Unconventional Edible Plants of the Amazon: Bioactive Compounds, Health Benefits, Challenges, and Future Trends
Abstract
:1. Introduction
| Botanical Family | Scientific Name | Common Name * | Parts Used | Forms of Consumption |
|---|---|---|---|---|
| Arecaceae | Euterpe oleracea Mart. | Açaí | Fruit | Raw, juice, wine, desserts, ice cream, and sauces |
| Malvaceae | Hibiscus sabdariffa | Vinagreira | Calyx and leaves | Soups, sauces, beverages, jams, and jellies |
| Malvaceae | Pachira aquatica | Monguba | Fruit pericarp, seeds, fruit, trunk, leaves, and flowers | Raw and used in confectionery, bakery, oils, and biofuels |
| Clusiaceae | Garcinia gardneriana | Bacopari, bacupari, abricó, and damasco | Fruit | Raw, juice, jams, and jellies |
| Fabaceae | Inga marginata Willd. | Angá-feijão, ingá-feijão, and angá | Fruit and seeds | Raw |
| Myrtaceae | Psidium cattleianum Sabine | Araçá, araçá-amarelo, araçá-roxo, araçá-vermelho, and araçá-manteiga | Fruit | Raw, juice, jams, and jellies |
| Marantaceae | Goeppertia allouia | Ariá | Tuber | Cooked or used as ingredients in culinary preparations |
| Arecaceae | Bactris gasipaes | Pupunha | Fruit pulp, peel, and seeds or stem | Cooked pulp, flour from pulp and peel, as a source of starch, in oil production, and heart of palm |
| Apiaceae | Eryngium foetidum | Chicória-do-Pará, chicória paraense, and coentrão | Leaves | Used as a condiment or seasoning for fish and as an ingredient in the preparation of typical meals, stews, omelets, and stir-fries |
| Humiriaceae | Endopleura uchi (Huber) Cuatrec | Uxi and uxipuçu | Pulp, peel, seed, and bark | Raw, tea, ice cream, and sweets; and in skincare products and as a powerful insecticide |
2. Chemical Composition and Biological Effects of UFPs Consumed in the Amazon
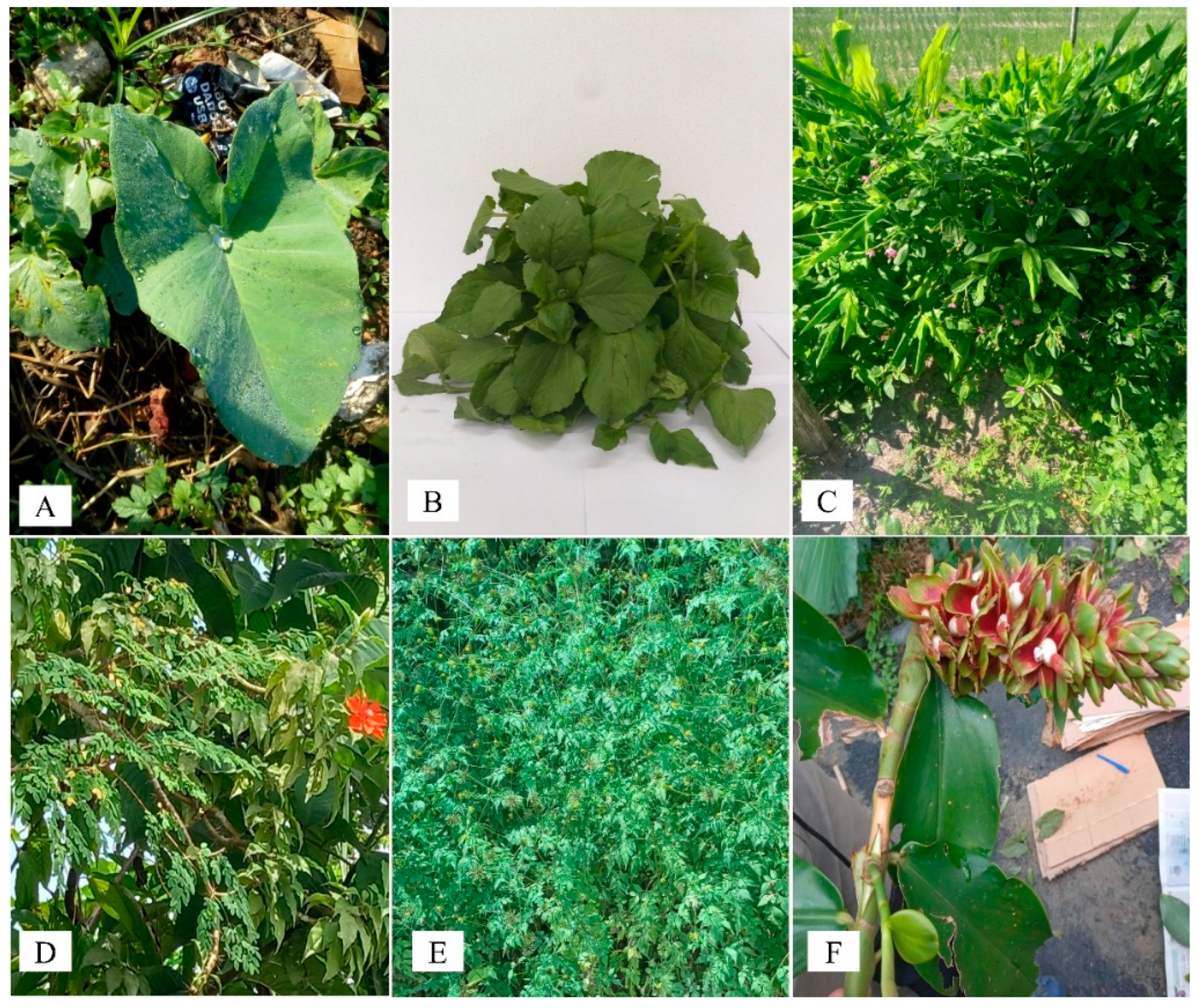
2.1. Xanthosoma sagittifolium (L.) Schott
2.2. Acmella oleracea (L.) R. K. Jansen
| Composition | Xanthosoma sagittifolium 1 | Acmella oleracea 2 | Talinum triangulare 3 | Pereskia bleo 4 | Costus spiralis 5 |
|---|---|---|---|---|---|
| Moisture | 88.58 g/100 g and 93.86 g/100 g (petiole) b | 89.87 g/100 g a | 87.13 g/100 g a | 91.39 g/100 g b | - |
| Ash | 13.77 g/100 g and 22.12 g/100 g (petiole) a | 1.11 g/100 g a | 7.92 g/100 g a | 2.15 g/100 g b | - |
| Lipids | 7.60 g/100 g and 5.86 g/100 g (petiole) a | 0.16 mg/100 g a | 1.98 g/100 g a | 0.41 g/100 g b | - |
| Proteins | 58.50 g/100 g and 30.90 g/100 g (petiole) a | 2.44 mg/100 g a | 14.65 g/100 g a | 3.25 g/100 g b | - |
| Soluble fibers | 3.50 g/100 g a | - | - | - | - |
| Insoluble fibers | 11.55 g/100 g a | - | - | - | - |
| Total fibers | 23.39 g/100 g and 16.66 g/100 g (petiole) a | 6.35 mg/100 g a | 7.92 g/100 g a | - | - |
| Sodium | 129 mg/100 g a | 1.62 mg/100 g a | 31.00 mg/100 g a | - | |
| Potassium | 3.03 g/100 g a | 594.44 mg/100 g a | 3546.00 mg/100 g a | 619.50 mg/100 g a | 1.40 mg/Kg a |
| Calcium | 1.79 g/100 g and 0.98 g/100 g (petiole) a | 260.00 mg/100 g a | 678.00 mg/100 g a | 480.71 mg/100 g a | 27.30 g/Kg a |
| Magnesium | 0.50 g/100 g and 0.25 g/100 g (petiole) a | 74.86 mg/100 g a | 1983.00 mg/100 g a | 88.27 mg/100 g a | 11.80 g/Kg a |
| Phosphorus | 41.99–43.89 mg/100 g a | - | 436.00 mg/100 g a | - | 3..50 g/Kg a |
| Iron | 7.22–7.89 mg/100 g a | 1.94 mg/100 g a | 14.33 mg/100 g a | 12.34 mg/100 g a | 545.00 mg/Kg a |
| Zinc | 4.15–4.60 mg/100 g a | 0.95 mg/100 g a | 4.24 mg/100 g a | 6.40 mg/100 g a | 22.00 mg/Kg a |
| Calcium oxalate | 648 mg/100 g and 846.72 mg/100 g (petiole) a | - | - | - | - |
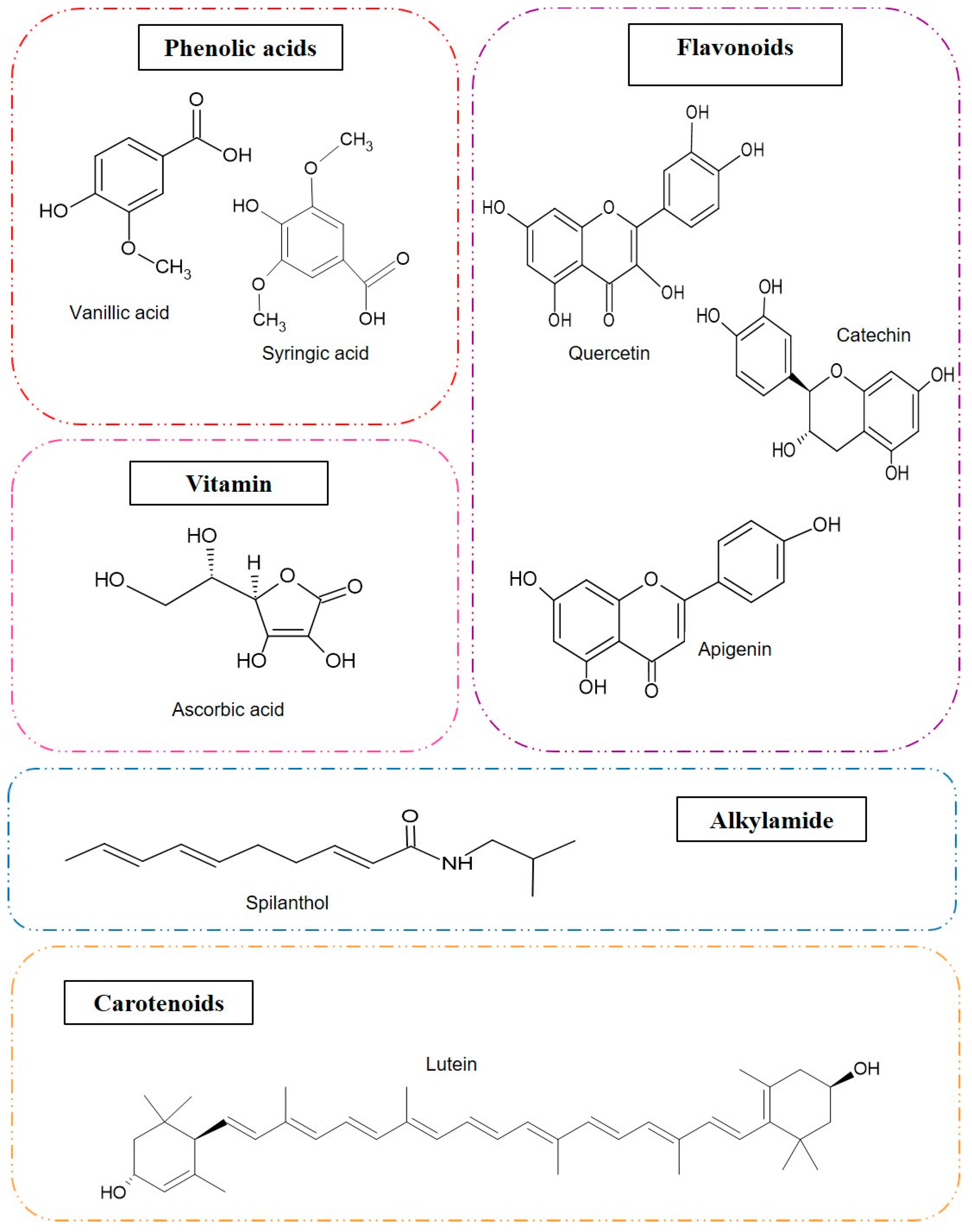
| Vitamin C | 87 mg/100 g and 83.00 mg/100 g (petiole) a | - | 1.11–1.36 g/100 g a | - | - |
| Total phenolics | 5.33 mg GAE/100 g and 2.80 mg GAE/100 g (petiole) a | 3.19 g GAE/g a | 0.61–1.09 g GAE/100 g a | 109.43 mg GAE/g | - |
| Total flavonoid | - | 11.45 mg rutin/g a | 0.33–3.52 g/100 g a | - | - |
| Total CHL | 8.94 mg/100 g and 7.00 mg/100 g (petiole) a | - | 92.26–584.19 g/100 g a | - | - |
| Tannins | 1.08–1.11 mg/100 g a | - | - | - | - |
| Total carotenoids | 83.19 mg/100 g and 54.07 mg/100 g (petiole) a | 618.00 μg/g a | - | - | - |
2.3. Talinum triangulare
2.4. Pereskia bleo
2.5. Bidens bipinnata L.
| Unconventional Food Plant | Source | Bioactivity | Method/Model | Related Compounds | Major Findings | References |
|---|---|---|---|---|---|---|
| Xanthosoma sagittifolium | Methanolic extract of the whole plant | Antioxidant | DPPH, ABTS, and FRAP assays | Not informed | -High antioxidant capacity in the DPPH (4173 g/g), ABTS (33.55 µM TE/g), and FRAP (0.0144 µM FS/g) assays; | [71] |
| Methanolic extract of the leaves | Antioxidant and antiproliferative | ORAC and HOCl assays | Not informed | -High antioxidant capacity in the ORAC (632.26 µM TE/g) and HOCl (35.21 µg/mL) assays. -Inhibited cell proliferation of human tumor cell lines (GI50): glioblastoma (205.1 µg/mL), melanoma (225.7 µg/mL), ovarian II (185.6 µg/mL), kidney (116.0 µg/mL), and leukemia (13.9 µg/mL). | [47] | |
| Methanolic extract of the corm | Anti-hypertensive | Forty normotensive male Wistar rats with induced hypertension from II of DOCA salt twice weekly and the daily inclusion of NaCl (1%) in their drinking water. The rats received 100 or 200 mg/kg of the extract | Not informed | -↓ in blood pressure and free protein thiols; -↓ in malondialdehyde levels and hydrogen peroxide activities; -↑ in total protein, gluthathione peroxidase, reduced glutathione, glutathione S-transferase, catalase, and nitric oxide in the heart, kidney, and liver. | [101] | |
| Acmella oleracea | Leaves’ essential oil by hydrodistillation | Cytotoxicity | MTT test | E-Caryophyllene | -Antiproliferative activity against cell lines of human cancer: gastric ascites (AGP-01), melanoma (SK-MEL-19), lung carcinoma (A549), and a healthy human kidney strain (HEK-293). | [102] |
| Hydroethanolic inflorescence extract | Cytotoxicity | MTT test and molecular docking against JAK1 and JAK2 proteins | Spilanthol | -Cytotoxicity against gastric cancer. | [103] | |
