Characterization of Peptide Profiles and the Hypoallergenic and High Antioxidant Activity of Whey Protein Hydrolysate Prepared Using Different Hydrolysis Modes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPHs
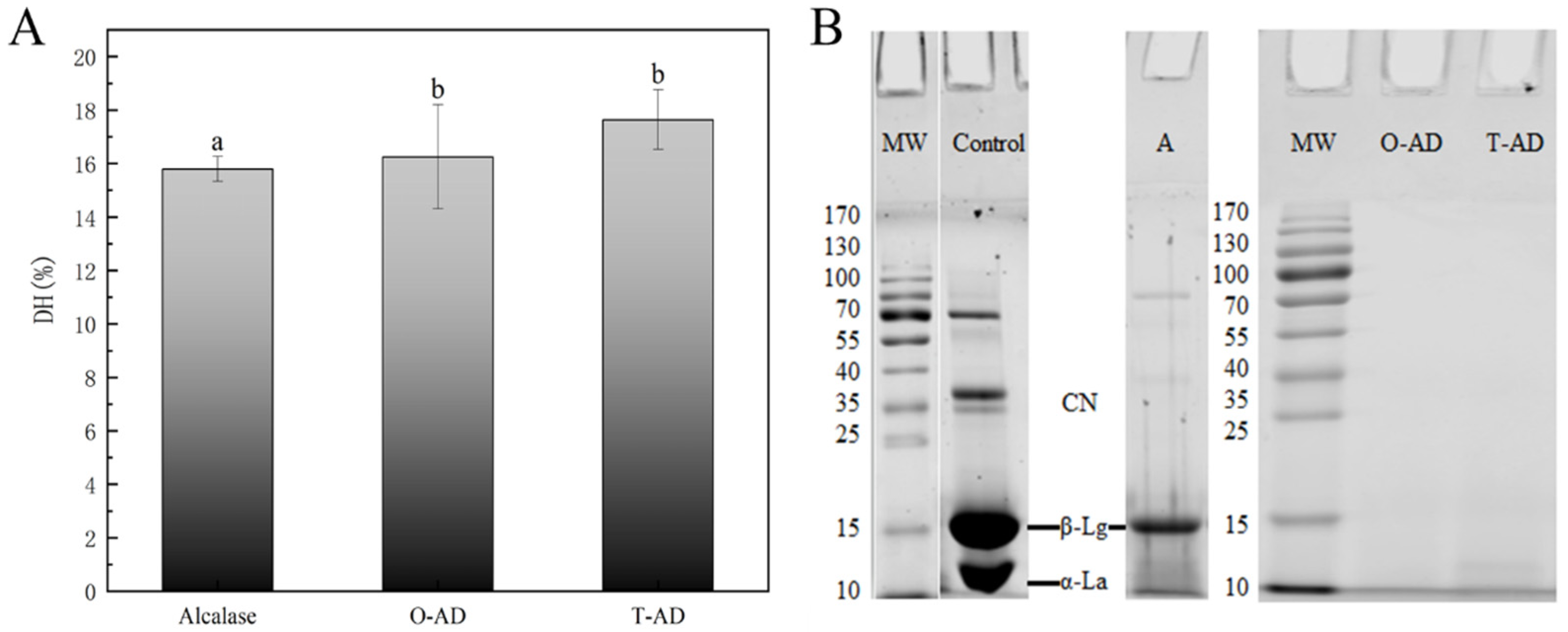
2.3. Degree of Hydrolysis (DH)
2.4. SDS-PAGE
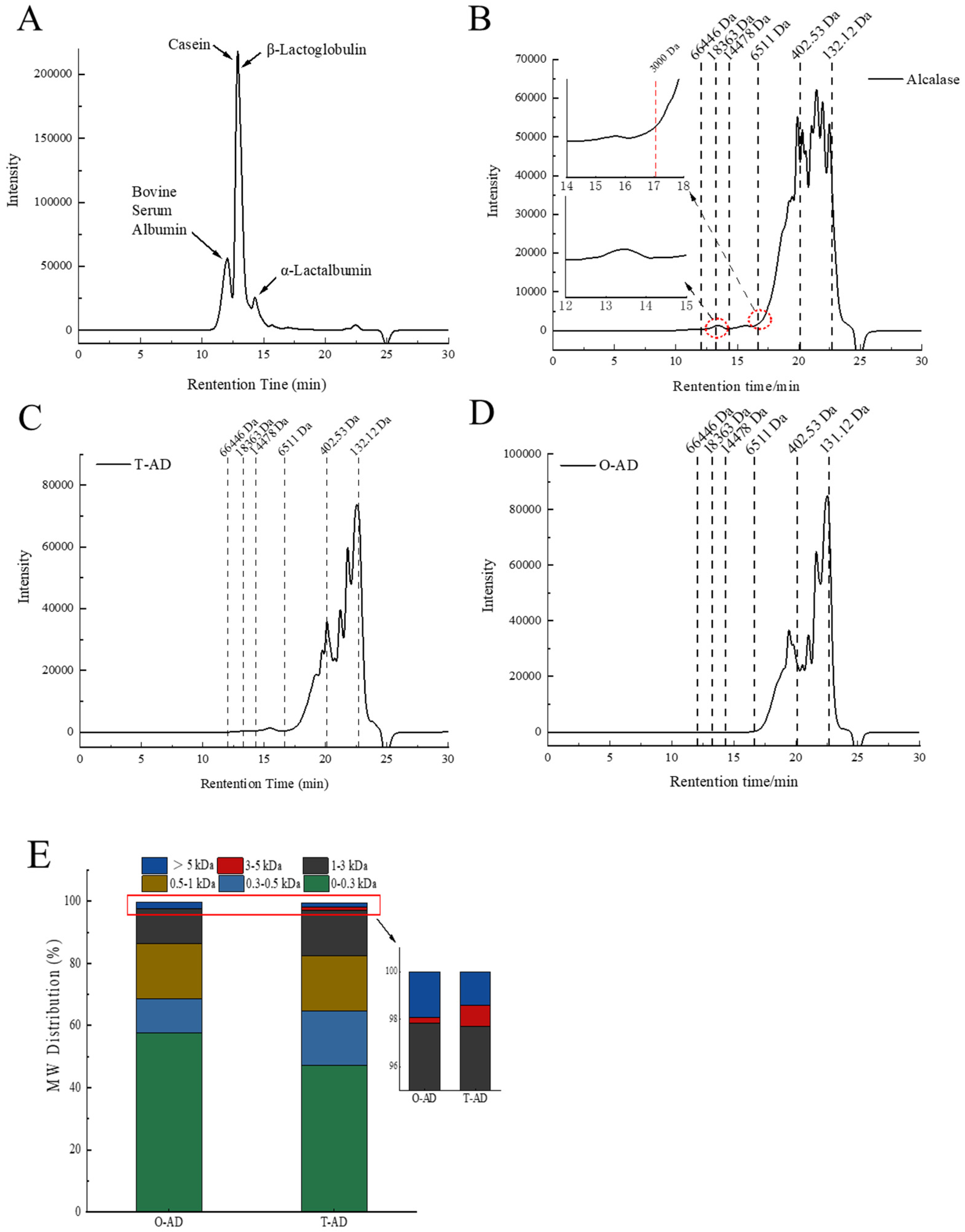
2.5. Molecular Weight Distribution
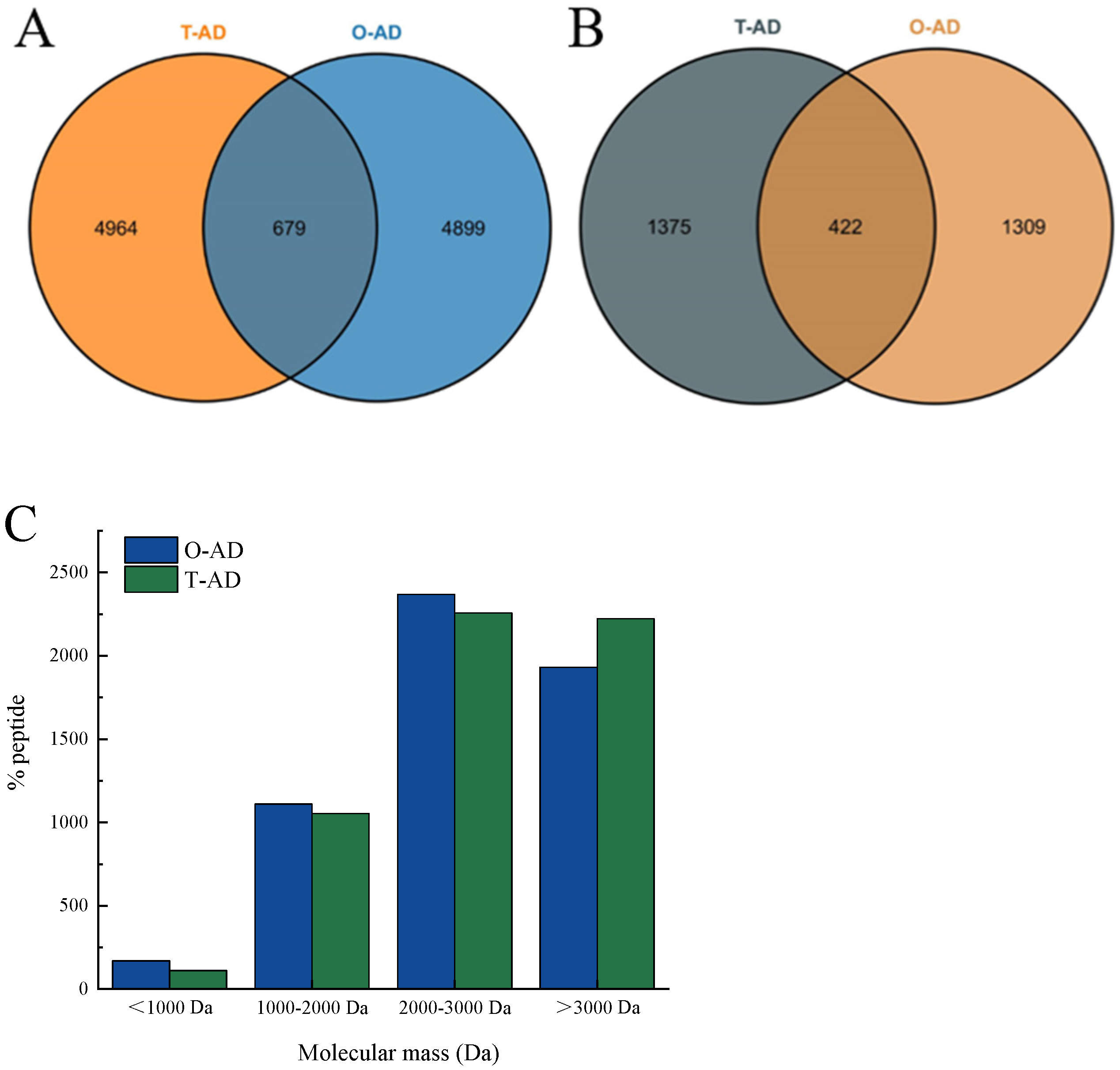
2.6. Peptidomics
2.6.1. EASY-nLC1200 Q Exactive Plus
2.6.2. Peptide Identification
2.7. Determination of Bioactive Properties
2.7.1. DPPH Radical Scavenging
2.7.2. ABTS Radical Scavenging Activity
2.7.3. Determination of In Vitro Antigenicity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Degree of Hydrolysis and SDS-PAGE
3.2. Molecular Weight Distribution
3.3. Peptidomics
3.4. Identification of the Bioactive and Allergen Peptides
3.4.1. Bioactive Peptides
3.4.2. Allergen Peptides
3.5. Functional Properties of WPH Samples
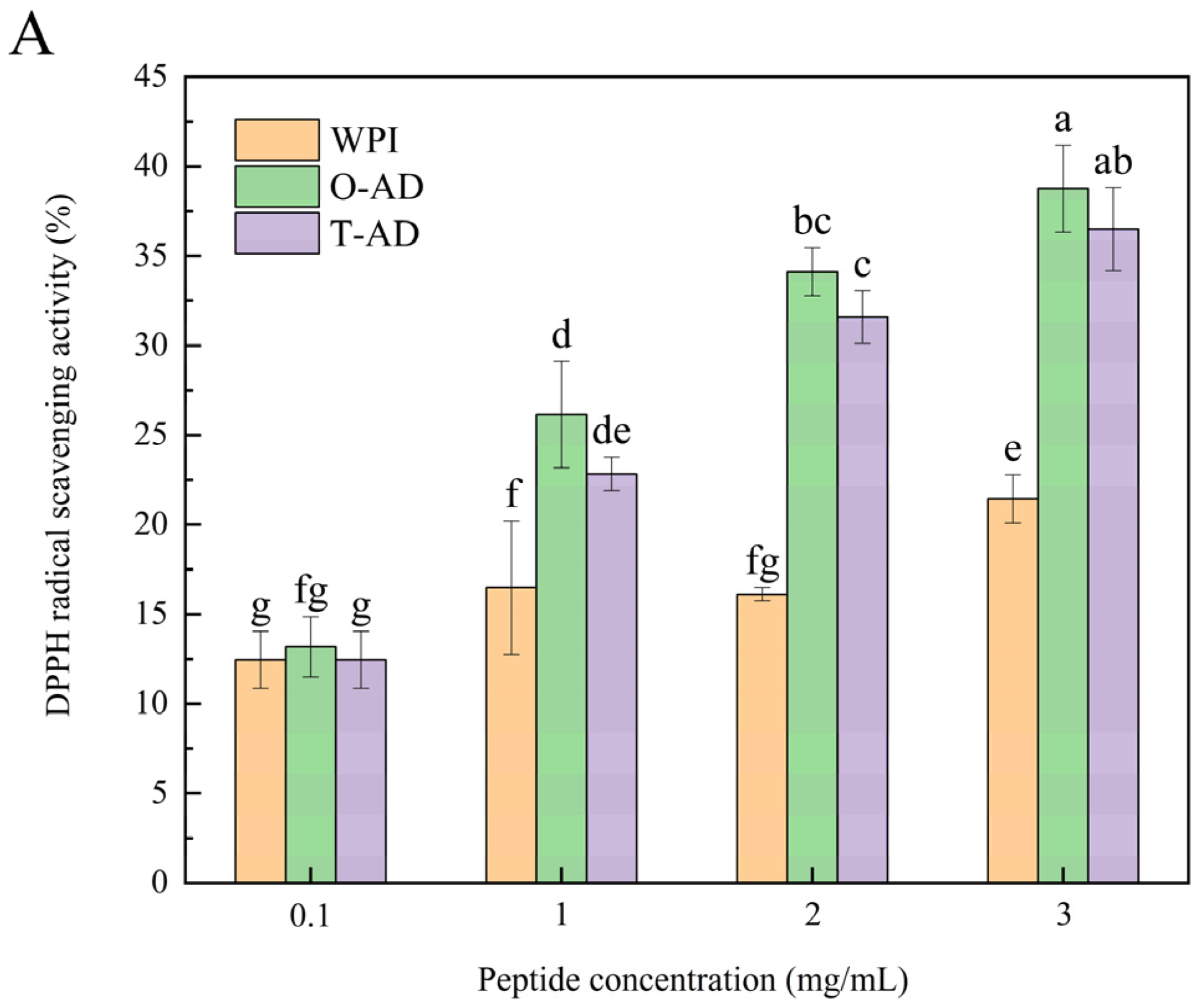
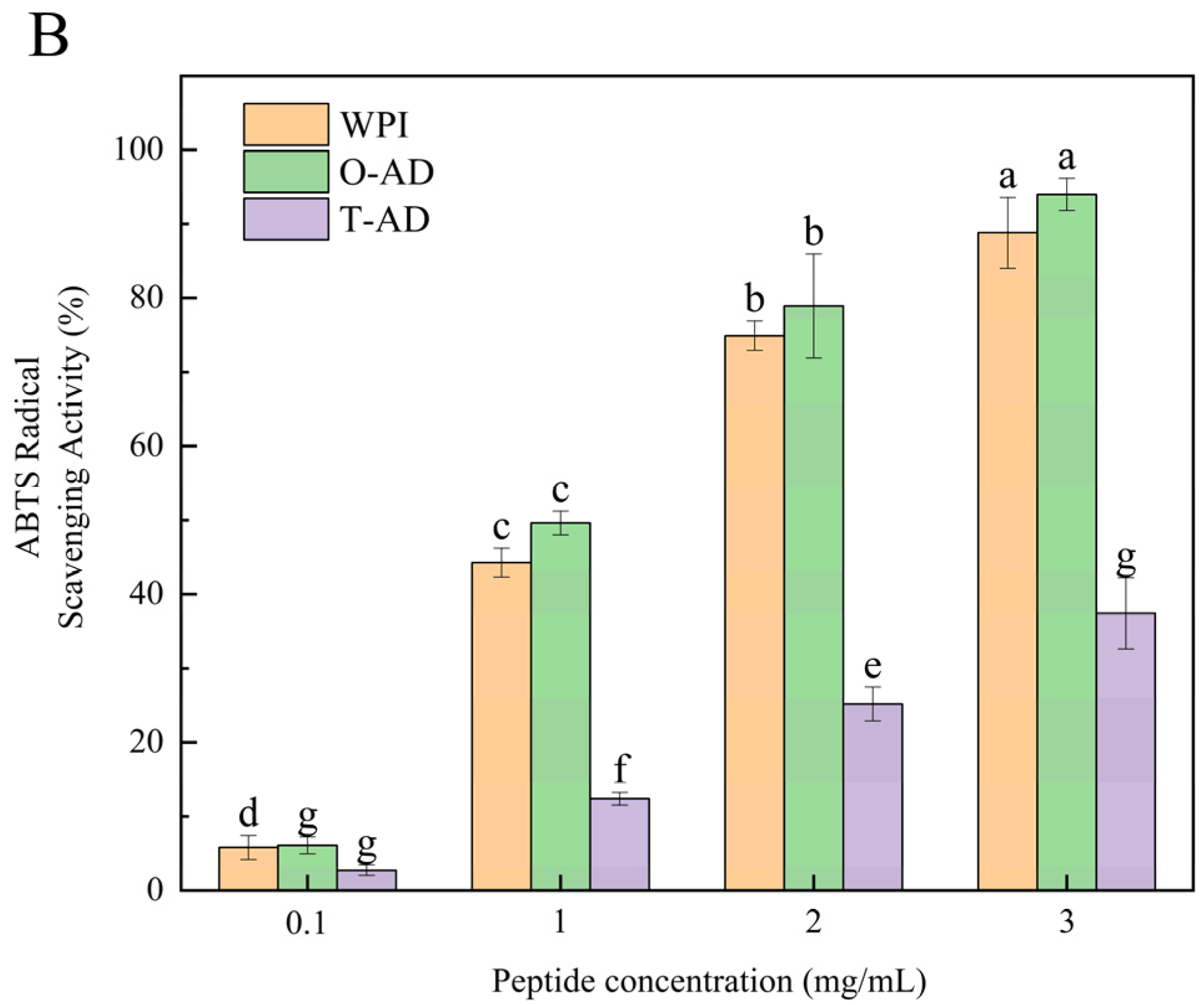
3.5.1. Antioxidant Activity
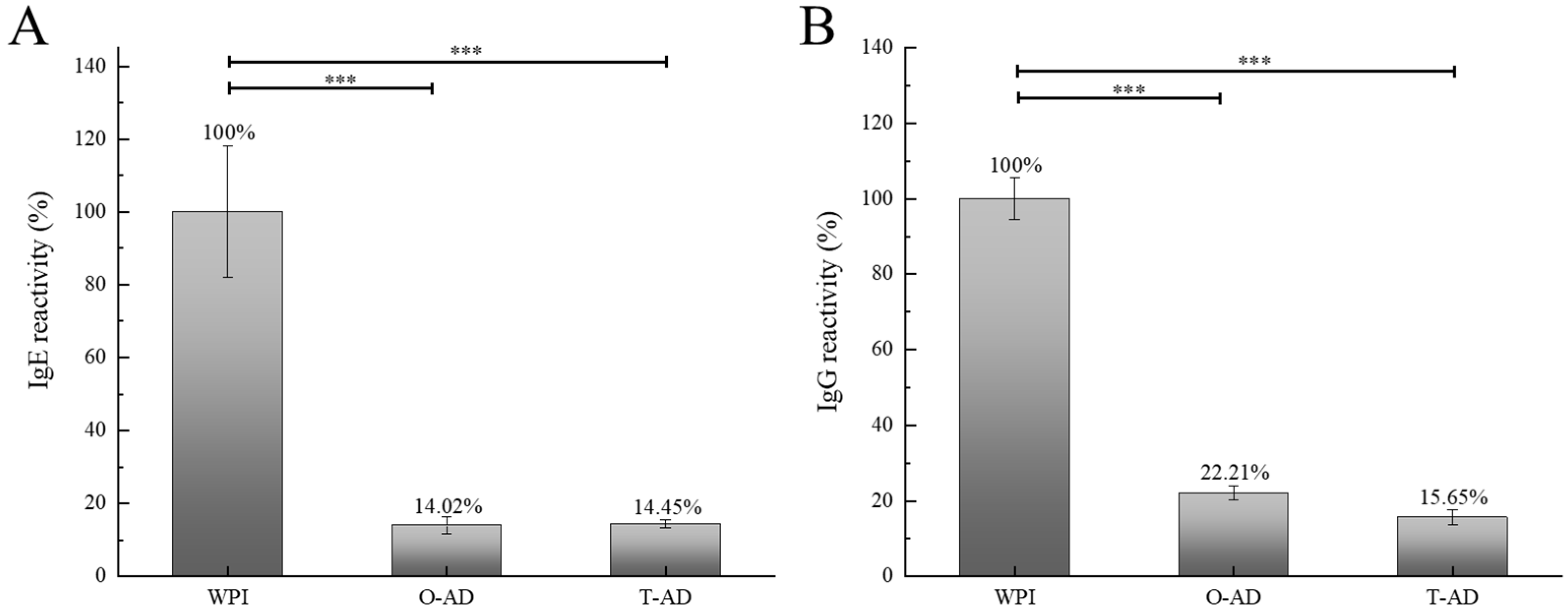
3.5.2. Antigenicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bae, E.K.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Kang, S.; Tian, J.; Li, M.; Liang, X.; Yang, M.; Yue, X. Interaction of xylitol with whey proteins: Multi-spectroscopic techniques and docking studies. Food Chem. 2022, 326, 126804. [Google Scholar] [CrossRef]
- Morris, G.A. The self-assembly and structure of caseins in solution. Biotechnol. Genet. Eng. Rev. 2002, 19, 357–376. [Google Scholar] [CrossRef]
