Effects of Soy Protein Isolate and Inulin Conjugate on Gel Properties and Molecular Conformation of Spanish Mackerel Myofibrillar Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MP
2.3. Preparation of SPI–Inulin Conjugates
2.4. Preparation of Mixed MP and Gel
2.5. Gel Properties
2.5.1. Determination of Gel Strength
2.5.2. Determination of TPA
2.5.3. Determination of WHC
2.5.4. Determination of Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.5.5. Determination of Chemical Forces
2.5.6. Determination of Raman Spectrum
2.5.7. Determination of Viscoelasticity
2.6. Determination of Endogenous Fluorescence Spectrum
2.7. Determination of Microstructure
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Additives on the Gel Properties of MP
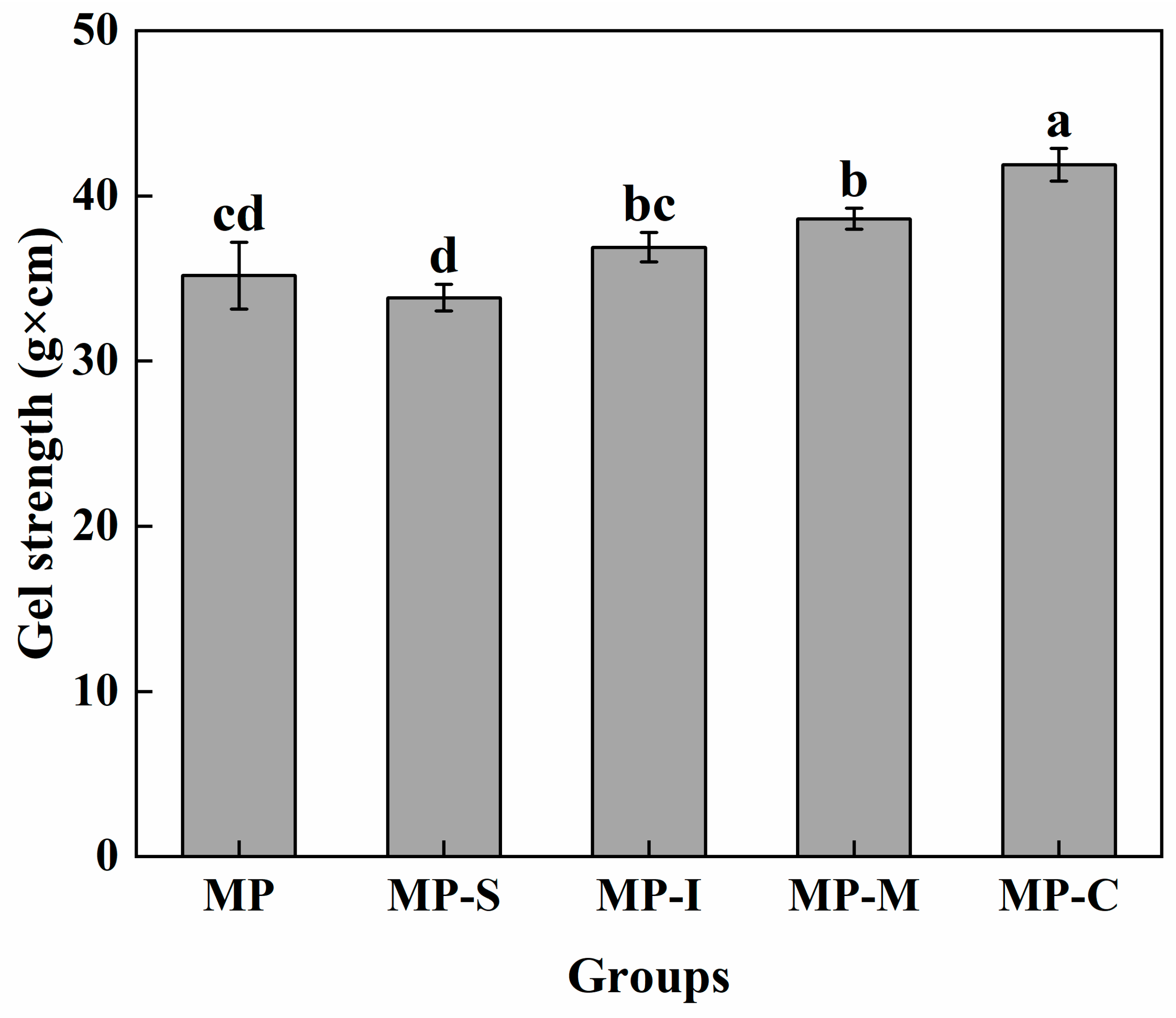
3.1.1. Gel Strength
3.1.2. Gel Textural Properties
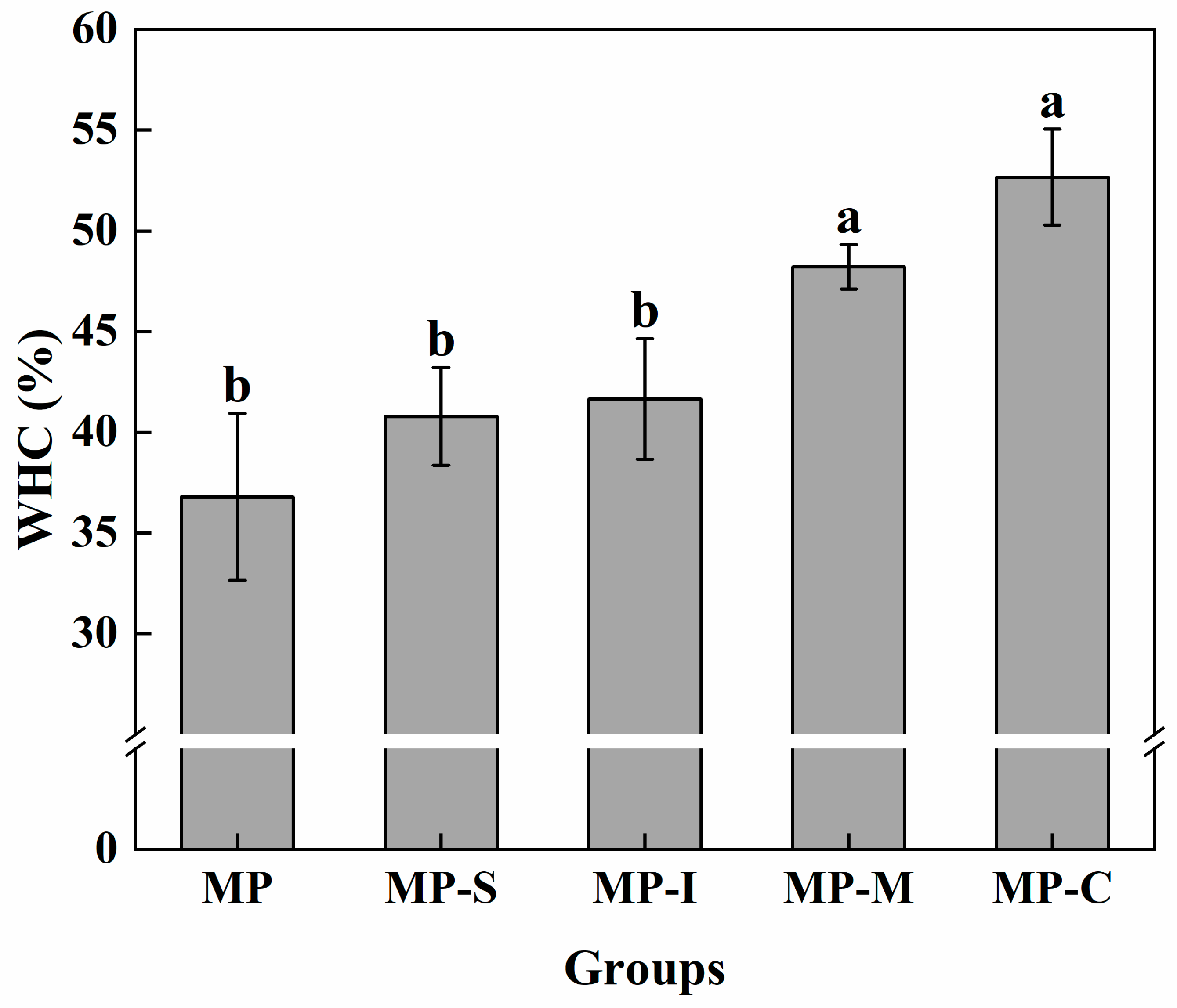
3.1.3. WHC
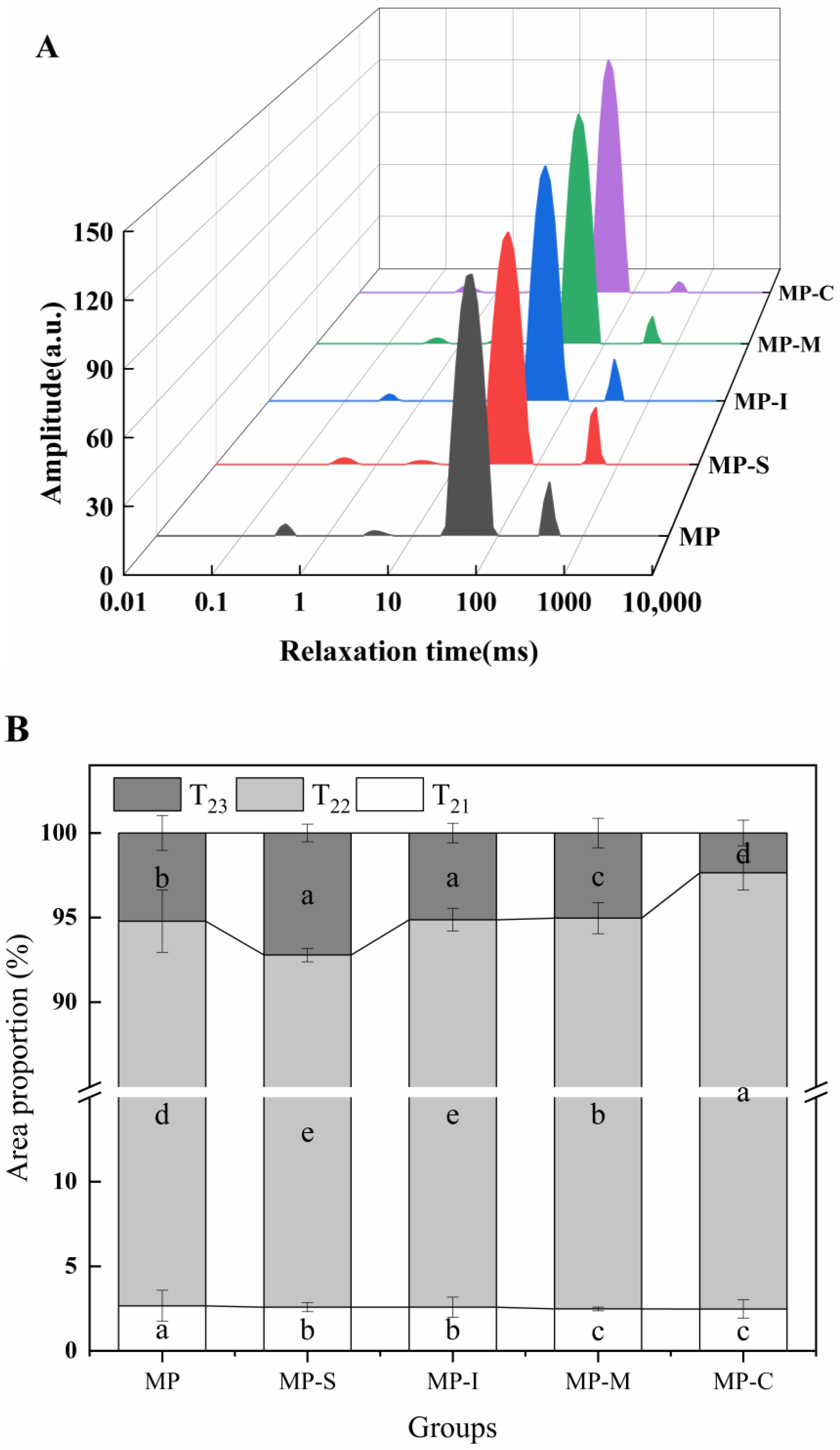
3.1.4. LF-NMR Proton Relaxation
3.1.5. Determination of Chemical Forces
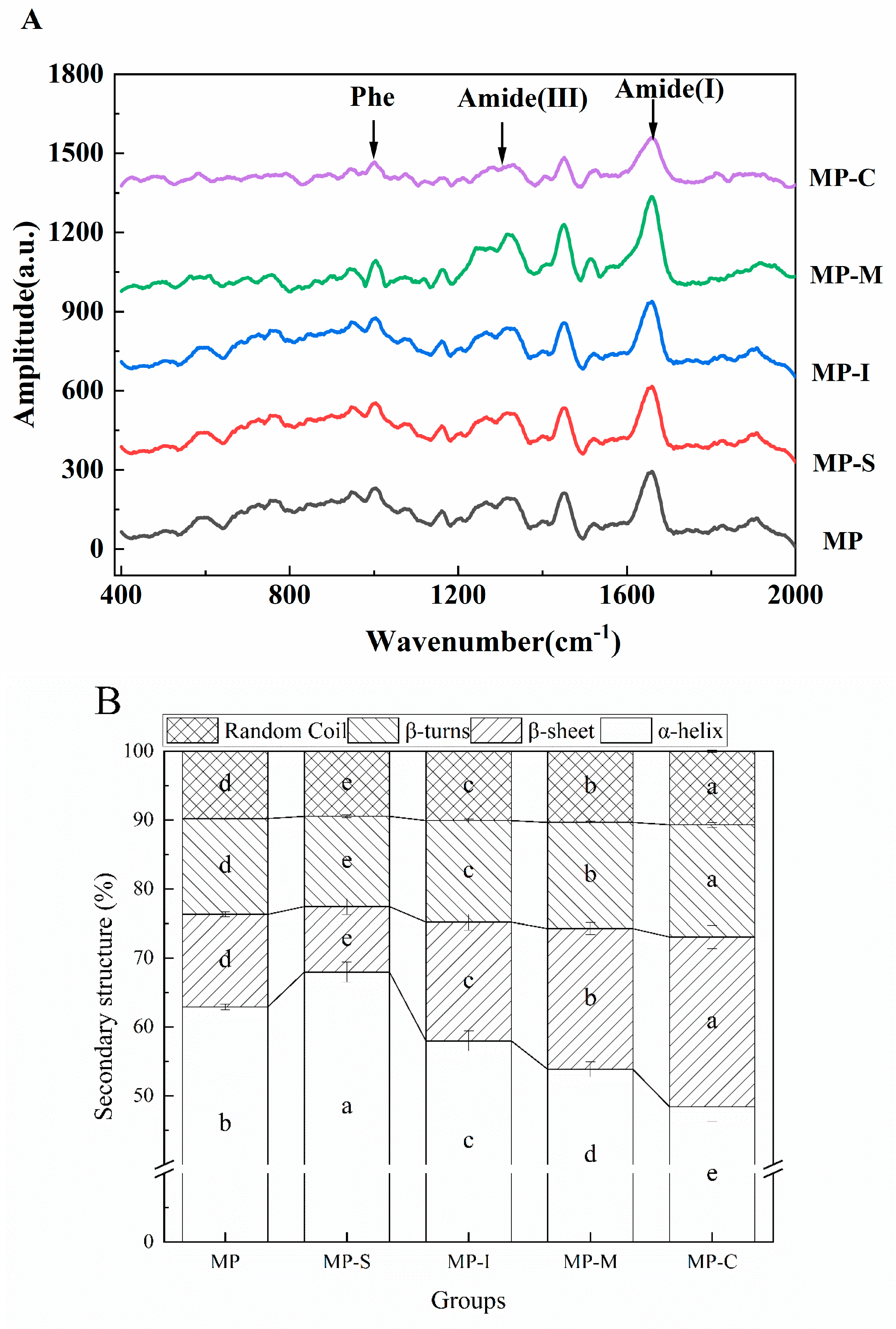
3.1.6. Raman Spectroscopy
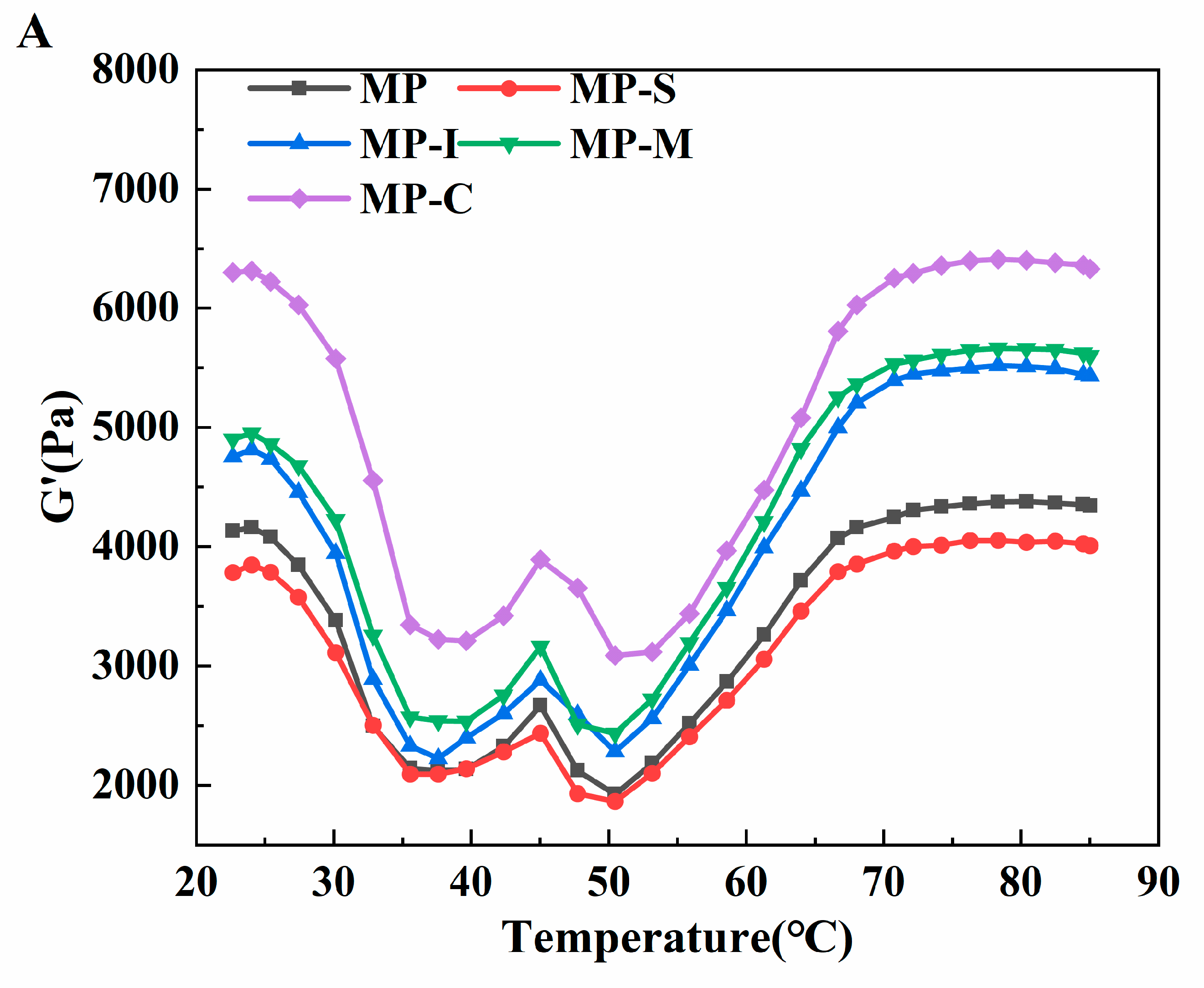
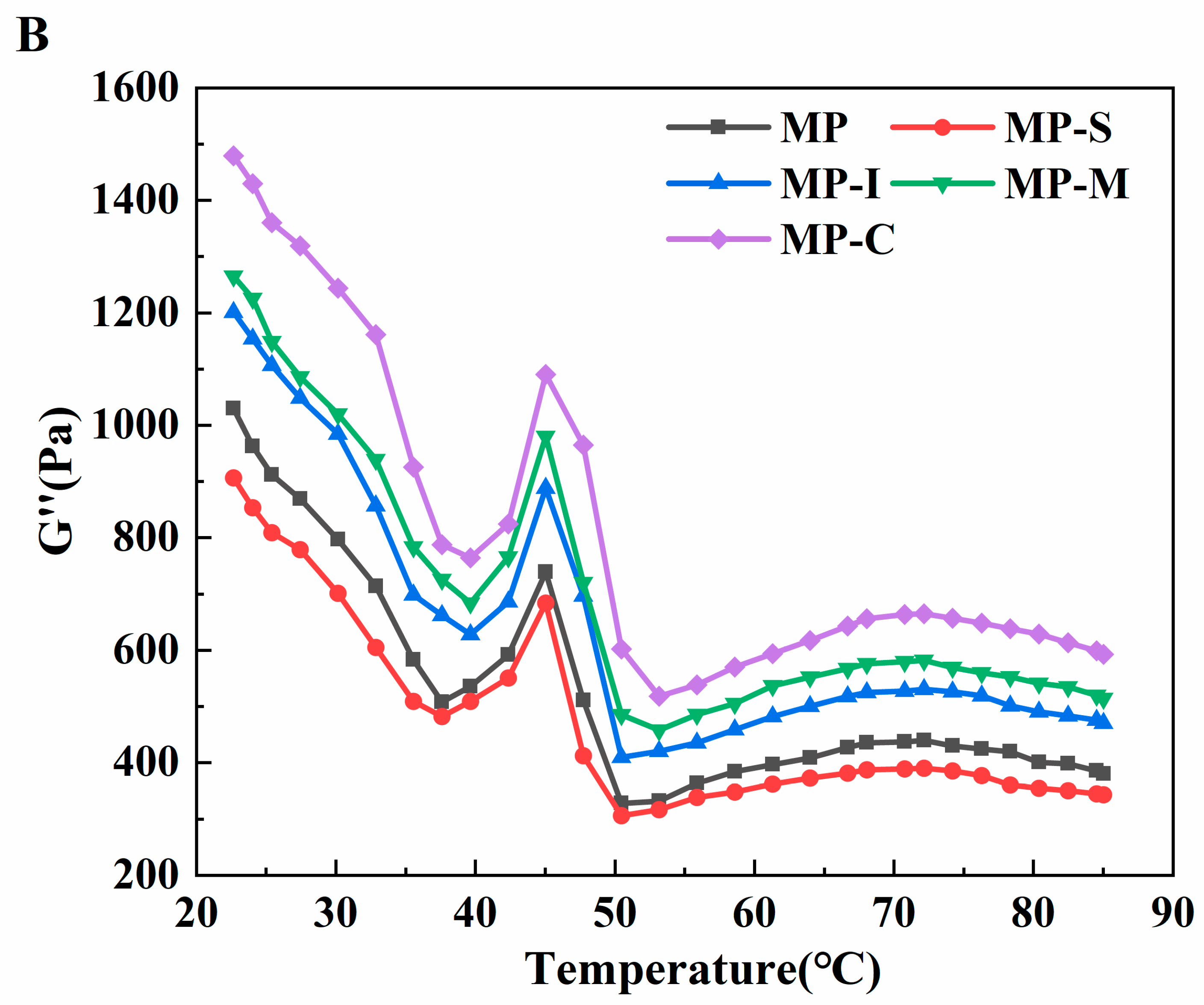
3.1.7. Viscoelasticity Measurements
3.2. Effect of Soybean Protein-Inulin Conjugates on the Conformation of MP of Spanish Mackerel
3.2.1. Fluorescence Spectroscopy
3.2.2. Atomic Force Microscopy
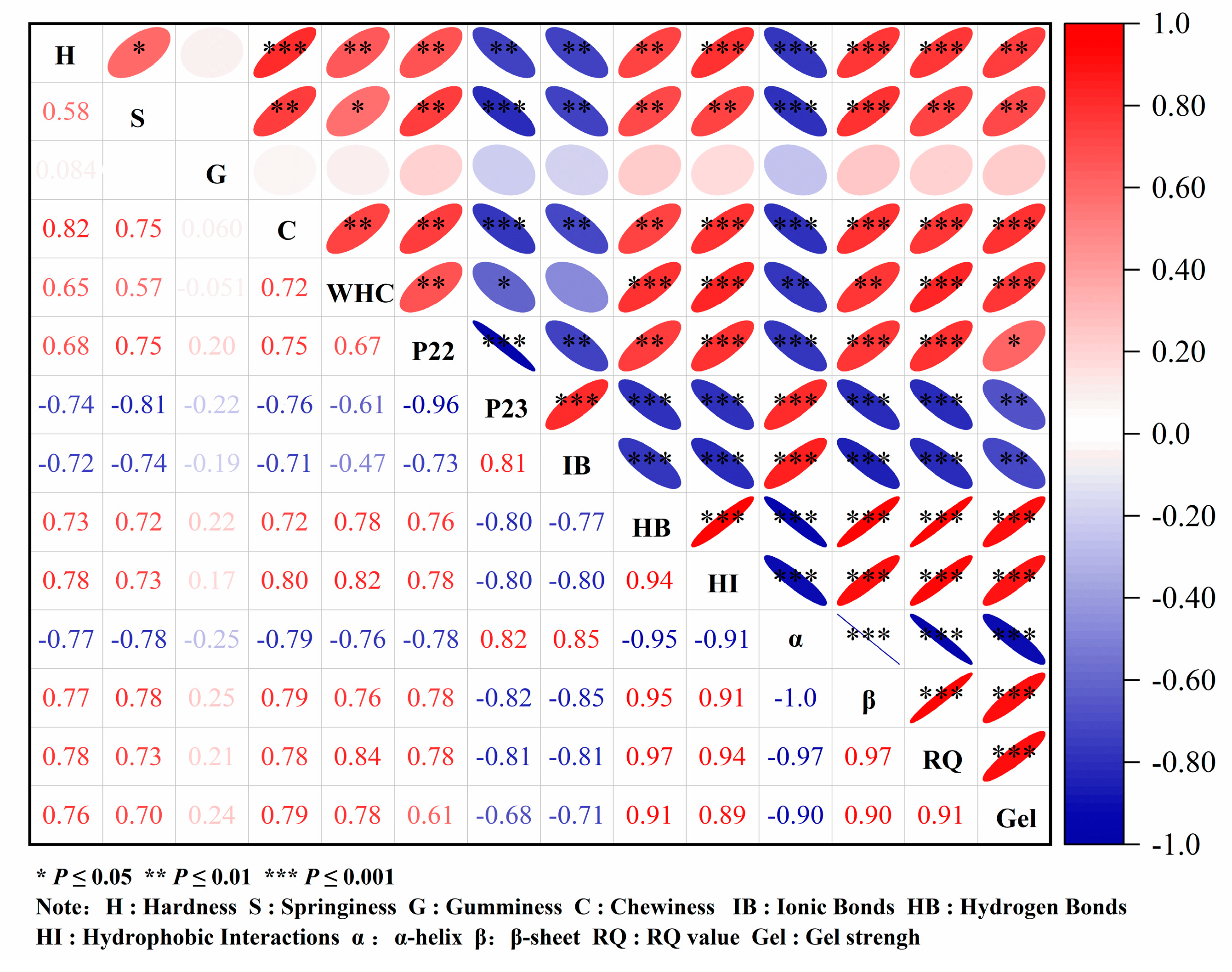
3.3. Correlation Analysis of MP Gel Strength and Various Factors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Lu, M.X.; Ai, C.; Cao, H.; Xiao, J.B.; Imran, M.; Chen, L.; Teng, H. Ultrasonic treatment combined with curdlan improves the gelation properties of low-salt Nemipterus virgatus surimi. Int. J. Biol. Macromol. 2023, 248, 125899. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Dong, H.L.; Wang, P.; Xu, X.L.; Zhang, Y.; Li, Y. Insight into the effect of charge regulation on the binding mechanism of curcumin to myofibrillar protein. Food Chem. 2021, 352, 129395. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, A.D.; Briggs, J.L.; Lonergan, S.M. Influence of pH on rheological properties of porcine myofibrillar protein during heat induced gelation. Meat Sci. 2005, 70, 293–299. [Google Scholar] [CrossRef]
