Occurrence, Molecular Serogroups, Antimicrobial Susceptibility and Identification by MALDI-TOF MS of Listeria monocytogenes Isolated from RTE Meat Products in Southern Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Samples
2.2. Multiplex PCR
2.3. Determination of Antimicrobial Susceptibility
2.4. MALDI TOF MS Identification
3. Results
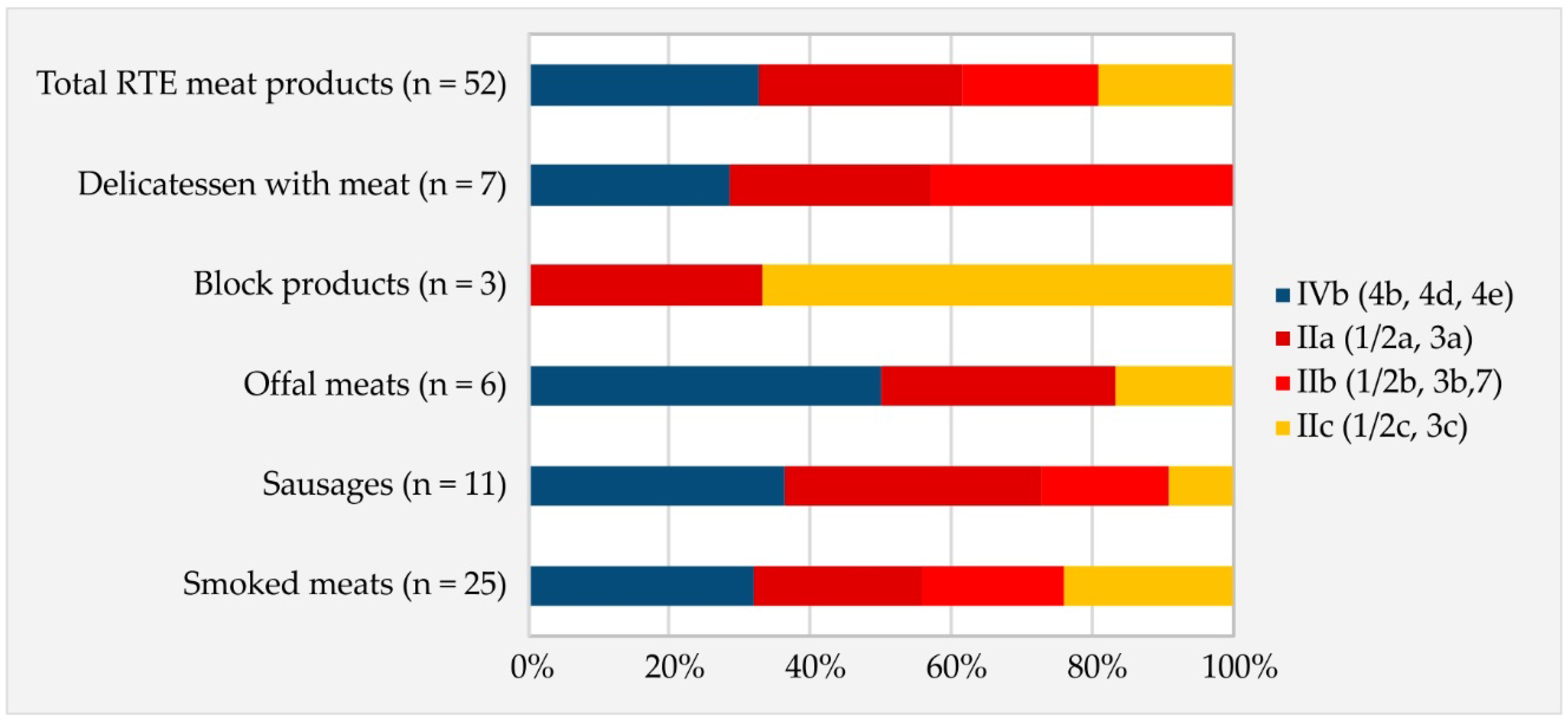
3.1. Occurrence and Serogroups of L. monocytogenes Isolates
3.2. Antimicrobial Susceptibility of L. monocytogenes Isolates
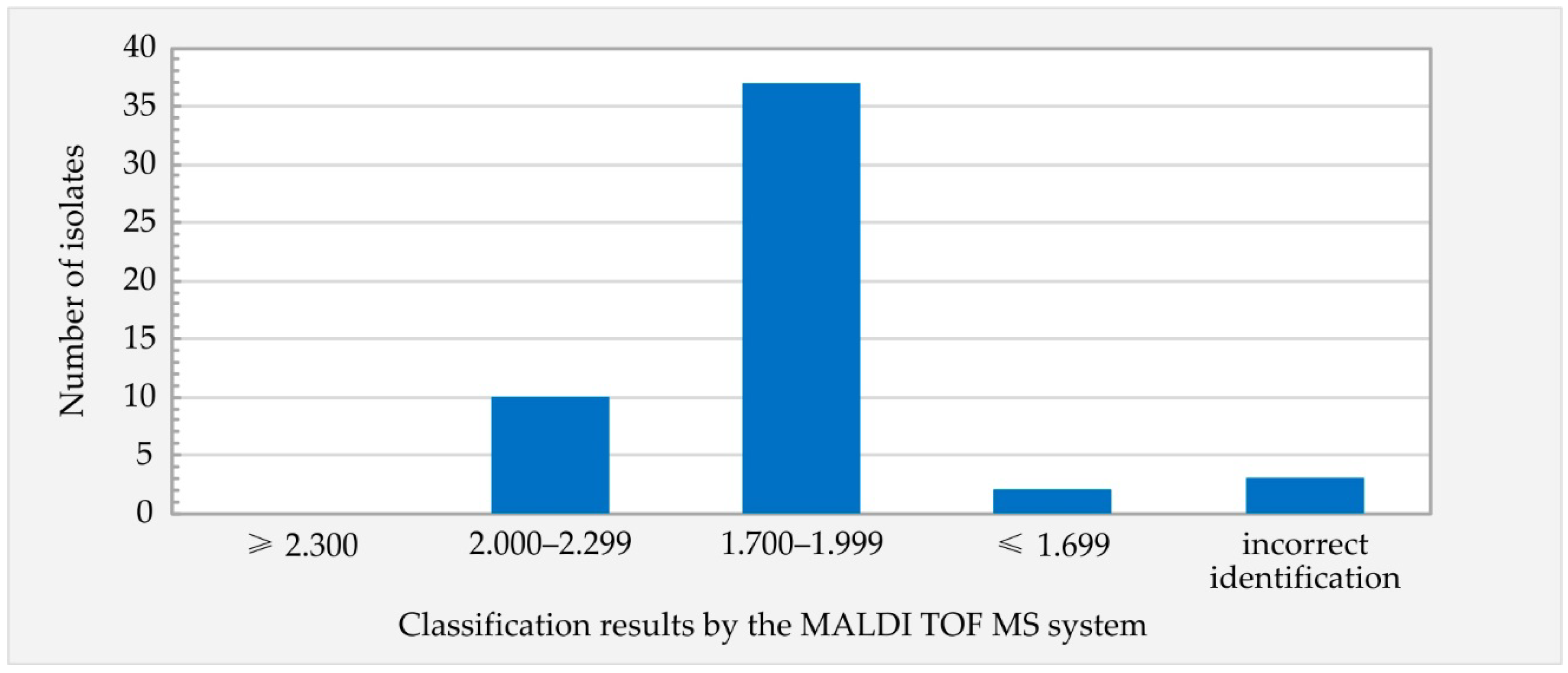
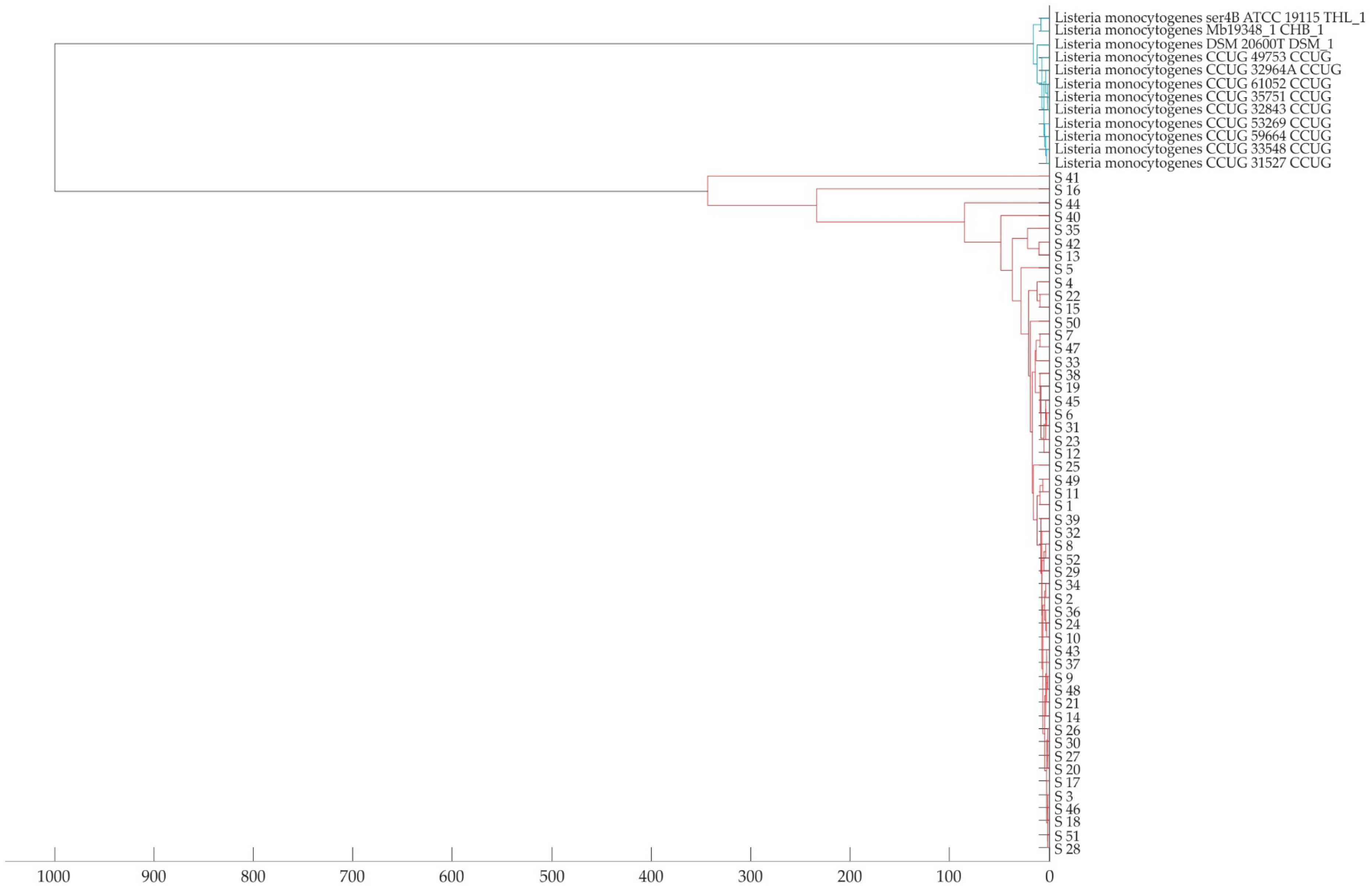
3.3. Identification of L. monocytogenes Isolates Using MALDI-TOF MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Koopmans, M.M.; Brouwer, M.C.; Vázquez-Boland, J.A.; van de Beek, D. Human listeriosis. Clin. Microbiol. Rev. 2023, 36, e0006019. [Google Scholar] [CrossRef] [PubMed]
- Księżak, E.; Sadkowska-Todys, M. Listeriosis in Poland in 2012–2021. Przegl. Epidemiol. 2023, 77, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Mpundu, P.; Mbewe, A.R.; Muma, J.B.; Mwasinga, W.; Mukumbuta, N.; Munyeme, M. A global perspective of antibiotic-resistant Listeria monocytogenes prevalence in assorted ready to eat foods: A systematic review. Vet. World 2021, 14, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Disson, O.; Moura, A.; Lecuit, M. Making sense of the biodiversity and virulence of Listeria monocytogenes. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-Del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and virulence of Listeria monocytogenes: A trip from environmental to medical microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef]
- Kuch, A.; Goc, A.; Belkiewicz, K.; Filipello, V.; Ronkiewicz, P.; Gołębiewska, A.; Wróbel, I.; Kiedrowska, M.; Waśko, I.; Hryniewicz, W.; et al. Molecular diversity and antimicrobial susceptibility of Listeria monocytogenes isolates from invasive infections in Poland (1997–2013). Sci. Rep. 2018, 8, 14562. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Alonso-Hernando, A.; Prieto, M.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control 2012, 23, 37–41. [Google Scholar] [CrossRef]
