Potential Use and Chemical Analysis of Some Natural Plant Extracts for Controlling Listeria spp. Growth In Vitro and in Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Test Strains
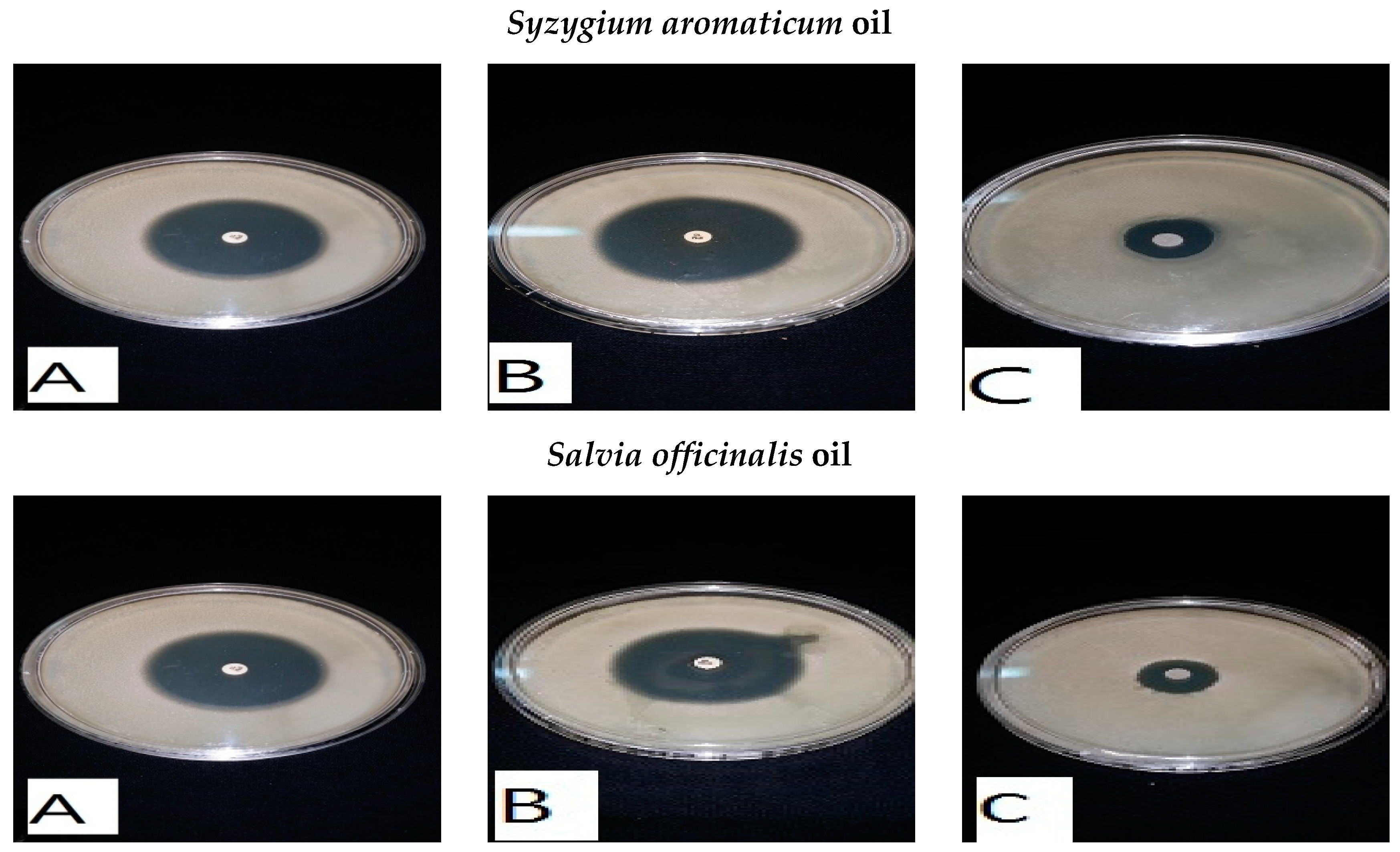
2.2. Essential Oils and Medicinal Plants
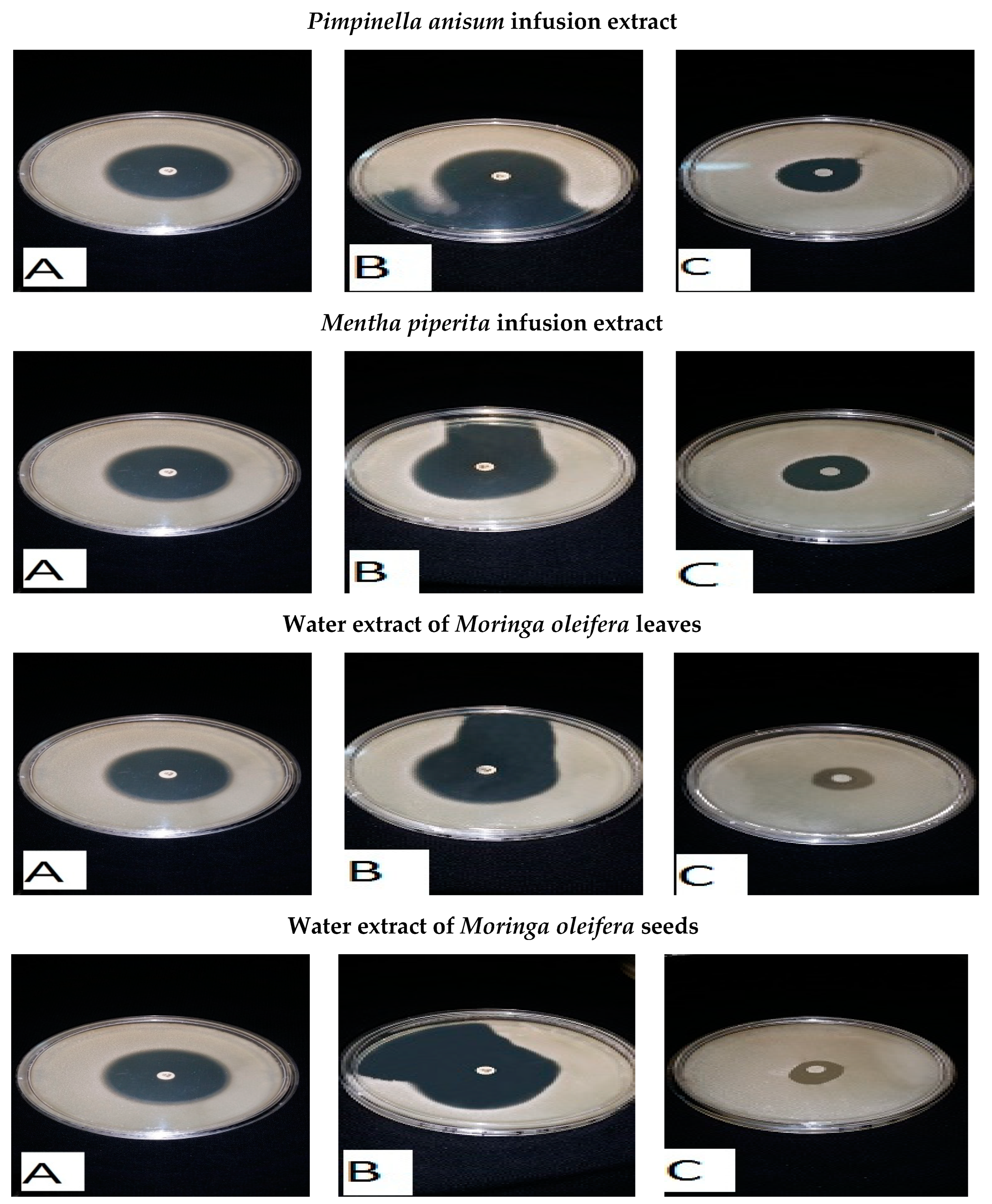
2.3. Preparation of the Moringa Oleifera Leaf (MLE) and Seed (MSE) Extracts
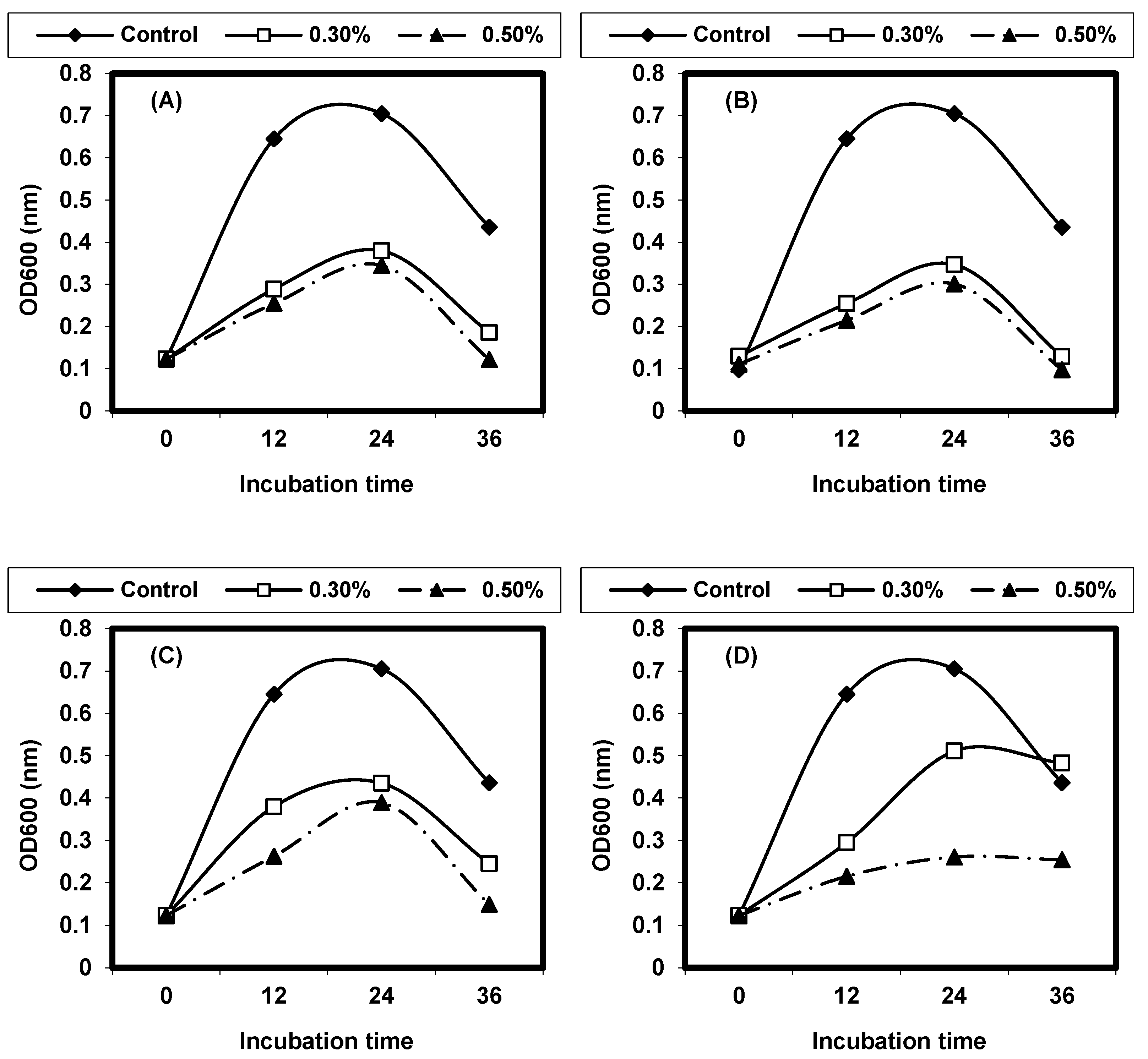
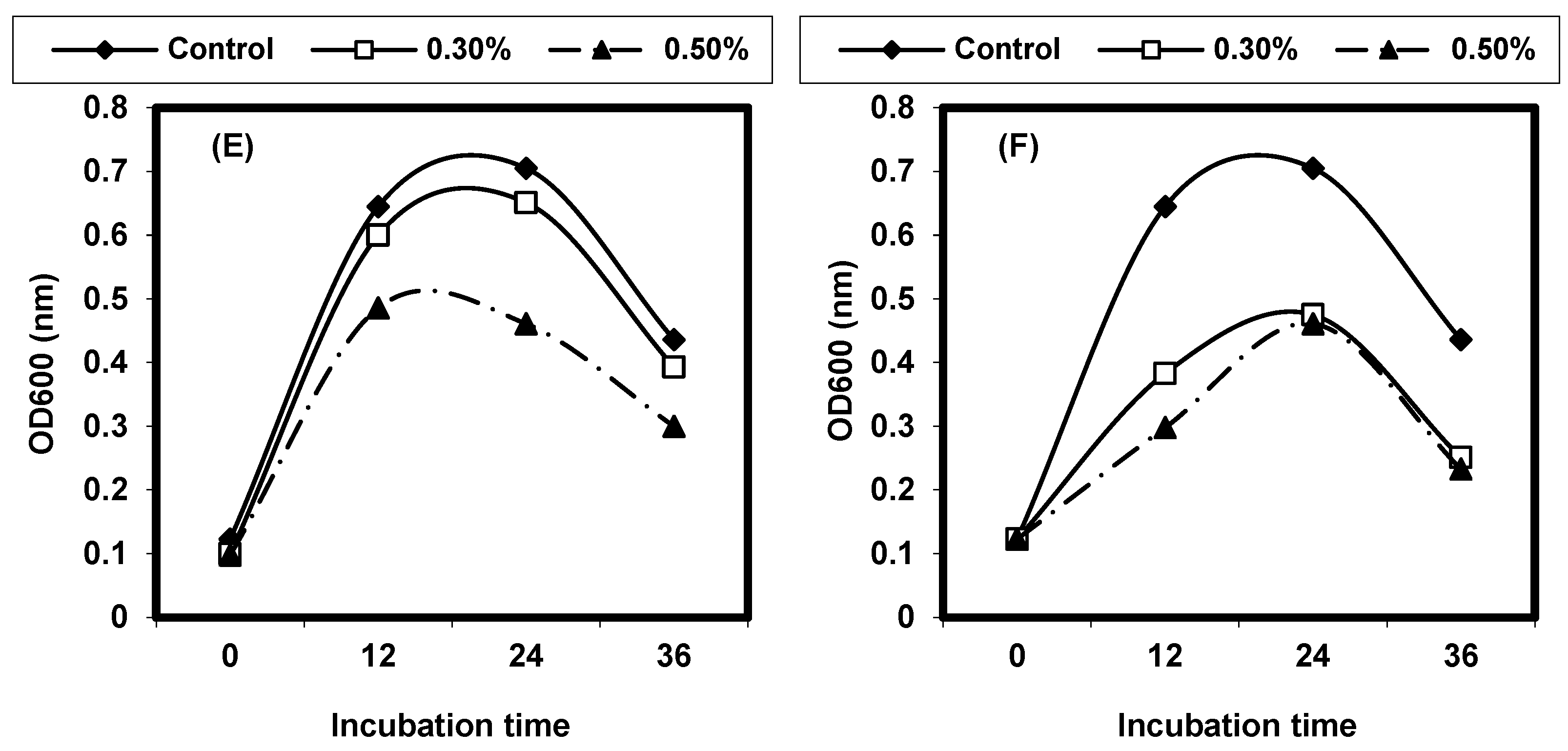
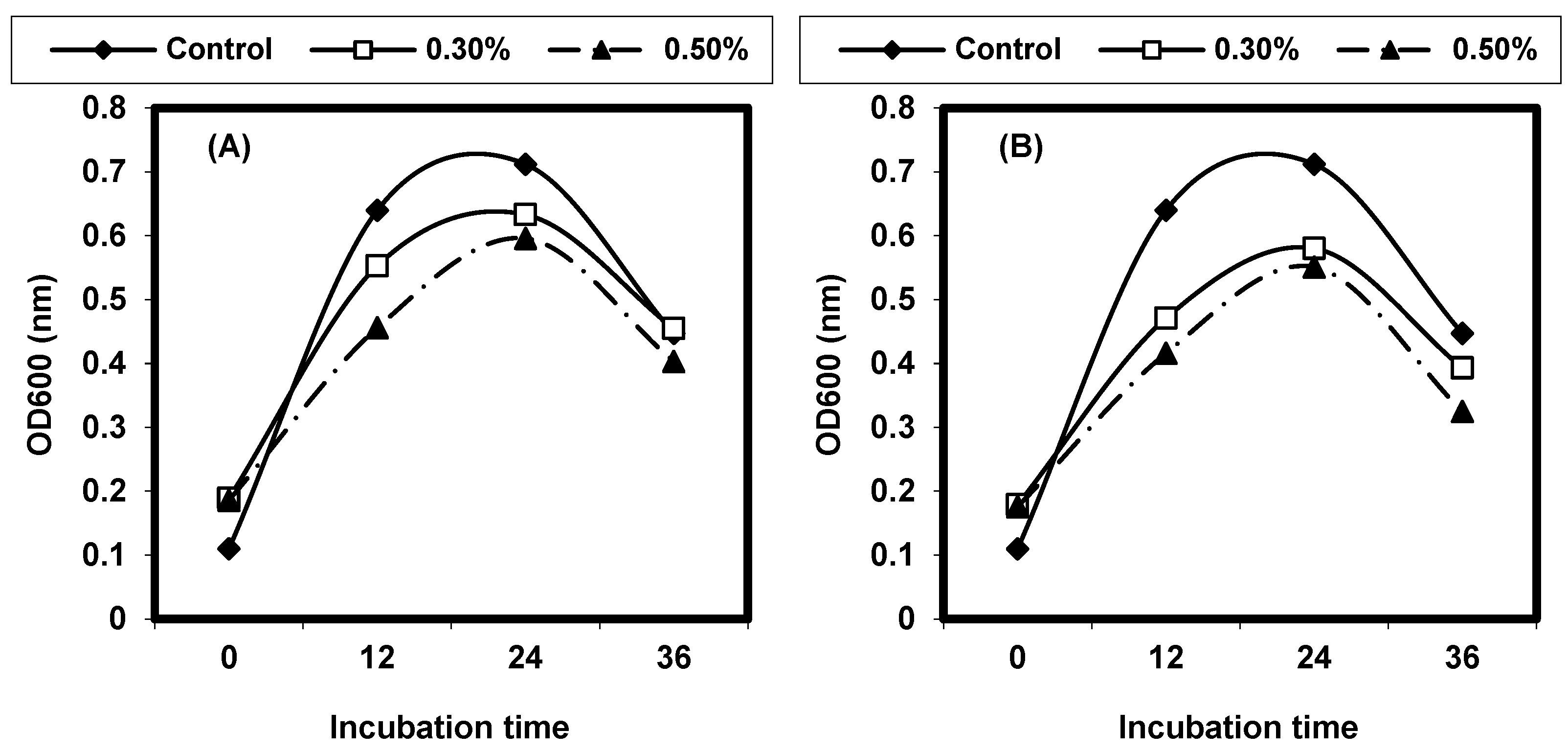
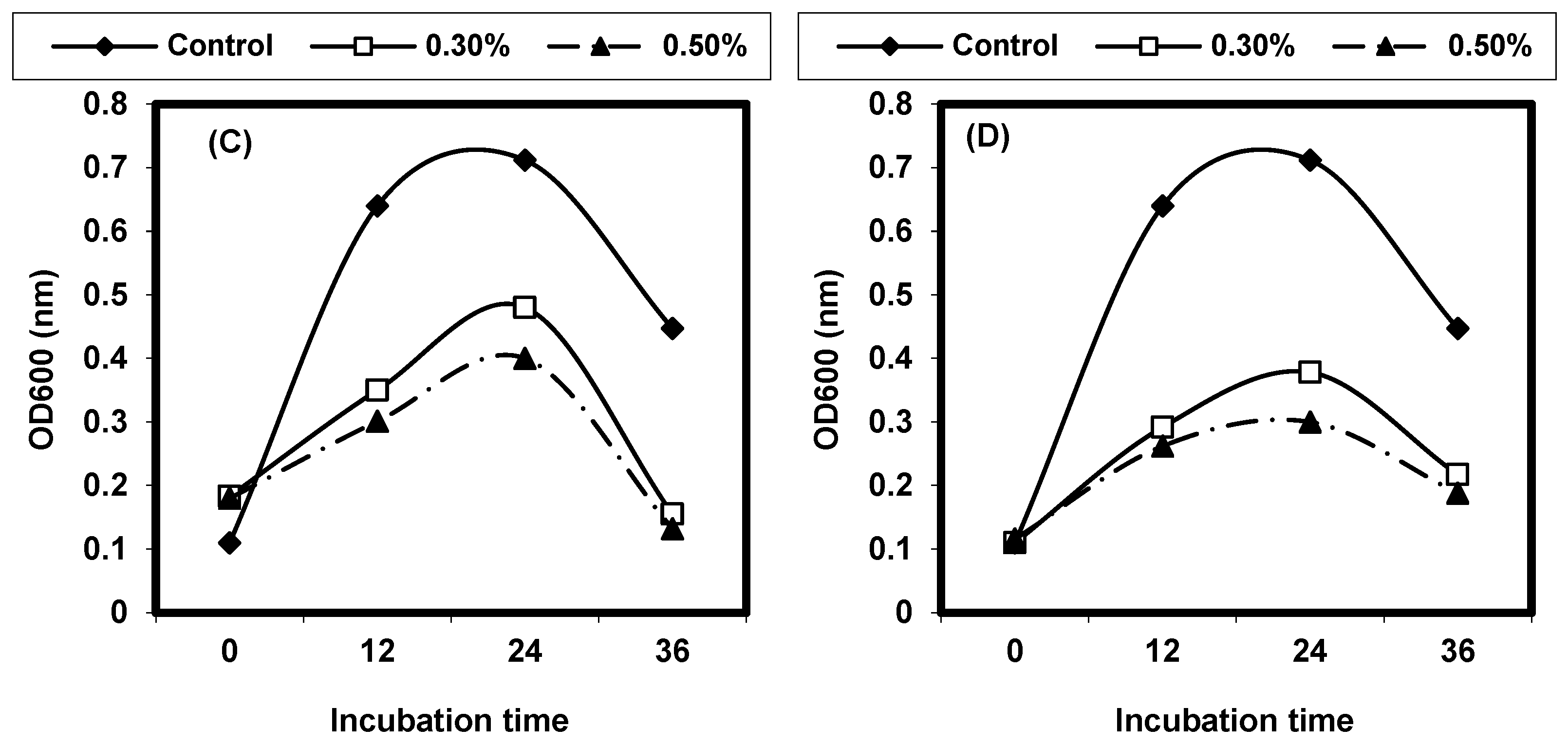
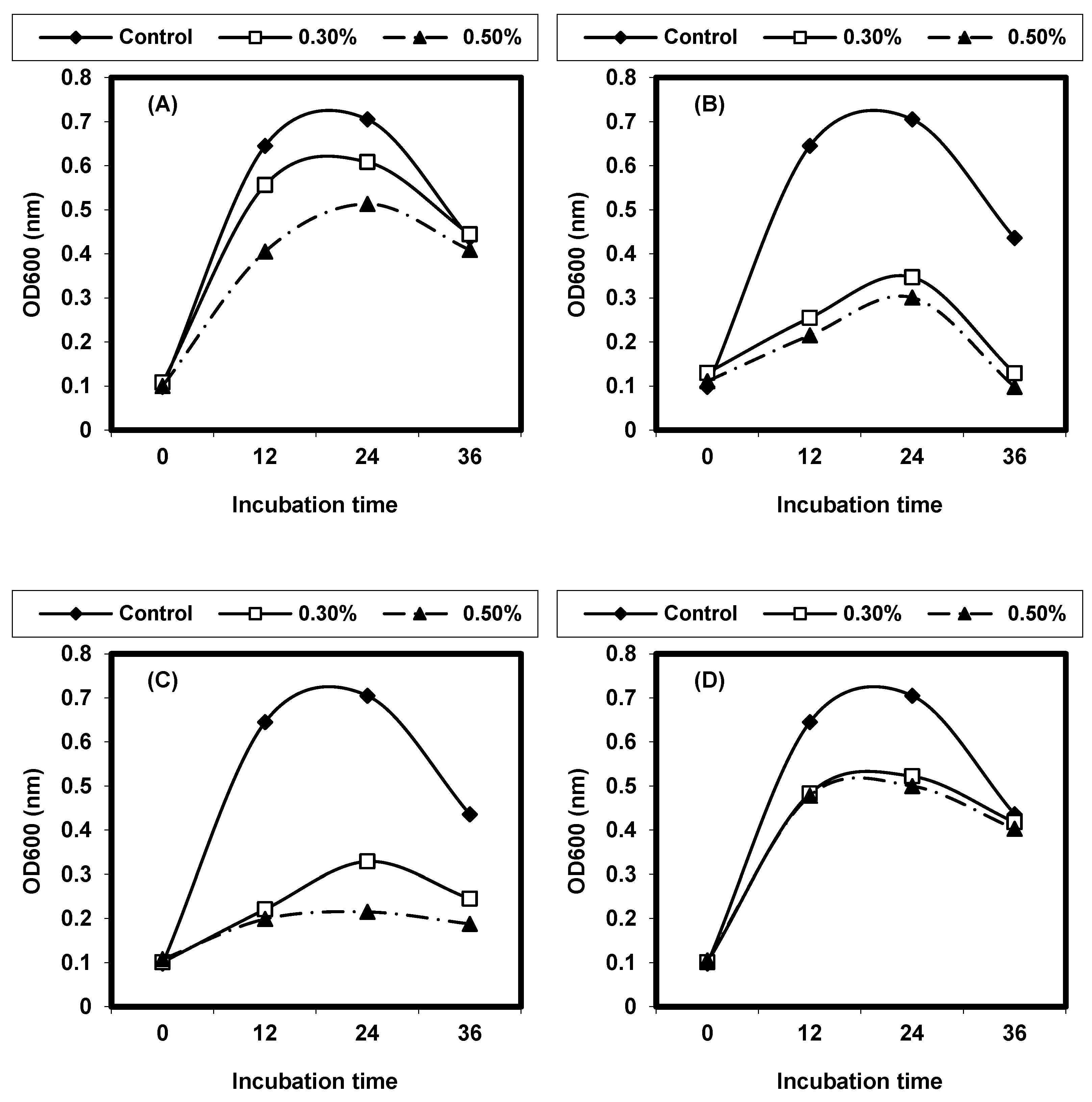
2.4. Effect of Different Physical Factors on Listeria spp.
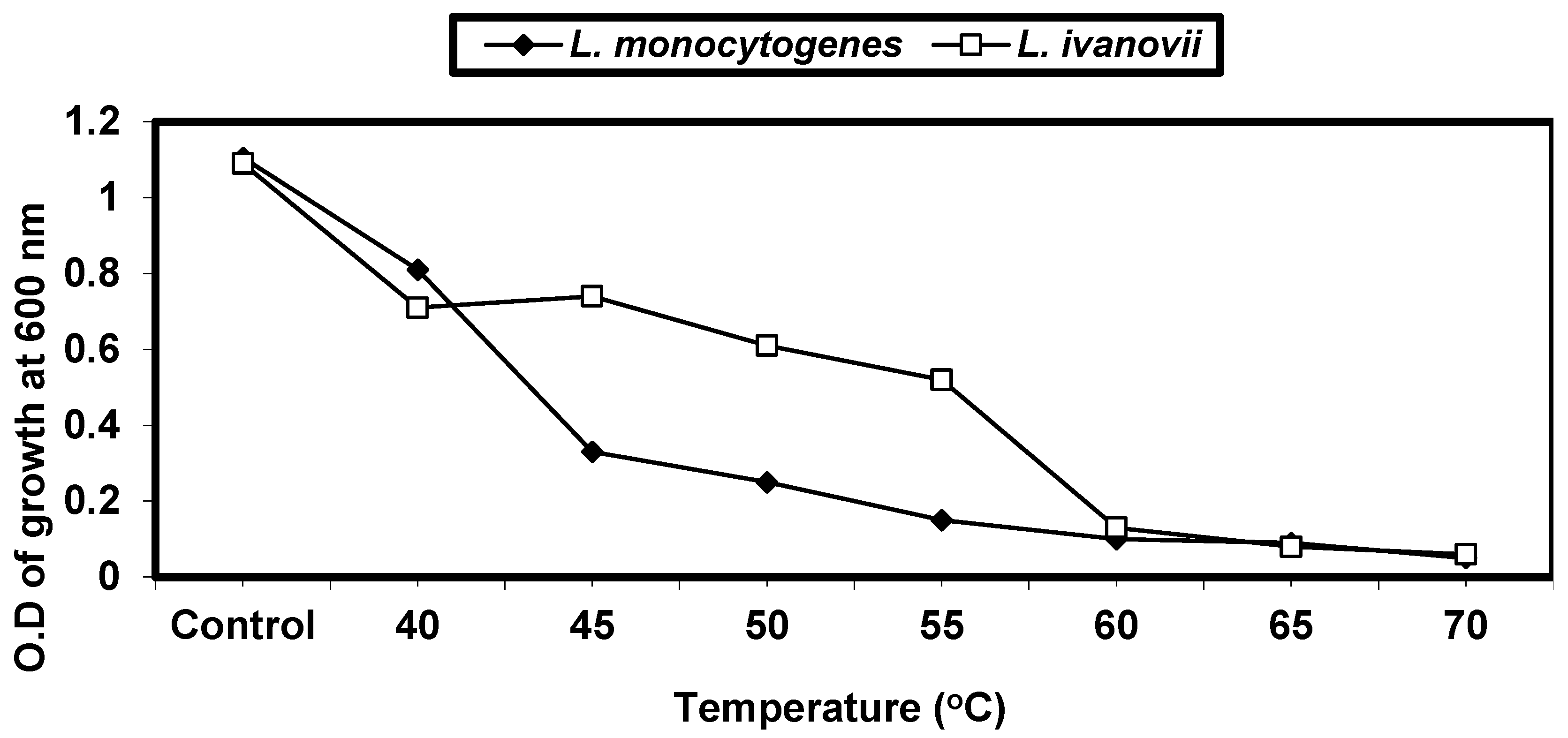
2.4.1. Effect of Different Temperatures on L. monocytogenes and L. ivanovii Growth
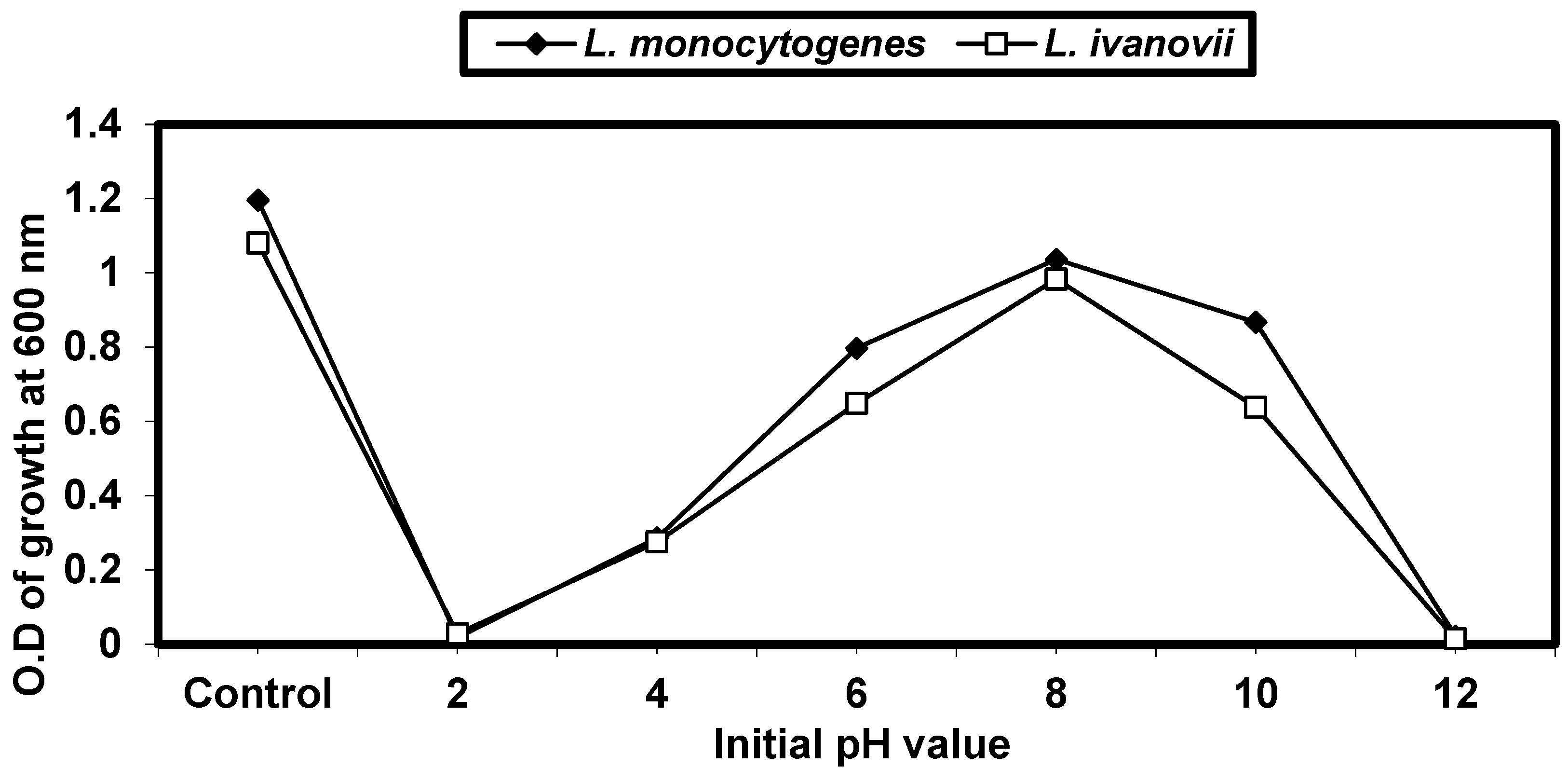