| Lyophilized ethanol extract from leaves and flowers | Healing | The calcaneal tendon of male Lewis rats was partially transected and treated at the site of injury with 64 mg of a topical application containing 20% A. oleracea | Not informed | -↑ in the molecular organization and content of collagen; -Potential application in tendon repair. | [67] | |
| Alkylamide-rich hexane fraction from flowers | Inflammatory pain | Swiss male adult mice pretreated, before the acute inflammatory response was induced by an injection of carrageenan into the right hind paw | Alkylamide | -↓ in the paw withdrawal threshold; -↓ in mechanical allodynia; -Effective and long-lasting antiallodynic and anti-oedematogenic activities; -↓ in MPO activity; TNF-α and IL-1β levels; SOD, CAT, and GSH contents; -Prevented the production of LOOH. | [104] | |
| Talinum triangulare | Powder | Prebiotic | Chicks of the SASSO strain fed with feed +2% powder | Possible synergy between proteins and phytochemicals | -↑ in significantly lower mortality rates; -↔ between the red and white blood cell values; -↑ in the concentration of butyric, valeric, and heptanoic acids. | [105] |
| Lyophilized aqueous extract | Antioxidant and anti-inflammatory | Matured male albino Wistar rats with AUC induced by 5% of DSS received 200 mg of lyophilized leaf/kg of body weight | Extract rich in phytochemicals | -↑ in body weight; -Ameliorated the toxic effect by length and weight of the colon; -↓ in the inflammatory marker’s levels in the colon; -It was more effective in inhibiting inflammation than sulphasalazine; -↓ in the colonic level of malondialdehyde; -↓ in H2O2 production; -↑ in the GSH and protein levels in the colon; -Reversed the DSS-induced inhibition of the cytoprotective enzymes. | [106] | |
| Pereskia bleo | Methanolic, hexane, and chloroform extracts from leaves | Antioxidant and antibacterial activities | DPPH assay, MIC, and MBC | Not informed | -IC50 values between 33.83 and 379.41 µg/mL; -Strong inhibitory action for Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, and Escherichia coli. | [89] |
| Bidens bipinnata | Hydroalcoholic extract from aerial parts | Hepatoprotective effect | Administration of extract of B. bipinnata (50, 100, and 200 mg/kg) in mice with an acute liver injury for seven days | Not informed | -↓ in liver weight, serum transaminases, and hepatic morphologic changes; -↑ in SOD and glutathione peroxidase; -Suppressed nitric oxide production and nuclear factor-kappaB activation. | [107] |
| Hexane extract from leaves, flowers, roots, stems, and fruit | Antifungal | Inoculation on YPD agar medium | Linoleic acid and dehydroabietic acid | -Inhibition of C. albicans, C. glabrata, C. tropicalis, C. krusei, and C. orthopsilosis. | [108] | |
| Costus spiralis | Ethyl acetate fraction from methanolic extract of leaves | Antihyperglycemic properties | Enzyme assay | Schaftoside and isoschaftoside | -IC50 1.95 times higher than acarbose. | [109] |
| Aqueous extract from leaves and stems | Antioxidant activity and cytogenotoxic effects | DPPH assay and mitotic index; and the frequency of chromosomal aberrations, micronuclei, and nuclear abnormalities | Not informed | -IC50 11.82 mg/mL to leaves and 15.38 mg/mL to stems; -Inhibitory effect on Allium cepa root’s growth. | [110] |
2.6. Costus spiralis (Jacq.) Roscoe
3. New Technologies
3.1. FoodTech
3.2. Market
3.3. Challenges and Future Trends for Food Technology Using UFPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; PAHO; UNICEF; WFP. Latin America and the Caribbean—Regional Overview of Food Security and Nutrition 2023; FAO: Santiago, Chile, 2023; ISBN 978-92-5-138358-2. [Google Scholar]
- Roberts, D.P.; Mattoo, A.K. Sustainable Crop Production Systems and Human Nutrition. Front. Sustain. Food Syst. 2019, 3, 72. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional Importance of Bioactive Compounds of Foods with Potential Health Benefits: A Review on Recent Trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Kinupp, V.F.; de Barros, I.B.I. Riqueza de Plantas Alimentícias Não-Convencionais Na Região Metropolitana de Porto Alegre, Rio Grande Do Sul. Rev. Bras. Biociências 2007, 5, 63–65. [Google Scholar]
- Milião, G.L.; de Oliveira, A.P.H.; Soares, L.D.S.; Arruda, T.R.; Vieira, É.N.R.; Leite Junior, B.R.d.C. Unconventional Food Plants: Nutritional Aspects and Perspectives for Industrial Applications. Futur. Foods 2022, 5, 100124. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Rebelo, K.S.; Bisconsin-Junior, A.; de Morais, J.S.; Magnani, M.; Maldonade, I.R.; Madeira, N.R.; Tiengo, A.; Maróstica, M.R.; Cazarin, C.B.B. The Use of Alternative Food Sources to Improve Health and Guarantee Access and Food Intake. Food Res. Int. 2021, 149, 110709. [Google Scholar] [CrossRef]
- Gomes, S.M.; Chaves, V.M.; de Carvalho, A.M.; da Silva, E.B.; de Menezes Neto, E.J.; de Farias Moura, G.; da Silva Chaves, L.; Alves, R.R.N.; de Albuquerque, U.P.; de Oliveira Pereira, F.; et al. Author Correction: Biodiversity Is Overlooked in the Diets of Different Social Groups in Brazil. Sci. Rep. 2023, 13, 9278. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Agricultura e Pecuária Hortaliças PANCs Atraem Agricultores Que Querem Diversificar Produção de Alimentos. Available online: https://www.gov.br/agricultura/pt-br/assuntos/noticias/hortalicas-pancs-atraem-a-atencao-de-agricultores-que-querem-diversificar-producao-de-alimentos (accessed on 9 August 2023).
- Brandão, D.O.; Barata, L.E.S.; Nobre, C.A. The Effects of Environmental Changes on Plant Species and Forest Dependent Communities in the Amazon Region. Forests 2022, 13, 466. [Google Scholar] [CrossRef]