- Noll, M.; Kleta, S.; Al Dahouk, S. Antibiotic susceptibility of 259 Listeria monocytogenes strains isolated from food, food-processing plants and human samples in Germany. J. Infect. Public Health 2018, 11, 572–577. [Google Scholar] [CrossRef]
- Ntshanka, Z.; Ekundayo, T.C.; du Plessis, E.M.; Korsten, L.; Okoh, A.I. Occurrence and molecular characterization of multidrug-resistant vegetable-borne Listeria monocytogenes isolates. Antibiotics 2022, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Lachtara, B.; Wieczorek, K.; Osek, J. Antimicrobial resistance of Listeria monocytogenes serogroups IIa and IVb from food and food-production environments in Poland. J. Vet. Res. 2023, 67, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Maćkiw, E.; Stasiak, M.; Kowalska, J.; Kucharek, K.; Korsak, D.; Postupolski, J. Occurrence and characteristics of Listeria monocytogenes in ready-to-eat meat products in Poland. J. Food Prot. 2020, 83, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Hou, X.; Xiao, M.; Zhang, L.; Cheng, J.W.; Zhou, M.L.; Huang, J.J.; Zhang, J.J.; Xu, Y.C.; Hsueh, P.R. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) analysis for the identification of pathogenic microorganisms: A review. Microorganisms 2021, 9, 1536. [Google Scholar] [CrossRef]
- Li, D.; Yi, J.; Han, G.; Qiao, L. MALDI-TOF mass spectrometry in clinical analysis and research. ACS Meas. Sci. Au 2022, 27, 385–404. [Google Scholar] [CrossRef]
- PN-EN ISO 11290-1:1999; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes—Part 1: Detection Method. PKN: Warszawa, Poland, 1999.
- PN-EN ISO 11290-1: 2017-07; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. PKN: Warszawa, Poland, 2017.
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST): Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 6 September 2024).
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–32.