2.4.2. Effect of Different pH on L. monocytogenes and L. ivanovii Growth
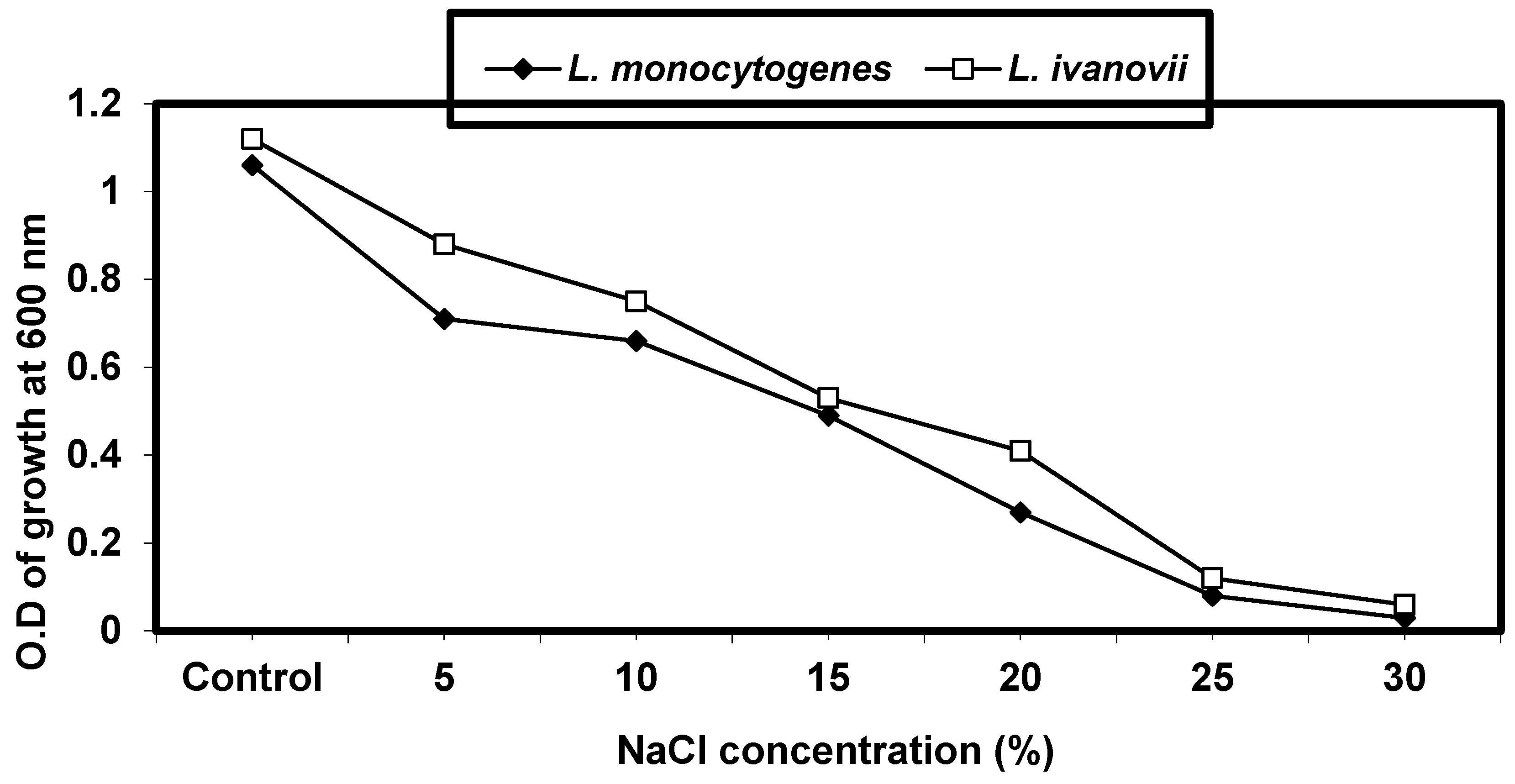
2.4.3. Effect of Sodium Chloride Concentrations on L. monocytogenes and L. ivanovii Growth
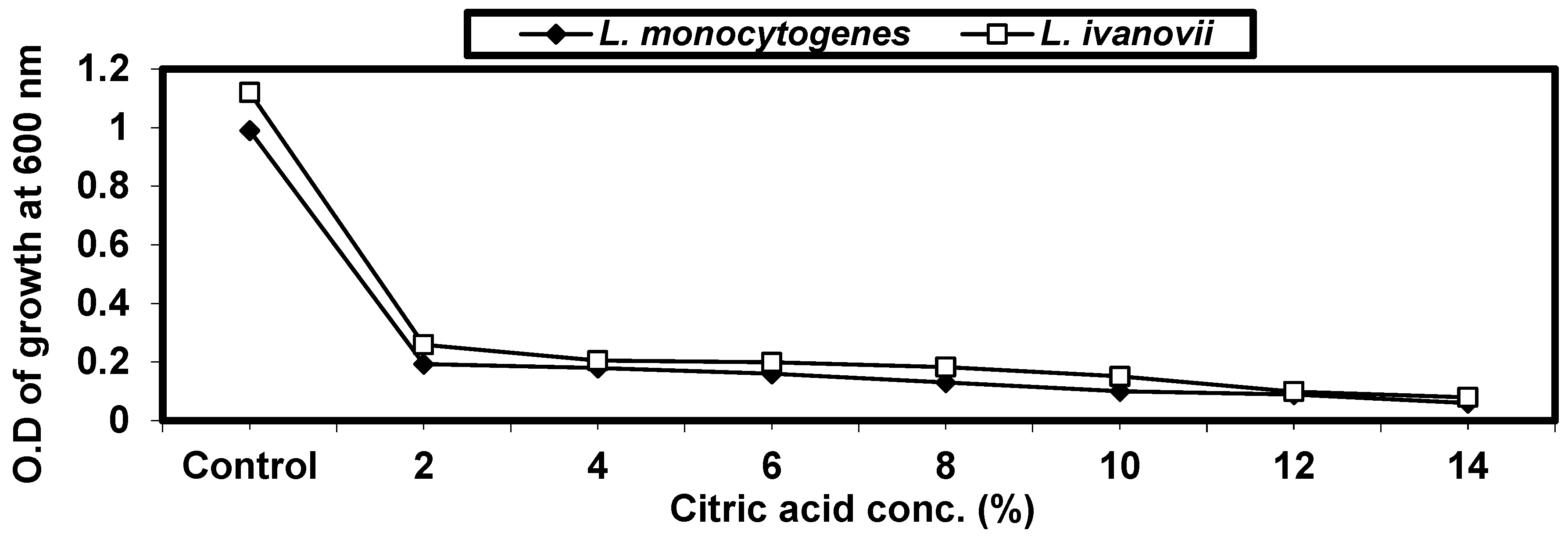
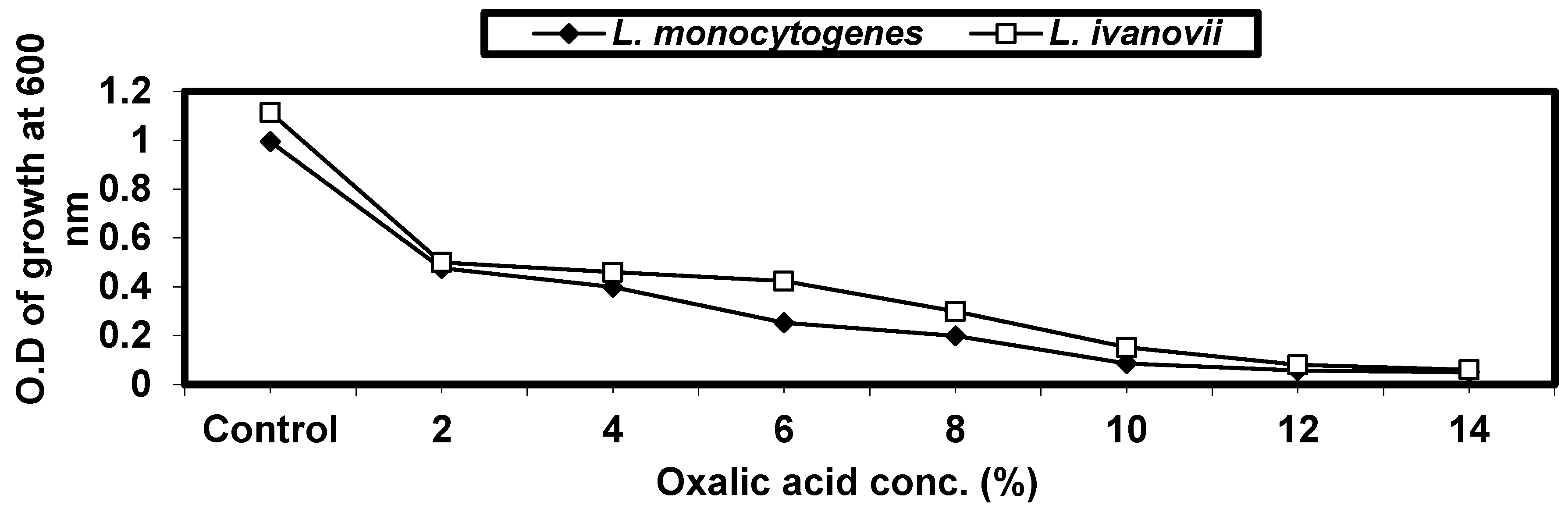
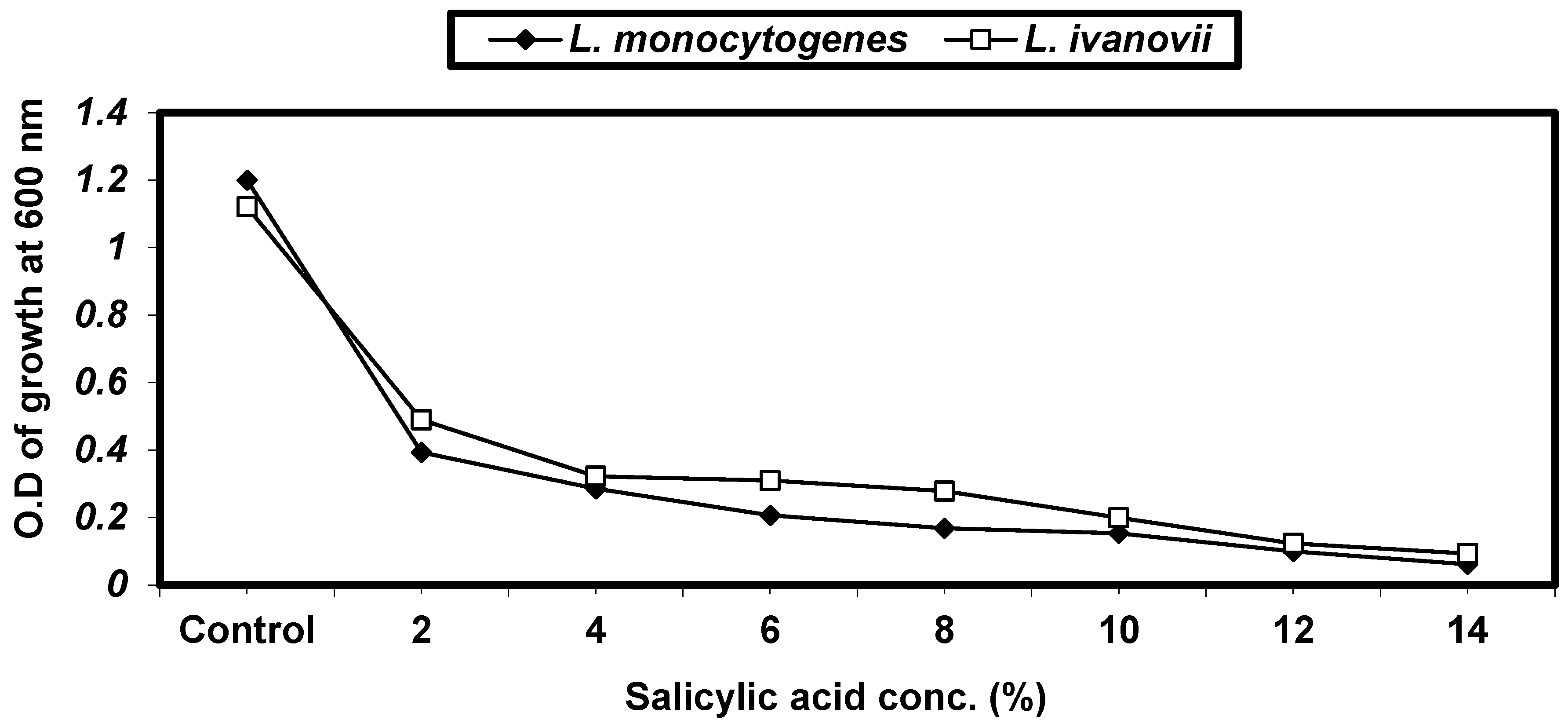
2.4.4. Effect of Some Organic Acids on L. monocytogenes and L. ivanovii Growth
2.5. Preparation of Infusion and Decoction Extracts
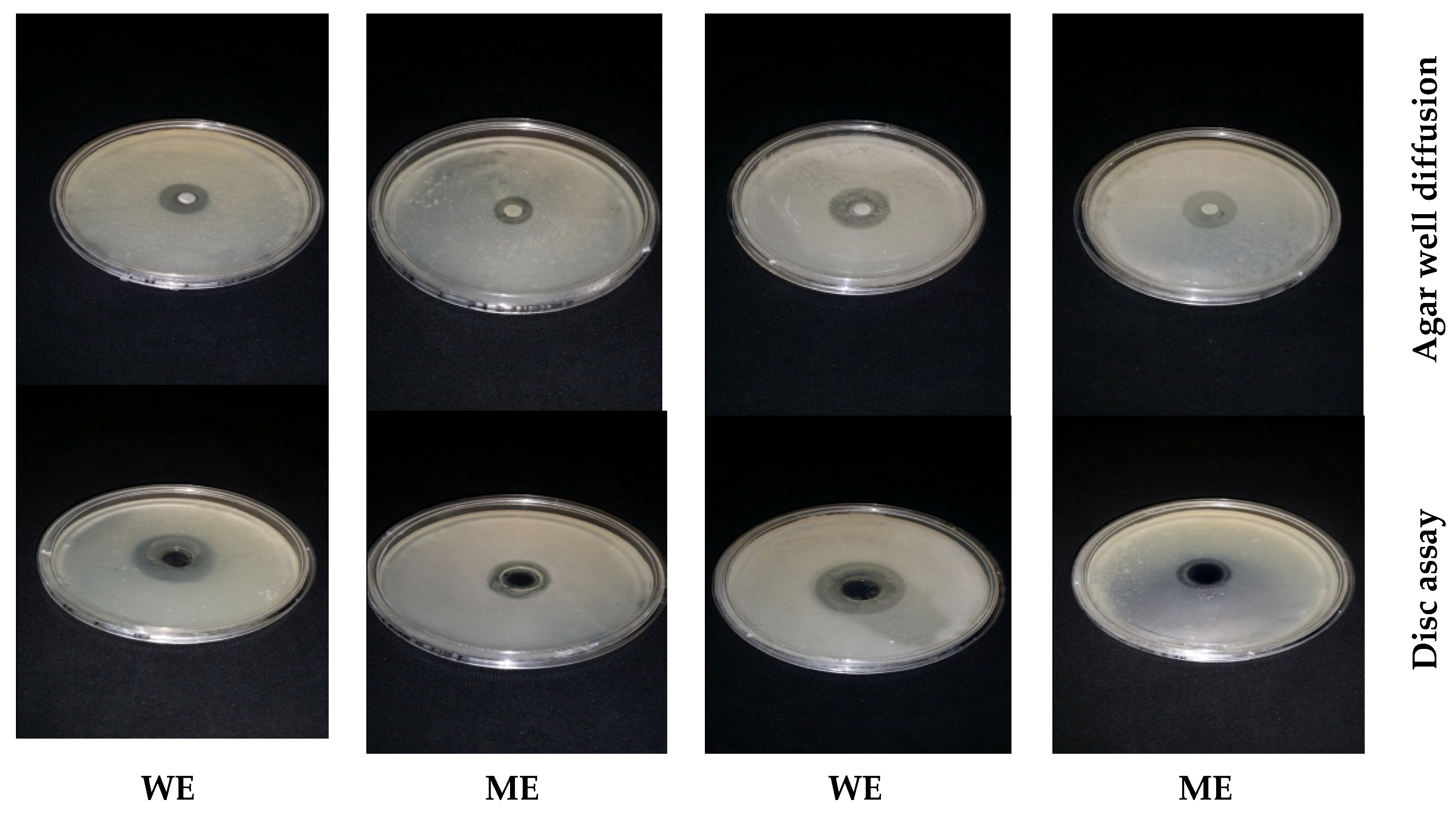
2.6. Antibacterial Bioassays of Essential Oils and Medicinal Plant Extracts
2.7. Determination of Minimum Inhibitory Concentration (MIC)
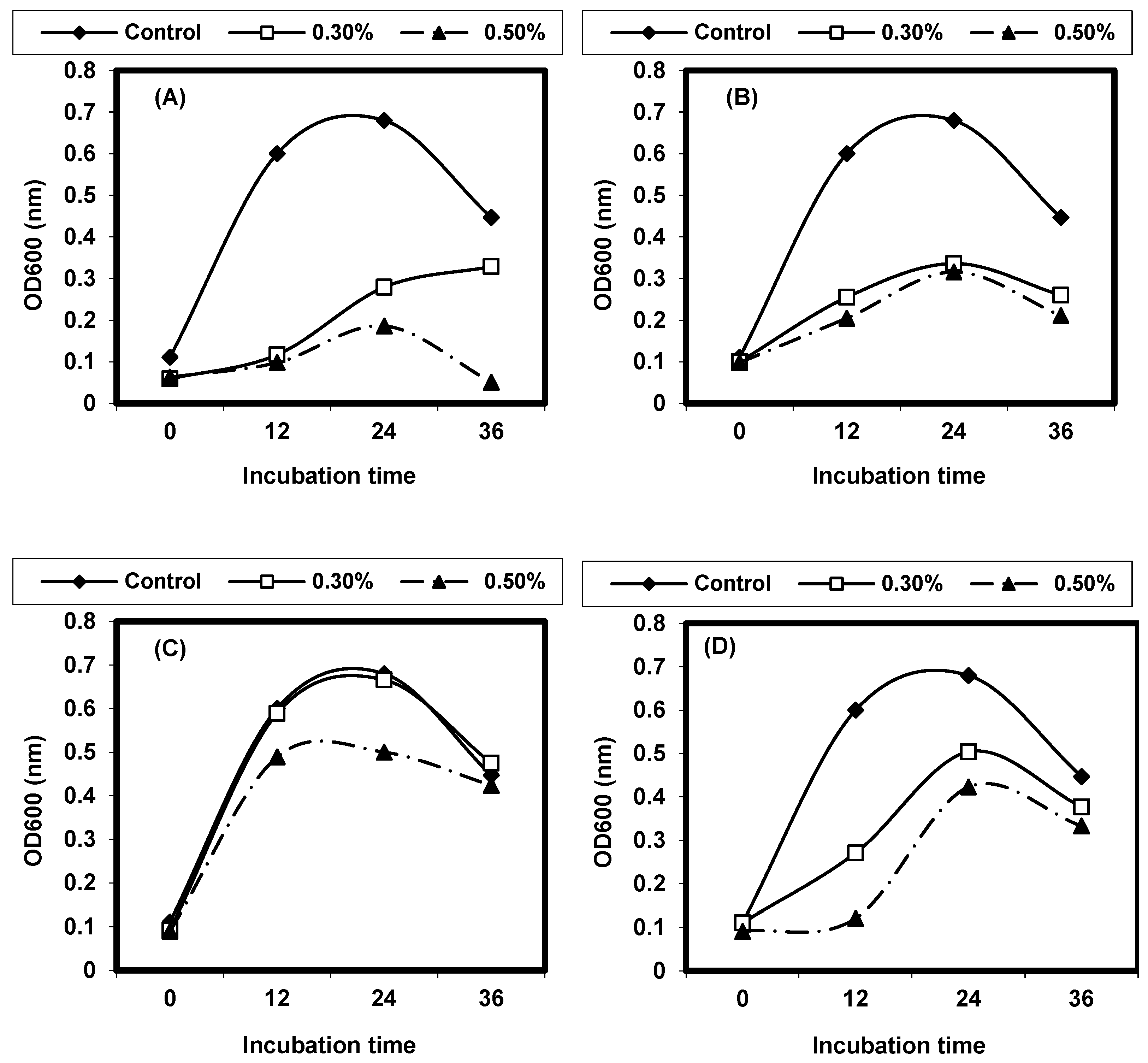
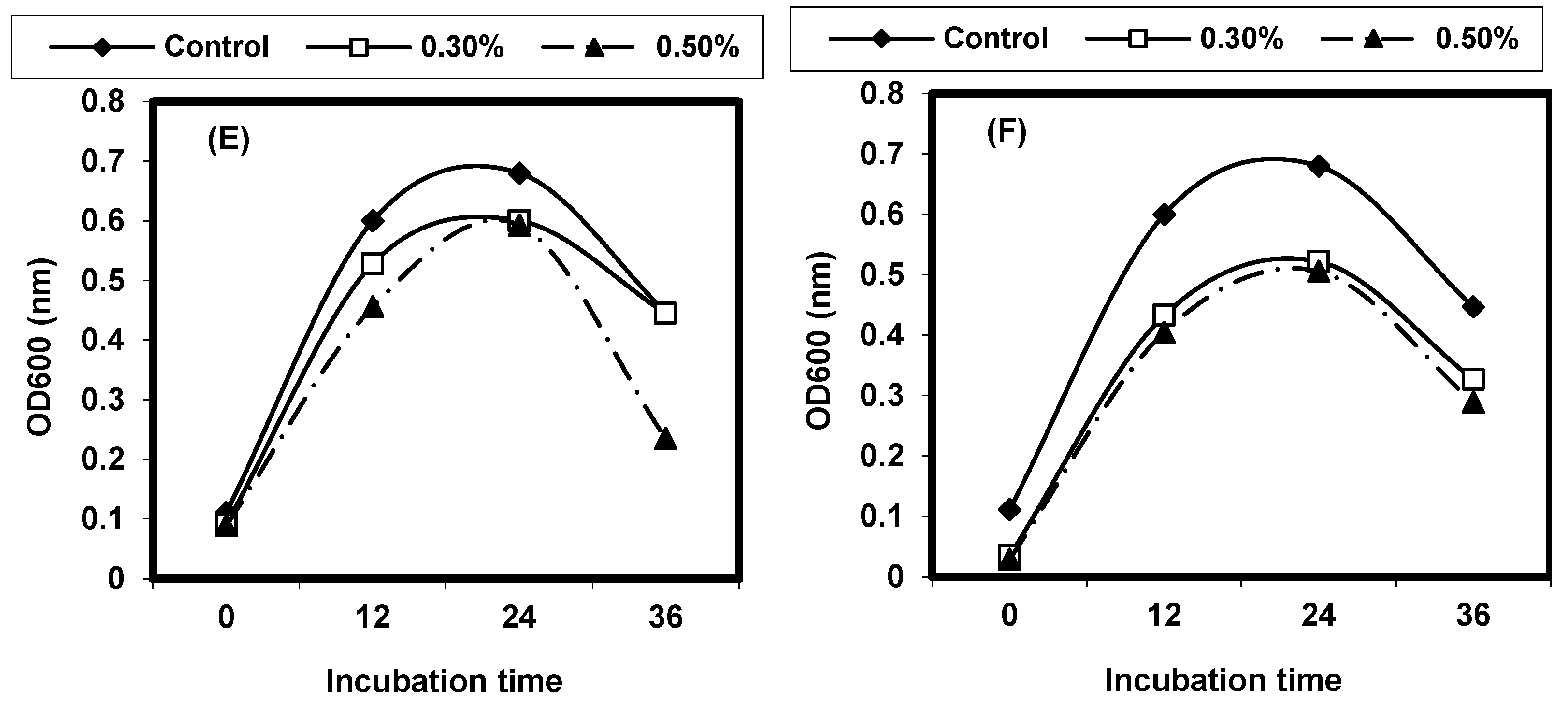
2.8. Quantitative Inhibition of Pathogenic Bacteria by Different Plant Extracts
2.9. Antibiotic Sensitivity Test and Antibacterial Activity of Natural Extract–Antibiotic Combinations by Disc Diffusion Assay