- da Silveira, J.T.; da Rosa, A.P.C.; de Morais, M.G.; Victoria, F.N.; Costa, J.A.V. An Integrative Review of Açaí (Euterpe Oleracea and Euterpe Precatoria): Traditional Uses, Phytochemical Composition, Market Trends, and Emerging Applications. Food Res. Int. 2023, 173, 113304. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Wang, C.; Qu, S.; Zhu, Y.; Yang, Z.; Wang, L. Açaí (Euterpe Oleracea Mart.) Attenuates Alcohol-induced Liver Injury in Rats by Alleviating Oxidative Stress and Inflammatory Response. Exp. Ther. Med. 2017, 15, 166–172. [Google Scholar] [CrossRef]
- da Silva Monteiro, C.E.; da Costa Filho, H.B.; Silva, F.G.O.; de Souza, M.d.F.F.; Sousa, J.A.O.; Franco, Á.X.; Resende, Â.C.; de Moura, R.S.; de Souza, M.H.L.; Soares, P.M.G.; et al. Euterpe Oleracea Mart. (Açaí) Attenuates Experimental Colitis in Rats: Involvement of TLR4/COX-2/NF-ĸB. Inflammopharmacology 2021, 29, 193–204. [Google Scholar] [CrossRef]
- de Oliveira, E.d.F.; Brasil, A.; Herculano, A.M.; Rosa, M.A.; Gomes, B.D.; de Farias Rocha, F.A. Neuroprotective Effects of Açaí (Euterpe oleracea Mart.) against Diabetic Retinopathy. Front. Pharmacol. 2023, 14, 1143923. [Google Scholar] [CrossRef] [PubMed]
- Interdonato, L.; Marino, Y.; Franco, G.A.; Arangia, A.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Impellizzeri, D.; Fusco, R.; Cuzzocrea, S.; et al. Açai Berry Administration Promotes Wound Healing through Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2023, 24, 834. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.-H.; Ho, C.-T.; Simon, J.E.; Wu, Q. Phytochemistry, Antioxidant Capacity, Total Phenolic Content and Anti-Inflammatory Activity of Hibiscus Sabdariffa Leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef]
- de Oliveira, R.T.; dos Santos Rolim, C.S.; do Nascimento Rolim, L.; de Sousa Gomes, M.L.; Martins, G.A.S.; de Castro, L.M.; do Nascimento, W.M.; Saraiva-Bonatto, E.C.; de Cássia Saraiva Nunomura, R.; Lamarão, C.V.; et al. Endopleura Uchi—A Review about Its Nutritional Compounds, Biological Activities and Production Market. Food Res. Int. 2021, 139, 109884. [Google Scholar] [CrossRef] [PubMed]
- Daim Costa, L.; Pereira Trindade, R.; da Silva Cardoso, P.; Barros Colauto, N.; Andrea Linde, G.; Murowaniecki Otero, D. Pachira Aquatica (Malvaceae): An Unconventional Food Plant with Food, Technological, and Nutritional Potential to Be Explored. Food Res. Int. 2023, 164, 112354. [Google Scholar] [CrossRef]
- da Silva, M.M.; Lemos, T.D.O.; Maria do Carmo, P.R.; de Araújo, A.M.S.; Gomes, A.M.M.; Pereira, A.L.F.; Abreu, V.K.G.; dos S. Araújo, E.; de S. Andrade, D. Sweet-and-Sour Sauce of Assai and Unconventional Food Plants with Functional Properties: An Innovation in Fruit Sauces. Int. J. Gastron. Food Sci. 2021, 25, 100372. [Google Scholar] [CrossRef]
- Oliveira, L.P.; Montenegro, M.D.A.; Lima, F.C.A.; Suarez, P.A.Z.; da Silva, E.C.; Meneghetti, M.R.; Meneghetti, S.M.P. Biofuel Production from Pachira Aquatic Aubl and Magonia Pubescens A St-Hil: Physical-Chemical Properties of Neat Vegetable Oils, Methyl-Esters and Bio-Oils (Hydrocarbons). Ind. Crops Prod. 2019, 127, 158–163. [Google Scholar] [CrossRef]
- Soares, S.D.; Dos Santos, O.V.; Nascimento, F.D.C.A.D.; da Silva Pena, R. A Review of the Nutritional Properties of Different Varieties and Byproducts of Peach Palm (Bactris gasipaes) and Their Potential as Functional Foods. Int. J. Food Prop. 2022, 25, 2146–2164. [Google Scholar] [CrossRef]
- Soares, S.D.; dos Santos, O.V.; da Conceição, L.R.V.; Costi, H.T.; Silva Júnior, J.O.C.; Nascimento, F.d.C.A.d.; Pena, R.d.S. Nutritional and Technological Properties of Albino Peach Palm (Bactris gasipaes) from the Amazon: Influence of Cooking and Drying. Foods 2023, 12, 4344. [Google Scholar] [CrossRef]
- Borah, G.; Bora, P.K.; Mahanta, B.P.; Saikia, S.P.; Haldar, S. Quality Control, Ontogenetic Variability and Sensory Profiling of ‘Cilantro-Mimic’ Spiny Coriander (Eryngium foetidum L.): A Flavour Perspective. Food Chem. Adv. 2023, 3, 100370. [Google Scholar] [CrossRef]
- Rodrigues, T.L.M.; Silva, M.E.P.; Gurgel, E.S.C.; Oliveira, M.S.; Lucas, F.C.A. Eryngium foetidum L. (Apiaceae): A Literature Review of Traditional Uses, Chemical Composition, and Pharmacological Activities. Evidence-Based Complement. Altern. Med. 2022, 2022, 2896895. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.R.; Carvalho, A.P.M.G.; da Silva, E.O.; Sampaio, U.M.; de Souza, S.M.; Sanches, E.A.; de Souza Sant’Ana, A.; Clerici, M.T.P.S.; Campelo, P.H. Ariá (Goeppertia allouia) Brazilian Amazon Tuber as a Non-Conventional Starch Source for Foods. Int. J. Biol. Macromol. 2021, 168, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.L.; Alves, R.P.; Hanazaki, N. Knowledge, Use, and Disuse of Unconventional Food Plants. J. Ethnobiol. Ethnomed. 2018, 14, 6. [Google Scholar] [CrossRef]
- da Silva, S.P.; Fernandes, J.A.L.; Santos, A.S.; Ferreira, N.R. Jambu Flower Extract (Acmella oleracea) Increases the Antioxidant Potential of Beer with a Reduced Alcohol Content. Plants 2023, 12, 1581. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Allegrini, P.; Faliva, M.A.; Naso, M.; Peroni, G.; Nichetti, M.; Gasparri, C.; Spadaccini, D.; Iannello, G.; et al. The Use of a New Food-Grade Lecithin Formulation of Highly Standardized Ginger (Zingiber officinale) and Acmella oleracea Extracts for the Treatment of Pain and Inflammation in a Group of Subjects with Moderate Knee Osteoarthritis. J. Pain Res. 2020, 13, 761–770. [Google Scholar] [CrossRef]
- Rahmawati, S.; Yassaroh, Y.; Theodora, M.; Tahril, T.; Afadil, A.; Santoso, T.; Suherman, S.; Nurmayanti, Y. Antioxidant Edible Films Derived from Belitung Taro Tubers (Xanthosoma sagittifolium) Incorporated with Moringa Leaf Extract (Moringa oleifera). Prev. Nutr. Food Sci. 2024, 29, 210–219. [Google Scholar] [CrossRef]
- Sartori, V.C.; Theodoro, H.; Minello, L.V.; Pansera, M.R.; Basso, A.; Scur, L. Plantas Alimentícias Não Convencionais, 2nd ed.; Educs: Caxias do Sul, Brazil, 2020; ISBN 9788570619921. [Google Scholar]