- Zhang, X.H.; Guo, Q.Y.; Shi, W.Z. Ultrasound-assisted processing: Changes in gel properties, water-holding capacity, and protein aggregation of low-salt Hypophthalmichthys molitrix surimi by soy protein isolate. Ultrason. Sonochem. 2023, 92, 106258. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Esua, O.J.; Sharma, N.; Thirumdas, R. Ultrasound-assisted modification of gelation properties of proteins: A review. J. Texture Stud. 2022, 53, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Gu, C.; Wei, R.R.; Luan, Y.; Liu, R.; Ge, Q.F.; Yu, H.; Wu, M. Enhanced gelling properties of myofibrillar protein by ultrasound-assisted thermal-induced gelation process: Give an insight into the mechanism. Ultrason. Sonochem. 2023, 94, 106349. [Google Scholar] [CrossRef]
- Zhuang, X.B.; Han, M.Y.; Bai, Y.; Liu, Y.F.; Xing, L.J.; Xu, X.L.; Zhou, G.H. Insight into the mechanism of myofibrillar protein gel improved by insoluble dietary fiber. Food Hydrocoll. 2018, 74, 219–226. [Google Scholar] [CrossRef]
- Hu, H.Y.; Pereira, J.; Xing, L.J.; Zhou, G.H.; Zhang, W.G. Thermal gelation and microstructural properties of myofibrillar protein gel with the incorporation of regenerated cellulose. LWT—Food Sci. Technol. 2017, 86, 14–19. [Google Scholar] [CrossRef]
- Chen, J.X.; Deng, T.Y.; Wang, C.; Mi, H.B.; Yi, S.M.; Li, X.P.; Li, J.R. Effect of hydrocolloids on gel properties and protein secondary structure of silver carp surimi. J. Sci. Food Agric. 2020, 100, 2252–2260. [Google Scholar] [CrossRef]
- Feng, J.; Bai, X.; Li, Y.; Kong, B.H.; Nuerjiang, M.; Wu, K.R.; Li, Z.; Xia, X. Improvement on gel properties of myofibrillar protein from chicken patty with potato dietary fiber: Based on the change in myofibrillar protein structure and water state. Int. J. Biol. Macromol. 2023, 230, 123228. [Google Scholar] [CrossRef]
- Niu, H.L.; Li, Y.; Han, J.C.; Liu, Q.; Kong, B.H. Gelation and rheological properties of myofibrillar proteins influenced by the addition of soybean protein isolates subjected to an acidic pH treatment combined with a mild heating. Food Hydrocoll. 2017, 70, 269–276. [Google Scholar] [CrossRef]
- Tao, F.; Jiang, H.; Chen, W.W.; Zhang, Y.Y.; Pan, J.R.; Jiang, J.X.; Jia, Z.B. Covalent modification of soy protein isolate by (-)-epigallocatechin-3-gallate: Effects on structural and emulsifying properties. J. Sci. Food Agric. 2018, 98, 5683–5689. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.M.; Zhu, J.Y.; Peng, X.H.; Feng, J.L.; Dong, H.X.; Tong, X.H.; Wang, H.; Jiang, L.Z. Effects of CaCl2 concentration on fibrils formation and characteristics of soybean protein isolate and β-conglycinin/glycinin. Food Hydrocoll. 2023, 142, 108769. [Google Scholar] [CrossRef]