2.10. Instrumental Analysis of Mentha and Ansium Infusion Extracts
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar-Veloz, L.M.; Calderón-Santoyo, M.; Vázquez González, Y.; Ragazzo-Sánchez, J.A. Application of essential oils and polyphenols as natural antimicrobial agents in postharvest treatments: Advances and challenges. Food Sci. Nutr. 2020, 8, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Khorshidian, N.; Hosseini, H. Potential application of essential oils for mitigation of Listeria monocytogenes in meat and poultry products. Front. Nutr. 2020, 7, 577287. [Google Scholar] [CrossRef] [PubMed]
- Rothrock, M.J., Jr.; Micciche, A.C.; Bodie, A.R.; Ricke, S.C. Listeria occurrence and potential control strategies in alternative and conventional poultry processing and retail. Front. Sustain. Food Syst. 2019, 3, 33. [Google Scholar] [CrossRef]
- Bahrami, A.; Baboli, Z.M.; Schimmel, K.; Jafari, S.M.; Williams, L. Efficiency of novel processing technologies for the control of Listeria monocytogenes in food products. Trends Food Sci. Technol. 2020, 96, 61–78. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Tiwari, R.; Zubair Shabbir, M.; Barbuddhe, S.; Singh Malik, S.V.; Kumar Singh, R. Listeriosis in animals, its public health significance (food-borne zoonosis) and advances in diagnosis and control: A comprehensive review. Vet. Q. 2015, 35, 211–235. [Google Scholar] [CrossRef]
- Wang, X.-M.; Lü, X.-F.; Yin, L.; Liu, H.-F.; Zhang, W.-J.; Si, W.; Yu, S.-Y.; Shao, M.-L.; Liu, S.-G. Occurrence and antimicrobial susceptibility of Listeria monocytogenes isolates from retail raw foods. Food Control 2013, 32, 153–158. [Google Scholar] [CrossRef]
- Ke, Y.; Ye, L.; Zhu, P.; Sun, Y.; Zhu, Z. Listeriosis during pregnancy: A retrospective cohort study. BMC Pregnancy Childbirth 2022, 22, 261. [Google Scholar] [CrossRef]
- George, S.M.; Lund, B.M.; Brocklehurst, T. The effect of pH and temperature on initiation of growth of Listeria monocytogenes. Lett. Appl. Microbiol. 1988, 6, 153–156. [Google Scholar] [CrossRef]
- Harris, L.J.; Fleming, H.P.; Klaenhammer, T.R. Sensitivity and resistance of Listeria monocytogenes ATCC 19115, Scott A, and UAL500 to nisin. J. Food Prot. 1991, 54, 836–840. [Google Scholar] [CrossRef]
- Food Safety and Inspection Service. Listeria monocytogenes. 2019. Available online: https://www.fsis.usda.gov/inspection/compliance-guidance/microbial-risk/listeria-monocytogenes (accessed on 13 February 2023).
