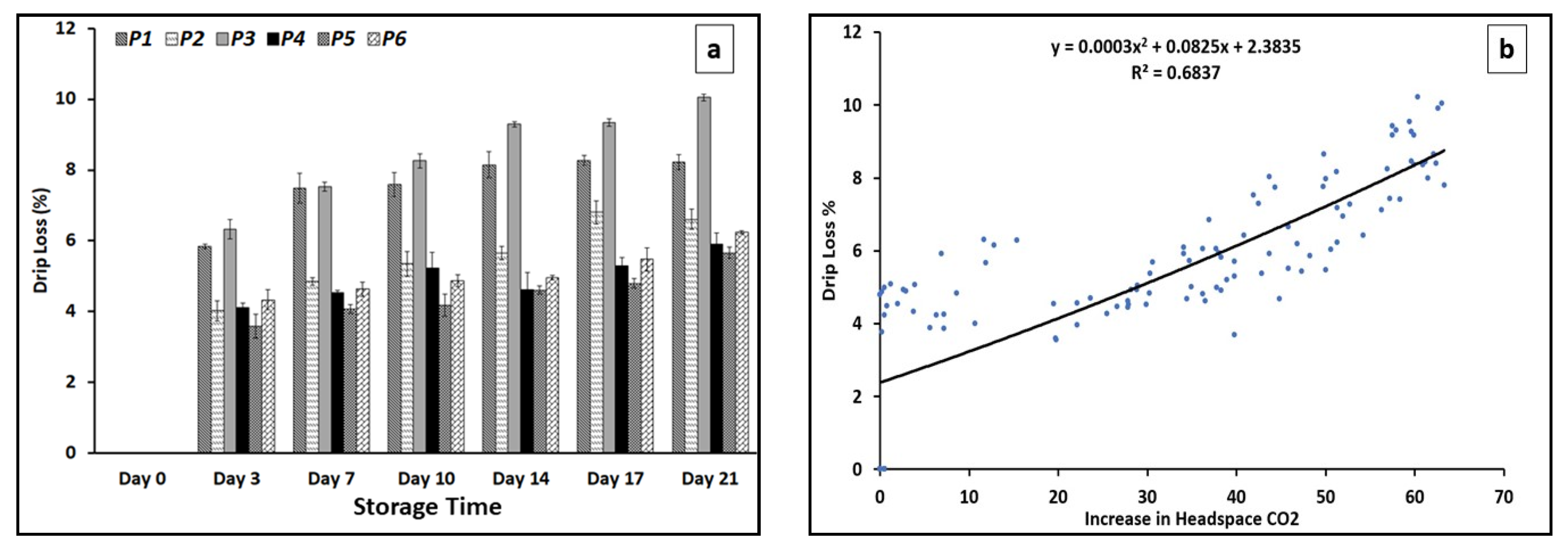
3.6. Drip Loss
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comi, G. Spoilage of meat and fish. In The microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 179–210. [Google Scholar]
- Wang, Z.; Tu, J.; Zhou, H.; Lu, A.; Xu, B. A comprehensive insight into the effects of microbial spoilage, myoglobin autoxidation, lipid oxidation, and protein oxidation on the discoloration of rabbit meat during retail display. Meat Sci. 2021, 172, 108359. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Stamatiou, A.; Skandamis, P.; Nychas, G.J. Development of a microbial model for the combined effect of temperature and pH on spoilage of ground meat, and validation of the model under dynamic temperature conditions. Appl. Environ. Microbiol. 2006, 72, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, I.; Slima, S.B.; Ktari, N.; Triki, M.; Abdehedi, R.; Abaza, W.; Moussa, H.; Abdeslam, A.; Salah, R.B. Incorporation of probiotic strain in raw minced beef meat: Study of textural modification, lipid and protein oxidation and color parameters during refrigerated storage. Meat Sci. 2019, 154, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Iulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat spoilage: A critical review of a neglected alteration due to ropy slime producing bacteria. Ital. J. Anim. Sci. 2015, 14, 4011. [Google Scholar] [CrossRef]
- Mortazavi, S.M.H.; Kaur, M.; Farahnaky, A.; Torley, P.J.; Osborn, A.M. The pathogenic and spoilage bacteria associated with red meat and application of different approaches of high CO2 packaging to extend product shelf-life. Crit. Rev. Food Sci. Nutr. 2023, 63, 1733–1754. [Google Scholar] [CrossRef]
- Conte-Junior, C.A.; Monteiro, M.L.G.; Patrícia, R.; Mársico, E.T.; Lopes, M.M.; Alvares, T.S.; Mano, S.B. The effect of different packaging systems on the shelf life of refrigerated ground beef. Foods 2020, 9, 495. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.; Zhu, L.; Luo, X.; Mao, Y.; Hopkins, D.L.; Zhang, Y.; Dong, P. Effect of modified atmosphere packaging on shelf life and bacterial community of roast duck meat. Food Res. Int. 2020, 137, 109645. [Google Scholar] [CrossRef]
- Wang, G.; Ma, F.; Zeng, L.; Bai, Y.; Wang, H.; Xu, X.; Zhou, G. Modified atmosphere packaging decreased Pseudomonas fragi cell metabolism and extracellular proteolytic activities on meat. Food Microbiol. 2018, 76, 443–449. [Google Scholar] [CrossRef]
- Ercolini, D.; Russo, F.; Torrieri, E.; Masi, P.; Villani, F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 2006, 72, 4663–4671. [Google Scholar] [CrossRef]
