Fundamentals of Edible Coatings and Combination with Biocontrol Agents: A Strategy to Improve Postharvest Fruit Preservation
Abstract
:1. Introduction
2. Fundamentals and Applications of EC
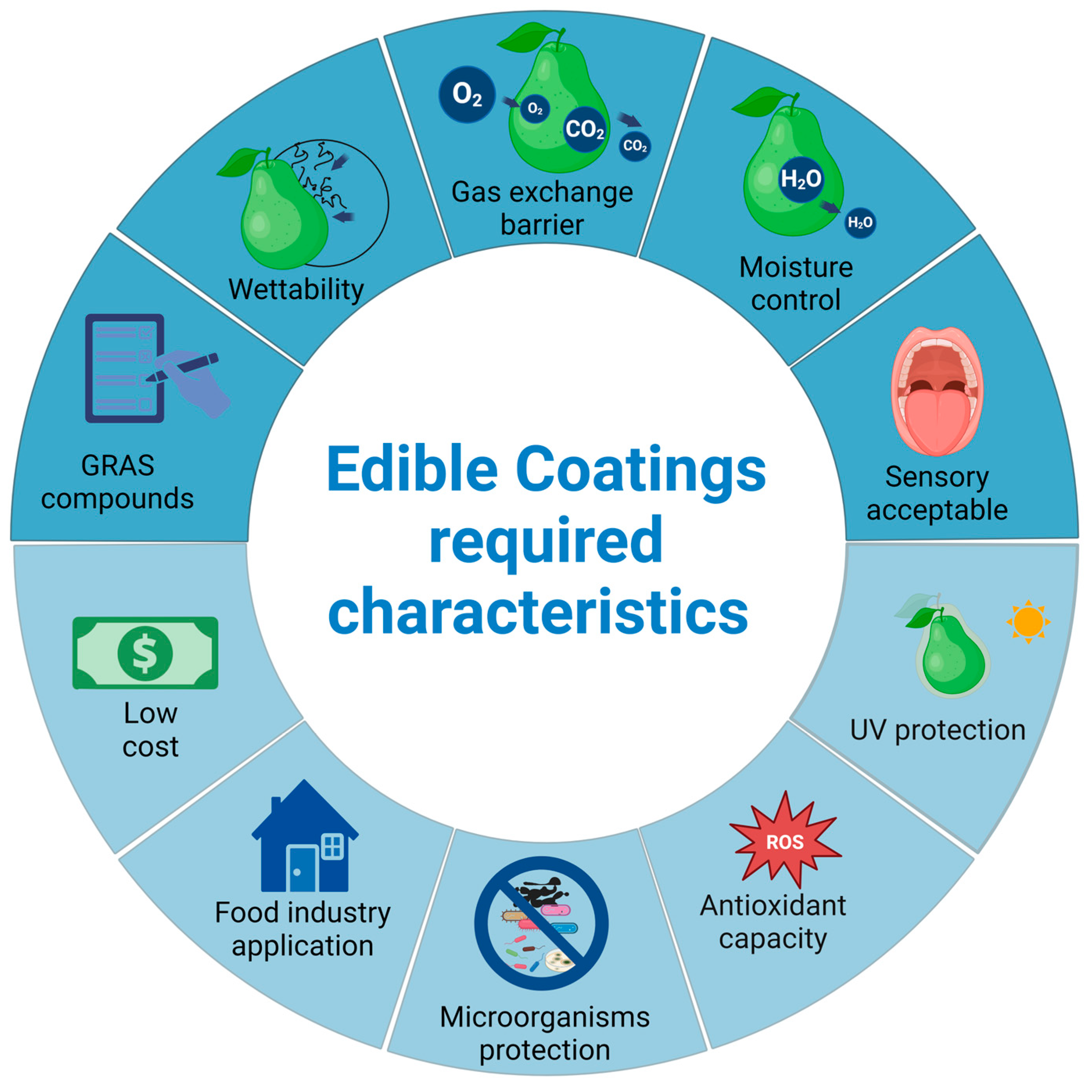
2.1. Matrices and Functionality
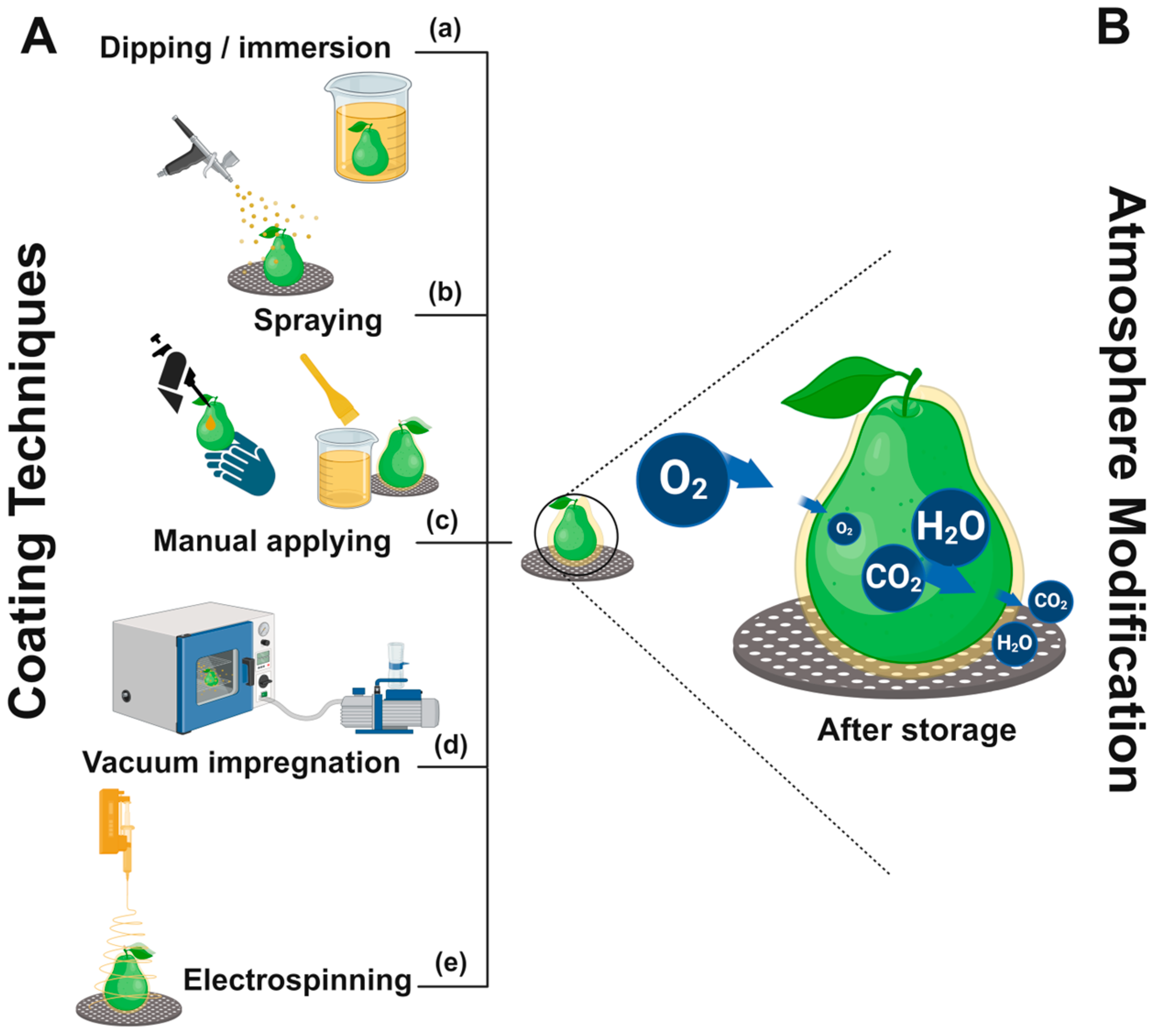
2.2. Application of Edible Coatings on Intact Fruits
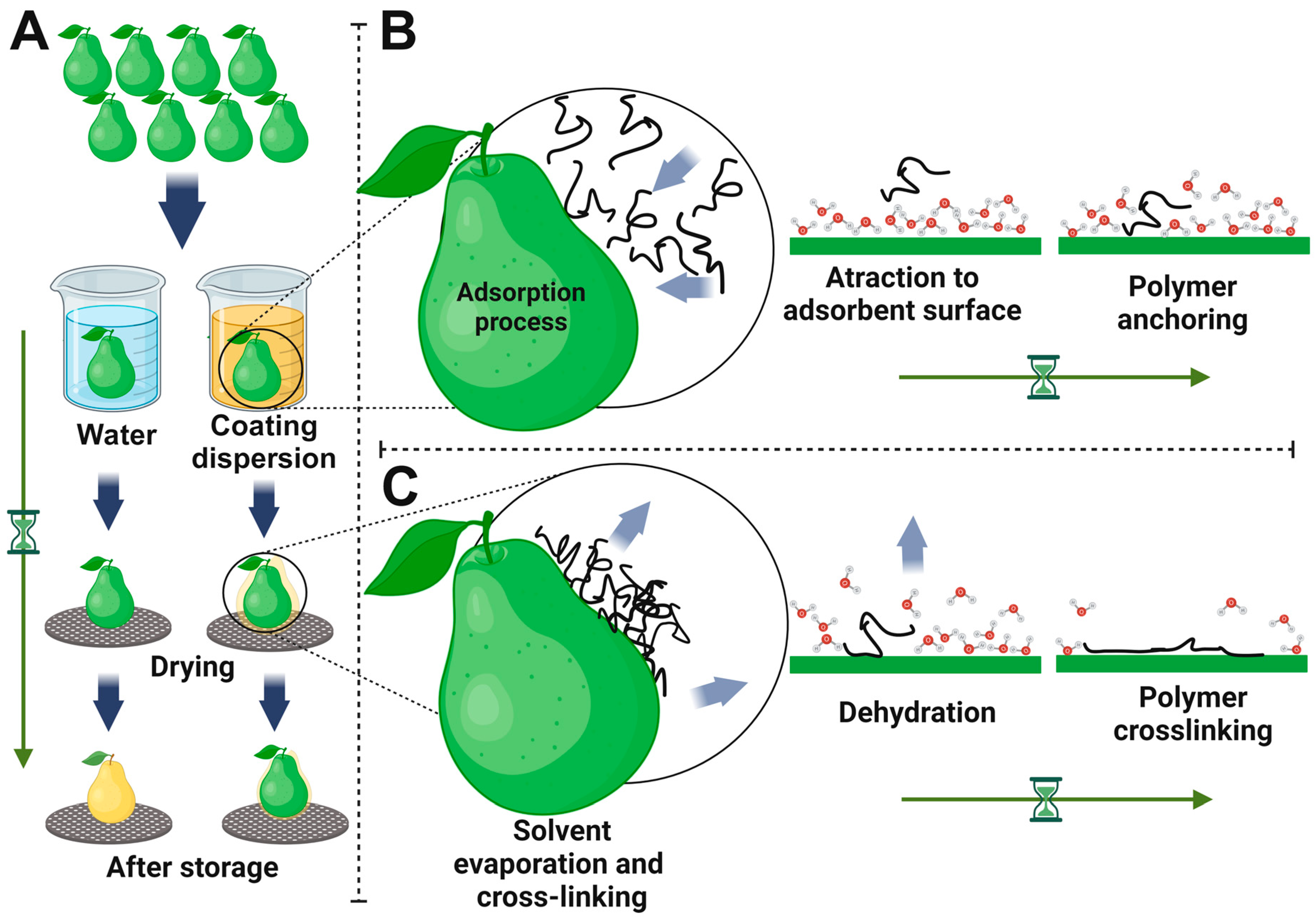
2.3. Coating Deposition and Curing Process
2.4. Coating Characterization
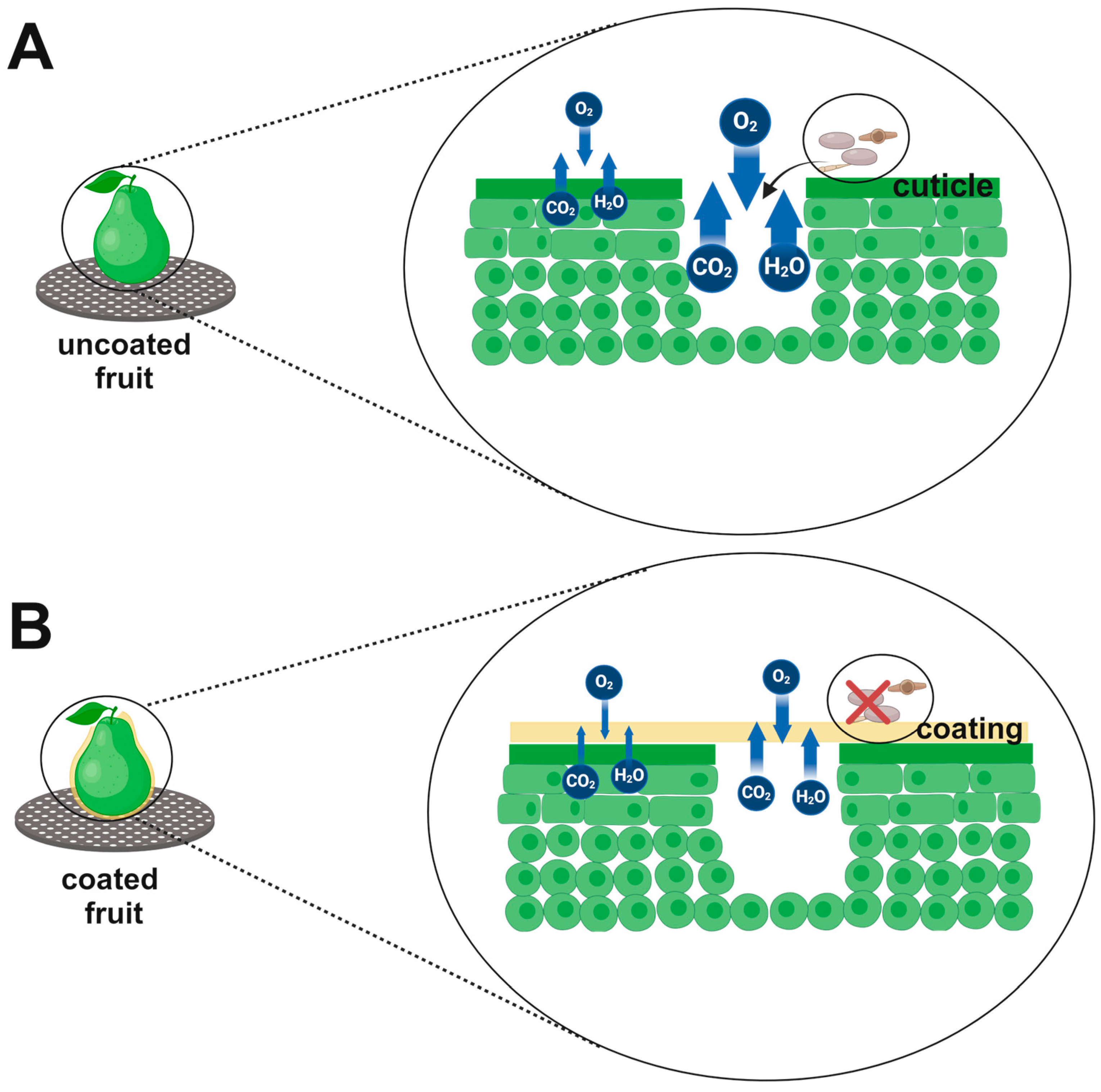
2.5. Mechanisms of Edible Coatings Action
3. Biocontrol Agents for Postharvest Disease Management
3.1. Enhancing Postharvest Disease Management by Biocontrol
3.2. Mode of Action of Biocontrol Agents
4. Combining Edible Coating and Biocontrol Agents
4.1. Relationship between Coating Matrix and Biocontrol Agents
4.2. Synergistic Effects and Impact on Fruit Quality and Postharvest Pathogen Control
5. Trends and Challenges in Edible Coatings Incorporating Biocontrol Agents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; OECD. Background Notes on Sustainable, Productive and Resilient Agro-Food Systems: Value Chains, Human Capital, and the 2030 Agenda; Organisation for Economic Co-Operation and Development: Paris, France, 2019. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the Evolution, Approaches and Perspectives on Alternative Uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.K.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of Agro-Industrial Waste for Sustainable Green Production: A Review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- El-Ramady, H.; Brevik, E.C.; Bayoumi, Y.; Shalaby, T.A.; El-Mahrouk, M.E.; Taha, N.; Elbasiouny, H.; Elbehiry, F.; Amer, M.; Abdalla, N.; et al. An Overview of Agro-Waste Management in Light of the Water-Energy-Waste Nexus. Sustainability 2022, 14, 15717. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste. 2011. Available online: https://www.fao.org/4/mb060e/mb060e00.pdf (accessed on 5 May 2024).
- Farm to Fork Strategy—European Commission. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 5 March 2024).
- Pashova, S. Application of Plant Waxes in Edible Coatings. Coatings 2023, 13, 911. [Google Scholar] [CrossRef]
- Miranda, M.; Sun, X.; Ference, C.; Plotto, A.; Bai, J.; Wood, D.; Assis, O.B.G.; Ferreira, M.D.; Baldwin, E. Nano- and Micro- Carnauba Wax Emulsions versus Shellac Protective Coatings on Postharvest Citrus Quality. J. Am. Soc. Hortic. Sci. 2021, 46, 40–49. [Google Scholar] [CrossRef]
- Hardenburg, R.E.; Hardenburg, R.E. Wax and Related Coatings for Horticultural Products; a Bibliography; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1967. [CrossRef]
- Winston, J.R. Harvesting and Handling Citrus Fruits in the Gulf States; U.S. Government Printing Office: Washington, DC, USA, 1950; p. 67. Available online: https://books.google.es/books/about/Harvesting_and_Handling_Citrus_Fruits_in.html?id=xOcIUEDpcxAC&redir_esc=y (accessed on 6 July 2024).
- Baldwin, E.A.; Hagenmaier, R.; Bai, J.; Krochta, J.M. Edible Coatings and Films to Improve Food Quality; Krochta, J.M., Baldwin, E.A., Nisperos Carriedo, M., Eds.; Edible coatings for fresh fruits and vegetables: Past, present, and future; CRC Press: Boca Raton, FL, USA, 1994; pp. 25–56. ISBN 978-1-56676-113-0. [Google Scholar]