- Drew, C.A.; Clydesdale, F.M. New food safety law: Effectiveness on the ground. Crit. Rev. Food Sci. Nutr. 2015, 55, 689–700. [Google Scholar] [CrossRef]
- Tompkin, R. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Gu, W.; McLandsborough, L. Low concentration of ethylenediaminetetraacetic acid (EDTA) affects biofilm formation of Listeria monocytogenes by inhibiting its initial adherence. Food Microbiol. 2012, 29, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, P.; Rodríguez-Herrera, J.J.; Vázquez-Sánchez, D.; Lopez Cabo, M. Current knowledge on Listeria monocytogenes biofilms in food-related environments: Incidence, resistance to biocides, ecology and biocontrol. Foods 2018, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Risk Assessment of Listeria monocytogenes in Ready-to-Eat Foods: Interpretative Summary; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Heir, E.; Liland, K.H.; Carlehög, M.; Holck, A.L. Reduction and inhibition of Listeria monocytogenes in cold-smoked salmon by Verdad N6, a buffered vinegar fermentate, and UV-C treatments. Int. J. Food Microbiol. 2019, 291, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohammadi, A.-R.; Ibrahim, R.A.; Moustafa, A.H.; Ismaiel, A.A.; Abou Zeid, A.; Enan, G. Chemical Constitution and Antimicrobial Activity of Kefir Fermented Beverage. Molecules 2021, 26, 2635. [Google Scholar] [CrossRef]
- Enan, G.; Abdel-Shafi, S.; Abdel-Haliem, M.F.; Negm, S. Characterization of probiotic lactic acid bacteria to be used as starter and protective cultures for dairy fermentations. Int. J. Probiotics Prebiotics 2013, 8, 157–163. [Google Scholar]
- Enan, G.; Abo-El-Khair, I.A.; Abdel-Shafi, S.; Al-Mohammadi, A.-R. Evaluation of the use of Enterococcus faecium NM2 as a probiotic for inhibition of some urogenital pathogens. J. Food Agric. Environ. 2015, 13, 2–7. [Google Scholar]
- Vosough, P.R.; Dovom, M.R.E.; Najafi, M.B.H.; Javadmanesh, A.; Mayo, B. Biodiversity of exopolysaccharide-producing lactic acid bacteria from Iranian traditional Kishk and optimization of EPS yield by Enterococcus spp. Food Biosci. 2022, 49, 101869. [Google Scholar]
- Angmo, K.; Kumari, A.; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Chen, L.; Dishisha, T.; Enshasy, H.E. Lactic acid bacteria: From starter cultures to producers of chemicals. FEMS microbiology letters 2018, 365, fny213. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.R.; Sitohy, M.; Mousa, B.; Ismaiel, A.; Enan, G.S.; Osman, A. Antimicrobial Activity and Chemical Constitution of the Crude, Phenolic-Rich Extracts of Hibiscus sabdariffa, Brassica oleracea and Beta vulgaris. Molecules 2019, 24, 4280. [Google Scholar] [CrossRef] [PubMed]
- Ed-Dra, A.; Filali, F.R.; Presti, V.L.; Zekkori, B.; Nalbone, L.; Bouymajane, A.; Trabelsi, N.; Lamberta, F.; Bentayeb, A.; Giuffrida, A.; et al. Chemical Composition, Antioxidant Capacity and Antibacterial Action of Five Moroccan Essential Oils against Listeria Monocytogenes and Different Serotypes of Salmonella Enterica. Microb. Pathog. 2020, 149, 104510. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P.; Omatola, C.A.; Olaniran, A.O. Applications of lactic acid bacteria and their bacteriocins against food spoilage microorganisms and foodborne pathogens. Molecules 2021, 26, 7055. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.T. Continuous Evolution: Perspective on the Epidemiology of Carbapenemase Resistance Among Enterobacterales and Other Gram-Negative Bacteria. Infect. Dis. Ther. 2021, 10, 75–92. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Enan, G.; Al-Mohammadi, A.R.; Mahgoub, S.; Abdel-Shafi, S.; Askar, E.; Ghaly, M.F.; Taha, M.A.; El-Gazzar, N. Inhibition of Staphylococcus aureus LC554891 by Moringa oleifera Seed Extract either Singly or in Combination with Antibiotics. Molecules 2020, 25, 4583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sitohy, M.; Enan, G.; Abdel-Shafi, S.; El-Wafa, N.A.; El-Gazzar, N.; Osman, A.; Sitohy, B. Mapping pathogenic bacteria resistance against common antibiotics and their potential susceptibility to methylated white kidney bean protein. BMC Microbiol. 2024, 24, 49. [Google Scholar] [CrossRef]
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef]
- Allerberger, F. Listeria: Growth, phenotypic differentiation and molecular microbiology. FEMS Immunol. Med. Microbiol. 2003, 35, 183–189. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.S.S.; Satharasinghe, D.A.; Tang, J.Y.H.; Rukayadi, Y.; Radu, K.R.; New, C.Y.; Son, R.; Premarathne, J.M.K.J.K. Persistence of Listeria monocytogenes in food commodities: Foodborne pathogenesis, virulence factors, and implications for public health. Food Res. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef] [PubMed]
- Arioli, S.; Montanari, C.; Magnani, M.; Tabanelli, G.; Patrignani, F.; Lanciotti, R.; Mora, D.; Gardini, F. Modelling of Listeria monocytogenes Scott A after a mild heat treatment in the presence of thymol and carvacrol: Effects on culturability and viability. J. Food Eng. 2019, 240, 73–82. [Google Scholar] [CrossRef]
- Rodríguez-Campos, D.; Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Persistent Listeria monocytogenes isolates from a poultry-processing facility form more biofilm but do not have a greater resistance to disinfectants than sporadic strains. Pathogens 2019, 8, 250. [Google Scholar] [CrossRef]
- Giotis, E.S.; Muthaiyan, A.; Natesan, S.; Wilkinson, B.J.; Blair, I.S.; McDowell, D.A. Transcriptome analysis of alkali shock and alkali adaptation in Listeria monocytogenes 10403S. Foodborne Path. Dis. 2010, 7, 1147–1157. [Google Scholar] [CrossRef]
- Shen, Q.; Pandare, P.; Soni, K.A.; Nannapaneni, R.; Mahmoud, B.S.M.; Sharma, C.S. Influence of temperature on alkali stress adaptation in Listeria monocytogenes. Food Control 2016, 62, 74–80. [Google Scholar] [CrossRef]
- Soni, K.A.; Nannapaneni, R.; Tasara, T. The contribution of transcriptomic and proteomic analysis in elucidating stress adaptation responses of Listeria monocytogenes. Foodborne Path. Dis. 2011, 8, 842–852. [Google Scholar] [CrossRef]
- Bae, D.; Liu, C.; Zhang, T.; Jones, M.; Peterson, S.N.; Wang, C. Global gene expression of Listeria monocytogenes to salt stress. J. Food Prot. 2012, 75, 906–912. [Google Scholar] [CrossRef]
- Li, M.; Carpenter, C.E.; Broadbent, J.R. Organic Acid Exposure Enhances Virulence in Some Listeria monocytogenes Strains Using the Galleria mellonella Infection Model. Front. Microbiol. 2021, 12, 675241. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; Martinez-Laorden, A.; Perez-Arnedo, I. Combined Effect of Organic Acids and Modified Atmosphere Packaging on Listeria monocytogenes in Chicken Legs. J. Anim. 2020, 10, 1818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khaleque, M.A.; Keya, C.A.; Hasan, K.N.; Hoque, M.M.; Inatsu, Y.; Bari, M.L. Use of cloves and cinnamon essential oil to inactivate Listeria monocytogenes in ground beef at freezing and refrigeration temperatures. LWT Food Sci. Technol. 2016, 74, 219–223. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Protect. 2002, 65, 1545–1560. [Google Scholar] [CrossRef] [PubMed]
- Jamilah, M.B.; Abbas, K.A.; Rahman, R.A. A review on some organic acids additives as shelf life extenders of fresh beef cuts. Am. J. Agric. Biol. Sci. 2008, 3, 566–574. [Google Scholar] [CrossRef]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Kacaniova, M.; Petrová, J.; Mellen, M.; Čuboň, J.; Haščík, P.; Hleba, L.; Terentjeva, M.; Kunova, S.; Blaškovičová, H. Impact of anise (Pimpinella anisum) and mint (Mentha piperita) essential oils to microbial activity in chicken meat. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 28–31. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant potential of mint (Mentha spicata L.) in radiation processed lamb meat. Food Chem. 2007, 100, 451–458. [Google Scholar] [CrossRef]
- Campana, R.; Tiboni, M.; Maggi, F.; Cappellacci, L.; Cianfaglione, K.; Morshedloo, M.R.; Frangipani, E.; Casettari, L. Comparative Analysis of the Antimicrobial Activity of Essential Oils and Their Formulated Microemulsions against Foodborne Pathogens and Spoilage Bacteria. Antibiotics 2022, 11, 447. [Google Scholar] [CrossRef]
- Dolghi, A.; Coricovac, D.; Dinu, S.; Pinzaru, I.; Dehelean, C.A.; Grosu, C.; Chioran, D.; Merghes, P.E.; Sarau, C.A. Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells. Molecules 2022, 27, 6106. [Google Scholar] [CrossRef]
- El-Hossary, D.; Mahdy, A.; Elariny, E.Y.T.; Askora, A.; Merwad, A.M.A.; Saber, T.; Dahshan, H.; Hakami, N.Y.; Ibrahim, R.A. Antibiotic Resistance, Virulence Gene Detection, and Biofilm Formation in Aeromonas spp. Isolated from Fish and Humans in Egypt. Biology 2023, 12, 421. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, W.-Q.; Zhou, X.-Z.; Shao, F.; Shen, T.; Guan, H.-Y.; Zheng, J.; Zhang, L.-M. Antibacterial and Antifungal Sesquiterpenoids: Chemistry, Resource, and Activity. Biomolecules 2022, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- The, J.S. Toxicity of Short Chain Fatty Acids towards Cladosporium Resinae. Appl. Microbiol. 1974, 28, 840–844. [Google Scholar]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.A.; Hammond, S.M. Potassium fluxes. First indications of membrane damage in microorganisms. Biochem. Biophys. Res. Commun. 1973, 54, 796–799. [Google Scholar] [CrossRef]
- Lei, Z.; Heyan, Z.; Tianyang, H.; Siran, L. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015, 187, 370–377. [Google Scholar]
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys. Chem. 1995, 55, 273–277. [Google Scholar] [CrossRef]
- Gold Book of IUPAC, Heterocyclic Compounds. 2017. Available online: http://goldbook.iupac.org/html/H/H02798.html (accessed on 15 December 2017).