- De Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; Dos Santos, J.G.A.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Davis, J.P.; Doucet, D.; Mcguffey, M.K. Advances in modifying and understanding whey protein functionality. Trends Food Sci. Technol. 2002, 13, 151–159. [Google Scholar] [CrossRef]
- Marcelo, P.A.; Rizvi, S.S.H. Physicochemical properties of liquid virgin whey protein isolate. Int. Dairy J. 2008, 18, 236–246. [Google Scholar] [CrossRef]
- Lamberti, C.; Baro, C.; Giribaldi, M.; Napolitano, L.; Cavallarin, L.; Giuffrida, M.G. Effects of two different domestic boiling practices on the allergenicity of cow’s milk proteins. J. Agric. Food Sci. 2018, 98, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Z.; Li, K.; Li, X.; Yang, A.; Tong, P.; Chen, H. Allergenicity assessment on thermally processed peanut influenced by extraction and assessment methods. Food Chem. 2019, 281, 130–139. [Google Scholar] [CrossRef]
- Yang, F.; Zou, L.; Wu, Y.; Wu, Z.; Yang, A.; Chen, H.; Li, X. Structure and allergenicity assessments of bovine beta-lactoglobulin treated by sonication-assisted irradiation. J. Dairy Sci. 2020, 103, 4109–4120. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Z.Q.; Li, M.L.; Zhou, M.; Sun, X.M. Peptide profiles and allergy-reactivity of extensive hydrolysates of milk protein. Food Chem. 2023, 411, 135544. [Google Scholar] [CrossRef]
- Nutten, S. Proteins, Peptides and Amino Acids: Role in Infant Nutrition. Nestle Nutr. Inst. Workshop 2016, 86, 1. [Google Scholar]