- Brooks, J.C.; Alvarado, M.; Stephens, T.P.; Kellermeier, J.D.; Tittor, A.W.; Miller, M.F.; Brashears, M.M. Spoilage and safety characteristics of ground beef packaged in traditional and modified atmosphere packages. J. Food Prot. 2008, 71, 293–301. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Y.; Hou, Q.; Huang, M.; Zhou, X. Dynamic changes of the bacterial communities in roast chicken stored under normal and modified atmosphere packaging. J. Food Sci. 2020, 85, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Berk, Z. Food Process Engineering and Technology; Academic Press: Cambridge, MA, USA, 2013; pp. 545–559. [Google Scholar]
- Greengrass, J. Packaging materials for MAP of foods. In Principles and Applications of Modified Atmosphere Packaging of Foods; Blakistone, B.A., Blakistone, B.A., Eds.; Springer: Boston, MA, USA, 1998; pp. 63–101. [Google Scholar]
- Murphy, K.M.; O’Grady, M.N.; Kerry, J.P. Effect of varying the gas headspace to meat ratio on the quality and shelf-life of beef steaks packaged in high oxygen modified atmosphere packs. Meat Sci. 2013, 94, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Chaix, E.; Couvert, O.; Guillaume, C.; Gontard, N.; Guillard, V. Predictive microbiology coupled with gas (O2/CO2) transfer in food/packaging systems: How to develop an efficient decision support tool for food packaging dimensioning. Compr. Rev. Food Sci. Food Saf. 2015, 14, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Manu-Tawiah, W. Microbiological, Physical and Chemical Studies on Fresh Red Meats Packaged under Different Modified Gas Atmospheres; Iowa State University: Ames, IA, USA, 1991. [Google Scholar]
- Gokoglu, N.; Yerlikaya, P.; Uran, H.; Topuz, O.K. The effect of modified atmosphere packaging on the quality and shelf life of frankfurter type-sausages. J. Food Qual. 2010, 33, 367–380. [Google Scholar] [CrossRef]
- Rodrigues, I.; Trindade, M.A.; Palu, A.F.; Baldin, J.C.; de Lima, C.G.; de Alvarenga Freire, M.T. Modified atmosphere packaging for lamb meat: Evaluation of gas composition in the extension of shelf life and consumer acceptance. J. Food Sci. Technol. 2018, 55, 3547–3555. [Google Scholar] [CrossRef]
- Vergara, H.; Berruga, M.I.; Linares, M.B. Effect of gas composition on rabbit meat quality in modified atmosphere packaging. J. Sci. Food Agric. 2005, 85, 1981–1986. [Google Scholar] [CrossRef]
- Bagdatli, A.; Kayaardi, S. Influence of storage period and packaging methods on quality attributes of fresh beef steaks. CyTA-J. Food 2015, 13, 124–133. [Google Scholar] [CrossRef]
- Clausen, I.; Madsen, N.T. Sensory evaluation of ground beef stored in different atmospheres. In Proceedings of the 51st International Congress of Meat Science and Technology, Baltimore, MD, USA, 7–12 August 2005. [Google Scholar]
- Boz, Z.; Welt, B.A.; Brecht, J.K.; Pelletier, W.; McLamore, E.; Kiker, G.A.; Butler, J.E. Review of challenges and advances in modification of food package headspace gases. J. Appl. Packag. Res. 2018, 10, 1. [Google Scholar]
- Ghris, S. Effect of the carbon dioxide grinding on modified atmosphere and color shelf life of ground beef. Available online: https://www.proquest.com/docview/304892671?fromopenview=true&pq-origsite=gscholar (accessed on 14 July 2023).