- Li, R.; Cui, Q.; Wang, G.R.; Liu, J.N.; Chen, S.; Wang, X.D.; Jiang, L.Z. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Utsumi, S.; Maruyama, N.; Satoh, R.; Adachi, M. Structure-function relationships of soybean proteins revealed by using recombinant systems. Enzym. Microb. Technol. 2002, 30, 284–288. [Google Scholar] [CrossRef]
- Huang, L.H.; Cai, Y.J.; Liu, T.X.; Zhao, X.J.; Chen, B.F.; Long, Z.; Deng, X.; Zhao, Q.Z. Stability of emulsion stabilized by low-concentration soybean protein isolate: Effects of insoluble soybean fiber. Food Hydrocoll. 2019, 97, 105232. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, A.Q.; Li, R.; Wang, X.B.; Sun, L.N.; Jiang, L.Z. Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates. Food Biosci. 2020, 38, 100747. [Google Scholar] [CrossRef]
- Niu, H.L.; Xia, X.F.; Wang, C.; Kong, B.H.; Liu, Q. Thermal stability and gel quality of myofibrillar protein as affected by soy protein isolates subjected to an acidic pH and mild heating. Food Chem. 2018, 242, 188–195. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, Y.L. Interaction of Myofibrillar and Preheated Soy Proteins. J. Food Sci. 2002, 67, 2851–2856. [Google Scholar] [CrossRef]
- Wang, J.M.; Na, X.K.; Navicha, W.B.; Wen, C.R.; Ma, W.C.; Xu, X.B.; Wu, C.; Du, M. Concentration-dependent improvement of gelling ability of soy proteins by preheating or ultrasound treatment. LWT—Food Sci. Technol. 2020, 134, 110170. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhao, X.; Zhou, C.; Chang, Y.; Wang, C.; Zheng, Y.Y.; Ye, K.P.; Li, C.B.; Zhou, G.H. Effects of gellan gum and inulin on mixed-gel properties and molecular structure of gelatin. Food Sci. Nutr. 2021, 9, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, Y.Y.; Ma, C.C.; Julian, M.D.; Liu, F.G.; Liu, X.B. Pea protein isolate-inulin conjugates prepared by pH-shift treatment and ultrasonic-enhanced glycosylation: Structural and functional properties. Food Chem. 2022, 384, 132511. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.F.; Kong, B.H.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Lv, Y.N.; Zhao, H.L.; He, X.L.; Li, X.P.; Yi, S.M.; Li, J.R. Diacylglycerol pre-emulsion prepared through ultrasound improves the gel properties of golden thread surimi. Ultrason. Sonochem. 2022, 82, 105915. [Google Scholar] [CrossRef]