- Krochta, J.M.; Mulder-Johnston, C.D. Edible and Biodegradable Polymer Films: Challenges and Opportunities. Food Technol. 1997, 51, 61–74. Available online: https://ucanr.edu/datastoreFiles/608-247.pdf (accessed on 10 July 2024).
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film Formation and Deposition Methods of Edible Coating on Food Products: A Review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Leon-Zapata, M.A.D.; Alvarez-Perez, O.B.; Torres-León, C.; Nieto-Oropeza, D.E.; Ventura-Sobrevilla, J.M.; Aguilar, M.A.; Ruelas-Chacón, X.; Rojas, R.; Elena, M.; et al. Basic and Applied Concepts of Edible Packaging for Foods. In Food Packaging and Preservation; Academic Press: Cambridge, MA, USA, 2018; pp. 1–61. [Google Scholar] [CrossRef]
- Hromiš, N.M.; Lazić, V.L.; Markov, S.L.; Vaštag, Ž.G.; Popović, S.Z.; Šuput, D.Z.; Džinić, N.R.; Velićanski, A.S.; Popović, L.M. Optimization of Chitosan Biofilm Properties by Addition of Caraway Essential Oil and Beeswax. J. Food Eng. 2015, 158, 86–93. [Google Scholar] [CrossRef]
- Dsouza, F.P.; Dinesh, S.; Sharma, S. Understanding the Intricacies of Microbial Biofilm Formation and Its Endurance in Chronic Infections: A Key to Advancing Biofilm-Targeted Therapeutic Strategies. Arch. Microbiol. 2024, 206, 85. [Google Scholar] [CrossRef]
- Assis, O.B.G.; Britto, D.D. Revisão: Coberturas comestíveis protetoras em frutas: Fundamentos e aplicações. Braz. J. Food Technol. 2014, 17, 87–97. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; González-González, R.B.; Pablo Pizaña-Aranda, J.J.; Parra-Arroyo, L.; Rodríguez-Aguayo, A.A.; Iñiguez-Moreno, M.; González-Meza, G.M.; Araújo, R.G.; Ramírez-Gamboa, D.; Parra-Saldívar, R.; et al. Agricultural Waste as a Sustainable Source for Nanoparticle Synthesis and Their Antimicrobial Properties for Food Preservation. Front. Nanotechnol. 2024, 6, 1346069. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Kumar, A.; Gupta, M.K. Extraction of Nanocellulose from Wheat Straw and its Characterization. Mater. Today Proc. 2023, 78, 48–54. [Google Scholar] [CrossRef]
- Koymeth, S.; S.V, A.K.; Thomas, S.; Parameswaranpillai, J.; C.D, M.D.; Susan George, J.; Reshmi, R.S. Biowaste-Derived Chitosan Nanocomposite Coatings for the Preservation of Banana. Biomass Convers. Biorefinery 2023, 1–13. [Google Scholar] [CrossRef]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; de Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosan Nanoparticle Coatings Reduce Microbial Growth on Fresh-Cut Apples While Not Affecting Quality Attributes. Int. J. Food Sci. Technol. 2015, 50, 440–448. [Google Scholar] [CrossRef]
- Miranda, M. Nano- and Micro-Sized Carnauba Wax Emulsions-Based Coatings Incorporated with Ginger Essential Oil and Hydroxypropyl Methylcellulose on Papaya: Preservation of Quality and Delay of Post-Harvest Fruit Decay. Food Chem. 2022, 13, 100249. [Google Scholar] [CrossRef]
- Filho, J.G.d.O.; Silva, G.d.C.; Oldoni, F.C.A.; Miranda, M.; Florencio, C.; de Oliveira, R.M.D.; Gomes, M.d.P.; Ferreira, M.D. Edible Coating Based on Carnauba Wax Nanoemulsion and Cymbopogon martinii Essential Oil on Papaya Postharvest Preservation. Coatings 2022, 12, 1700. [Google Scholar] [CrossRef]