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Hansen, A.Å.; Høy, M.; Pettersen, M.K. Prediction of optimal CO2 emitter capacity developed for modified atmosphere packaging of fresh salmon fillets (Salmo salar L.). Packag. Technol. Sci. Int. J. 2009, 22, 199–208. [Google Scholar] [CrossRef]
- Tsironi, T.; Ntzimani, A.; Gogou, E.; Tsevdou, M.; Semenoglou, I.; Dermesonlouoglou, E.; Taoukis, P. Modeling the effect of active modified atmosphere packaging on the microbial stability and shelf life of gutted sea bass. Appl. Sci. 2019, 9, 5019. [Google Scholar] [CrossRef]
- Hansen, A.A.; Moen, B.; Rødbotten, M.; Berget, I.; Pettersen, M.K. Effect of vacuum or modified atmosphere packaging (MAP) in combination with a CO2 emitter on quality parameters of cod loins (Gadus morhua). Food Packag. Shelf Life 2016, 9, 29–37. [Google Scholar] [CrossRef]
- Holck, A.L.; Pettersen, M.K.; Moen, M.H.; Sørheim, O. Prolonged shelf life and reduced drip loss of chicken filets by the use of carbon dioxide emitters and modified atmosphere packaging. J. Food Prot. 2014, 77, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, M.K.; Hansen, A.Å.; Mielnik, M. Effect of different packaging methods on quality and shelf life of fresh reindeer meat. Packag. Technol. Sci. 2014, 27, 987–997. [Google Scholar] [CrossRef]
- Hur, S.J.; Jin, S.K.; Park, J.H.; Jung, S.W.; Lyu, H.J. Effect of modified atmosphere packaging and vacuum packaging on quality characteristics of low grade beef during cold storage. Asian-Australas. J. Anim. Sci. 2013, 26, 1781–1789. [Google Scholar] [CrossRef]
- Łopacka, J.; Półtorak, A.; Wierzbicka, A. Effect of MAP, vacuum skin-pack and combined packaging methods on physicochemical properties of beef steaks stored up to 12 days. Meat Sci. 2016, 119, 147–153. [Google Scholar] [CrossRef]
- ISO 6887-2; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination Part 2: Specific Rules for the Preparation of Meat and Meat Products. International Organization for Standardization: Geneva, Switzerland, 2017.
- Xu, M.M.; Kaur, M.; Pillidge, C.J.; Torley, P.J. Evaluation of the potential of protective cultures to extend the microbial shelf-life of chilled lamb meat. Meat Sci. 2021, 181, 108613. [Google Scholar] [CrossRef]
- Dumont, M.J.; Orsat, V.; Raghavan, V. Reducing postharvest losses. In Emerging Technologies for Promoting Food Security; Madramootoo, C., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 135–156. [Google Scholar]
- Jakobsen, M.; Bertelsen, G. Solubility of carbon dioxide in fat and muscle tissue. J. Muscle Foods 2006, 17, 9–19. [Google Scholar] [CrossRef]
- Kolbeck, S.; Reetz, L.; Hilgarth, M.; Vogel, R.F. Quantitative oxygen consumption and respiratory activity of meat spoiling bacteria upon high oxygen modified atmosphere. Front. Microbiol. 2019, 10, 2398. [Google Scholar] [CrossRef]
- Esmer, O.K.; Irkin, R.; Degirmencioglu, N.; Degirmencioglu, A. The effects of modified atmosphere gas composition on microbiological criteria, color and oxidation values of minced beef meat. Meat Sci. 2011, 88, 221–226. [Google Scholar] [CrossRef]
- Rouger, A.; Moriceau, N.; Prévost, H.; Remenant, B.; Zagorec, M. Diversity of bacterial communities in French chicken cuts stored under modified atmosphere packaging. Food Microbiol. 2018, 70, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.B.; van de Ven, R.J.; Mao, Y.; Coombs, C.E.; Hopkins, D.L. Using instrumental (CIE and reflectance) measures to predict consumers’ acceptance of beef colour. Meat Sci. 2017, 127, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Luo, X.; Zhang, Y.; Zhu, L.; Xu, B.; Hopkins, D.L.; Liang, R. Influence of oxygen concentration on the fresh and internal cooked color of modified atmosphere packaged dark-cutting beef stored under chilled and superchilled conditions. Meat Sci. 2022, 188, 108773. [Google Scholar] [CrossRef] [PubMed]
- Danijela, Š.Z.; Vera, L.L.; Ljubinko, L.B.; Lato, P.L.; Vladimir, T.M.; Nevena, H.M. Effect of specific packaging conditions on myoglobin and meat color. Food Feed. Res. 2013, 40, 1–10. [Google Scholar]
- Carse, W.A.; Locker, R.H. A survey of pH values at the surface of beef and lamb carcasses, stored in a chiller. J. Sci. Food Agric. 1974, 25, 1529–1535. [Google Scholar] [CrossRef]
- Meat Standards Australia, Beef. Available online: https://www.mla.com.au/marketing-beef-and-lamb/meat-standards-australia/. (accessed on 13 September 2024).