- Lu, Y.F.; Zhu, Y.J.; Ye, T.; Nie, Y.T.; Jiang, S.T.; Lin, L.; Lu, J.F. Physicochemical properties and microstructure of composite surimi gels: The effects of ultrasonic treatment and olive oil concentration. Ultrason. Sonochem. 2022, 88, 106065. [Google Scholar] [CrossRef]
- Han, G.; Li, Y.X.; Liu, Q.; Chen, Q.; Liu, H.T.; Kong, B.H. Improved water solubility of myofibrillar proteins by ultrasound combined with glycation: A study of myosin molecular behavior. Ultrason. Sonochem. 2022, 89, 106140. [Google Scholar] [CrossRef]
- Ramírez, J.A.; Uresti, R.M.; Velazquez, G.; Vázquez, M. Food hydrocolloids as additives to improve the mechanical and functional properties of fish products: A review. Food Hydrocoll. 2011, 25, 1842–1852. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.J.; Wang, Y.M.; Xue, C.H. Effects of deacetylation of konjac glucomannan on Alaska Pollock surimi gels subjected to high-temperature (120 °C) treatment. Food Hydrocoll. 2015, 43, 125–131. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.Q.; Li, X.X.; Sun, L.J.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Nieto-Nieto, T.V.; Wang, Y.X.; Ozimek, L.; Chen, L.Y. Inulin at low concentrations significantly improves the gelling properties of oat protein—A molecular mechanism study. Food Hydrocoll. 2015, 50, 116–127. [Google Scholar] [CrossRef]
- Ma, X.B.; Hou, F.R.; Zhao, H.H.; Wang, D.; Chen, W.J.; Miao, S.; Liu, D.H. Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocoll. 2020, 108, 106056. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Swedlund, P.J.; Hemar, Y. Physical and rheological properties of fish gelatin gel as influenced by κ-carrageenan. Food Biosci. 2017, 20, 88–95. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.L. Extreme pH treatments enhance the structure-reinforcement role of soy protein isolate and its emulsions in pork myofibrillar protein gels in the presence of microbial transglutaminase. Meat Sci. 2013, 93, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Geng, R.; Zhao, J.Y.; Chen, Q.; Kong, B.H. Structural and Gel Textural Properties of Soy Protein Isolate When Subjected to Extreme Acid pH-Shifting and Mild Heating Processes. J. Agric. Food Chem. 2015, 63, 4853–4861. [Google Scholar] [CrossRef]