- Metcalfe, D.D.S.H.; Simon, R.A.; Lack, G. Food Allergy—Adverse Reaction to Foods and Food Additives, 5th ed.; Malden WileyBlackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Cui, Q.; Sun, Y.; Cheng, J.; Guo, M. Effect of two-step enzymatic hydrolysis on the antioxidant properties and proteomics of hydrolysates of milk protein concentrate. Food Chem. 2021, 366, 130711. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Duan, Y.Q.; Zhang, M.J.; Liang, S.X.; Sun, Y.X.; Cheng, J.J.; Guo, M.R. Peptide profiles and antioxidant capacity of extensive hydrolysates of milk protein concentrate. J. Dairy Sci. 2022, 105, 7972–7985. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Song, X.; Guo, M. Effects of kefir grains from different origins on proteolysis and volatile profile of goat milk kefir. Food Chem. 2021, 339, 128099. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Guerrero, A.; Parker, E.A.; Robinson, R.C.; Gan, J.; German, J.B.; Lebrilla, C.B. Current peptidomics: Applications, purification, identification, quantification, and functional analysis. Proteomics 2015, 15, 1026–1038. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Q.H.; He, G.Q. Effect of Ultrasonic-ionic liquid pretreatment on the hydrolysis degree and antigenicity of enzymatic hydrolysates from whey protein. Ultrason. Sonochemistry 2019, 63, 104926. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.; Wang, G.; Zhang, A.; Wang, X.; Zhao, X.H. Effect of freeze-thaw treatment on the structure and texture of soy protein-dextran conjugate gels crosslinked by transglutaminase. LWT-Food Sci. Technol. 2022, 153, 112443. [Google Scholar] [CrossRef]