- Feiner, G. The microbiology of specific bacteria. In Meat Products Handbook: Practical Science and Technology; Feiner, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 595–615. [Google Scholar]
- Borch, E.; Kant-Muermans, M.L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef]
- Muhlisin; Panjono; Kim, D.S.; Song, Y.R.; Lee, S.J.; Lee, J.K.; Lee, S.K. Effects of gas composition in the modified atmosphere packaging on the shelf-life of Longissimus dorsi of Korean native black pigs-duroc crossbred during refrigerated storage. Asian-Australas. J. Anim. Sci. 2014, 27, 1157–1163. [Google Scholar]
- Gill, A.O.; Gill, C.O. Preservative packaging for fresh meats, poultry, and fin fish. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 204–226. [Google Scholar]
- Wang, Q.; Chen, Q.; Xu, J.; Sun, F.; Liu, H.; Kong, B. Effects of modified atmosphere packaging with various CO2 concentrations on the bacterial community and shelf-life of smoked chicken Legs. Foods 2022, 11, 559. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Paramithiotis, S.; Kagkli, D.M.; Nychas, G.J.E. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Food Microbiol. 2010, 27, 1028–1034. [Google Scholar] [CrossRef]
- Jaberi, R.; Kaban, G.; Kaya, M. Effects of vacuum and high-oxygen modified atmosphere packaging on physico-chemical and microbiological properties of minced water buffalo meat. Asian-Australas. J. Anim. Sci. 2019, 32, 421–429. [Google Scholar] [CrossRef]
- Egan, A.F. Lactic acid bacteria of meat and meat products. Antonie Van Leeuwenhoek 1983, 49, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Tamplin, M.; Chandry, P.S. Insight into the genome of Brochothrix thermosphacta, a problematic meat spoilage bacterium. Appl. Environ. Microbiol. 2017, 83, e02786-16. [Google Scholar] [CrossRef] [PubMed]
- Clemens, R.M.; Adam, K.H.; Brightwell, G. Contamination levels of Clostridium estertheticum spores that result in gaseous spoilage of vacuum-packaged chilled beef and lamb meat. Lett. Appl. Microbiol. 2010, 50, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Broda, D.M.; Bell, R.G.; Boerema, J.A.; Musgrave, D.R. The abattoir source of culturable psychrophilic Clostridium spp. causing ‘blown pack’ spoilage of vacuum-packed chilled venison. J. Appl. Microbiol. 2002, 93, 817–824. [Google Scholar] [CrossRef]