- Bolocan, A.S.; Oniciuc, E.A.; Alvarez-Ordóñez, A.; Wagner, M.; Rychli, K.; Jordan, K.; Nicolau, A.I. Putative cross-contamination routes of Listeria monocytogenes in a meat processing facility in Romania. J. Food Prot. 2015, 78, 1664–1674. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, J.; Zhang, J.; Chen, Y.; Zeng, H.; Xue, L.; Lei, T.; Pang, R.; Wu, S.; Wu, H.; et al. Isolation, potential virulence, and population diversity of Listeria monocytogenes from meat and meat products in China. Front. Microbiol. 2019, 10, 946. [Google Scholar] [CrossRef]
- Henriques, A.R.; Cristino, J.M.; Fraqueza, M.J. Genetic characterization of Listeria monocytogenes isolates from industrial and retail ready-to-eat meat-based foods and their relationship with clinical strains from human listeriosis in Portugal. J. Food Prot. 2017, 80, 551–560. [Google Scholar] [CrossRef]
- Lüth, S.; Halbedel, S.; Rosner, B.; Wilking, H.; Holzer, A.; Roedel, A.; Dieckmann, R.; Vincze, S.; Prager, R.; Flieger, A.; et al. Backtracking and forward checking of human listeriosis clusters identified a multiclonal outbreak linked to Listeria monocytogenes in meat products of a single producer. Emerg. Microbes Infect. 2020, 9, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Que, F.; Xu, B.; Sun, L.; Zhu, Y.; Chen, W.; Ye, Y.; Dong, Q.; Liu, H.; Zhang, X. Identification of Listeria monocytogenes contamination in a ready-to-eat meat processing plant in China. Front. Microbiol. 2021, 12, 628204. [Google Scholar] [CrossRef]
- Muhterem-Uyar, M.; Dalmasso, M.; Bolocan, A.S.; Hernandez, M.; Kapetanakou, A.E.; Kuchta, T.; Manios, S.G.; Melero, B.; Minarovičová, J.; Nicolau, A.I.; et al. Environmental sampling for Listeria monocytogenes control in food processing facilities reveals three contamination scenarios. Food Control 2015, 51, 94–107. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernàndez Escàmez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; et al. Scientific opinion on the Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar] [CrossRef]