- dos Santos, O.V.; da Cunha, N.S.R.; Duarte, S.d.P.d.A.; Soares, S.D.; da Costa, R.S.; Mendes, P.M.; Martins, M.G.; das C.A. do Nascimento, F.; de S. Figueira, M.; Teixeira-Costa, B.E. Determination of Bioactive Compounds Obtained by the Green Extraction of Taioba Leaves (Xanthosoma taioba) on Hydrothermal Processing. Food Sci. Technol. 2022, 42, e22422. [Google Scholar] [CrossRef]
- Costa, A.; Da Silva, E.C.; De Almeida Carlos, L.; Moreira Martins, L.; Mascarenhas Maciel, G.; Nunes de Mendonça, T.F. Cultivation of Taioba in Hydroponic System (Ebb and Flow) Using Different Substrates. Sci. Plena 2020, 16, 060201. [Google Scholar] [CrossRef]
- Wada, E.; Feyissa, T.; Tesfaye, K.; Asfaw, Z.; Potter, D. Genetic Diversity of Ethiopian Cocoyam (Xanthosoma sagittifolium (L.) Schott) Accessions as Revealed by Morphological Traits and SSR Markers. PLoS ONE 2021, 16, e0245120. [Google Scholar] [CrossRef]
- Siqueira, M.V.B.M.; do Nascimento, W.F.; Pedrosa, M.W.; Veasey, E.A. Agronomic Characteristics (Varieties or Landraces) and Potential of Xanthosoma sagittifolium as Food and Starch Source. In Varieties and Landraces; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 261–272. ISBN 9780323900577. [Google Scholar]
- Ukom, A.; Nwanagba, N.; Okereke, D. Effect of Drying Methods on the Chemical Composition and Anti-Nutrtional Properties of a Cocoyam (Xanthosoma Maffafa Schott) Tuber Flour and Leaf Powder. EAS J. Nutr. Food Sci. 2020, 1873, 197–203. [Google Scholar] [CrossRef]
- Hernandez, P.; Rojas, V.; Mata, C. Methodology for Adding Glycemic Index Values to a Venezuelan Food Composition Database. Meas. Food 2022, 7, 100048. [Google Scholar] [CrossRef]
- Barbosa, T.P.; Lins, J.A.S.; Valente, E.C.N.; de Lima, A.S.T. Rescuing Popular Knowledge and Using Unconventional Food Plants as a Possibility of Nutritional Security. Res. Soc. Dev. 2022, 11, e43111628325. [Google Scholar] [CrossRef]
- Budiarti, M.; Maruzy, A.; Mujahid, R.; Sari, A.N.; Jokopriyambodo, W.; Widayat, T.; Wahyono, S. The Use of Antimalarial Plants as Traditional Treatment in Papua Island, Indonesia. Heliyon 2020, 6, e05562. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Butaud, J.F.; Torrente, F.; Conte, E.; Ho, R.; Raharivelomanana, P. Polynesian Medicine Used to Treat Diarrhea and Ciguatera: An Ethnobotanical Survey in Six Islands from French Polynesia. J. Ethnopharmacol. 2022, 292, 115186. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Spiegler, V.; Asase, A.; Scholz, M.; Hempel, G.; Hensel, A. An Ethnopharmacological Survey of Medicinal Plants Traditionally Used for Cancer Treatment in the Ashanti Region, Ghana. J. Ethnopharmacol. 2018, 212, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.L.E.; Souza, D.C.; Resende, L.V.; Nassur, R.d.C.M.R.; Samartini, C.Q.; Gonçalves, W.M. Nutritional Evaluation of Non-Conventional Vegetables in Brazil. An. Acad. Bras. Cienc. 2018, 90, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Moura, H.F.S.; de Souza Dias, F.; Souza e Souza, L.B.; de Magalhães, B.E.A.; de Aragão Tannus, C.; de Carvalho, W.C.; Brandão, G.C.; dos Santos, W.N.L.; Graças Andrade Korn, M.; Cristina Muniz Batista dos Santos, D.; et al. Evaluation of Multielement/Proximate Composition and Bioactive Phenolics Contents of Unconventional Edible Plants from Brazil Using Multivariate Analysis Techniques. Food Chem. 2021, 363, 129995. [Google Scholar] [CrossRef]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Serna, J.; Bergwitz, C. Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients 2020, 12, 3001. [Google Scholar] [CrossRef]
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of Phenolic Compounds, Ascorbic Acid and Vitamin E to Antioxidant Activity of Currant (Ribes L.) and Gooseberry (Ribes uva-crispa L.) Fruits. Food Chem. 2019, 284, 323–333. [Google Scholar] [CrossRef]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of Ascorbic Acid in Chitosan-Based Edible Coating Improves Postharvest Quality and Storability of Strawberry Fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Trifunschi, S.; Zugravu, C.A.; Munteanu, M.F.; Borcan, F.; Pogurschi, E.N. Determination of the Ascorbic Acid Content and the Antioxidant Activity of Different Varieties of Vegetables Consumed in Romania, from Farmers and Supermarkets. Sustainability 2022, 14, 13749. [Google Scholar] [CrossRef]
- de Souza, T.C.L.; da Silveira, T.F.F.; Rodrigues, M.I.; Ruiz, A.L.T.G.; Neves, D.A.; Duarte, M.C.T.; Cunha-Santos, E.C.E.; Kuhnle, G.; Ribeiro, A.B.; Godoy, H.T. A Study of the Bioactive Potential of Seven Neglected and Underutilized Leaves Consumed in Brazil. Food Chem. 2021, 364, 130350. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic Acid: The Chemistry Underlying Its Antioxidant Properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Attia, M.; Essa, E.A.; Zaki, R.M.; Elkordy, A.A. An Overview of the Antioxidant Effects of Ascorbic Acid and Alpha Lipoic Acid (In Liposomal Forms) as Adjuvant in Cancer Treatment. Antioxidants 2020, 9, 359. [Google Scholar] [CrossRef]
- Moskowitz, A.; Huang, D.T.; Hou, P.C.; Gong, J.; Doshi, P.B.; Grossestreuer, A.V.; Andersen, L.W.; Ngo, L.; Sherwin, R.L.; Berg, K.M.; et al. Effect of Ascorbic Acid, Corticosteroids, and Thiamine on Organ Injury in Septic Shock: The ACTS Randomized Clinical Trial. JAMA 2020, 324, 642–650. [Google Scholar] [CrossRef]
- Nam, S.; Seo, M.; Seo, J.; Rhim, H.; Nahm, S.; Cho, I.-H.; Chang, B.-J.; Kim, H.-J.; Choi, S.-H.; Nah, S. Ascorbic Acid Mitigates D-Galactose-Induced Brain Aging by Increasing Hippocampal Neurogenesis and Improving Memory Function. Nutrients 2019, 11, 176. [Google Scholar] [CrossRef]
- Moncayo, S.; Cornejo, X.; Castillo, J.; Valdez, V. Preliminary Phytochemical Screening for Antioxidant Activity and Content of Phenols and Flavonoids of 18 Species of Plants Native to Western Ecuador. Trends Phytochem. Res. 2021, 5, 92–104. [Google Scholar] [CrossRef]