- Gurunathan, K.; Tahseen, A.; Manyam, S. Effect of aerobic and modified atmosphere packaging on quality characteristics of chicken leg meat at refrigerated storage. Poult. Sci. 2022, 101, 102170. [Google Scholar] [CrossRef]
- Rodriguez-Aguilera, R.; Oliveira, J.C.; Montanez, J.C.; Mahajan, P.V. Gas exchange dynamics in modified atmosphere packaging of soft cheese. J. Food Eng. 2009, 95, 438–445. [Google Scholar] [CrossRef]
- Watanabe, G.; Motoyama, M.; Nakajima, I.; Sasaki, K. Relationship between water-holding capacity and intramuscular fat content in Japanese commercial pork loin. Asian-Australas. J. Anim. Sci. 2018, 31, 914–918. [Google Scholar] [CrossRef]
- Marcinkowska-Lesiak, M.; Zdanowska-Sąsiadek, Ż.; Stelmasiak, A.; Damaziak, K.; Michalczuk, M.; Poławska, E.; Wyrwisz, J.; Wierzbicka, A. Effect of packaging method and cold-storage time on chicken meat quality. CyTA-J. Food 2016, 14, 41–46. [Google Scholar] [CrossRef]
- Payne, S.R.; Durham, C.J.; Scott, S.M.; Devine, C.E. The effects of non-vacuum packaging systems on drip loss from chilled beef. Meat Sci. 1998, 49, 277–287. [Google Scholar] [CrossRef]
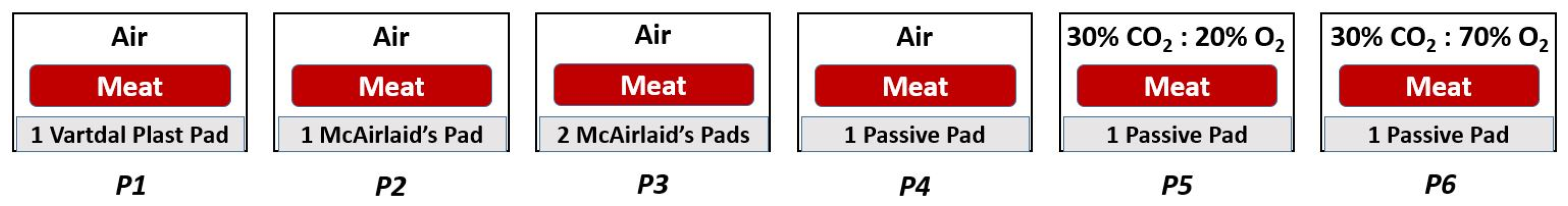


| Code | Packaging | Meat Side | Storage Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (Headspace/Pad) | Day 0 | Day 3 | Day 7 | Day 10 | Day 14 | Day 17 | Day 21 | ||
| P1 | Air/1 VP | Top | 5.3 ± 0.0 | 5.6 ± 0.0 | 5.7 ± 0.0 | 5.8 ± 0.1 | 5.8 ± 0.0 | 5.8 ± 0.0 | 5.7 ± 0.0 |
| Down | 5.3 ± 0.0 | 5.6 ± 0.1 | 5.9 ± 0.0 | 5.9 ± 0.0 | 6.0 ± 0.1 | 5.9 ± 0.0 | 5.7 ± 0.0 | ||
| P2 | Air/1 MA | Top | 5.3 ± 0.0 | 5.6 ± 0.0 | 5.7 ± 0.0 | 5.8 ± 0.0 | 5.8 ± 0.0 | 5.7 ± 0.0 | 5.7 ± 0.0 |
| Down | 5.3 ± 0.0 | 5.6 ± 0.0 | 5.7 ± 0.0 | 5.9 ± 0.0 | 5.9 ± 0.0 | 5.8 ± 0.1 | 5.6 ± 0.0 | ||
| P3 | Air/2 MA | Top | 5.3 ± 0.0 | 5.6 ± 0.0 | 5.7 ± 0.1 | 5.7 ± 0.1 | 5.8 ± 0.0 | 5.9 ± 0.0 | 5.7 ± 0.0 |
| Down | 5.3 ± 0.0 | 5.7 ± 0.0 | 5.7 ± 0.0 | 5.9 ± 0.1 | 5.9 ± 0.0 | 5.9 ± 0.0 | 5.7 ± 0.0 | ||
| P4 | Air/1 PP | Top | 5.3 ± 0.0 | 5.6 ± 0.1 | 5.7 ± 0.1 | 5.6 ± 0.0 | 5.6 ± 0.1 | 5.6 ± 0.1 | 5.5 ± 0.0 |