- Liu, P.; Huang, M.; Song, S.; Hayat, K.; Zhang, X.; Xia, S.; Jia, C. Sensory characteristics and antioxidant activities of maillard reaction products from soy protein hydrolysates with different molecular weight distribution. Food Bioprocess Technol. 2010, 5, 1775–1789. [Google Scholar] [CrossRef]
- Silveira Coelho, M.; de Araujo Aquino, S.; Machado Latorres, J.; de las Mercedes Salas-Mellado, M. In vitro and in vivo antioxidant capacity of chia protein hydrolysates and peptides. Food Hydrocoll. 2019, 91, 19–25. [Google Scholar] [CrossRef]
- Yu, X.X.; Liu, C.; Lu, M.H.; Liu, Y.L.; Yin, J.Y.; Zhang, Y.H. Impact of enzymatic hydrolysis followed by transglutaminase-induced cross-linking on decreasing antigenicity and reserving partial interfacial properties of whey protein isolate. Food Funct. 2019, 10, 1653. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Doucet, D.; Otter, D.E.; Gauthier, S.F.; Foegeding, E.A. Enzyme-induced gelation of extensively hydrolyzed whey proteins by Alcalase: Peptide identification and determination of enzyme specificity. J. Agric. Food Chem. 2003, 51, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Kamnerdpetch, C.; Weiss, M.; Kasper, C.; Scheper, T. An improvement of potato pulp protein hydrolyzation process by the combination of protease enzyme systems. Enzym. Microb. Technol. 2007, 40, 508–514. [Google Scholar] [CrossRef]
- Banach, J.C.; Lin, Z.; Lamsal, B.P. Enzymatic modification of milk protein concentrate and characterization of resulting functional properties. LWT-Food Sci. Technol. 2013, 54, 397–403. [Google Scholar] [CrossRef]