- Mofor Elvis, G.; Boudjeko, T. Different Flavonoid Profiles in Xanthosoma sagittifolium L. Schott Leaves (White and Red CV) During Growth under the Influence of Poultry Manure and NPK Fertilizers Antitumor Activities of Some African Herbal Medicine View Project. Int. J. Sci. Res. Methodol. Hum. 2019, 13, 101–117. [Google Scholar]
- de Medeiros, P.M.; dos Santos, G.M.C.; Barbosa, D.M.; Gomes, L.C.A.; Santos, É.M.C.; da Silva, R.R.V. Local Knowledge as a Tool for Prospecting Wild Food Plants: Experiences in Northeastern Brazil. Sci. Rep. 2021, 11, 594. [Google Scholar] [CrossRef]
- Akonor, P.T.; Tortoe, C.; Buckman, E.S. Evaluation of Cocoyam-Wheat Composite Flour in Pastry Products Based on Proximate Composition, Physicochemical, Functional, and Sensory Properties. J. Culin. Sci. Technol. 2018, 16, 52–65. [Google Scholar] [CrossRef]
- Caxito, M.L.C.; Correia, R.R.; Gomes, A.C.C.; Justo, G.; Coelho, M.G.P.; Sakuragui, C.M.; Kuster, R.M.; Sabino, K.C.C. In Vitro Antileukemic Activity of Xanthosoma sagittifolium (Taioba) Leaf Extract. Evid.-Based Complement. Altern. Med. 2015, 2015, 384267. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Jackix, E.; Monteiro, E.B.; Raposo, H.F.; Vanzela, E.C.; Amaya-Farfán, J. Taioba (Xanthosoma sagittifolium) Leaves: Nutrient Composition and Physiological Effects on Healthy Rats. J. Food Sci. 2013, 78, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, E.; Ferrati, M.; Baldassarri, C.; Cappellacci, L.; Marmugi, M.; Caselli, A.; Benelli, G.; Maggi, F.; Petrelli, R. A Review of the Chemistry and Biological Activities of Acmella oleracea (“Jambù”, Asteraceae), with a View to the Development of Bioinsecticides and Acaricides. Plants 2022, 11, 2721. [Google Scholar] [CrossRef]
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea Plant; Identification, Applications and Use as an Emerging Food Source—Review. Food Rev. Int. 2021, 37, 399–414. [Google Scholar] [CrossRef]
- Paes, A.S.; Koga, R.d.C.R.; Sales, P.F.; Santos Almeida, H.K.; Teixeira, T.A.C.C.; Carvalho, J.C.T. Phytocompounds from Amazonian Plant Species against Acute Kidney Injury: Potential Nephroprotective Effects. Molecules 2023, 28, 6411. [Google Scholar] [CrossRef] [PubMed]
- Anju, T.; Rai, N.K.S.R.; Uthirchamkavu, I.; Sreedharan, S.; Ndhlala, A.R.; Singh, P.; Kumar, A. Analysis of Nutritional and Antioxidant Potential of Three Traditional Leafy Vegetables for Food Security and Human Wellbeing. S. Afr. J. Bot. 2022, 145, 99–110. [Google Scholar] [CrossRef]
- Arumugam, R.; Elanchezhian, B.; Sarikurkcu, C.; Jayakumar, S.; Amirthaganesan, K.; Sudhakar, S. Nutraceutical Assessment of Conventional Leafy Vegetables of South India. S. Afr. J. Bot. 2023, 152, 304–312. [Google Scholar] [CrossRef]
- Rondanelli, M.; Fossari, F.; Vecchio, V.; Braschi, V.; Riva, A.; Allegrini, P.; Petrangolini, G.; Iannello, G.; Faliva, M.A.; Peroni, G.; et al. Acmella oleracea for Pain Management. Fitoterapia 2020, 140, 104419. [Google Scholar] [CrossRef]
- Nascimento, L.E.S.; Arriola, N.D.A.; da Silva, L.A.L.; Faqueti, L.G.; Sandjo, L.P.; de Araújo, C.E.S.; Biavatti, M.W.; Barcelos-Oliveira, J.L.; Dias de Mello Castanho Amboni, R. Phytochemical Profile of Different Anatomical Parts of Jambu (Acmella oleracea (L.) R.K. Jansen): A Comparison between Hydroponic and Conventional Cultivation Using PCA and Cluster Analysis. Food Chem. 2020, 332, 127393. [Google Scholar] [CrossRef]
- Weintraub, L.; Naftzger, T.; Parr, T.; Henning, S.; Soendergaard, M. Antioxidant Activity and Antiproliferative Effects of Acmella alba, Acmella oleracea, and Acmella calirrhiza. FASEB J. 2020, 34, 1-1. [Google Scholar] [CrossRef]
- Rahim, R.A.; Jayusman, P.A.; Muhammad, N.; Mohamed, N.; Lim, V.; Ahmad, N.H.; Mohamad, S.; Hamid, Z.A.A.; Ahmad, F.; Mokhtar, N.; et al. Potential Antioxidant and Anti-Inflammatory Effects of Spilanthes Acmella and Its Health Beneficial Effects: A Review. Int. J. Environ. Res. Public Health 2021, 18, 3532. [Google Scholar] [CrossRef] [PubMed]
- de Souza Moro, S.D.; de Oliveira Fujii, L.; Teodoro, L.F.R.; Frauz, K.; Mazoni, A.F.; Esquisatto, M.A.M.; Rodrigues, R.A.F.; Pimentel, E.R.; de Aro, A.A. Acmella oleracea Extract Increases Collagen Content and Organization in Partially Transected Tendons. Microsc. Res. Tech. 2021, 84, 2588–2597. [Google Scholar] [CrossRef]
- Huang, W.-C.; Huang, C.-H.; Hu, S.; Peng, H.-L.; Wu, S.-J. Topical Spilanthol Inhibits MAPK Signaling and Ameliorates Allergic Inflammation in DNCB-Induced Atopic Dermatitis in Mice. Int. J. Mol. Sci. 2019, 20, 2490. [Google Scholar] [CrossRef] [PubMed]
- Radhika, N.P.; Malini, S.; Raj, K.; Anantharaju, K.S.; Shylaja, K.R.; Appaji, A. Acmella oleracea Induced Nanostructured Ca2Fe2O5 for Evaluation of Photo Catalytic Degradation of Cardiovascular Drugs and Bio Toxicity. Heliyon 2023, 9, e15933. [Google Scholar] [CrossRef]
- Araújo, S.d.S.; Araújo, P.d.S.; Giunco, A.J.; Silva, S.M.; Argandoña, E.J.S. Bromatology, Food Chemistry and Antioxidant Activity of Xanthosoma sagittifolium (L.) Schott. Emirates J. Food Agric. 2019, 31, 188–195. [Google Scholar] [CrossRef]
- de Jesus Benevides, C.M.; da Silva, H.B.M.; Lopes, M.V.; Montes, S.d.S.; da Silva, A.S.L.; Matos, R.A.; de Freitas Santos Júnior, A.; dos Santos Souza, A.C.; de Almeida Bezerra, M. Multivariate Analysis for the Quantitative Characterization of Bioactive Compounds in “Taioba” (Xanthosoma sagittifolium) from Brazil. J. Food Meas. Charact. 2022, 16, 1901–1910. [Google Scholar] [CrossRef]
- Neves, D.A.; Schmiele, M.; Pallone, J.A.L.; Orlando, E.A.; Risso, E.M.; Cunha, E.C.E.; Godoy, H.T. Chemical and Nutritional Characterization of Raw and Hydrothermal Processed Jambu (Acmella oleracea (L.) R.K. Jansen). Food Res. Int. 2019, 116, 1144–1152. [Google Scholar] [CrossRef]
- Brasileiro, B.G.; Barbosa, J.B.; Masrouh Jama, C.; Leão Coelho, O.G.; Ronchi, R.; Ramos Pizziolo, V. Caracterização Anatômica, Composição Química e Atividade Citotóxica de Talinum triangulare (Jacq.) Willd (Portulacaceae). Ciência e Nat. 2016, 38, 665–674. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Nagbhushan, P.; Giridhar, P.; Shetty, N.P.; Yannam, S.K.; Mahadevappa, P. Evaluation of Various Drying Methods on Bioactives, Ascorbic Acid and Antioxidant Potentials of Talinum triangulare L., Foliage. Plant Foods Hum. Nutr. 2020, 75, 283–291. [Google Scholar] [CrossRef]
