Solid-State Fermentation of Cereal Waste Improves the Bioavailability and Yield of Bacterial Cellulose Production by a Novacetimonas sp. Isolate
Abstract
:1. Introduction
2. Materials and Methods
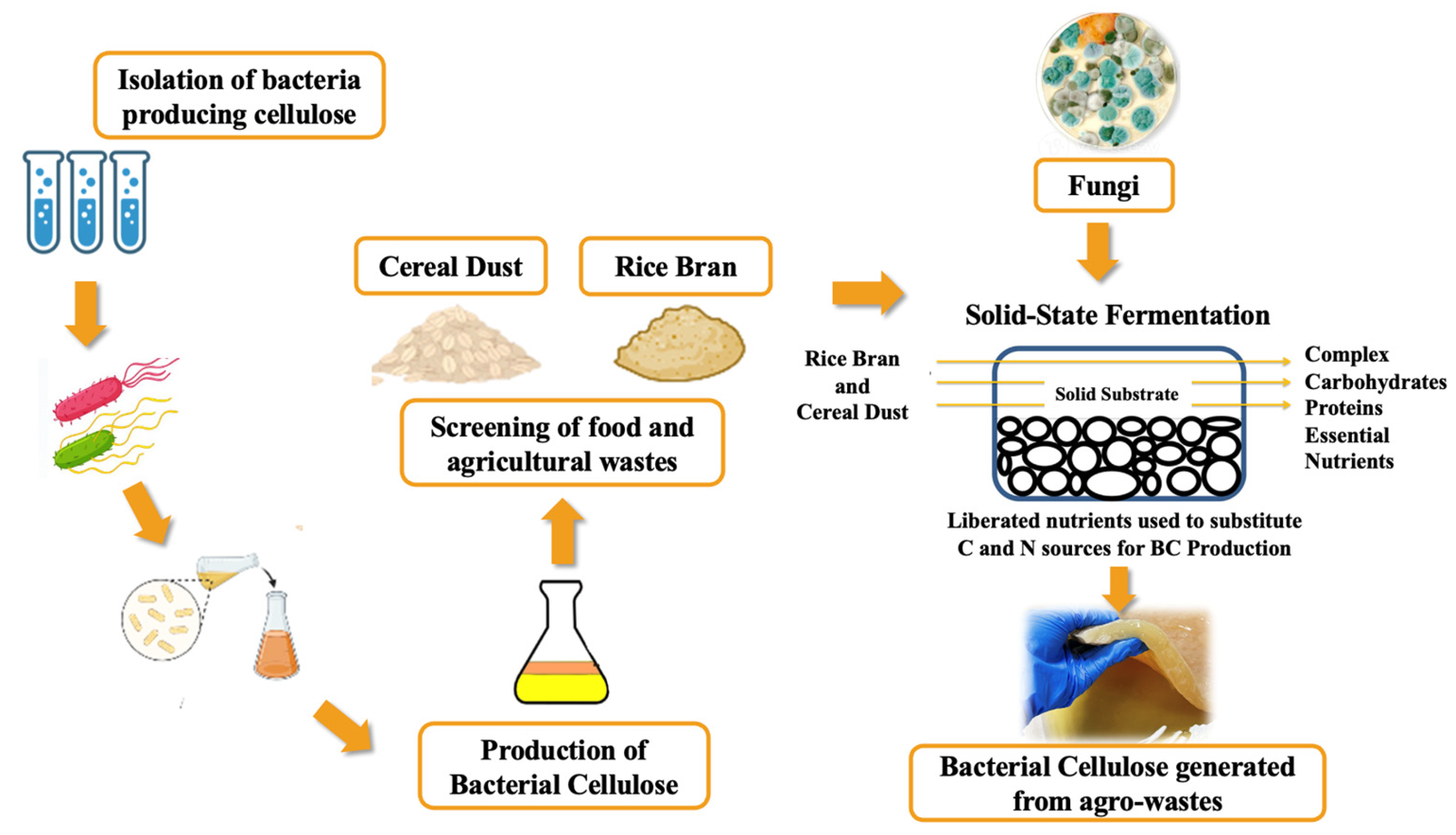
2.1. Microorganisms and Preparation of Inoculum
2.2. Waste Materials Used for the Reformulation of Bacterial Cellulose Media
2.3. Reformulation of HS Culture Medium Using Agro Wastes for BC Production
2.4. Solid-State Fermentation of Rice Bran (RB) and Cereal Dust (CD)
2.5. BC Production from Treated and Untreated Waste Substrates
2.6. Characterisation of BC Produced from SSF-Treated Substrates
2.7. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.8. Fourier-Transformed Infrared Spectroscopy (FTIR)
2.9. X-ray Diffraction (XRD)
2.10. Thermogravimetric Analysis (TGA)
3. Results
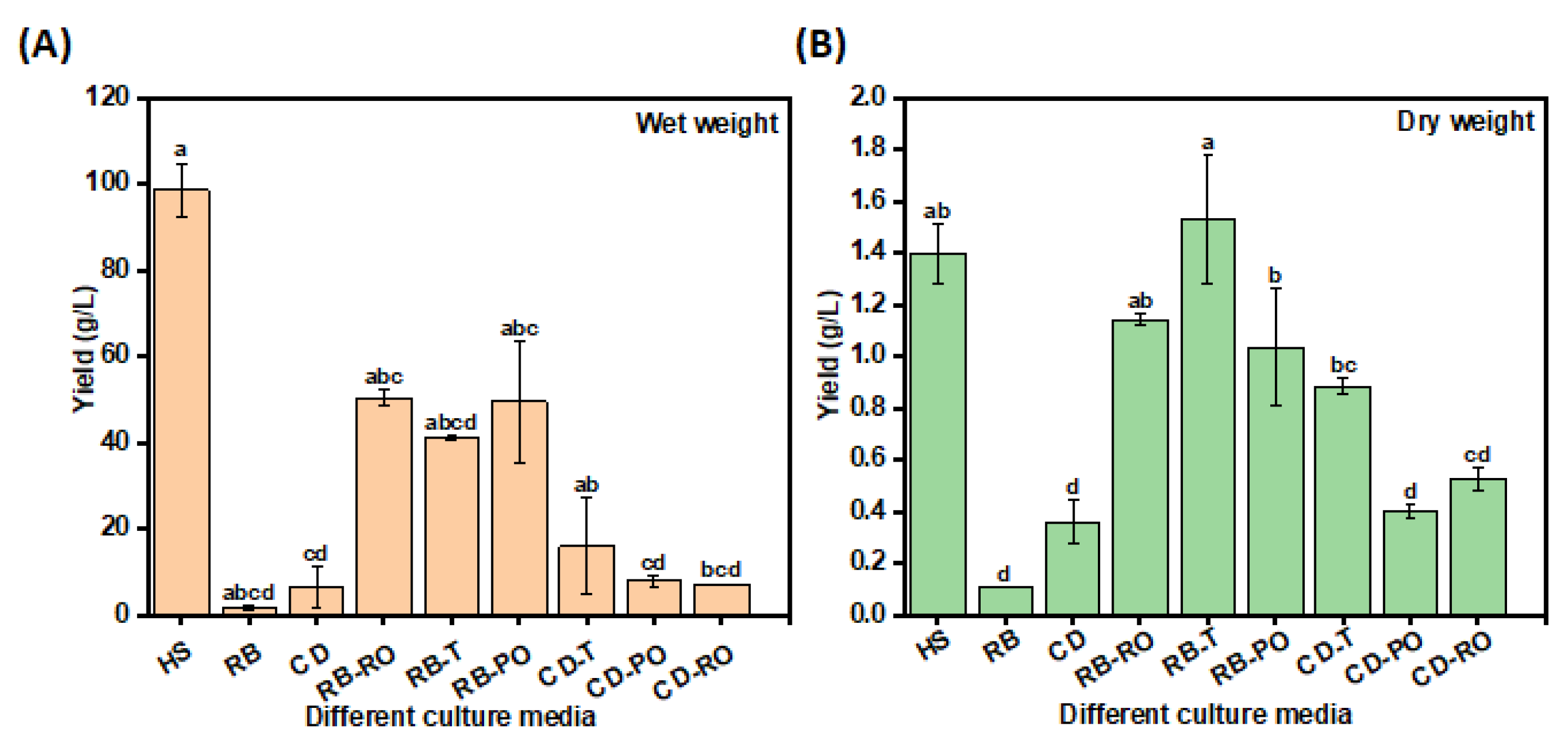
3.1. Solid-State Fermentation of Rice Bran and Cereal Dust
3.2. Media Substitution Using SSF-Treated Rice Bran and Cereal Dust
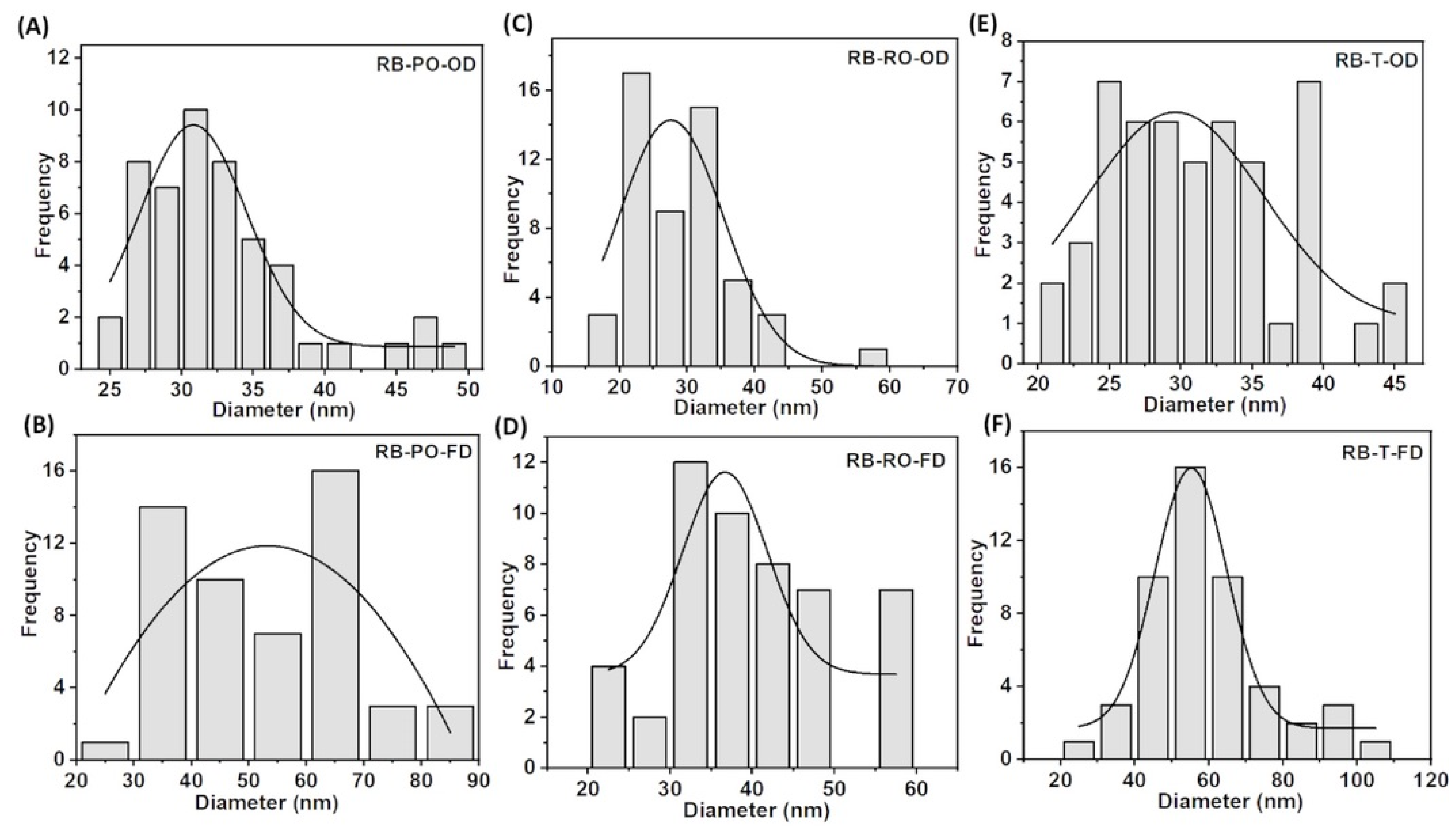
3.3. Comparative Analyses of the BC Produced from SSF-Treated Rice Bran and Cereal Dust
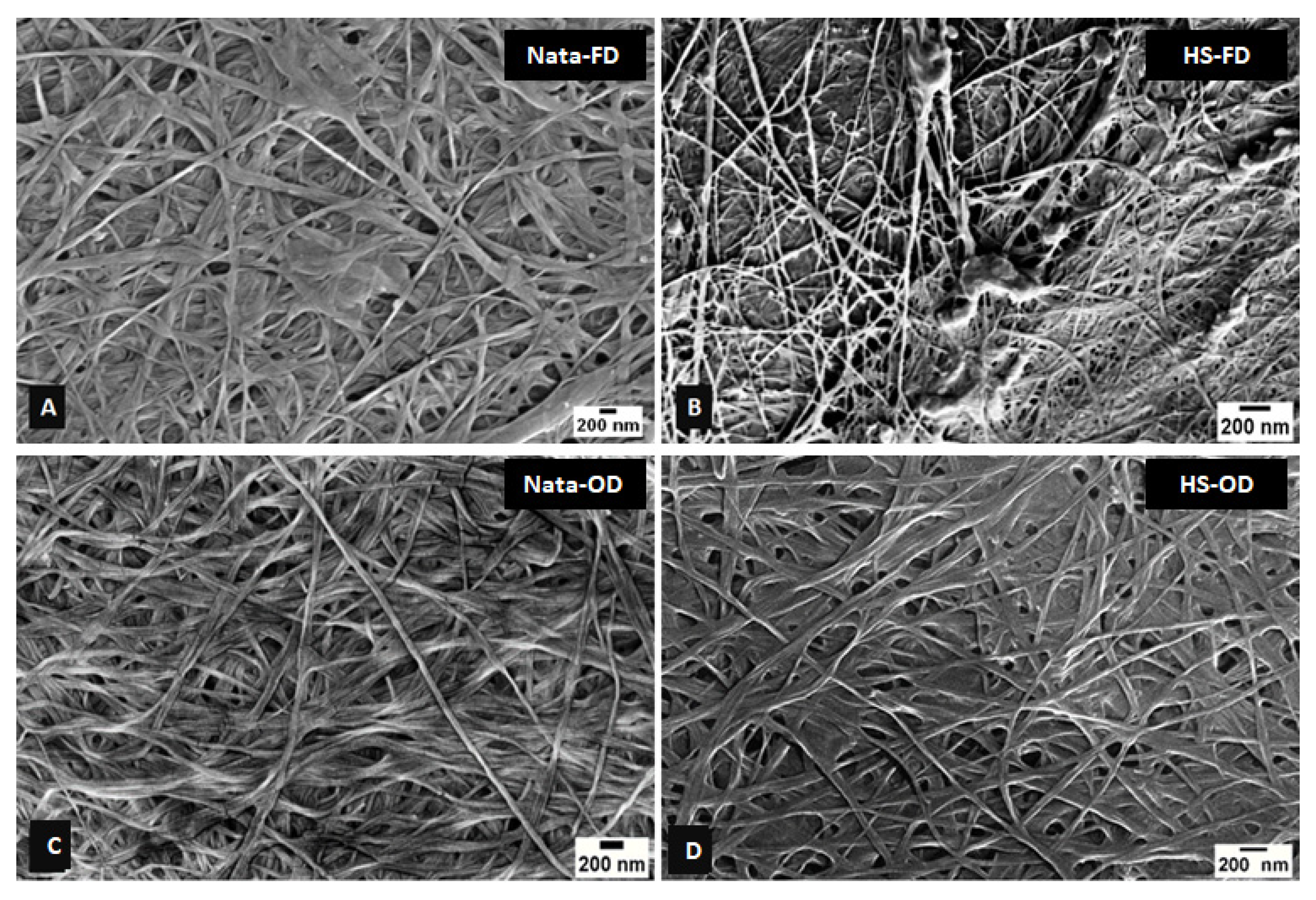
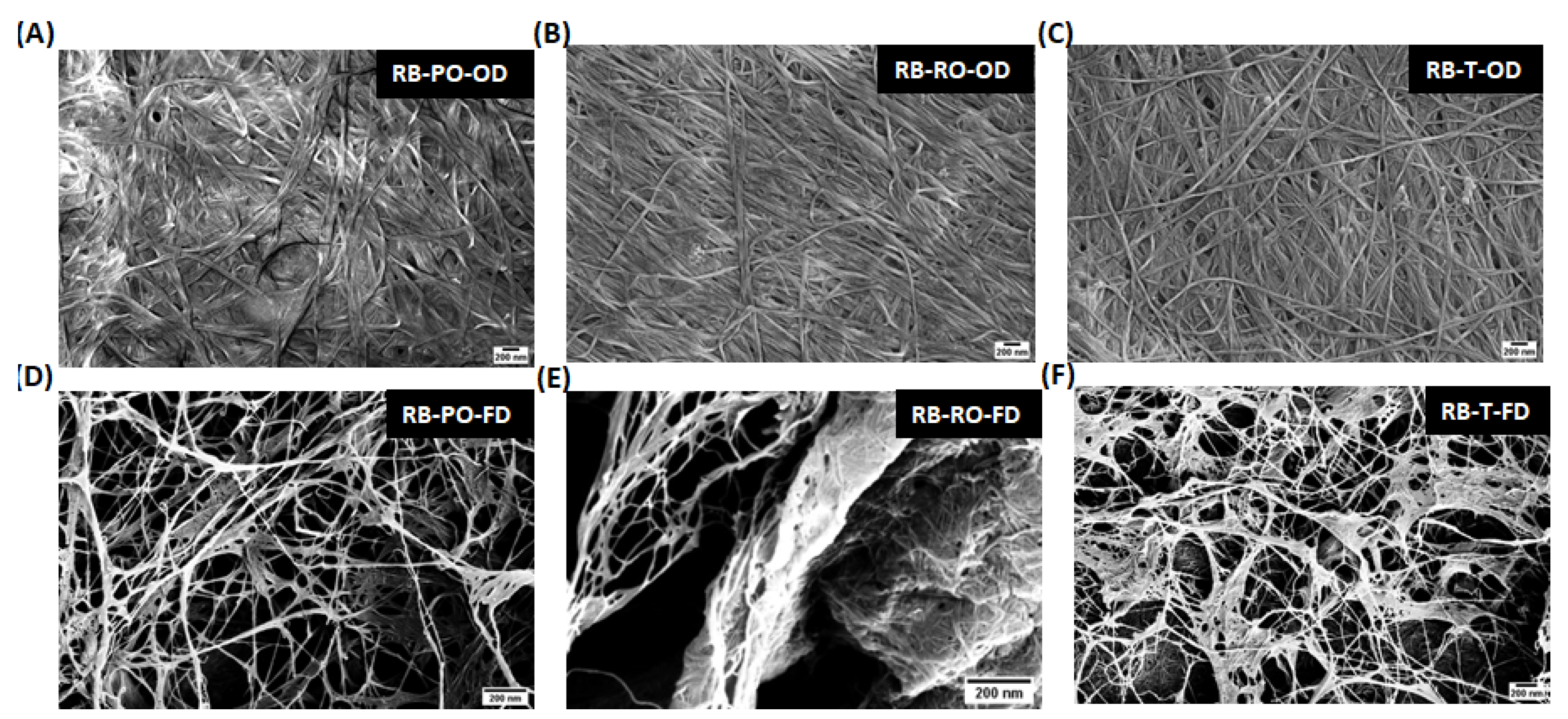
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Energy-Dispersive X-ray Spectroscopy (EDS) Analysis
3.3.3. Fourier-Transformed Infrared Spectroscope (FTIR)
3.3.4. X-ray Diffraction (XRD)
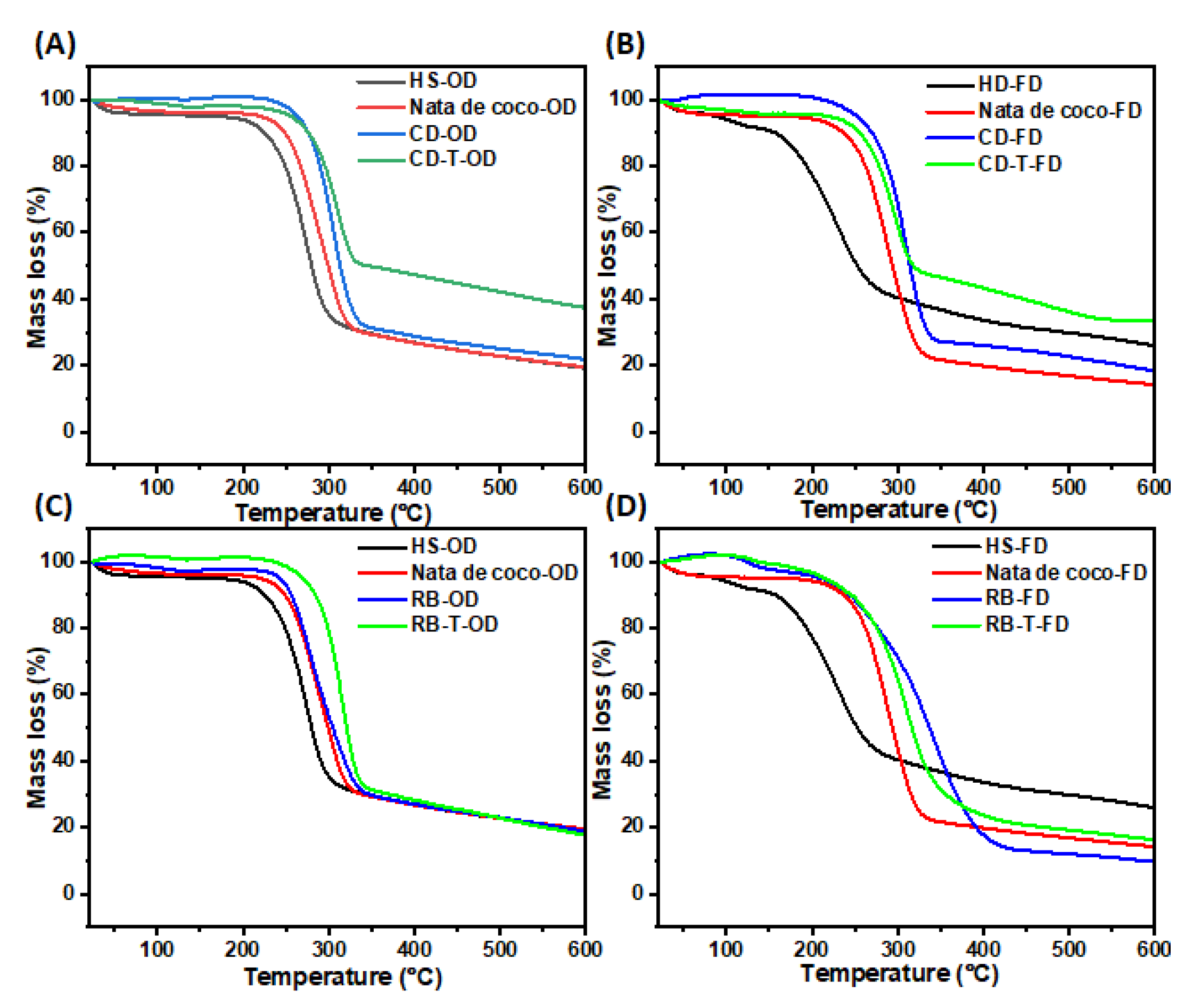
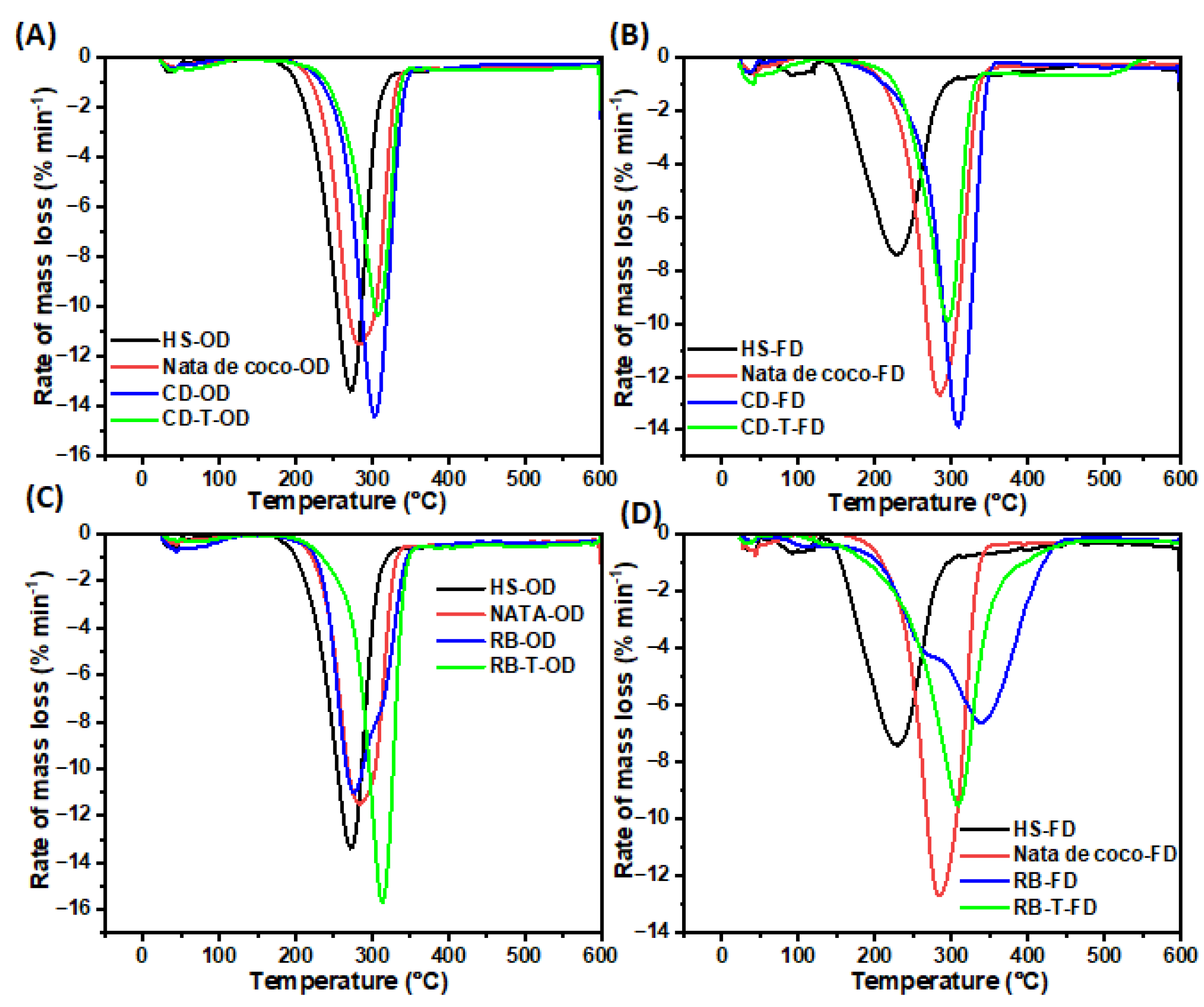
3.3.5. Thermogravimetric Analysis (TGA)
3.3.6. Cost Analysis of Growth Media Input