- Amorim, F.G.; Coitinho, L.B.; Dias, A.T.; Friques, A.G.F.; Monteiro, B.L.; de Rezende, L.C.D.; Pereira, T.D.M.C.; Campagnaro, B.P.; De Pauw, E.; Vasquez, E.C.; et al. Identification of new bioactive peptides from kefir milk through proteopeptidomics: Bioprospection of antihypertensive molecules. Food Chem. 2019, 282, 109–119. [Google Scholar] [CrossRef]
- Manguy, J.; Jehl, P.; Dillon, E.T.; Davey, N.E.; Shields, D.C.; Holton, T.A. Peptigram: A web-based application for peptidomics data visualization. J. Proteome Res. 2016, 16, 712–719. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A.; Koskinen, P.; Piilola, K.; Tupasela, T.; Korhonen, H. Angiotensin I-converting enzyme inhibitory properties of whey protein digests: Concentration and characterization of active peptides. J. Dairy Res. 2000, 67, 53–64. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martinez-Maqueda, D.; Recio, I.; Hernandez-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in beta-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef]
- Tavares, T.; Contreras Mdel, M.; Amorim, M.; Pintado, M.; Recio, I.; Malcata, F.X. Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: In vitro effect and stability to gastrointestinal enzymes. Peptides 2011, 32, 1013–1019. [Google Scholar] [CrossRef]
- Zheng, L.; Su, G.; Ren, J.; Gu, L.; You, L.; Zhao, M. Isolation and characterization of an oxygen radical absorbance activity peptide from defatted peanut meal hydrolysate and its antioxidant properties. J. Agric. Food Chem. 2012, 60, 5431–5437. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, P.; Betzel, C.; Singh, T.P. Structure and function of proteins involved in milk allergies. J. Chromatogr. B Biomed. Sci. Appl. 2001, 756, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Van, B.; Meijer, R.; Schmidt, D.G. Residual antigenicity of hypoallergenic infant formulas and the occurrence of milk-specific IgE antibodies in patients with clinical allergy. J. Allergy Clin. Immunol. 