- Amusat, A.I.; Adedokun, M.A.; Tairu, H.M.; Amuzat, A.I.; Adaramola, K.A.; Olabamiji, S.O. Proximate Analysis, Phyto-Chemical Screening and Mineral Composition of Water Leaves (Talinum triangulare) Harvested in Oyo State College of Agriculture and Technology Igboora. Int. J. Agric. Environ. Sci. 2018, 5, 7–10. [Google Scholar] [CrossRef]
- Bezerra, K.d.O.; Figueiredo, G.d.L.; Mendonça, L.R.; Marques, M.N.; Ferreira, A.C.G.; Aguiar, J.P.L.; Souza, F.d.C.d.A. Efeito Do Tratamento Térmico Nos Compostos Nutricionais e Anti-Nutricionais de Plantas Alimentícias Não Convencionais (PANC). Res. Soc. Dev. 2022, 11, e382111335074. [Google Scholar] [CrossRef]
- Hassanbaglou, B. Antioxidant Activity of Different Extracts from Leaves of Pereskia bleo (Cactaceae). J. Med. Plants Res. 2012, 6, 2932–2937. [Google Scholar] [CrossRef]
- Duarte, R.C.; Taleb-Contini, S.H.; Pereira, P.S.; Oliveira, C.F.; Miranda, C.E.S.; Bertoni, B.W.; Coppede, J.S.; Willrich, G.B.; Crevelin, E.J.; França, S.C.; et al. Effect of Costus spiralis (Jacq.) Roscoe Leaves, Methanolic Extract and Guaijaverin on Blood Glucose and Lipid Levels in a Type II Diabetic Rat Model. Chem. Biodivers. 2019, 16, e1800365. [Google Scholar] [CrossRef] [PubMed]
- Brilhaus, D.; Bräutigam, A.; Mettler-Altmann, T.; Winter, K.; Weber, A.P.M. Reversible Burst of Transcriptional Changes during Induction of Crassulacean Acid Metabolism in Talinum triangulare. Plant Physiol. 2016, 170, 102–122. [Google Scholar] [CrossRef]
- Pavithra, M.; Sridhar, K.R.; Greeshma, A.A. Nutraceutical Profile of the Ceylon Spinach (Talinum triangulare). J. Health Allied Sci. NU 2023, 13, 38–45. [Google Scholar] [CrossRef]
- Okpalanma, F.E.; Ojimelukwe, P.C. Evaluation of Effects of Storage Condition and Processing on Carotenoids, Chlorophyll, Vitamins and Minerals in a Water Leaf (Talinum triangulare). Asian Food Sci. J. 2018, 2, 1–14. [Google Scholar] [CrossRef]
- Airaodion, A.I.; Ogbuagu, E.O.; Ekenjoku, J.A.; Ogbuagu, U.; Airaodion, E.O. Haematopoietic Potential of Ethanolic Leaf Extract of Talinum triangulare in Wistar Rats. Asian J. Res. Biochem. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Airaodion, A.I.; Akinmolayan, J.D.; Ogbuagu, E.O.; Airaodion, E.O.; Ogbuagu, U.; Awosanya, O.O. Effect of Methanolic Extract of Corchorus Olitorius Leaves on Hypoglycemic and Hypolipidaemic Activities in Albino Rats. Asian Plant Res. J. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Oluba, O.M.; Adebiyi, F.D.; Dada, A.A.; Ajayi, A.A.; Adebisi, K.E.; Josiah, S.J.; Odutuga, A.A. Effects of Talinum triangulare Leaf Flavonoid Extract on Streptozotocin-induced Hyperglycemia and Associated Complications in Rats. Food Sci. Nutr. 2019, 7, 385–394. [Google Scholar] [CrossRef]
- Mathala, N.; Rao, V.B.; Vemuri, V.D.; Gowri, G.; Swathi, N.; Sasikala, A. Modulation of Oxidative Stress Induced Cerebral Ischemia in Wistar Rats by Hydroalcoholic Extract of Talinum triangulare. Indian J. Pharm. Educ. Res. 2022, 56, 503–510. [Google Scholar] [CrossRef]
- Airaodion, A.I.; Akinmolayan, J.D.; Ogbuagu, E.O.; Esonu, C.E.; Ogbuagu, U. Preventive and Therapeutic Activities of Methanolic Extract of Talinum triangulare Leaves against Ethanol-Induced Oxidative Stress in Wistar Rats. Int. J. Bio-Sci. Bio-Technol. 2019, 11, 85–96. [Google Scholar]
- Afolabi, O.B.; Olasehinde, O.R.; Owolabi, O.V.; Jaiyesimi, K.F.; Adewumi, F.D.; Idowu, O.T.; Mabayoje, S.O.; Obajuluwa, A.O.; Akpor, O.B. Insight into Antioxidant-like Activity and Computational Exploration of Identified Bioactive Compounds in Talinum triangulare (Jacq.) Aqueous Extract as Potential Cholinesterase Inhibitors. BMC Complement. Med. Ther. 2024, 24, 134. [Google Scholar] [CrossRef]
- Vijayablan, S.; Chigurupati, S.; Alhowail, A.; Das, S. A Retrospective Review of Pereskia bleo (Kunth) DC on Its Properties and Preclinical Insights for Future Drug Discovery Trends. Ann. Rom. Soc. Cell Biol. 2021, 25, 2123–2132. [Google Scholar]
- Johari, M.A.; Khong, H.Y. Total Phenolic Content and Antioxidant and Antibacterial Activities of Pereskia bleo. Adv. Pharmacol. Sci. 2019, 2019, 1–4. [Google Scholar] [CrossRef]
- Mohd-Salleh, S.F.; Ismail, N.; Wan-Ibrahim, W.S.; Tuan Ismail, T.N.N. Phytochemical Screening and Cytotoxic Effects of Crude Extracts of Pereskia bleo Leaves. J. Herbs. Spices Med. Plants 2020, 26, 291–302. [Google Scholar] [CrossRef]
- Rani, A.A.; Mahmud, R.; Amran, N.; Asmawi, M.; Mohamed, N.; Perumal, S. In Vivo Hypoglycemic Investigation, Antihyperglycemic and Antihyperlipidemic Potentials of Pereskia bleo Kunth. in Normal and Streptozotocin-Induced Diabetic Rats. Asian Pac. J. Trop. Biomed. 2019, 9, 73. [Google Scholar] [CrossRef]
- Siew, Y.-Y.; Yew, H.-C.; Neo, S.-Y.; Seow, S.-V.; Lew, S.-M.; Lim, S.-W.; Lim, C.S.E.-S.; Ng, Y.-C.; Seetoh, W.-G.; Ali, A.; et al. Evaluation of Anti-Proliferative Activity of Medicinal Plants Used in Asian Traditional Medicine to Treat Cancer. J. Ethnopharmacol. 2019, 235, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Siska, S.; Hanani, E.; Bariroh, T.; Febrianto, B.; Pratiwi, A.D.A.P.; Yaner, N.N.; Fitri, N.A. Effect of the Ethanol Extract of Pereskia bleo (Kunth) DC. on the Blood Pressure and Electrolyte Levels of Hypertensive Rats. J. Herbmed Pharmacol. 2023, 12, 448–452. [Google Scholar] [CrossRef]
- Zhuang, G.; Wang, Y.; Li, S.; Jiang, X.; Wang, X. Tissue Distribution and Molecular Docking Research on the Active Components of Bidens bipinnata L. against Hyperlipidemia. Biomed. Chromatogr. 2021, 35, e5026. [Google Scholar] [CrossRef]
- Bringel, J.B.A., Jr.; Reis-Silva, G.A.; Barbosa, M. Bidens in Flora e Funga Do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB103746 (accessed on 8 March 2024).