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Dungani, R.; Karina, M.; Subyakto; Sulaeman, A.; Hermawan, D.; Hadiyane, A. Agricultural waste fibers towards sustainability and advanced utilization: A review. Asian J. Plant Sci. 2016, 15, 42–55. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Gullo, M.; La China, S.; Falcone, P.M.; Giudici, P. Biotechnological production of cellulose by acetic acid bacteria: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6885–6898. [Google Scholar] [CrossRef]
- Salihu, R.; Foong, C.Y.; Razak, S.I.A.; Kadir, M.R.A.; Yusof, H.; Nayan, G.H.M. Overview of Inexpensive Production Routes of Bacterial Cellulose and Its Applications in Biomedical Engineering. Cellul. Chem. Technol. 2019, 53, 1–13. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Fatima, A.; Ullah, M.W.; Manan, S. Cost-Effective Synthesis of Bacterial Cellulose and Its Applications in the Food and Environmental Sectors. Gels 2022, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Brunschwiler, C.; Heine, D.; Kappeler, S.; Conde-Petit, B.; Nyström, L. Direct measurement of rice bran lipase activity for inactivation kinetics and storage stability prediction. J. Cereal Sci. 2013, 58, 272–277. [Google Scholar] [CrossRef]
- Narh, C.; Frimpong, C.; Mensah, A.; Wei, Q. Rice Bran, an Alternative Nitrogen Source for Acetobacter xylinum Bacterial Cellulose Synthesis. Bioresources 2018, 13, 4346–4363. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: Sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P. Changes in rice bran bioactives, their bioactivity, bioaccessibility and bioavailability with solid-state fermentation by Rhizopus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101510. [Google Scholar] [CrossRef]