- Sun, H.; Cao, Y.; Kim, D.; Marelli, B. Biomaterials Technology for AgroFood Resilience. Adv. Funct. Mater. 2022, 32, 2201930. [Google Scholar] [CrossRef]
- Petrônio, M.S.; Barros-Alexandrino, T.T.; Lima, A.M.F.; Assis, O.B.G.; Nagata, A.K.I.; Nakasu, E.Y.T.; Tiera, M.J.; Pilon, L. Physicochemical and Toxicity Investigation of Chitosan- Based dsRNA Nanocarrier Formation. Biointerface Res. Appl. Chem. 2022, 12, 5266–5279. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Miranda, M.; Ferreira, M.D.; Plotto, A. Nanoemulsions as Edible Coatings: A Potential Strategy for Fresh Fruits and Vegetables Preservation. Foods 2021, 10, 2438. [Google Scholar] [CrossRef]
- Marín, A.; Cháfer, M.; Atarés, L.; Chiralt, A.; Torres, R.; Usall, J.; Teixidó, N. Effect of Different Coating-Forming Agents on the Efficacy of the Biocontrol Agent Candida sake CPA-1 for Control of Botrytis cinerea on Grapes. Biol. Control 2016, 96, 108–119. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible Films from Essential-Oil-Loaded Nanoemulsions: Physicochemical Characterization and Antimicrobial Properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple Puree-Alginate Edible Coating as Carrier of Antimicrobial Agents to Prolong Shelf-Life of Fresh-Cut Apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Remolif, G.; Buonsenso, F.; Schiavon, G.; Garello, M.; Spadaro, D. Efficacy of Essential Oil Vapours in Reducing Postharvest Rots and Effect on the Fruit Mycobiome of Nectarines. J. Fungi 2024, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The Science, Development, and Commercialization of Postharvest Biocontrol Products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Usall, J.; Marín, A.; Chiralt, A.; Teixidó, N. Novel Film-Forming Formulations of the Biocontrol Agent Candida sake CPA-1: Biocontrol Efficacy and Performance at Field Conditions in Organic Wine Grapes. Pest Manag. Sci. 2018, 75, 959–968. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Teixidó, N.; Usall, J.; Medina, A.; Magan, N. Impact of Climate Change Environmental Conditions on the Resilience of Different Formulations of the Biocontrol Agent Candida sake CPA-1 on Grapes. Lett. Appl. Microbiol. 2018, 67, 2–8. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of Biocontrol Products for Postharvest Diseases of Fruit: The Importance of Elucidating the Mechanisms of Action of Yeast Antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Oregel-Zamudio, E.; Angoa-Pérez, M.V.; Oyoque-Salcedo, G.; Aguilar-González, C.N.; Mena-Violante, H.G. Effect of Candelilla Wax Edible Coatings Combined with Biocontrol Bacteria on Strawberry Quality during the Shelf-Life. Sci. Hortic. 2017, 214, 273–279. [Google Scholar] [CrossRef]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Lactic Acid Bacteria Incorporated into Edible Coatings to Control Fungal Growth and Maintain Postharvest Quality of Grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef]