- Khalaf, A.J. Synthesis and antibacterial activity of metronidazole and imidiazole derivatives. Molecules 2009, 14, 2431–2446. [Google Scholar]
- Beale, J.M.; Block, J.H. Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; A Wolters Kluwer Business; Lippincot & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Trombetta, D.; Bisignano, G.; Arena, S. Study on the Mechanisms of the Antibacterial Action of Some Plant unsaturated Aldehydes. Lett. Appl. Microbiol. 2002, 35, 285–290. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Megeed, K.N.A.; Hassan, S.E.; Abdelaziz, M.M.; Toaleb, N.I.; El Shanawany, E.E.; Aboelsoued, D. Comparative ovicidal activity of Moringa oleifera leaf extracts on Fasciola gigantica eggs. Vet. World 2018, 11, 215–220. [Google Scholar] [CrossRef]
- El-Kholy, K.H.; Barakat, S.A.; Morsy, W.A.; Abdel-Maboud, K.; Seif-Elnaser, M.I.; Ghazal, M.N. Effect of aqueous extract of Moringa oleifera leaves on some production performance and microbial ecology of the gastrointestinal tract in growing rabbits. Pak. J. Nutr. 2018, 17, 1–7. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Armstrong, T.; Fenn, S.J.; Hardie, K.R. JMM Profile: Carbapenems: A broad-spectrum antibiotic. J. Med. Microbiol. 2021, 70, 001462. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Rodríguez, A.; Delgado, J.; Córdoba, J.J. Strategies for Biocontrol of Listeria monocytogenes Using Lactic Acid Bacteria and Their Metabolites in Ready-to-Eat Meat- and Dairy-Ripened Products. Foods 2022, 11, 542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef]
- Abd Rani, N.Z.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Patton, T.; Barrett, J.; Brennan, J.; Moran, N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J. Microbiol. Methods 2006, 64, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Al-Mijalli, S.H.; Mrabti, N.N.; Ouassou, H.; Sheikh, R.A.; Abdallah, E.M.; Assaggaf, H.; Bakrim, S.; Alshahrani, M.M.; Awadh, A.A.A.; Qasem, A.; et al. Phytochemical Variability, In Vitro and In Vivo Biological Investigations, and In Silico Antibacterial Mechanisms of Mentha piperita Essential Oils Collected from Two Different Regions in Morocco. Foods 2022, 11, 3466. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; Volume 39. [Google Scholar]
- Abdel-Shafi, S.; Al-Mohammadi, A.; Hamdi, S.; Moustafa, A.H.; Enan, G. Biological characterization and inhibition of Streptococcus pyogenesZUH1 causing chronic cystitis by both Crocus sativus methanol extract; bee honey singly or in combination with antibiotics: An in vitro study. Molecules 2019, 24, 2903. [Google Scholar] [CrossRef]


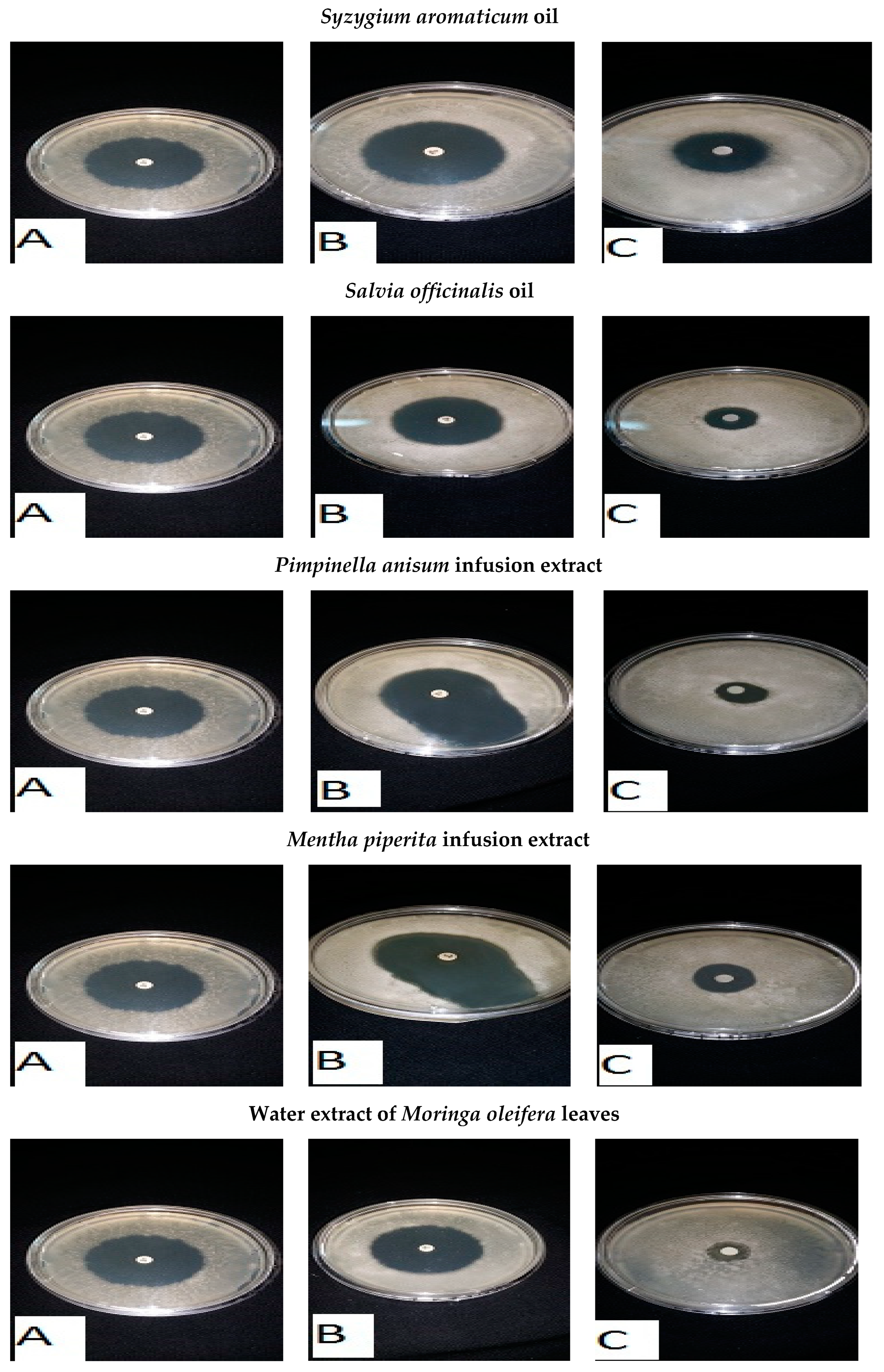
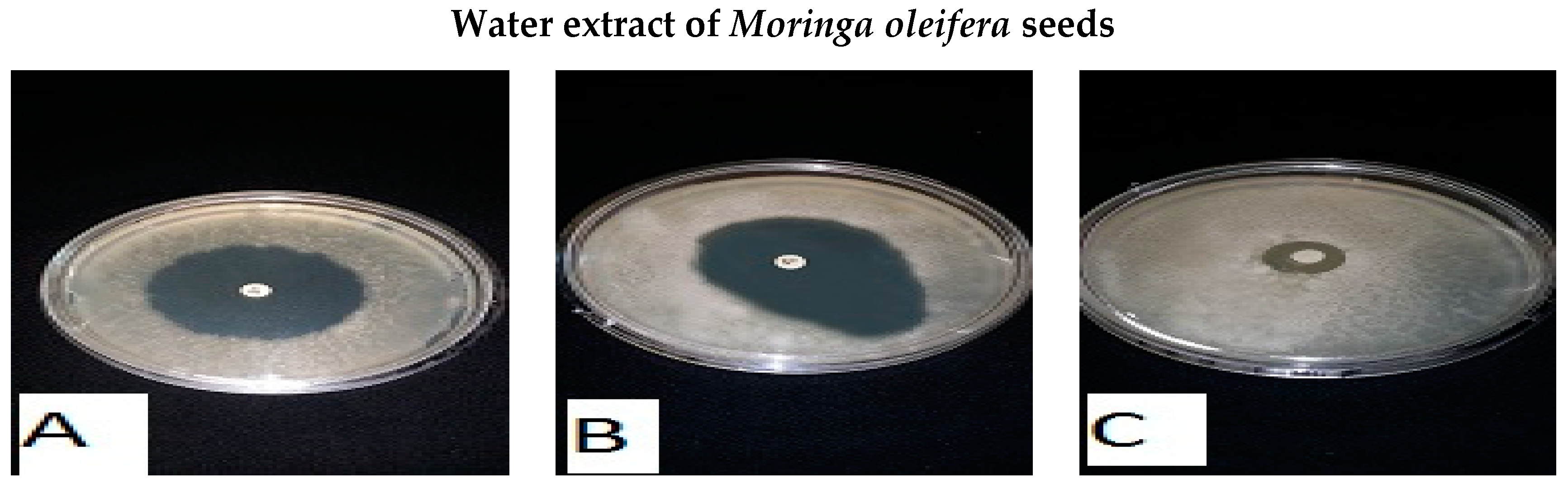
| Common Name | Scientific Name | Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|---|
| L. monocytogenes | L. ivanovii | ||||
| Disc | Wells | Disc | Wells | ||
| Clove | Syzygium aromaticum | 14 ± 0.9 | 15 ± 1.0 | 13 ± 0.8 | 14 ± 0.9 |
| Chamomile | Martricaria chamomilla | - | - | - | - |
| Rosemary | Rosemarinus officinalis | - | - | - | - |
| Mint | Mentha piperita | - | - | - | - |
| Black cumin | Nigella sativa | - | - | - | - |
| Anise | Pimpinella anisum | - | - | - | - |
| Thyme | Thymus vulgaris | - | - | - | - |
| Ginger | Zingiber officinalis | N | N | N | N |
| Sage | Salvia officinalis | 10 ± 2 | 17 ± 3 | 3 ± 1.0 | 9 ± 1.5 |
| Cinnamon | Cinnamum zeylanieum | - | - | - | - |
| Common Name | Scientific Name | Inhibition Zone Diameter (mm) | |
|---|---|---|---|
| L. monocytogenes | L. ivanovii | ||
| Disc | Disc | ||
| Clove | Syzygium aromaticum | 3 ± 0.2 | 2 ± 0.2 |
| Chamomile | Martricaria chamomilla | 3 ± 0.2 | 9 ± 0.5 |
| Rosemary | Rosemarinus officinalis | 14 ± 1.0 | 18 ± 2.0 |
| Mint | Mentha piperita | 32 ± 2.0 | 29 ± 2.0 |
| Black cumin | Nigella sativa | 13 ± 3.0 | 10 ± 1.0 |
| Anise | Pimpinella anisum | 32 ± 2.0 | 27 ± 2.0 |
| Thyme | Thymus vulgaris | 2 ± 0.2 | 20 ± 0.3 |
| Ginger | Zingiber officinalis | 25 ± 1.0 | 24 ± 4.0 |
| Sage | Salvia officinalis | 5 ± 0.3 | 29 ± 4.0 |
| Cinnamon | Cinnamum zeylanieum | 3 ± 0.3 | 4 ± 0.5 |
| Decoction of Medicinal Plants | Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|
| L. monocytogenes | L. ivanovii | |||
| Disc | Wells | Disc | Wells | |
| Syzygium aromaticum | 8 | 9 | 5 | 7 |
| Martricaria chamomilla | - | - | - | - |
| Rosemarinus officinalis | 1 | 1 | 2 | 2 |
| Mentha piperita | - | - | - | - |
| Nigella sativa | - | - | - | - |
| Pimpinella anisum | - | - | - | - |
| Thymus vulgaris | - | - | - | - |
| Zingiber officinalis | - | - | - | - |
| Salvia officinalis | - | - | - | - |
| Cinnamum zeylanieum | 3 | 3 | 5 | 8 |
| Microorganisms | MIC (μg/mL) of Infusion Extracts | MIC (μg/mL) of Decoction Extracts | ||
|---|---|---|---|---|
| Pimpinella anisum (g/100 mL) | Mentha piperita (g/100 mL) | Cinnamum zeylanieum (g/100 mL) | Syzygium aromaticum (g/100 mL) | |
| L. monocytogenes | 0.62 | 2.5 | 5 | 5 |
| L. ivanovii | 2.5 | 2.5 | 2.5 | 5 |
| Names of Antibiotics μg | Inhibition Zone Diameter (mm) | |
|---|---|---|
| L. Monocytogenes | L. ivanovii | |
| Imipenem (10 μg) | 40 ± 3.0 | 31 ± 2.5 |
| Levofloxacin (5 μg) | 20 ± 2.5 | 14 ± 2.0 |
| Amikacin (30 μg) | 24 ± 1.0 | 20 ± 2.0 |
| Ampicillin/sulbactam (10/10) μg | 21 ± 2.5 | 27 ± 1.0 |
| Amoxicillin (20/10) μg | 12 ± 2.0 | 27 ± 2.0 |
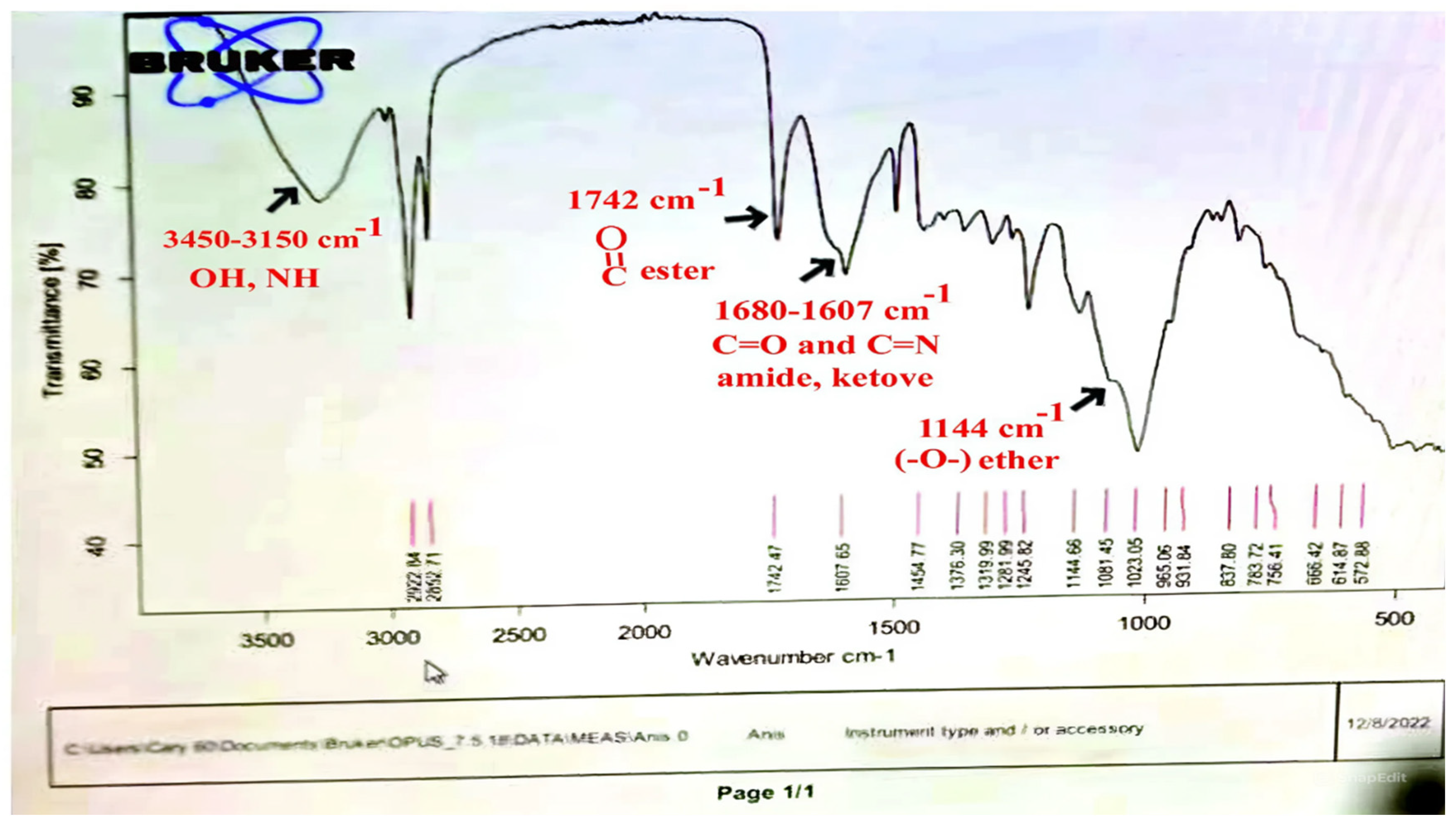