- Ali, H.K.Q.; Zulkali, M.M.D. Utilization of Agro-Residual Ligno-Cellulosic Substances by Using Solid State Fermentation: A Review. Croat. J. Food Technol. Biotechnol. 2011, 6, 5–12. [Google Scholar]
- Kupski, L.; Cipolatti, E.; Rocha, M.D.; Oliveira, M.D.S.; Souza-Soares, L.D.A.; Badiale-Furlong, E. Solid-state fermentation for the enrichment and extraction of proteins and antioxidant compounds in rice bran by Rhizopus oryzae. Braz. Arch. Biol. Technol. 2012, 55, 937–942. [Google Scholar] [CrossRef]
- Ellaiah, P.; Prabhakar, T.; Ramakrishna, B.; Taleb, A.T.; Adinarayana, K. Production of lipase by immobilized cells of Aspergillus niger. Process Biochem. 2004, 39, 525–528. [Google Scholar] [CrossRef]
- Adinarayana, K.; Ellaiah, P.; Prasad, D.S. Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS PharmSciTech 2003, 4, E56. [Google Scholar] [CrossRef] [PubMed]
- Baysal, Z.; Uyar, F.; Aytekin, Ç. Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochem. 2003, 38, 1665–1668. [Google Scholar] [CrossRef]
- Sodhi, H.K.; Sharma, K.; Gupta, J.K.; Soni, S.K. Production of a thermostable α-amylase from Bacillus sp. PS-7 by solid state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production. Process Biochem. 2005, 40, 525–534. [Google Scholar] [CrossRef]
- Ritthibut, N.; Oh, S.-J.; Lim, S.-T. Enhancement of bioactivity of rice bran by solid-state fermentation with Aspergillus strains. LWT 2021, 135, 110273. [Google Scholar] [CrossRef]