| Down | 5.3 ± 0.0 | 5.6 ± 0.0 | 5.7 ± 0.1 | 5.6 ± 0.0 | 5.6 ± 0.0 | 5.5 ± 0.1 | 5.5 ± 0.0 | ||
| P5 | 30% CO2: 20% O2/1 PP | Top | 5.3 ± 0.0 | 5.6 ± 0.0 | 5.6 ± 0.0 | 5.7 ± 0.0 | 5.7 ± 0.0 | 5.6 ± 0.0 | 5.5 ± 0.0 |
| Down | 5.3 ± 0.0 | 5.6 ± 0.0 | 5.6 ± 0.0 | 5.7 ± 0.0 | 5.7 ± 0.1 | 5.6 ± 0.0 | 5.4 ± 0.0 | ||
| P6 | 30% CO2: 70% O2/1 PP | Top | 5.3 ± 0.0 | 5.5 ± 0.0 | 5.6 ± 0.0 | 5.6 ± 0.0 | 5.3 ± 0.0 | 5.3 ± 0.0 | 5.1 ± 0.0 |
| Down | 5.3 ± 0.0 | 5.6 ± 0.0 | 5.6 ± 0.0 | 5.6 ± 0.1 | 5.3 ± 0.0 | 5.2 ± 0.0 | 5.1 ± 0.0 | ||
| Code | Packaging (P) | Storage Time (T) | LSD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Headspace/Pad) | Day 0 | Day 3 | Day 7 | Day 10 | Day 14 | Day 17 | Day 21 | (S × T) | ||
| Aerobic Counts | P1 | Air/1 VP | 2.3 ± 0.2 | 2.7 ± 0.1 | 2.8 ± 0.2 | 3.9 ± 0.2 | 5.1 ± 0.2 | 5.4 ± 0.0 | 5.5 ± 0.1 | 0.63 |
| P2 | Air/1 MA | 2.3 ± 0.2 | 3.0 ± 0.2 | 3.1 ± 0.5 | 4.2 ± 0.1 | 5.2 ± 0.1 | 5.8 ± 0.1 | 5.2 ± 0.5 | ||
| P3 | Air/2 MA | 2.3 ± 0.2 | 2.4 ± 0.3 | 2.8 ± 0.3 | 3.6 ± 0.5 | 5.1 ± 0.1 | 5.6 ± 0.1 | 5.7 ± 0.4 | ||
| P4 | Air/1 PP | 2.3 ± 0.2 | 4.3 ± 0.4 | 4.3 ± 0.2 | 4.6 ± 0.0 | 5.8 ± 0.1 | 5.9 ± 0.1 | 6.3 ± 0.1 | ||
| P5 | 30% CO2: 20% O2/1 PP | 2.3 ± 0.2 | 2.5 ± 0.1 | 2.6 ± 0.1 | 3.6 ± 0.5 | 4.7 ± 0.1 | 5.5 ± 0.2 | 5.7 ± 0.3 | ||
| P6 | 30% CO2: 70% O2/1 PP | 2.3 ± 0.2 | 3.5 ± 0.1 | 3.5 ± 0.2 | 4.3 ± 0.1 | 4.8 ± 0.4 | 6.4 ± 0.1 | 6.4 ± 0.1 | ||
| Lactic acid bacteria | P1 | Air/1 VP | 1.8 ± 0.1 | 2.1 ± 0.2 | 3.8 ± 0.3 | 5.2 ± 0.2 | 5.9 ± 0.1 | 6.4 ± 0.0 | 6.8 ± 0.1 | 0.62 |
| P2 | Air/1 MA | 1.8 ± 0.1 | 2.1 ± 0.1 | 3.7 ± 0.2 | 5.4 ± 0.3 | 5.5 ± 0.2 | 5.9 ± 0.2 | 6.6 ± 0.1 | ||
| P3 | Air/2 MA | 1.8 ± 0.1 | 1.9 ± 0.3 | 3.5 ± 0.5 | 4.4 ± 0.5 | 5.3 ± 0.1 | 6.0 ± 0.1 | 6.5 ± 0.1 | ||
| P4 | Air/1 PP | 1.8 ± 0.1 | 2.8 ± 0.4 | 4.3 ± 0.2 | 4.9 ± 0.2 | 5.5 ± 0.1 | 5.9 ± 0.1 | 6.3 ± 0.2 | ||
| P5 | 30% CO2: 20% O2/1 PP | 1.8 ± 0.1 | 2.4 ± 0.4 | 3.5 ± 0.5 | 4.9 ± 0.2 | 5.5 ± 0.2 | 5.7 ± 0.2 | 6.1 ± 0.2 | ||
| P6 | 30% CO2: 70% O2/1 PP | 1.7 ± 0.1 | 3.2 ± 0.0 | 5.0 ± 0.2 | 5.8 ± 0.1 | 6.1 ± 0.1 | 6.7 ± 0.1 | 7.1 ± 0.2 | ||
| Brochothrix thermosphacta | P1 | Air/1 VP | 0.0 ± 0.0 | 0.6 ± 0.1 | 1.7 ± 0.0 | 2.4 ± 0.2 | 2.5 ± 0.2 | 2.6 ± 0.1 | 2.3 ± 0.1 | 0.91 |