- Mu, L.X.; Zhao, M.M.; Yang, B.; Zhao Hai, F.; Cui, C.; Zhao, Q.Z. Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J. Agric. Food Chem. 2010, 58, 4494–4499. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Dong, M.; Zhang, X.Y.; Hu, Y.J.; Han, M.Y.; Xu, X.L.; Zhou, G.H. Effects of inulin on the gel properties and molecular structure of porcine myosin: A underlying mechanisms study. Food Hydrocoll. 2020, 108, 105974. [Google Scholar] [CrossRef]
- Sánchez-González, I.; Rodríguez-Casado, A.; Careche, M.; Carmona, P. Raman analysis of surimi gelation by addition of wheat dietary fibre. Food Chem. 2008, 112, 162–168. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liang, J.; Jiang, L.Z.; Li, Y.; Wang, J.; Zhang, H.; Li, D.; Han, F.; Li, Q.; Wang, R.; et al. Effect of the interaction between myofibrillar protein and heat-induced soy protein isolates on gel properties. CyTA—J. Food 2015, 13, 527–534. [Google Scholar] [CrossRef]
- Zhao, C.B.; Chu, Z.J.; Miao, Z.C.; Liu, J.; Liu, J.S.; Xu, X.Y.; Wu, Y.Z.; Qi, B.K.; Yan, J.N. Ultrasound heat treatment effects on structure and acid-induced cold set gel properties of soybean protein isolate. Food Biosci. 2021, 39, 100827. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Bertram, H.C.; Andersen, H.J.; Karlsson, A.H. Comparative study of low-field NMR relaxation measurements and two traditional methods in the determination of water holding capacity of pork. Meat Sci. 2001, 57, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cen, K.; Yu, X.; Gao, C.; Yang, Y.; Tang, X.; Feng, X. Effects of quinoa protein Pickering emulsion on the properties, structure and intermolecular interactions of myofibrillar protein gel. Food Chem. 2022, 394, 133456. [Google Scholar] [CrossRef] [PubMed]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2007, 86, 281–287. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Q.; Xiong, S.B.; Fu, Y.C.; Chen, L. Effects of high intensity unltrasound on structural and physicochemical properties of myosin from Silver Carp. Ultrason. Sonochem. 