| Classification and Compound Name | Mol.wt and Mol. Formula | Parent Ion (M+) | Area | Base Peak (m/z) (100%) | |
|---|---|---|---|---|---|
| Group A: Ethers | |||||
| 1. | Estragole | C10H20O (148.0) | 148.0 | 0.71 | 77.00 |
| 2. | Anethole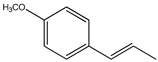 | C10H12O (148.0) | 148.0 | 43.64 | 77.00 |
| 3 | 1,2-Dimethoxy-4-(2-propenyl)- benzene or Methyleugenol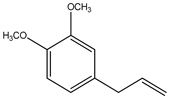 | C11H14O2 (178.0) | 178.0 | 0.60 | 91.00 |
| 4 | 1,2-Dimethoxy-4-n-propylbenzene | C11H16O2 (180.0) | 180.0 | 0.35 | 151.0 |
| Group B: Heterocyclic compound | |||||
| 1. | 5-Hydroxymethyl furfural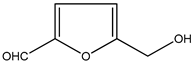 | C6H6O3 (126.0) | 126.0 | 1.21 | 97.00 |
| Group C:Aromatic aldehydes | |||||
| 1. | 4-Methoxybenzaldehyde | C8H8O2 (136.0) | 136.0 | 0.99 | 77.00 |
| Group D: Condensed Heterocyclic cpd | |||||
| 1. | 6-Methoxy-3-methyl-1-benzofuran | C10H10 O2 (162.0) | 162.0 | 0.68 | 147.0 |
| Group E: ketone | |||||
| 1. | p-Methoxyphenyl-2-propanene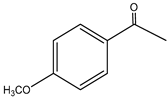 | C10H12O2 (164.0) | 164.0 | 1.49 | 121.0 |
| Group F: Alicyclic compounds | |||||
| 1. | ongifolene (V4) | C15H24 (204.0) | 204.0 | 10.15 | 105.0 |
| 2. | α-logipinene | C15H24 (204.0) | 204.0 | 0.97 | 119.0 |
| Group G: Aromatics | |||||
| 1. | 1-(1,5-Dimethyl-4-hexenyl)-4-methylbenzene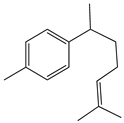 | C15H22 (202.0) | 202.0 | 1.29 | 119.0 |
| 2. | B-Bisabolene | C15H24 (204.0) | 204.0 | 2.37 | 69.00 |
| Group H: Esters | |||||
| 1. | 2-Methyl 4-methoxy-2-(1E)-1-propen-1-phenylbutanoate | C15 H20O3 (248.0) | 248.0 | 11.10 | 164.0 |
| 2. | Methyl(3,4-dimethoxy-phenyl)(hydroxy) acetate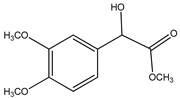 | C11 H14O5 (226.0) | 226.0 | 3.06 | 167.0 |
| 3. | Bis(2-ethylhexyl)phthalate | C24 H38O4 (390.0) | 390.0 | 2.48 | 149.0 |
| Group I: Herbicides | |||||
| 1. | 3-Hydroxycarbofuran | C12H15NO4 (237.0) | 237.0 | 7.62 | 137.0 |
| Group J: saturated fatty acids | |||||
| 1. | Hexadecanoic acid CH3(CH2)14-COOH | C16H32O2 (256.0) | 256.0 | 1.43 | 60.00 |
| Group k: unsaturated fatty acid | |||||
| 1. | Cis-9,Cis-12-Octadecadiienoic acid | C18H32O2 (280.0) | 280.0 | 3.20 | 67.00 |
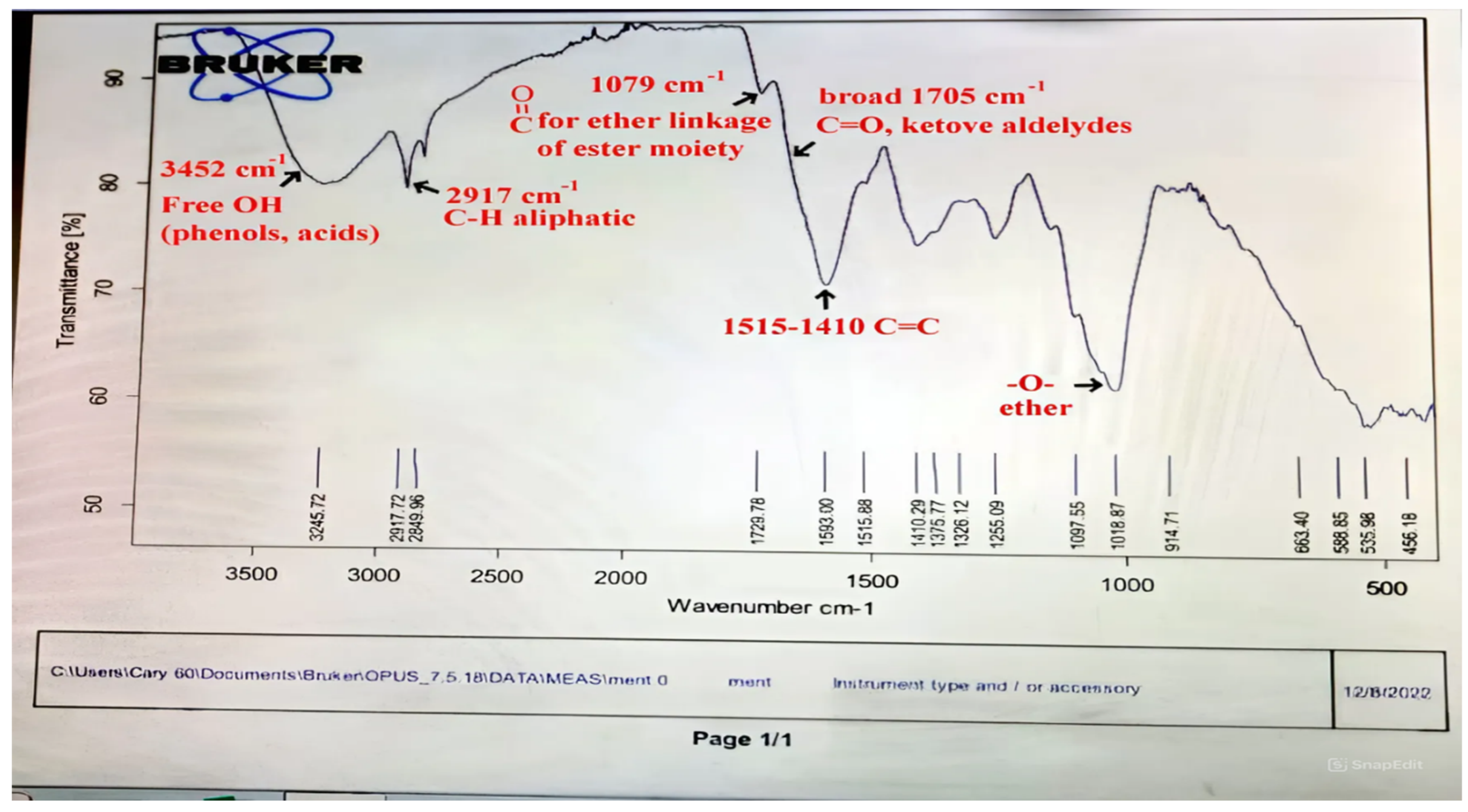
| Classification and Compound Name | Mol.wt and Mol. Formula | Parent Ion (M+) | Area | Base Peak (m/z) (100%) | |
|---|---|---|---|---|---|
| Group A: Alkenes | |||||
| 1. | 2,6-Dimethyl-1,3,5,7-octatetraene | C10H14 (134.0) | 134.0 | 2.50 | 91.00 |
| 2. | Meophytadiene | C20H28 (278.0) | 278.0 | 6.99 | 68.00 |
| Group B: Alkaloides | |||||
| 1. | Limonene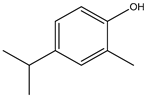 | C10H16 (126.0) | 136.0 | 0.90 | 68.00 |
| 2. | Cis-p-menthan-3-one | C10H18O (154.0) | 154.0 | 1.46 | 41.00 |
| 3. | Citronellal | C10H18 O (154.0) | 154.0 | 1.33 | 69&41 |
| 4. | (-)-Carvone | C10H14O (150.0) | 150.0 | 56.39 | 82.00 |
| Group C: Cyclic ether | |||||
| 1. | Cineole | C10H18O (154.0) | 154.0 | 1.30 | 43.00 |
| Group D: Heterocyclic cpd | |||||
| 1. | 2-Furylmethanol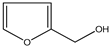 | C5H6O2 (98.0) | 98.0 | 1.07 | 39.00 |
| Group E: ketone | |||||
| 1. | 5-Methyl-2-(1-methyl-ethylidene) cyclo-hexanone | C10H16O (152.0) | 152.0 | 1.57 | 81.00 |
| Group F: Aldehydes | |||||
| 1. | 4-(2,2-Dimethyl-6-methylenecyclohexyl)butanal | C13H22O (194.0) | 194.0 | 0.73 | 69.00 |
| Group F: Phenols | |||||
| 1. | 5-Isopropyl-2-methylphenol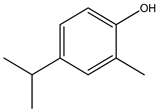 | C10H14O (150.0) | 150.0 | 0.77 | 135.0 |
| 2. | 3- Allyl-2-methoxyphenol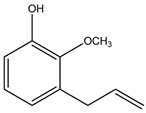 | C10H12O2 (164.0) | 164.0 | 0.91 | 91.00 |
| Group F: Alicyclic compounds | |||||
| 1. | α-Bourbonene | C15H24 (204.0) | 204.0 | 0.94 | 81.00 |
| 2. | 2,6,10,10- Tetramethyl-bicyclo [7.2.0]undeca-1,6-diene | C15H24 (204.0) | 204.0 | 1.71 | 41.00 |
| Group G:Polynuclears | |||||
| 1. | 1,2,4,5,6,8-Hexahydro-1-isopropyl-4,7-dimethyl naphthalene | C15H24 (204.0) | 204.0 | 0.98 | 105.0 |
| 2. | Cis-calamenene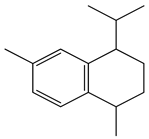 | C15H22 (202.0) | 202.0 | 1.72 | 159.0 |
| Group H: saturated fatty ester | |||||
| 1. | Methylpalmitate | C17 H34O2 (270.0) | 270.0 | 0.65 | 74.00 |
| Group I: Saturated fatty acid | |||||
| 1. | Hexadecanoic acid | C16 H32O2 (256.0) | 256. 0 | 2.07 | 73.00 |
| Group J: unsaturated fatty acid | |||||
| 1. | Linolenic acid | C18H30O2 (278.0) | 278.0 | 1.26 | 79.00 |
| Group H: Esters | |||||
| 1. | Bis (2-ethylhexyl) phthalate | C24H38O4 (390.0) | 390.0 | 7.73 | 149.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mohammadi, A.-R.; Abdel-Shafi, S.; Moustafa, A.H.; Fouad, N.; Enan, G.; Ibrahim, R.A. Potential Use and Chemical Analysis of Some Natural Plant Extracts for Controlling Listeria spp. Growth In Vitro and in Food. Foods 2024, 13, 2915. https://doi.org/10.3390/foods13182915
Al-Mohammadi A-R, Abdel-Shafi S, Moustafa AH, Fouad N, Enan G, Ibrahim RA. Potential Use and Chemical Analysis of Some Natural Plant Extracts for Controlling Listeria spp. Growth In Vitro and in Food. Foods. 2024; 13(18):2915. https://doi.org/10.3390/foods13182915
Chicago/Turabian StyleAl-Mohammadi, Abdul-Raouf, Seham Abdel-Shafi, Ahmed H. Moustafa, Nehal Fouad, Gamal Enan, and Rehab A. Ibrahim. 2024. "Potential Use and Chemical Analysis of Some Natural Plant Extracts for Controlling Listeria spp. Growth In Vitro and in Food" Foods 13, no. 18: 2915. https://doi.org/10.3390/foods13182915