1995, 96, 365–374. [Google Scholar]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Arise, A.K.; Alashi, A.M.; Nwachukwu, I.D.; Ijabadeniyi, O.A.; Aluko, R.E.; Amonsou, E.O. Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions. Food Funct. 2016, 7, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- Wal, J.M. Bovine milk allergenicity. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2004, 93 (Suppl. S3), S2–S11. [Google Scholar] [CrossRef]
- Huby, R.D.J.; Dearman, R.J.; Ian, K. Why are some proteins allergens? Toxicol. Sci. Off. J. Soc. Toxicol. 2000, 2, 235. [Google Scholar] [CrossRef]
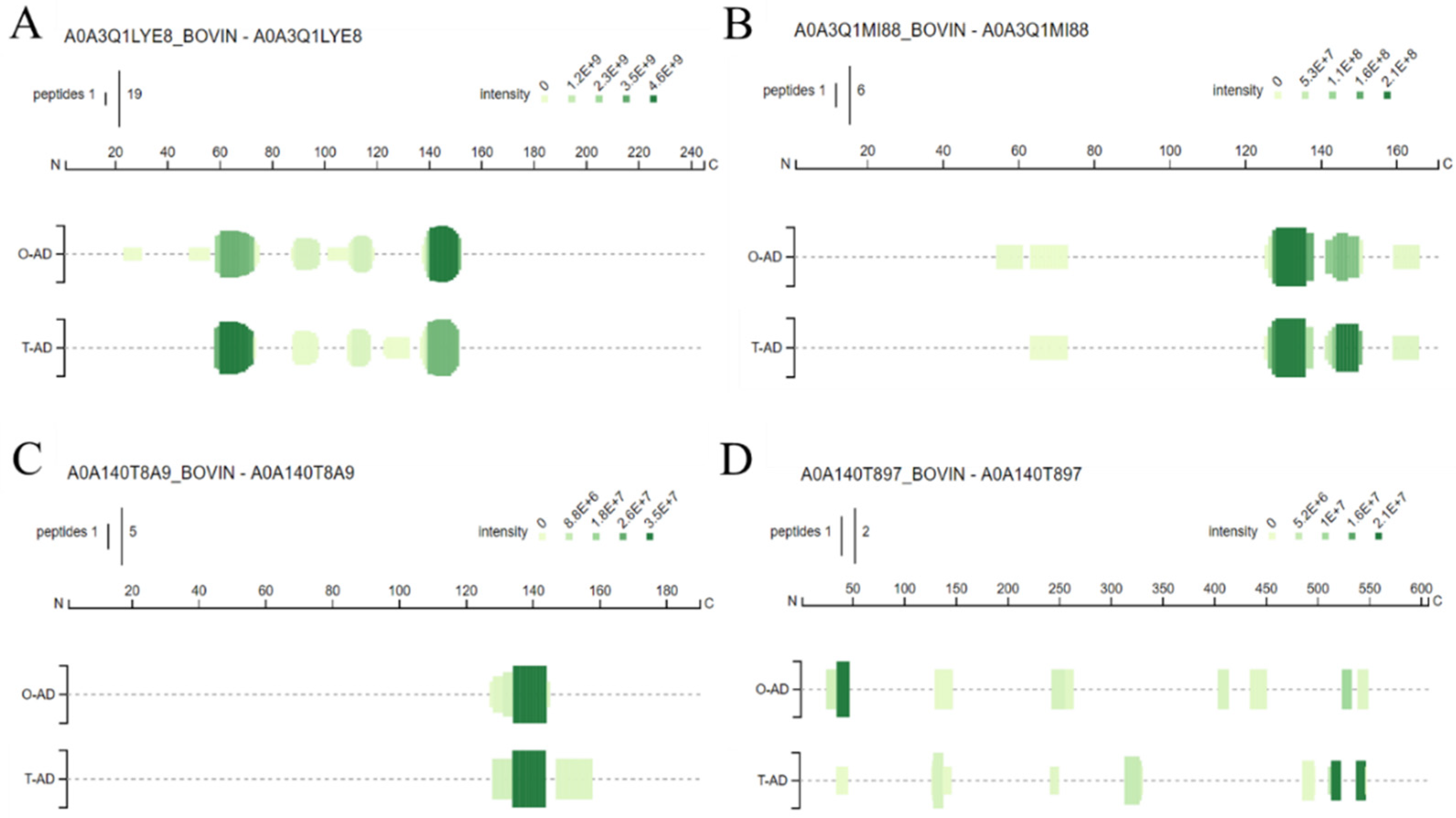
| Protein | Protein Groups | O-AD | T-AE |
|---|---|---|---|
| Shisa family member 6 A0A3Q1M188 | Coverage [%] | 29 | 27 |
| # Peptides | 16 | 14 | |
| # PSMs | 67 | 64 | |
| Score Sequest HT | 158.93 | 149.23 | |
| Albumin A0A140T897 | Coverage [%] | 18 | 15 |
| # Peptides | 12 | 17 | |
| # PSMs | 25 | 43 | |
| Score Sequest HT | 70.25 | 115.88 | |
| κ-casein A0A140T8A9 | Coverage [%] | 9 | 14 |
| # Peptides | 5 | 3 | |
| # PSMs | 16 | 21 | |
| Score Sequest HT | 37.79 | 53.33 | |
| β-casein A0A452DHW7 | Coverage [%] | 15 | 5 |
| # Peptides | 4 | 1 | |
| # PSMs | 6 | 1 | |
| Score Sequest HT | 15.36 | 2.2 | |
| Progestagen-associated endometrial protein A0A3Q1M701 | Coverage [%] | 38 | 32 |
| # Peptides | 59 | 48 | |
| # PSMs | 506 | 378 | |
| Score Sequest HT | 1508.64 | 1086.96 |