- Sukma, A.C.T.; Oktavianty, H.; Sumardiono, S. Optimization of solid-state fermentation condition for crude protein enrichment of rice bran using Rhizopus oryzae in tray bioreactor. Indones. J. Biotechnol. 2021, 26, 33–40. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kim, S.-M.; Lee, J.H.; Lim, S.-T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Henry, S. Production of Bacterial Cellulose from Agricultural Wastes for Tissue Scaffold Application. Ph.D. Thesis, Swinburne University of Technology, Hawthorn, Australia, 2024. [Google Scholar]
- Yuan, Y.; Feng, F.; Chen, L.; Yao, Q.; Chen, K. Directional isolation of ethanol-tolerant acetic acid bacteria from industrial fermented vinegar. Eur. Food Res. Technol. 2013, 236, 573–578. [Google Scholar] [CrossRef]
- Oliveira, M.d.S.; Feddern, V.; Kupski, L.; Cipolatti, E.P.; Badiale-Furlong, E.; de Souza-Soares, L.A. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 2011, 102, 8335–8338. [Google Scholar] [CrossRef] [PubMed]
- Borzani, W.; Souza, S.J.d. Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitated liquid media. Biotechnol. Lett. 1995, 17, 1271–1272. [Google Scholar] [CrossRef]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr. Polym. 2012, 89, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Kodali, B.; Pogaku, R. Pretreatment studies of rice bran for the effective production of cellulose. Electron. J. Environ. Agric. Food Chem. 2006, 5, 1253–1264. [Google Scholar]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Holmes, D. Bacterial Cellulose. Master’s Thesis, Department of Chemical and Process Engineering University of Canterbury Christchurch, Christchurch, New Zealand, 2004. [Google Scholar]