2016, 37, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Xu, Y.; Qi, J.; Xu, X.L.; Zhao, X. Effect of high intensity ultrasound on the gelation properties of wooden breast meat with different NaCl contents. Food Chem. 2021, 347, 129031. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.M.; Xie, B.J.; Xiong, S.B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Xiong, Z.Y.; Walayat, N.; Lorenzo, J.M.; Nawaz, A.; Xiong, H.G. Effects of the Mixture of Xylooligosaccharides and Egg White Protein on the Physicochemical Properties, Conformation, and Gel-Forming Ability of Culter alburnus Myofibrillar Protein during Multiple Freeze–Thaw Cycles. Foods 2021, 10, 2007. [Google Scholar] [CrossRef]
- Liu, H.M.; Gao, L.L.; Ren, Y.X.; Zhao, Q. Chemical Interactions and Protein Conformation Changes During Silver Carp (Hypophthalmichthys molitrix) Surimi Gel Formation. Int. J. Food Prop. 2014, 17, 1702–1713. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.J.; Liu, J.B.; Yu, X.H. Effects of rice bran feruloyl oligosaccharides on gel properties and microstructure of grass carp surimi. Food Chem. 2023, 407, 135003. [Google Scholar] [CrossRef]
- Mi, H.B.; Su, Q.; Chen, J.X.; Yi, S.M.; Li, X.P.; Li, J.R. Starch-fatty acid complexes improve the gel properties and enhance the fatty acid content of Nemipterus virgatus surimi under high-temperature treatment. Food Chem. 2021, 362, 130253. [Google Scholar] [CrossRef]
- Sánchez-González, I.; Carmona, P.; Moreno, P.; Borderías, J.; Sánchez-Alonso, I.; Rodríguez-Casado, A.; Careche, M. Protein and water structural changes in fish surimi during gelation as revealed by isotopic H/D exchange and Raman spectroscopy. Food Chem. 2007, 106, 56–64. [Google Scholar] [CrossRef]
- Yu, N.N.; Xu, Y.S.; Jiang, Q.X.; Xia, W.S. Molecular forces involved in heat-induced freshwater surimi gel: Effects of various bond disrupting agents on the gel properties and protein conformation changes. Food Hydrocoll. 2017, 69, 193–201. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S.; Konno, K. Improvement of Gel Quality of Sardine Surimi with Low Setting Phenomenon by Ethanolic Coconut Husk Extract. J. Texture Stud. 2017, 48, 47–56. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.Z.; Yang, F.J.; Wu, J.H.; Huang, D.; Huang, J.L.; Wang, S.Y. Effects and mechanism of antifreeze peptides from silver carp scales on the freeze-thaw stability of frozen surimi. Food Chem. 2022, 396, 133717. [Google Scholar] [CrossRef]