- Cañamás, T.P.; Viñas, I.; Torres, R.; Usall, J.; Solsona, C.; Teixidó, N. Field Applications of Improved Formulations of Candida sake CPA-1 for Control of Botrytis cinerea in Grapes. Biol. Control 2011, 56, 150–158. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Elmer, P.A.G. Biological Control of Botrytis Bunch Rot in Organic Wine Grapes with the Yeast Antagonist Candida sake CPA-1. Plant Pathol. 2013, 62, 510–519. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Sandoval-Contreras, T.; Calderón-Santoyo, M. Sodium Alginate Coatings Added with Meyerozyma caribbica: Postharvest Biocontrol of Colletotrichum gloeosporioides in avocado (Persea americana Mill. cv. Hass). Postharvest Biol. Technol. 2020, 163, 111123. [Google Scholar] [CrossRef]
- Bauchot, A.D.; John, P.; Soria, Y.; Recasens, I. Sucrose Ester-Based Coatings Formulated with Food-Compatible Antioxidants in the Prevention of Superficial Scald in Stored Apples. J. Am. Soc. Hortic. Sci. 1995, 120, 491–496. [Google Scholar] [CrossRef]
- Fernández-Cancelo, P. A Hydroxypropyl Methylcellulose (HPMC)-Based Coating Inhibits Ethylene-Dependent Quality Changes and Reduces Superficial Scald Incidence and Blue Mould Severity during Postharvest Handling of Two Apple Varieties. Postharvest Biol. Technol. 2024, 207, 112610. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; González-González, R.B.; Flores-Contreras, E.A.; Araújo, R.G.; Chen, W.N.; Alfaro-Ponce, M.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldívar, R. Nano and Technological Frontiers as a Sustainable Platform for Postharvest Preservation of Berry Fruits. Foods 2023, 12, 3159. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Marilene De Mori, M.R.; Spricigo, P.C.; Pilon, L.; Mitsuyuki, M.C.; Correa, D.S.; Ferreira, M.D. Carnauba Wax Nanoemulsion Applied as an Edible Coating on Fresh Tomato for Postharvest Quality Evaluation. Heliyon 2022, 8, e09803. [Google Scholar] [CrossRef]
- Sapper, M.; Martin-Esparza, M.E.; Chiralt, A.; Gonzalez Martinez, C. Antifungal Polyvinyl Alcohol Coatings Incorporating Carvacrol for the Postharvest Preservation of Golden Delicious Apple. Coatings 2020, 10, 1027. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Bezerra, C.C.D.O.N.; Albiero, B.R.; Oldoni, F.C.A.; Miranda, M.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. New Approach in the Development of Edible Films: The Use of Carnauba Wax Micro- or Nanoemulsions in Arrowroot Starch-Based Films. Food Packag. Shelf Life 2020, 26, 100589. [Google Scholar] [CrossRef]
- Ranjith, F.H.; Adhikari, B.; Muhialdin, B.J.; Yusof, N.L.; Mohammed, N.K.; Ariffin, S.H.; Meor Hussin, A.S. Peptide-Based Edible Coatings to Control Postharvest Fungal Spoilage of Mango (Mangifera indica L.) Fruit. Food Control 2022, 135, 108789. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Goodner, K.; Rousseff, R.; Dou, H. Storage of “Marsh” Grapefruit and “Valencia” Oranges with Different Coatings. Proc. Fla. State Hortic. Soc. 2002, 115, 303–308. Available online: https://journals.flvc.org/fshs/article/download/86482/83398/0 (accessed on 28 July 2024).
- Pedrosa, V.M.; Sanches, A.G.; da Silva, M.B.; Gratão, P.L.; Isaac, V.L.; Gindri, M.; Teixeira, G.H. Production of Mycosporine-like Amino Acid (MAA)-Loaded Emulsions as Chemical Barriers to Control Sunscald in Fruits and Vegetables. J. Sci. Food Agric. 2022, 102, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- BioRender. Figure Adapted from BioRenders Templates “Agricultural Sustainability Variables”. Available online: https://app.biorender.com (accessed on 28 July 2024).