| Amino Acid Sequence | O-AD | T-AE | Mass (Da) | Reported Bioactivity | Start–End Position | Protein Accession |
|---|---|---|---|---|---|---|
| VLDTDYK | √ | √ | 852.92 | ACE inhibitor | [94–100] | P02666 |
| TPEVDDEALEK | √ | √ | 1244.56 | DPP IV inhibitor | [125–135) | A0A3Q1LYE8 |
| DKVGINY | √ | √ | 807.39 | ACE inhibitor | [97–103] | A0A3Q1MI88 |
| YPFPGPIPN | √ | 1000.48 | ACE inhibitor | [75–83] | P02666 | |
| LKPTPEGDLEIL | √ | 1323.71 | DPP IV inhibitor | [62–73] | A0A3Q1M701 |
| Amino Acid Sequence | O-AD | T-AE | Allergen | Protein | A * | Start–End Position | Mass (Da) |
|---|---|---|---|---|---|---|---|
| TKLTEEEKNR | √ | √ | Bos d 8 alpha s2 | αS2-casein | 0.8468 | [166–175] | 1247.65 |
| TPEVDDEALEK | √ | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [141–151] | 1245.58 |
| EVDDEALEKF | √ | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [143–152] | 1194.55 |
| LDDDLTDDIM | √ | √ | Bos d 4 | α-lactalbumin | 0.4648 | [129–138] | 1165.49 |
| RTPEVDDEAL | √ | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [140–149] | 1144.54 |
| TPEVDDEALE | √ | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [141–150] | 1117.48 |
| AEKTKIPAVF | √ | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [89–98] | 1103.64 |
| VEELKPTPEG | √ | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [59–68] | 1098.56 |
| TDYKKY | √ | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [113–118] | 817.41 |
| RTPEVDDEALE | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [140–150] | 1273.59 | |
| TPEGDLEILL | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [65–74] | 1099.58 | |