- Szymczak, B.; Szymczak, M.; Trafiałek, J. Prevalence of Listeria species and L. monocytogenes in ready-to-eat foods in the West Pomeranian region of Poland: Correlations between the contamination level, serogroups, ingredients, and producers. Food Microbiol. 2020, 91, 103532. [Google Scholar] [CrossRef]
- Nemati, V.; Khomeiri, M.; Sadeghi Mahoonak, A.; Moayedi, A. Prevalence and antibiotic susceptibility of Listeria monocytogenes isolated from retail ready-to-eat meat products in Gorgan, Iran. Nutr. Food Sci. Res. 2020, 7, 41–46. [Google Scholar] [CrossRef][Green Version]
- Rippa, A.; Bilei, S.; Peruzy, M.F.; Marrocco, M.G.; Leggeri, P.; Bossù, T.; Murru, N. Antimicrobial resistance of Listeria monocytogenes strains isolated in food and food-processing environments in Italy. Antibiotics 2024, 13, 525. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- Rychert, J. Benefits and limitations of MALDI-TOF mass spectrometry for the identification of microorganisms. J. Infect. Epidemiol. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Farfour, E.; Leto, J.; Barritault, M.; Barberis, C.; Meyer, J.; Dauphin, B.; Le Guern, A.S.; Leflèche, A.; Badell, E.; Guiso, N.; et al. Evaluation of the Andromas matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of aerobically growing Gram-positive bacilli. J. Clin. Microbiol. 2012, 50, 2702–2707. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Szabó, J.; Dombrádi, Z.; Kovács, S.; Pócsi, I. Foodborne Listeria monocytogenes: A real challenge in quality control. Scientifica 2016, 2016, 5768526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Barbuddhe, S.B.; Maier, T.; Schwarz, G.; Kostrzewa, M.; Hof, H.; Domann, E.; Chakraborty, T.; Hain, T. Rapid identification and typing of Listeria species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2008, 74, 5402–5407. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, L.; Butler-Wu, S.M. MALDI-TOF mass spectrometry for microorganism identification. Methods Microbiol. 2015, 42, 37–85. [Google Scholar] [CrossRef]