- Hu, H.-M.; Bai, S.-M.; Chen, L.-J.; Hu, W.-Y.; Chen, G. Chemical Constituents from Bidens bipinnata Linn. Biochem. Syst. Ecol. 2018, 79, 44–49. [Google Scholar] [CrossRef]
- Yang, X.; Bai, Z.; Zhang, D.; Zhang, Y.; Cui, H.; Zhou, H. Enrichment of Flavonoid-rich Extract from Bidens bipinnata L. by Macroporous Resin Using Response Surface Methodology, UHPLC–Q-TOF MS/MS-assisted Characterization and Comprehensive Evaluation of Its Bioactivities by Analytical Hierarch. Biomed. Chromatogr. 2020, 34, e4933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Li, S.-J.; Man, Y.-H.; Zhuang, G. Serum Metabonomics Coupled with HPLC-LTQ/Orbitrap MS and Multivariate Data Analysis on the Ameliorative Effects of Bidens bipinnata L. in Hyperlipidemic Rats. J. Ethnopharmacol. 2020, 262, 113196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Si, C.; Hong, W.; Xia, C.; Yang, Y.; He, Y.; Su, M.; Long, X.; Zhang, H. Identification of the Chemical Components of Ethanol Extract of Chenopodium Ambrosioides and Evaluation of Their in Vitro Antioxidant and Anti Tumor Activities. Trop. J. Pharm. Res. 2022, 21, 1689–1697. [Google Scholar] [CrossRef]
- Yang, X.; Bai, Z.-F.; Zhang, Y.; Cui, H.; Zhou, H.-L. Flavonoids-Rich Extract from Bidens bipinnata L. Protects Pancreatic β-Cells against Oxidative Stress-Induced Apoptosis through Intrinsic and Extrinsic Pathways. J. Ethnopharmacol. 2021, 275, 114097. [Google Scholar] [CrossRef]
- Oridupa, O.; Omobowale, T.O.; Oyagbemi, A.A.; Danjuma, N.O.; Obisesan, A.D.; Olakojo, T.A.; Saba, A.B. Antioxidant Activity Enhancement and Oxidative Damage Inhibition by Lagenaria breviflora Fruit and Xanthosoma sagittifolium Corm in Hypertensive Wistar Rats. Niger. J. Physiol. Sci. 2023, 38, 101–106. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; Lima Santos, P.V.; Pinto, L.C.; da Costa, J.S.; Andrade, E.H.d.A.; Setzer, W.N.; da Silva, J.K.d.R.; de Araújo, J.A.C.; Figueiredo, P.L.B. Acmella oleracea (L.) R.K. Jansen Essential Oils: Chemical Composition, Antioxidant, and Cytotoxic Activities. Biochem. Syst. Ecol. 2024, 112, 104775. [Google Scholar] [CrossRef]
- Pinheiro, M.S.d.S.; Moysés, D.A.; Galucio, N.C.R.; Santos, W.O.; Pina, J.R.S.; Oliveira, L.C.; Silva, S.Y.S.; Silva, S.d.C.; Frazão, N.F.; Marinho, P.S.B.; et al. Cytotoxic and Molecular Evaluation of Spilanthol Obtained from Acmella oleracea (L.) R. K. Jansen (Jambu) in Human Gastric Cancer Cells. Nat. Prod. Res. 2024, 38, 1806–1811. [Google Scholar] [CrossRef]
- Dallazen, J.L.; Maria-Ferreira, D.; da Luz, B.B.; Nascimento, A.M.; Cipriani, T.R.; de Souza, L.M.; Felipe, L.P.G.; Silva, B.J.G.; Nassini, R.; de Paula Werner, M.F. Pharmacological Potential of Alkylamides from Acmella oleracea Flowers and Synthetic Isobutylalkyl Amide to Treat Inflammatory Pain. Inflammopharmacology 2020, 28, 175–186. [Google Scholar] [CrossRef]
- Sodjinou, B.D.; Leno, P.F.; Millimono, G.; Akpavi, S.; Tona, K.; Houndonougbo, F.M. Prebiotic Effects of Talinum triangulare and Mangifera indica on Slow Growing Broiler Chickens (SASSO). Heliyon 2024, 10, e25557. [Google Scholar] [CrossRef]
- Oladele, O.T.; Oladele, J.O.; Ajayi, E.I.O.; Alabi, K.E.; Oyeleke, O.M.; Atolagbe, O.S.; Olowookere, B.D.; Bamigboye, M.O. Bioactive Composition and Protective Properties of Talium Triangulare in Dextran Sodium Sulphate-Induced Ulcerative Colitis in Rats. Pharmacol. Res. Mod. Chin. Med. 2024, 10, 100344. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, F.; Yuan, L.; Wang, X.; Wu, F.; Yuan, F.; Cheng, W. Protective Effect of Total Flavonoids from Bidens bipinnata L. against Carbon Tetrachloride-Induced Liver Injury in Mice. J. Pharm. Pharmacol. 2010, 59, 1017–1025. [Google Scholar] [CrossRef]
- Zahara, K.; Bibi, Y.; Masood, S.; Nisa, S.; Qayyum, A.; Ishaque, M.; Shahzad, K.; Ahmed, W.; Shah, Z.H.; Alsamadany, H.; et al. Using HPLC–DAD and GC–MS Analysis Isolation and Identification of Anticandida Compounds from Gui Zhen Cao Herbs (Genus Bidens): An Important Chinese Medicinal Formulation. Molecules 2021, 26, 5820. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.P.; Coppede, J.S.; Bertoni, B.W.; Crotti, A.E.M.; França, S.C.; Pereira, A.M.S.; Taleb-Contini, S.H. Costus spiralis (Jacq.) Roscoe: A Novel Source of Flavones with α-Glycosidase Inhibitory Activity. Chem. Biodivers. 2018, 15, 1–6. [Google Scholar] [CrossRef]
- De Sousa, W.C.; Paz, A.T.S.; Rocha, J.D.; Da Conceição, E.C.; De Almeida, L.M.; Chen, L.C.; Borges, L.L.; Bailão, E.F.L.C. In Vivo Assessment of Cyto/Genotoxic, Antigenotoxic and Antifungal Potential of Costus spiralis (Jacq.) Roscoe Leaves and Stems. Anais da Academia Brasileira de Ciências 2018, 90, 1565–1577. [Google Scholar] [CrossRef]
- Fernandes, J.M. Morfologia de Costus spiralis (Jacq.) Roscoe (Costaceae): Uma Espécie Medicinal Em Alta Floresta, Mato Grosso. Enciclopédia Biosf. 2021, 18, 530–543. [Google Scholar] [CrossRef]
- Rahmawati, N.; Widiyastuti, Y.; Purwanto, R.; Lestari, S.S.; Sene, I.H.A.; Bakari, Y. Medicinal Plants Used by Traditional Healers for the Treatment of Various Diseases in Ondae Sub-Ethnic of Poso District in Indonesia. In Proceedings of the 4th International Symposium on Health Research (ISHR 2019), Bali, Indonesia, 28–30 November 2019; Atlantis Press: Paris, France, 2020. [Google Scholar]
- Neto Galvão, M.; Villas Bôas, G.; Machado, M.; Silva, M.F.; Boscolo, O. Ethnobotany Applied to the Selection of Medicinal Plants for Agroecological Crops in Rural Communities in the Southern End of Bahia, Brazil. Rev. Fitos 2021, 15, 40–57. [Google Scholar] [CrossRef]
- Carmona, F.; Pereira, A.M.S. Prescription Patterns of Herbal Medicines at a Brazilian Living Pharmacy: The Farmácia Da Natureza Experience, 2013–2019. J. Herb. Med. 2022, 36, 100597. [Google Scholar] [CrossRef]
- Amorim, J.M.; Ribeiro de Souza, L.C.; Lemos de Souza, R.A.; da Silva Filha, R.; de Oliveira Silva, J.; de Almeida Araújo, S.; Tagliti, C.A.; Simões e Silva, A.C.; Castilho, R.O. Costus spiralis Extract Restores Kidney Function in Cisplatin-Induced Nephrotoxicity Model: Ethnopharmacological Use, Chemical and Toxicological Investigation. J. Ethnopharmacol. 2022, 299, 115510. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Q.; Liu, T.; Zhao, H.; Wang, R.; Li, H.; Zhang, Y.; Shan, L.; He, B.; Wang, X.; et al. Effect of Vicenin-2 on Ovariectomy-Induced Osteoporosis in Rats. Biomed. Pharmacother. 2020, 129, 110474. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Zhang, M.; Wang, Z.-L.; Qiao, X.; Ye, M. Advances in Plant-Derived C-Glycosides: Phytochemistry, Bioactivities, and Biotechnological Production. Biotechnol. Adv. 2022, 60, 108030. [Google Scholar] [CrossRef] [PubMed]
- de Farias Silva, D.; Simões Bezerra, P.H.; Lopes de Sousa Ribeiro, L.; Viana, M.D.M.; de Lima, A.A.; da Silva Neto, G.J.; Teixeira, C.S.; Machado, S.S.; Alexandre Moreira, M.S.; Delatorre, P.; et al. Costus spiralis (Jacq.) Roscoe Leaves Fractions Have Potential to Reduce Effects of Inflammatory Diseases. J. Ethnopharmacol. 2021, 268, 113607. [Google Scholar] [CrossRef] [PubMed]
- Arruda Filho, E.J.M.; De Muylder, C.F.; Cançado, A.C.; Dholakia, R.R.; Paladino, A. Technology Perspectives and Innovative Scenarios Applied in the Amazon Region. Rev. Adm. Contemp. 2019, 23, 607–618. [Google Scholar] [CrossRef]
- Allen, D. Agttech: How Technology Is Giving Yield to Growth. Available online: https://neuronicworks.com/blog/agritech/ (accessed on 9 August 2023).