- Zhang, F.H.; Fang, L.; Wang, C.J.; Shi, L.; Chang, T.; Yang, H.; Cui, M. Effects of starches on the textural, rheological, and color properties of surimi–beef gels with microbial tranglutaminase. Meat Sci. 2013, 93, 533–537. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Proteolysis and Its Control Using Protease Inhibitors in Fish and Fish Products: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef]
- Velazquez, G.; Méndez-Montealvo, M.G.; Welti-Chanes, J.; Ramírez, J.A.; Martínez-Maldonado, M.A. Effect of high pressure processing and heat treatment on the gelation properties of blue crab meat proteins. LWT—Food Sci. Technol. 2021, 146, 111389. [Google Scholar] [CrossRef]
- Shi, J.; Lei, Y.T.; Shen, H.X.; Hong, H.; Yu, X.P.; Zhu, B.W.; Luo, Y.K. Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenocera melantho) during frozen storage. Food Chem. 2019, 272, 604–612. [Google Scholar] [CrossRef]
- Li, D.Y.; Tan, Z.F.; Liu, Z.Q.; Wu, C.; Liu, H.L.; Guo, C.; Zhou, D.Y. Effect of hydroxyl radical induced oxidation on the physicochemical and gelling properties of shrimp myofibrillar protein and its mechanism. Food Chem. 2021, 351, 129344. [Google Scholar] [CrossRef]
- Xu, M.F.; Ni, X.X.; Liu, Q.W.; Chen, C.C.; Deng, X.H.; Wang, X.; Yu, R.R. Ultra-high pressure improved gelation and digestive properties of Tai Lake whitebait myofibrillar protein. Food Chem. X 2024, 21, 101061. [Google Scholar] [CrossRef]
- Gao, Y.F.; Wang, S.C.; Liu, H.Y.; Gu, Y.Y.; Zhu, J. Design and characterization of low salt myofibrillar protein-sugar beet pectin double-crosslinked gels pretreated by ultrasound and konjac glucomannan: Conformational and gelling properties. Food Hydrocoll. 2023, 141, 108717. [Google Scholar] [CrossRef]
- Kirby, A.R.; MacDougall, A.J.; Morris, V.J. Sugar Beet Pectin–Protein Complexes. Food Biophys. 2006, 1, 51–56. [Google Scholar] [CrossRef]
- Wei, Z.H.; Zhu, P.; Huang, Q.R. Investigation of ovotransferrin conformation and its complexation with sugar beet pectin. Food Hydrocoll. 2019, 87, 448–458. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.L.; Shen, C.L.; Deng, S.G. Understanding the influence of carrageenan oligosaccharides and xylooligosaccharides on ice-crystal growth in peeled shrimp (Litopenaeus vannamei) during frozen storage. Food Funct. 2018, 9, 4394–4403. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Fu, C.L.; Yang, H.S. Gelatin addition improves the nutrient retention, texture and mass transfer of fish balls without altering their nanostructure during boiling. LWT—Food Sci. Technol. 2017, 77, 142–151. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, X.D.; Yan, B.W.; Meng, L.L.; Cao, H.W.; Huang, J.L.; Zhao, J.X.; Zhang, H.; Chen, W.; Fan, D.M. Inhibitory effect of microwave heating on cathepsin l-induced degradation of myofibrillar protein gel. Food Chem. 2021, 357, 129745. [Google Scholar] [CrossRef]
- Chen, H.Y.; Han, M.Y. Raman spectroscopic study of the effects of microbial transglutaminase on heat-induced gelation of pork myofibrillar proteins and its relationship with textural characteristics. Food Res. Int. 2011, 44, 1514–1520. [Google Scholar] [CrossRef]
- Yan, B.W.; Jiao, X.D.; Zhu, H.P.; Wang, Q.; Huang, J.L.; Zhao, J.X.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; et al. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]



| Groups | Hardness (g) | Springiness | Gumminess | Chewiness (g) |
|---|---|---|---|---|
| MP | 627.081 ± 12.273 bc | 0.916 ± 0.015 ab | 0.726 ± 0.084 a | 392.770 ± 18.868 b |
| MP-S | 570.489 ± 22.885 c | 0.890 ± 0.012 b | 0.681 ± 0.049 a | 375.400 ± 19.489 b |
| MP-I | 648.890 ± 12.263 bc | 0.919 ± 0.013 ab | 0.729 ± 0.072 a | 420.275 ± 26.135 b |
| MP-M | 677.893 ± 42.694 ab | 0.924 ± 0.007 a | 0.730 ± 0.081 a | 429.929 ± 26.317 b |
| MP-C | 748.947 ± 65.197 a | 0.945 ± 0.013 a | 0.742 ± 0.058 a | 496.459 ± 37.582 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Ma, S.; Shao, Q.; Yi, S. Effects of Soy Protein Isolate and Inulin Conjugate on Gel Properties and Molecular Conformation of Spanish Mackerel Myofibrillar Protein. Foods 2024, 13, 2920. https://doi.org/10.3390/foods13182920
Wang W, Ma S, Shao Q, Yi S. Effects of Soy Protein Isolate and Inulin Conjugate on Gel Properties and Molecular Conformation of Spanish Mackerel Myofibrillar Protein. Foods. 2024; 13(18):2920. https://doi.org/10.3390/foods13182920
Chicago/Turabian StyleWang, Wei, Sirui Ma, Qing Shao, and Shumin Yi. 2024. "Effects of Soy Protein Isolate and Inulin Conjugate on Gel Properties and Molecular Conformation of Spanish Mackerel Myofibrillar Protein" Foods 13, no. 18: 2920. https://doi.org/10.3390/foods13182920