- Aytekin, A.Ö.; Demirbağ, D.D.; Bayrakdar, T. The statistical optimization of bacterial cellulose production via semi-continuous operation mode. J. Ind. Eng. Chem. 2016, 37, 243–250. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef]
- Omarini, A.B.; Labuckas, D.; Zunino, M.P.; Pizzolitto, R.; Fernández-Lahore, M.; Barrionuevo, D.; Zygadlo, J.A. Upgrading the Nutritional Value of Rice Bran by Solid-State Fermentation with Pleurotus sapidus. Fermentation 2019, 5, 44. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Zhao, F.; Peng, Q.; Zhou, Z.; Han, Y. Production and characterization of bacterial cellulose produced by Gluconacetobacter xylinus isolated from Chinese persimmon vinegar. Carbohydr. Polym. 2018, 194, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Han, Y.-H.; Ye, Y.-X.; Shi, X.-X.; Xiang, P.; Chen, D.-L.; Li, M. Physicochemical characterization of high-quality bacterial cellulose produced by Komagataeibacter sp. strain W1 and identification of the associated genes in bacterial cellulose production. RSC Adv. 2017, 7, 45145–45155. [Google Scholar] [CrossRef]
- Dórame-Miranda, R.F.; Gámez-Meza, N.; Medina-Juárez, L.Á.; Ezquerra-Brauer, J.M.; Ovando-Martínez, M.; Lizardi-Mendoza, J. Bacterial cellulose production by Gluconacetobacter entanii using pecan nutshell as carbon source and its chemical functionalization. Carbohydr. Polym. 2019, 207, 91–99. [Google Scholar] [CrossRef]
- Rani, M.U.; Appaiah, A. Optimization of culture conditions for bacterial cellulose production from Gluconacetobacter hansenii UAC09. Ann. Microbiol. 2011, 61, 781–787. [Google Scholar] [CrossRef]
- Slapšak, N.; Cleenwerck, I.; De Vos, P.; Trček, J. Gluconacetobacter maltaceti sp. nov., a novel vinegar producing acetic acid bacterium. Syst. Appl. Microbiol. 2013, 36, 17–21. [Google Scholar] [CrossRef]