- Saguy, I.S.; Silva, C.L.M.; Cohen, E. Author Correction: Emerging Challenges and Opportunities in Innovating Food Science Technology and Engineering Education. NPJ Sci. Food 2024, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Numa, I.A.N.; Wolf, K.E.; Pastore, G.M. FoodTech Startups: Technological Solutions to Achieve SDGs. Food Humanit. 2023, 1, 358–369. [Google Scholar] [CrossRef]
- StartUs Insights. Discover Top 10 Food Technology Trends & Innovations in 2023. 2022. Available online: https://www.startus-insights.com/innovators-guide/top-10-food-technology-trends-innovations-in-2021/ (accessed on 9 August 2023).
- Khaliq, A.; Chughtai, M.F.J.; Mehmood, T.; Ahsan, S.; Liaqat, A.; Nadeem, M.; Sameed, N.; Saeed, K.; Rehman, J.U.; Ali, A. High-Pressure Processing; Principle, Applications, Impact, and Future Prospective. In Sustainable Food Processing and Engineering Challenges; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–108. [Google Scholar]
- Hwang, H.-J.; Yee, S.-Y.; Chung, M.-S. Decontamination of Powdery Foods Using an Intense Pulsed Light (IPL) Device for Practical Application. Appl. Sci. 2021, 11, 1518. [Google Scholar] [CrossRef]
- Eslamipoor, R.; Sepehriar, A. Enhancing Supply Chain Relationships in the Circular Economy: Strategies for a Green Centralized Supply Chain with Deteriorating Products. J. Environ. Manag. 2024, 367, 121738. [Google Scholar] [CrossRef] [PubMed]
- Kakani, V.; Nguyen, V.H.; Kumar, B.P.; Kim, H.; Pasupuleti, V.R. A Critical Review on Computer Vision and Artificial Intelligence in Food Industry. J. Agric. Food Res. 2020, 2, 100033. [Google Scholar] [CrossRef]
- Eslamipoor, R.; Sepehriyar, A. Promoting Green Supply Chain under Carbon Tax, Carbon Cap and Carbon Trading Policies. Bus. Strateg. Environ. 2024, 33, 4901–4912. [Google Scholar] [CrossRef]
- da Silva, R.R.V.; de Medeiros, P.M.; Gomes, D.L. Potentials of Value Chains of Unconventional Food Plants in Brazil. In Local Food Plants; Jacob, M., Albuquerque, U., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 351–360. [Google Scholar]
- de Oliveira, W.Q.; Neri-Numa, I.A.; Arruda, H.S.; Lopes, A.T.; Pelissari, F.M.; Barros, F.F.C.; Pastore, G.M. Special Emphasis on the Therapeutic Potential of Microparticles with Antidiabetic Effect: Trends and Possible Applications. Trends Food Sci. Technol. 2021, 111, 442–462. [Google Scholar] [CrossRef]
- Ferreira Júnior, W.S.; Campos, L.Z.d.O.; de Medeiros, P.M. Unconventional Food Plants: Food or Medicine. In Local Food Plants; Jacob, M., Albuquerque, U., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 29–47. [Google Scholar]
- Blanco-Gutiérrez, I.; Manners, R.; Varela-Ortega, C.; Tarquis, A.M.; Martorano, L.G.; Toledo, M. Examining the Sustainability and Development Challenge in Agricultural-Forest Frontiers of the Amazon Basin through the Eyes of Locals. Nat. Hazards Earth Syst. Sci. 2020, 20, 797–813. [Google Scholar] [CrossRef]
- Smith, D.J.; Helmy, M.; Lindley, N.D.; Selvarajoo, K. The Transformation of Our Food System Using Cellular Agriculture: What Lies Ahead and Who Will Lead It? Trends Food Sci. Technol. 2022, 127, 368–376. [Google Scholar] [CrossRef]
- Tachie, C.; Nwachukwu, I.D.; Aryee, A.N.A. Trends and Innovations in the Formulation of Plant-Based Foods. Food Prod. Process. Nutr. 2023, 5, 16. [Google Scholar] [CrossRef]
- Embrapa. Mais Do Que Matos, Elas São as Plantas Alimentícias Não Convencionais (PANCs). Available online: https://www.embrapa.br/busca-de-noticias/-/noticia/33580014/mais-do-que-matos-elas-sao-as-plantas-alimenticias-nao-convencionais-pancs (accessed on 14 August 2024).
- Inovativa. Rede Inovativa. Available online: https://www.inovativa.online/sobre-o-hub/ (accessed on 14 August 2024).
- Seed Startups and Entrepreneurship Ecosystem Development. Available online: https://seed.mg.gov.br/ (accessed on 14 August 2024).
- Imazon. Cooperativa Mista Dos Povos e Comunidades Tradicionais Da Calha Norte (Coopaflora). Available online: https://imazon.org.br/imprensa/cooperativa-dos-povos-da-calha-norte-do-para-comercializa-35-ton-de-cumaru-com-apoio-do-imazon/ (accessed on 14 August 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, C.T.C.d.S.; Soares, S.D.; de Oliveira, W.Q.; Lima, A.d.S.; Neri Numa, I.A.; Pastore, G.M. Unconventional Edible Plants of the Amazon: Bioactive Compounds, Health Benefits, Challenges, and Future Trends. Foods 2024, 13, 2925. https://doi.org/10.3390/foods13182925
Miranda CTCdS, Soares SD, de Oliveira WQ, Lima AdS, Neri Numa IA, Pastore GM. Unconventional Edible Plants of the Amazon: Bioactive Compounds, Health Benefits, Challenges, and Future Trends. Foods. 2024; 13(18):2925. https://doi.org/10.3390/foods13182925
Chicago/Turabian StyleMiranda, Cynthia Tereza Corrêa da Silva, Stephanie Dias Soares, Williara Queiroz de Oliveira, Adriana de Souza Lima, Iramaia Angélica Neri Numa, and Gláucia Maria Pastore. 2024. "Unconventional Edible Plants of the Amazon: Bioactive Compounds, Health Benefits, Challenges, and Future Trends" Foods 13, no. 18: 2925. https://doi.org/10.3390/foods13182925