- Assis, O.B.G.; de Britto, D.; Forato, L.A. The Use of Biopolymers as Edible Coatings to Protect and Preserve Fresh and Minimally Processed Fruits. In Research and Development Bulletin; Embrapa Instrumentação Agropecuária: Sao Carlos, Brazil, 2009; Volume 29, 23p, Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPDIA-2010/12624/1/BPD29-2009.pdf (accessed on 20 May 2024).
- Sun, H.; Marelli, B. Growing Silk Fibroin in Advanced Materials for Food Security. MRS Commun. 2021, 11, 31–45. [Google Scholar] [CrossRef]
- Miranda, M.; Gozalbo, A.M.; Sun, X.; Plotto, A.; Bai, J.; de Assis, O.B.G.; Ferreira, M.D.; Baldwin, E. Effect of Mono and Bilayer af Carnauba Wax Based Nano-Emulsion and Hpmc Coatings on Post-Harvest Quality of ‘Redtainung’ Papaya. In Proceedings of the SIAGRO 2019 Simpósio Nacional de Instrumentação Agropecuária, São Carlos, Brazil, 3–5 December 2019; p. 5. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/206747/1/P-EFFECT-OF-MONO-AND-BILAYER-OF-CARNAUBA-WAX-BASED-NANO-EMULSION.pdf (accessed on 20 May 2024).
- Vinod, B.R.; Asrey, R.; Sethi, S.; Menaka, M.; Meena, N.K.; Shivaswamy, G. Recent Advances in Vacuum Impregnation of Fruits and Vegetables Processing: A Concise Review. Heliyon 2024, 10, e28023. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Aliabadi, M.; Ansarifar, E. Zein Multilayer Electrospun Nanofibers Contain Essential Oil: Release Kinetic, Functional Effectiveness, and Application to Fruit Preservation. Foods 2024, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Ayón-Macías, K.D.; Castañeda-Andrade, A.J.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. Application of Nanofibers with Jackfruit Leaf Extract via Electrospinning to Control Phytopathogens in Averrhoa carambola L. Polym. Bull. 2024, 81, 2601–2626. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, L.L.P.; Dam, M.S.; Baranyai, L. Application of Edible Coating in Extension of Fruit Shelf Life: Review. AgriEngineering 2023, 5, 520–536. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.; Ritenour, M.; Hagenmaier, R.; Bai, J. Formulating a Natural Colorant Containing Wax for a One-Step Color-Add Application for Fresh Citrus. HortScience 2017, 52, 408–412. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Schutyser, M.; Schroën, K.; Boom, R. Barrier Properties and Storage Stability of Edible Coatings Prepared with Electrospraying. Innov. Food Sci. Emerg. Technol. 2014, 23, 182–187. [Google Scholar] [CrossRef]
- Shu, C.; Kim-Lee, B.; Sun, X. Chitosan Coating Incorporated with Carvacrol Improves Postharvest Guava (Psidium guajava) Quality. Horticulturae 2024, 10, 80. [Google Scholar] [CrossRef]
- Valero, D.; Zapata, P.J.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Serrano, M. Vacuum Impregnation of Aloe Vera Gel Maintains Postharvest Quality of Peach and Sweet Cherry Fruit. Acta Hortic. 2013, 1012, 399–403. [Google Scholar] [CrossRef]
- Neri, L.; Di Biase, L.; Sacchetti, G.; Di Mattia, C.; Santarelli, V.; Mastrocola, D.; Pittia, P. Use of Vacuum Impregnation for the Production of High Quality Fresh-like Apple Products. J. Food Eng. 2016, 179, 98–108. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of advanced electrospinning techniques: A critical review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional Bio-Nanocomposite Coatings for Perishable Fruits. Adv. Mater. 2020, 32, e1908291. [Google Scholar] [CrossRef]
- Assis, O.B.G.; Britto, D. Coberturas Comestíveis sobre Frutas s Hortaliças: Fundamentos e Prática; Ferreira, M.D., Ed.; Instrumentação Pós-Colheita em Frutas e Hortaliças; Embrapa: Brasília, Brazil, 2017; pp. 37–55. Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/1079109/coberturas-comestiveis-sobre-frutas-e-hortalicas-fundamentos-e-pratica (accessed on 5 May 2024).
- Calbo, A.G.; Moretti, C.L.; Henz, G.P. Respiração de Frutas d Hortaliças. Technical communication. In Embrapa Hortaliças; Embrapa: Brasília, Brazil, 2007; ISSN 1414-9850. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/103079/1/cot-46.pdf (accessed on 4 July 2024).
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Kader, A.A. Regulation of Fruit Physiology by Controlled/Modified Atmospheres. Acta Hortic. 1995, 398, 59–70. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible Films and Coatings to Prevent the Detrimental Effect of Oxygen on Food Quality: Possibilities and Limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Alabbosh, K.F.; Manan, S.; Khan, S.; Ahmad, F.; Ullah, M.W. Chitosan-Based Nanostructured Biomaterials: Synthesis, Properties, and Biomedical Applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 79–99. [Google Scholar] [CrossRef]
- Sapper, M.; Palou, L.; Pérez-Gago, M.B.; Chiralt, A. Antifungal Starch–Gellan Edible Coatings with Thyme Essential Oil for the Postharvest Preservation of Apple and Persimmon. Coatings 2019, 9, 333. [Google Scholar] [CrossRef]
- Martínez-Blay, V.; Pérez-Gago, M.B.; de la Fuente, B.; Carbó, R.; Palou, L. Edible Coatings Formulated with Antifungal GRAS Salts to Control Citrus Anthracnose Caused by Colletotrichum gloeosporioides and Preserve Postharvest Fruit Quality. Coatings 2020, 10, 730. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Pérez-Gago, M.B.; Taberner, V.; Settier-Ramírez, L.; Martínez-Blay, V.; Palou, L. Postharvest Application of Novel Bio-Based Antifungal Composite Edible Coatings to Reduce Sour Rot and Quality Losses of ‘Valencia’ Oranges. Coatings 2023, 13, 1412. [Google Scholar] [CrossRef]
- Almeida, C.L.; Figueiredo, L.R.F.; Ribeiro, D.V.M.; Santos, A.M.C.; Souza, E.L.; Oliveira, K.A.R.; Oliveira, J.E.; Medeiros, E.S. Antifungal Edible Coatings for Fruits Based on Zein and Chitosan Nanowhiskers. J. Food Sci. 2023, 89, 404–418. [Google Scholar] [CrossRef]
- De Oliveira Caretta, T.; Baldo, C.; Silveira, V.A.I.; Hipólito, A.; Costa, N.J.A.; Mali, S.; Celligoi, M.A.P.C. Synthesis of Novel Antimicrobial Bioactive Films for Strawberry Coating Based on Sophorolipids and Fructooligosaccharides-Modified Starch. Polym. Bull. 2024, 81, 3563–3581. [Google Scholar] [CrossRef]
- Martínez-González, M.D.C.; Bautista-Baños, S.; Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Del Río-García, J.C.; Ramos-García, M.D.L. Effect of nanostructured chitosan/propolis coatings on the quality and antioxidant capacity of strawberries during storage. Coatings 2020, 10, 90. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When Is It Biological Control? A Framework of Definitions, Mechanisms, and Classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Droby, S.; Zhimo, V.Y.; Wisniewski, M.; Freilich, S. The Pathobiome Concept Applied to Postharvest Pathology and Its Implication on Biocontrol Strategies. Postharvest Biol. Technol. 2022, 189, 111911. [Google Scholar] [CrossRef]