- Bi, J.C.; Liu, S.X.; Li, C.F.; Li, J.; Liu, L.X.; Deng, J.; Yang, Y.C. Morphology and structure characterization of bacterial celluloses produced by different strains in agitated culture. J. Appl. Microbiol. 2014, 117, 1305–1311. [Google Scholar] [CrossRef]
- Kongruang, S. Bacterial Cellulose Production by Acetobacter xylinum Strains from Agricultural Waste Products. In Biotechnology for Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2007; pp. 763–774. [Google Scholar]
- De Souza, K.C.; Dos Santos, G.R.; Trindade, F.C.; Costa, A.F.D.S.; De Almeida, Y.M.; Sarubbo, L.A.; Vinhas, G.M. Production of bacterial cellulose biopolymers in media containing rice and corn hydrolysate as carbon sources. Polym. Polym. Compos. 2021, 29, S1466–S1474. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Popescu, M.-C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral Characterization of Eucalyptus Wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.J.; Hossain, L.; Browne, C.; Raghuwanshi, V.S.; Simon, G.P.; Garnier, G. Controlling the transparency and rheology of nanocellulose gels with the extent of carboxylation. Carbohydr. Polym. 2020, 245, 116566. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.-L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Akintunde, M.; Sanusi, J. Effect of different fruit juice media on bacterial cellulose production by Acinetobacter sp. BAN1 and Acetobacter pasteurianus PW1. J. Adv. Biol. Biotechnol. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-Functionalized Bacterial Cellulose as Antibacterial Membrane for Wound-Healing Applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, K.M.; Gea, S.; Ilyas, S.; Tamrin, T.; Radecka, I. Characterization of Bacterial Cellulose-Based Wound Dressing in Different Order Impregnation of Chitosan and Collagen. Biomolecules 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]


| Samples | Oven-Dried | Freeze-Dried | ||
|---|---|---|---|---|
| C | O | C | O | |
| Rice bran | 68.0% | 32.0% | 75.1% | 24.9% |
| Rice bran (Pleurotus osteratus) | 68.7% | 31.3% | 63.7% | 36.3% |
| Rice bran (Rhizopus oryzae) | 67.0% | 33.0% | 57.6% | 42.4% |
| Rice bran (Rhizopus oligosporus) | 65.2% | 34.8% | 59.6% | 40.4% |
| Cereal dust | 65.4% | 34.6% | 59.8% | 40.2% |
| Cereal dust (Pleurotus osteratus) | 64.3% | 35.7% | 57.1% | 42.9% |
| Cereal dust (Rhizopus oryzae) | 63.2% | 36.8% | 53.8% | 46.2% |
| Cereal dust (Rhizopus oligosporus) | 59.9% | 40.1% | 53.8% | 46.2% |
| Price | Hestrin-Schramm Media/L | Rice Bran/L | Cereal Dust/L | SSF-Treated RB/L | SSF-Treated CD/L | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Media Composition | Unit | AU $/Unit | AU $/g | Quantity (g) | Cost ($/g) | Quantity (g) | Cost ($/g) | Quantity (g) | Cost ($/g) | Quantity (g) | Cost ($/g) | Quantity (g) | Cost ($/g) |
| Glucose | 1 kg | 63.5 | 0.06 | 20 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yeast extract | 1 kg | 360 | 0.36 | 5 | 1.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peptone | 500 g | 210 | 0.42 | 5 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Di-sodium hydrogen phosphate | 500 g | 93.13 | 0.19 | 2.7 | 0.51 | 2.7 | 0.51 | 0.51 | 0.51 | 2.7 | 0.51 | 2.7 | 0.51 |