- Lau, A.F.; Drake, S.K.; Calhoun, L.B.; Henderson, C.M.; Zelazny, A.M. Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 828–834. [Google Scholar] [CrossRef]
| Gene * | Primer Name | Sequence (5′→3′) | Amplicon Size (bp) |
|---|---|---|---|
| lmo0737 | lmo0737F | AGGGCTTCAAGGACTTACCC | 691 |
| lmo0737R | ACGATTTCTGCTTGCCATTC | ||
| lmo1118 | lmo1118F | AGGGGTCTTAAATCCTGGAA | 906 |
| lmo1118R | CGGCTTGTTCGGCATACTTA | ||
| ORF2819 | ORF2819F | AGCAAAATGCCAAAACTCGT | 471 |
| ORF2819R | CATCACTAAAGCCTCCCATTG | ||
| ORF2110 | ORF2110F | AGTGGACAATTGATTGGTGAA | 597 |
| ORF2110R | CATCCATCCCTTACTTTGGAC | ||
| prs | prsR | GCTGAAGAGATTGCGAAAGAAG | 307 |
| prsF | CAAAGAAACCTTGGATTTGCGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyz-Łukasik, R.; Piróg-Komorowska, A.; Policht, A. Occurrence, Molecular Serogroups, Antimicrobial Susceptibility and Identification by MALDI-TOF MS of Listeria monocytogenes Isolated from RTE Meat Products in Southern Poland. Foods 2024, 13, 2950. https://doi.org/10.3390/foods13182950
Pyz-Łukasik R, Piróg-Komorowska A, Policht A. Occurrence, Molecular Serogroups, Antimicrobial Susceptibility and Identification by MALDI-TOF MS of Listeria monocytogenes Isolated from RTE Meat Products in Southern Poland. Foods. 2024; 13(18):2950. https://doi.org/10.3390/foods13182950
Chicago/Turabian StylePyz-Łukasik, Renata, Anna Piróg-Komorowska, and Agata Policht. 2024. "Occurrence, Molecular Serogroups, Antimicrobial Susceptibility and Identification by MALDI-TOF MS of Listeria monocytogenes Isolated from RTE Meat Products in Southern Poland" Foods 13, no. 18: 2950. https://doi.org/10.3390/foods13182950
APA StylePyz-Łukasik, R., Piróg-Komorowska, A., & Policht, A. (2024). Occurrence, Molecular Serogroups, Antimicrobial Susceptibility and Identification by MALDI-TOF MS of Listeria monocytogenes Isolated from RTE Meat Products in Southern Poland. Foods, 13(18), 2950. https://doi.org/10.3390/foods13182950