- Li, X.; Zeng, S.; Wisniewski, M.; Droby, S.; Yu, L.; An, F.; Leng, Y.; Wang, C.; Li, X.; He, M.; et al. Current and Future Trends in the Biocontrol of Postharvest Diseases. Crit. Rev. Food Sci. Nutr. 2024, 64, 5672–5684. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.; Stentiford, G.D.; Wang, H.-C.; Koskella, B.; Tyler, C.R. The Pathobiome in Animal and Plant Diseases. Trends Ecol. Evol. 2019, 34, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Cañamás, T.P.; Viñas, I.; Usall, J.; Casals, C.; Solsona, C.; Teixidó, N. Control of postharvest diseases on citrus fruit by preharvest application of the biocontrol agent Pantoea agglomerans CPA-2: Part I. Study of different formulation strategies to improve survival of cells in unfavourable environmental conditions. Postharvest Biol. Technol. 2008, 49, 86–95. [Google Scholar] [CrossRef]
- Cañamás, T.P.; Viñas, I.; Usall, J.; Torres, R.; Anguera, M.; Teixidó, N. Control of postharvest diseases on citrus fruit by preharvest applications of biocontrol agent Pantoea agglomerans CPA-2: Part II. Effectiveness of different cell formulations. Postharvest Biol. Technol. 2008, 49, 96–106. [Google Scholar] [CrossRef]
- Taha, N.A.; Elsharkawy, M.M.; Shoughy, A.A.; El-Kazzaz, M.K.; Khedr, A.A. Biological Control of Postharvest Tomato Fruit Rots Using Bacillus spp. and Pseudomonas spp. Egypt. J. Biol. Pest Control 2023, 33, 106. [Google Scholar] [CrossRef]
- Casals, C.; Guijarro, B.; Usall, J.; Perdrix, V.; Hilscher, U.; Ladurner, E.; Smets, T.; Teixidó, N. Field Validation of Biocontrol Strategies to Control Brown Rot on Stone Fruit in Several European Countries. Pest Manag. Sci. 2021, 77, 2502–2511. [Google Scholar] [CrossRef]
- Lassois, L.; de Lapeyre de Bellaire, L.; Jijakli, M.H. Biological Control of Crown Rot of Bananas with Pichia anomala Strain K and Candida oleophila Strain O. Biol. Control 2008, 45, 410–418. [Google Scholar] [CrossRef]
- Jijakli, M.H.; Lepoivre, P. Characterization of an Exo-β-1,3-Glucanase Produced by Pichia anomala Strain K, Antagonist of Botrytis cinerea on Apples. Phytopathology 1998, 88, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Nujthet, Y.; Kaewkrajay, C.; Kijjoa, A.; Dethoup, T. Biocontrol Efficacy of Antagonists Trichoderma and Bacillus against Post-Harvest Diseases in Mangos. Eur. J. Plant Pathol. 2024, 168, 315–327. [Google Scholar] [CrossRef]
- Shi, Y. Aureobasidium pullulans S2 Controls Tomato Gray Mold and Produces Volatile Organic Compounds and Biofilms. Postharvest Biol. Technol. 2023, 204, 112450. [Google Scholar] [CrossRef]
- Sezer, B. The Use of Bacteriophage-Based Edible Coatings for the Biocontrol of Salmonella in Strawberries. Food Control. 2022, 135, 108812. [Google Scholar] [CrossRef]
- Casals, C.; Segarra, J.; Torres, R.; Teixidó, N.; De Cal, A.; Usall, J. Validation of a Warning System to Control Brown Rot in Peach and Nectarine. Agronomy 2023, 13, 254. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of Microbial Antagonists for the Control of Postharvest Diseases of Fruits: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Vola, R.; Piano, S.; Gullino, M.L. Mechanisms of Action and Efficacy of Four Isolates of the Yeast Metschnikowia pulcherrima Active against Postharvest Pathogens on Apples. Postharvest Biol. Technol. 2002, 24, 123–134. [Google Scholar] [CrossRef]
- Droby, S.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.E.; Porat, R. Induction of Resistance to Penicillium digitatum in Grapefruit by the Yeast Biocontrol Agent Candida oleophila. Phytopathology 2002, 92, 393–399. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, C.; Zhang, T.; Spadaro, D.; Liu, D.; Liu, W. Candida pruni sp. nov. Is a New Yeast Species with Antagonistic Potential against Brown Rot of Peaches. Arch. Microbiol. 2014, 196, 525–530. [Google Scholar] [CrossRef]
- López-Cruz, R.; Segarra, G.; Torres, R.; Teixidó, N.; Ragazzo-Sanchez, J.A.; Calderon-Santoyo, M. Biocontrol Efficacy of Meyerozyma guilliermondii LMA-Cp01 against Post-Harvest Pathogens of Fruits. Arch. Phytopathol. Plant Prot. 2023, 56, 1003–1020. [Google Scholar] [CrossRef]
- Khadiri, M.; Boubaker, H.; Lahmamsi, H.; Taoussi, M.; Ezzouggari, R.; Askarne, L.; Farhaoui, A.; Barka, E.A.; Lahlali, R. Challenges in Apple Preservation: Fungicide Resistance and Emerging Biocontrols. Physiol. Mol. Plant Pathol. 2024, 127, 102205. [Google Scholar] [CrossRef]
- de O Caretta, T.; I Silveira, V.A.; Andrade, G.; Macedo, F., Jr. Antimicrobial Activity of Sophorolipids Produced by Starmerella bombicola against Phytopathogens from Cherry Tomato. J. Sci. Food Agric. 2021, 102, 1245–1254. [Google Scholar] [CrossRef]
- Ezzouggari, R.; Bahhou, J.; Taoussi, M.; Kallali, N.S.; Aberkani, K.; Barka, E.A.; Lahlali, R. Yeast Warriors: Exploring the Potential of Yeasts for Sustainable Citrus Post-Harvest Disease Management. Agronomy 2024, 14, 288. [Google Scholar] [CrossRef]
- Weller, D.M. Pseudomonas Biocontrol Agents of Soilborne Pathogens: Looking Back Over 30 Years. Phytopathology 2007, 97, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Biles, C.; Droby, S.; McLaughlin, R.; Wilson, C.; Chalutz, E. Mode of Action of the Postharvest Biocontrol Yeast, Pichia guilliermondii. I. Characterization of Attachment to Botrytis cinerea. Physiol. Mol. Plant Pathol. 1991, 39, 245–258. [Google Scholar] [CrossRef]
- Lieu, M.D.; Phuong, T.V.; Nguyen, T.T.B.; Dang, T.K.T.; Nguyen, T.H. A Review of Preservation Approaches for Extending Avocado Fruit Shelf-Life. J. Agric. Food Res. 2024, 16, 101102. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Solís-Pacheco, J.R.; Calderón-Santoyo, M. Characterization of Sodium Alginate Coatings with Meyerozyma caribbica and Impact on Quality Properties of Avocado Fruit. LWT 2021, 152, 112346. [Google Scholar] [CrossRef]
- Vonasek, E.L.; Choi, A.H.; Sanchez, J., Jr.; Nitin, N. Incorporating Phage Therapy into WPI Dip Coatings for Applications on Fresh Whole and Cut Fruit and Vegetable Surfaces. Food Eng. 2018, 83, 1871–1879. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Bambace, M.F.; Quintana, G.; Gomez-Zavaglia, A.; del Rosario Moreira, M. Prebiotic-Alginate Edible Coating on Fresh-Cut Apple as a New Carrier for Probiotic Lactobacilli and Bifidobacteria. LWT 2021, 137, 110483. [Google Scholar] [CrossRef]
- Qualified Presumption of Safety (QPS) | European Food Safety Authority (EFSA). Available online: https://www.efsa.europa.eu/en/applications/qps-assessment (accessed on 22 August 2024).