| Citric acid monohydrate | 1 kg | 185 | 0.18 | 1.85 | 0.33 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 |
| Magnesium sulphate heptahydrate | 1 kg | 287 | 0.28 | 0.5 | 0.14 | 0.5 | 0.15 | 0.15 | 0.15 | 0.5 | 0.15 | 0.5 | 0.15 |
| Ethanol | 1 L | 117 | 0.11 | 100 mL | 11 | 100 | 11 | 0 | 11 | 0 | 0 | 0 | 0 |
| Rice bran | 10 kg | 0 | 0 | 0 | 0 | 76.9 | 0 | 0 | 0 | 76.9 | 0 | 0 | 0 |
| Cereal Dust | 5 kg | 0 | 0 | 0 | 0 | 0 | 0 | 58.5 | 0 | 0 | 0 | 58.5 | 0 |
| Total cost | 17.08 | 13.51 | 13.51 | 2.51 | 2.51 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, S.; Dhital, S.; Sumer, H.; Butardo, V., Jr. Solid-State Fermentation of Cereal Waste Improves the Bioavailability and Yield of Bacterial Cellulose Production by a Novacetimonas sp. Isolate. Foods 2024, 13, 3052. https://doi.org/10.3390/foods13193052
Henry S, Dhital S, Sumer H, Butardo V Jr. Solid-State Fermentation of Cereal Waste Improves the Bioavailability and Yield of Bacterial Cellulose Production by a Novacetimonas sp. Isolate. Foods. 2024; 13(19):3052. https://doi.org/10.3390/foods13193052
Chicago/Turabian StyleHenry, Shriya, Sushil Dhital, Huseyin Sumer, and Vito Butardo, Jr. 2024. "Solid-State Fermentation of Cereal Waste Improves the Bioavailability and Yield of Bacterial Cellulose Production by a Novacetimonas sp. Isolate" Foods 13, no. 19: 3052. https://doi.org/10.3390/foods13193052
APA StyleHenry, S., Dhital, S., Sumer, H., & Butardo, V., Jr. (2024). Solid-State Fermentation of Cereal Waste Improves the Bioavailability and Yield of Bacterial Cellulose Production by a Novacetimonas sp. Isolate. Foods, 13(19), 3052. https://doi.org/10.3390/foods13193052