| PTPEGDLEIL | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [64–73] | 1083.55 | |
| ELKPTPEGDL | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [61–70] | 1098.56 | |
| ENSAEPEQSL | √ | Bos d 5 beta-lg | β-lactoglobulin | 1.7022 | [124–133] | 1103.48 | |
| KFLDDDLTDD | √ | Bos d 4 | α-lactalbumin | 0.4648 | [127–136] | 1196.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Q.; Li, Y.; Li, T.; Yu, J.; Shen, G.; Sun, X.; Zhou, M.; Zhang, Z. Characterization of Peptide Profiles and the Hypoallergenic and High Antioxidant Activity of Whey Protein Hydrolysate Prepared Using Different Hydrolysis Modes. Foods 2024, 13, 2978. https://doi.org/10.3390/foods13182978
Cui Q, Li Y, Li T, Yu J, Shen G, Sun X, Zhou M, Zhang Z. Characterization of Peptide Profiles and the Hypoallergenic and High Antioxidant Activity of Whey Protein Hydrolysate Prepared Using Different Hydrolysis Modes. Foods. 2024; 13(18):2978. https://doi.org/10.3390/foods13182978
Chicago/Turabian StyleCui, Qiang, Yuting Li, Tingli Li, Jie Yu, Guanghui Shen, Xiaomeng Sun, Man Zhou, and Zhiqing Zhang. 2024. "Characterization of Peptide Profiles and the Hypoallergenic and High Antioxidant Activity of Whey Protein Hydrolysate Prepared Using Different Hydrolysis Modes" Foods 13, no. 18: 2978. https://doi.org/10.3390/foods13182978
APA StyleCui, Q., Li, Y., Li, T., Yu, J., Shen, G., Sun, X., Zhou, M., & Zhang, Z. (2024). Characterization of Peptide Profiles and the Hypoallergenic and High Antioxidant Activity of Whey Protein Hydrolysate Prepared Using Different Hydrolysis Modes. Foods, 13(18), 2978. https://doi.org/10.3390/foods13182978