- Strano, M.C.; Restuccia, C.; De Leo, R.; Mangiameli, S.; Bedin, E.; Allegra, M.; Quartieri, A.; Cirvilleri, G.; Pulvirenti, A. Efficacy of an Antifungal Edible Coating for the Quality Maintenance of Tarocco Orange Fruit during Cold Storage. Crop. Prot. 2021, 148, 105719. [Google Scholar] [CrossRef]
- Panel, E.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Updated List of QPS-Recommended Microorganisms for Safety Risk Assessments Carried out by European Food Safety Authority (EFSA). 2024. Available online: https://zenodo.org/records/10534041 (accessed on 3 July 2024).
- McGuire, R.G.; Dimitroglou, D.A. Evaluation of Shellac and Sucrose Ester Fruit Coating Formulations That Support Biological Control of Post-Harvest Grapefruit Decay. Biocontrol Sci. Technol. 1999, 9, 53–65. [Google Scholar] [CrossRef]
- Zibaei-Rad, A.; Rahmati-Joneidabad, M.; Alizadeh Behbahani, B.; Taki, M. Probiotic-Loaded Seed Mucilage-Based Edible Coatings for Fresh Pistachio Fruit Preservation: An Experimental and Modeling Study. Sci. Rep. 2024, 14, 509. [Google Scholar] [CrossRef]
- Amarillas, L.; Lightbourn-Rojas, L.; Angulo-Gaxiola, A.K.; Basilio Heredia, J.; González-Robles, A.; León-Félix, J. The Antibacterial Effect of Chitosan-based Edible Coating Incorporated with a Lytic Bacteriophage against Escherichia coli O157:H7 on the Surface of Tomatoes. J. Food Saf. 2018, 38, e12571. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Usall, J.; Solsona, C.; Teixidó, N. Fluidised-Bed Spray-Drying Formulations of Candida sake CPA-1 by Adding Biodegradable Coatings to Enhance their Survival Under Stress Conditions. Appl. Microbiol. Biotechnol. 2017, 101, 7865–7876. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, F.H.; Ariffin, S.H.; Muhialdin, B.J.; Yusof, N.L.; Mohammed, N.K.; Marzlan, A.A.; Hussin, A.S.M. Influence of Natural Antifungal Coatings Produced by Lacto-Fermented Antifungal Substances on Respiration, Quality, Antioxidant Attributes, and Shelf Life of Mango (Mangifera indica L.). Postharvest Biol. Technol. 2022, 189, 111904. [Google Scholar] [CrossRef]
- Settier-Ramírez, L.; López-Carballo, G.; Hernández-Muñoz, P.; Fontana-Tachon, A.; Strub, C.; Schorr-Galindo, S. Apple-Based Coatings Incorporated with Wild Apple Isolated Yeast to Reduce Penicillium expansum Postharvest Decay of Apples. Postharvest Biol. Technol. 2022, 185, 111805. [Google Scholar] [CrossRef]

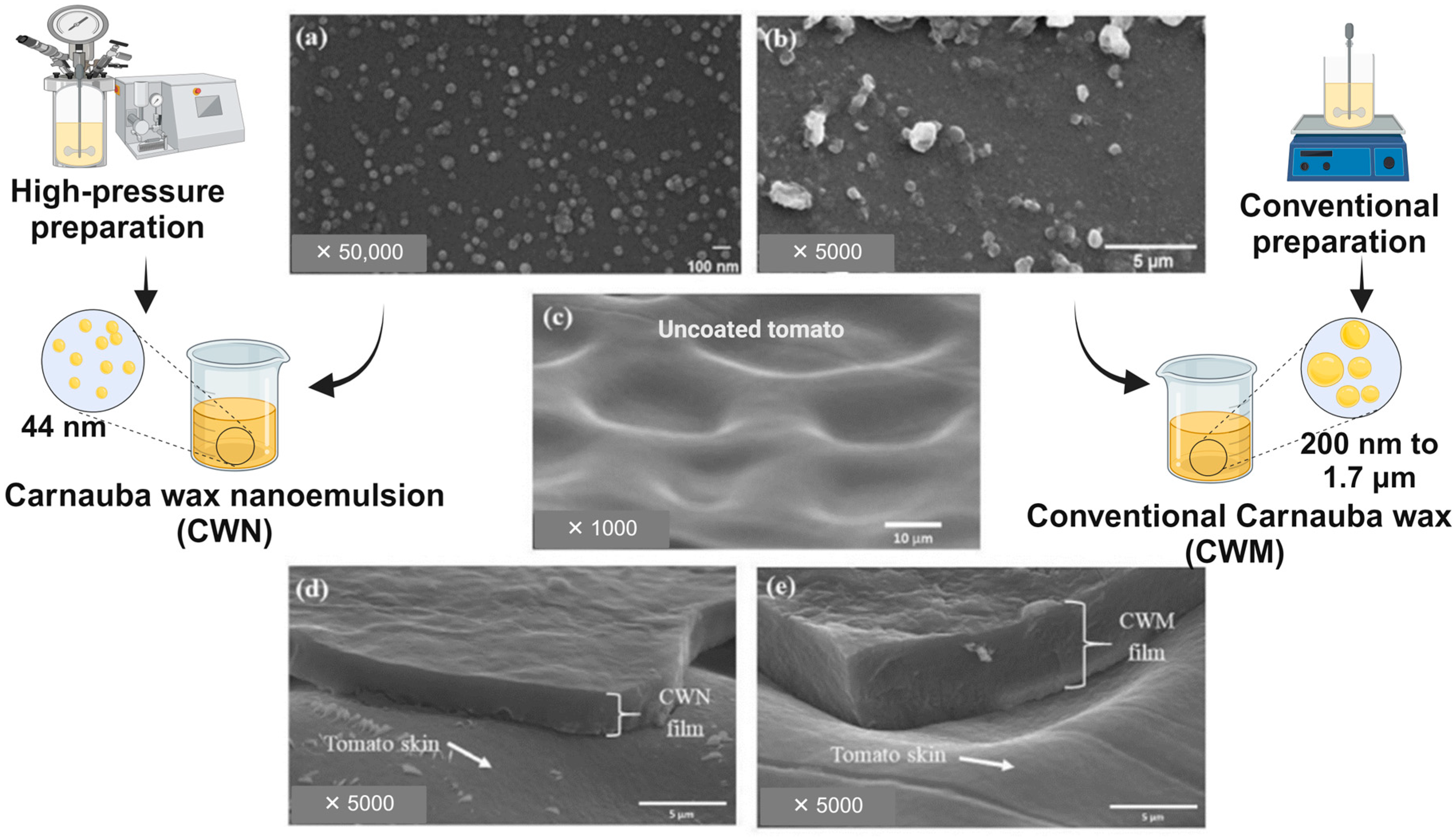
| Fruit | Matrix | Additives | Coating Technique | Storage Condition | Finds/Benefits | References |
|---|---|---|---|---|---|---|
| Apple | Polyvinyl alcohol-native potato starch | Carvacrol | Dipping | 25 °C, 65% RH, 14 days | Did not preserve fruit quality; exhibited high black and green mold control | [45] |
| Apple and Persimmon | Cassava starch-gellan gum | Thyme essential oil | Manual | 25 °C, 65% RH, 14 days | Decreased water loss for persimmons and reduced incidence and severity of black spot and gray mold | [74] |
| Citrus | Hydroxypropyl-methylcellulose and beeswax | GRAS salts | 5 °C, 90% RH, 28 and 56 days + shelf life | Reduce anthracnose severity and enhance CO2 without compromising sensory | [75] | |
| Citrus | Carnauba wax nanoemulsion (CWN) | - | Manual | Cold storage + shelf life | Decreased water loss, increased CO2, and low O2 while producing less ethanol and volatile profile alterations | [8] |
| Citrus | Citrus pectin—beeswax | Essential oils | Dipping | 20 °C, 80% RH, 14 days | Reduced weight loss and maintained firmness | [76] |
| Guava | Zein | Chitosan nanowhiskers | Dipping | 26 °C, 65% RH, 8–11 days | Reduce Colletotrichum species severity while preserving quality and extending shelf life | [77] |
| Papaya | CWN–HPMC | Ginger oil | Manual | Shelf life, Cold storage + shelf life | Decreased water loss, color development, and ripening while showing positive effects against fungal diseases | [23] |
| Strawberry | Cassava starch | Sophorolipid fructooligosaccharides | Dipping | 4 °C, 85% RH, 15 days | Delayed fruit senescence, mold, and yeast growth inhibition | [78] |
| Strawberry | Nanochitosan | Propolis | Dipping | 4 °C, −RH, 8 days | Increased levels of total phenols, flavonoids, and antioxidant capacity | [79] |
| Strawberry, Papaya, Avocado, Banana | Egg-protein | Curcumin and cellulose nanocrystals | Dipping | −°C, −RH, 8–11 days | Reduced enzymatic browning, delayed pulp ripening, and maintained firmness | [66] |
| Tomato | CWN | - | Dipping | 23 °C, 80% RH, 15 days | Decreased water loss, improved gloss, and higher consumer acceptance | [44] |