| P2 | Air/1 MA | 0.0 ± 0.0 | 1.2 ± 0.6 | 2.3 ± 0.1 | 2.8 ± 0.0 | 2.7 ± 0.3 | 2.4 ± 0.1 | 2.0 ± 0.3 | ||
| P3 | Air/2 MA | 0.0 ± 0.0 | 1.1 ± 0.5 | 1.6 ± 0.8 | 2.0 ± 0.2 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.2 | ||
| P4 | Air/1 PP | 0.0 ± 0.0 | 2.2 ± 0.5 | 3.4 ± 0.2 | 3.1 ± 0.1 | 3.0 ± 0.1 | 2.5 ± 0.2 | 2.1 ± 0.3 | ||
| P5 | 30% CO2: 20% O2/1 PP | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.4 ± 0.7 | 2.1 ± 0.4 | 2.1 ± 0.3 | 2.0 ± 0.1 | ||
| P6 | 30% CO2: 70% O2/1 PP | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.6 ± 0.8 | 1.9 ± 0.1 | 1.8 ± 0.0 | 1.9 ± 0.1 | ||
| Pseudomonas spp. | P1 | Air/1 VP | 1.7 ± 0.1 | 2.6 ± 0.0 | 2.4 ± 0.4 | 2.0 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.2 | 0.79 |
| P2 | Air/1 MA | 1.7 ± 0.1 | 1.9 ± 0.9 | 2.3 ± 0.2 | 2.7 ± 0.2 | 2.2 ± 0.2 | 2.4 ± 0.3 | 1.7 ± 0.1 | ||
| P3 | Air/2 MA | 1.7 ± 0.1 | 1.6 ± 0.8 | 2.1 ± 0.3 | 2.2 ± 0.2 | 2.6 ± 0.2 | 2.1 ± 0.0 | 2.1 ± 0.3 | ||
| P4 | Air/1 PP | 1.7 ± 0.1 | 3.4 ± 0.2 | 4.0 ± 0.1 | 3.9 ± 0.0 | 3.9 ± 0.1 | 3.7 ± 0.1 | 3.6 ± 0.1 | ||
| P5 | 30% CO2: 20% O2/1 PP | 1.7 ± 0.1 | 1.8 ± 0.0 | 1.9 ± 0.1 | 2.2 ± 0.3 | 2.4 ± 0.2 | 2.8 ± 0.1 | 2.4 ± 0.2 | ||
| P6 | 30% CO2: 70% O2/1 PP | 1.7 ± 0.1 | 2.8 ± 0.0 | 3.2 ± 0.1 | 3.8 ± 0.3 | 3.7 ± 0.1 | 3.4 ± 0.1 | 3.3 ± 0.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortazavi, S.M.H.; Kaur, M.; Farahnaky, A.; Torley, P.J.; Osborn, A.M. Microbial and Quality Attributes of Beef Steaks under High-CO2 Packaging: Emitter Pads versus Gas Flushing. Foods 2024, 13, 2913. https://doi.org/10.3390/foods13182913
Mortazavi SMH, Kaur M, Farahnaky A, Torley PJ, Osborn AM. Microbial and Quality Attributes of Beef Steaks under High-CO2 Packaging: Emitter Pads versus Gas Flushing. Foods. 2024; 13(18):2913. https://doi.org/10.3390/foods13182913
Chicago/Turabian StyleMortazavi, Seyed Mohammad Hassan, Mandeep Kaur, Asgar Farahnaky, Peter Joseph Torley, and Andrew Mark Osborn. 2024. "Microbial and Quality Attributes of Beef Steaks under High-CO2 Packaging: Emitter Pads versus Gas Flushing" Foods 13, no. 18: 2913. https://doi.org/10.3390/foods13182913