| Antagonist | Fruit | Matrix and Additives | Target Microorganism | Storage Condition | Finds | Ref. |
|---|---|---|---|---|---|---|
| Metschnikowia pulcherrima | Apple | Apple residues | Penicillium expansum | 21 °C, − % RH, 17 days | Reduced fungus growth and mycotoxin (patulin). | [116] |
| Meyerozyma caribbica | Avocado | Sodium alginate | Colletotrichum gloeosporioides | Cold storage + self-life, self-life | Decreased disease severity and incidence. | [40] |
| Lacticaseibacillus casei (Lbc1.5 or Lbc3) | Fresh pistachio fruit | Seed mucilage-based (Lallemantia royleana mucilage) | - | 4 °C, 85% RH, 35 days | EC retarded mold and yeast growth, with a stronger effect when loaded with BCA. EC also preserved physicochemical and sensory properties. | [112] |
| Lactobacillus rhamnosus (L) or Bifidobacterium animalis subsp. Lactis (B) | Fresh-cut apple | Alginate + calcium chloride and prebiotics (inulin and oligofructose) | Escherichia coli Listeria innocua | 5 °C, − % RH, 8 days | L + EC 1 and B + EC 1 reduced E. coli viability and showed bactericidal effects against L. innocua. | [107] |
| Candida sake (C) | Grape | Maltodextrin, skimmed milk and sucrose formulation (M), or pregelatinized potato starch (PS) | Botrytis cinerea | 20 °C, 85% RH, 10 days | PS + C reduced fungal severity by over 65%, while M + C achieved a reduction of approximately 40%. | [114] |
| C. sake | Grape | HPMC 2, starch, sodium caseinate (SC) or pea protein | B. cinerea | 20 °C, 85% RH, 7 and 12 days | EC enhanced C. sake survival and efficacy, while SC and starch were recommended for cost-effective control of B. cinerea. | [28] |
| Lactobacillus plantarum subsp. plantarum (LP) | Grape | Pregelatinized potato starch (PS) or sodium caseinate | B. cinerea | 20 °C, 85% RH, 7 and 9 days | Coating minimally affected quality but boosted LP’s anti-fungal performance, while PS + LP showed the greatest incidence reduction | [37] |
| Candida oleophila | Grapefruit | Shellac-based free alcohol or morpholine; or sucrose ester and surfactants | P. digitatum | 13 °C, − % RH, 30 and 90 days | Optimized EC improved yeast survival on fruit and boosted disease control. | [111] |
| Wickerhamomyces anomalus (W) | Oranges | Pectin + calcium chloride | P. digitatum; P. italicum | Inoculated fruits: 23 °C, 90% RH, 7 days. Quality: 5 °C, 90% RH, 60 days. Then: then: 20 °C, 75% RH, 7 days | Viability of W inserted into EC was maintained during storage and modified chilling injury index. | [109] |
| Bacteriophage cocktail (Phages: S. Enteritidis F5–4, S. Typhimurium L2–1 and S. Typhimurium ICB1–1) | Strawberries | Whey protein (WP), carboxymethyl cellulose, chitosan, or sodium alginate | S. enterica subsp. enterica serovars Enteritidis and Typhimurium | 4 °C, 90% RH, 5 days | WP + phage preserved fruit quality and showed the highest antimicrobial activity against S. Enterica | [92] |
| Bacillus subtilis HFC103 | Strawberries | Candelilla wax + guar gum | Rhizopus stolonifer | 25 °C, − % RH, 6 days | Preserve fruit quality parameters, reduced moisture loss and fungal decay | [36] |
| Bacteriophage (V) (vB_EcoMH2W), Caudovirales order | Tomato | Chitosan-based commercialized by FreshSeal® BASF Corporation, NJ, USA | Escherichia coli | 20 °C, − % RH, 6 days | V + EC 1 reduced bacterial growth by three logarithmic units compared to chitosan coating alone. | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, M.; Bai, J.; Pilon, L.; Torres, R.; Casals, C.; Solsona, C.; Teixidó, N. Fundamentals of Edible Coatings and Combination with Biocontrol Agents: A Strategy to Improve Postharvest Fruit Preservation. Foods 2024, 13, 2980. https://doi.org/10.3390/foods13182980
Miranda M, Bai J, Pilon L, Torres R, Casals C, Solsona C, Teixidó N. Fundamentals of Edible Coatings and Combination with Biocontrol Agents: A Strategy to Improve Postharvest Fruit Preservation. Foods. 2024; 13(18):2980. https://doi.org/10.3390/foods13182980
Chicago/Turabian StyleMiranda, Marcela, Jinhe Bai, Lucimeire Pilon, Rosario Torres, Carla Casals, Cristina Solsona, and Neus Teixidó. 2024. "Fundamentals of Edible Coatings and Combination with Biocontrol Agents: A Strategy to Improve Postharvest Fruit Preservation" Foods 13, no. 18: 2980. https://doi.org/10.3390/foods13182980
APA StyleMiranda, M., Bai, J., Pilon, L., Torres, R., Casals, C., Solsona, C., & Teixidó, N. (2024). Fundamentals of Edible Coatings and Combination with Biocontrol Agents: A Strategy to Improve Postharvest Fruit Preservation. Foods, 13(18), 2980. https://doi.org/10.3390/foods13182980








