Potato Protein-Based Vegan Burgers Enriched with Different Sources of Iron and Fiber: Nutrition, Sensory Characteristics, and Antioxidants before and after In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.1.1. PBBs Ingredients
2.1.2. Chemical Reagents and Standards
2.2. PBBs Manufacturing
2.3. Nutritional Value and Digestibility
2.4. Amino Acids, Fatty Acids, and Mineral Contents
2.4.1. Determination of Amino Acids
2.4.2. Determination of Fatty Acid Profiles and Their Nutritional Indices
2.4.3. Determination of Mineral Profiles
2.5. Simulated In Vitro Digestion Process
2.6. Analysis of Changes in Antioxidant Activity Resulting from the Digestion Process
2.6.1. Extraction Process of Antioxidants
2.6.2. Total Phenolic Compounds Content Analysis
2.6.3. Antioxidant Activity Determined by ABTS Method
2.6.4. FRAP (Ferric Reducing Antioxidant Power) Assay
2.7. Analysis of Changes in the Content of Glycoalkaloids and Phenolic Acids before and after the Digestion Process
2.7.1. Glycoalkaloid Contents
2.7.2. Phenolic Acid Contents
2.8. Consumer Study
2.9. Statistical Analyses
3. Results and Discussion
3.1. Basic Nutritional Value
3.2. Amino Acid Profile
3.3. Fat Content and Fatty Acids Composition
3.4. Dietary Fiber Analysis Results
3.5. Minerals
3.6. Antioxidant Activity
3.7. Changes in Glycoalcaloids
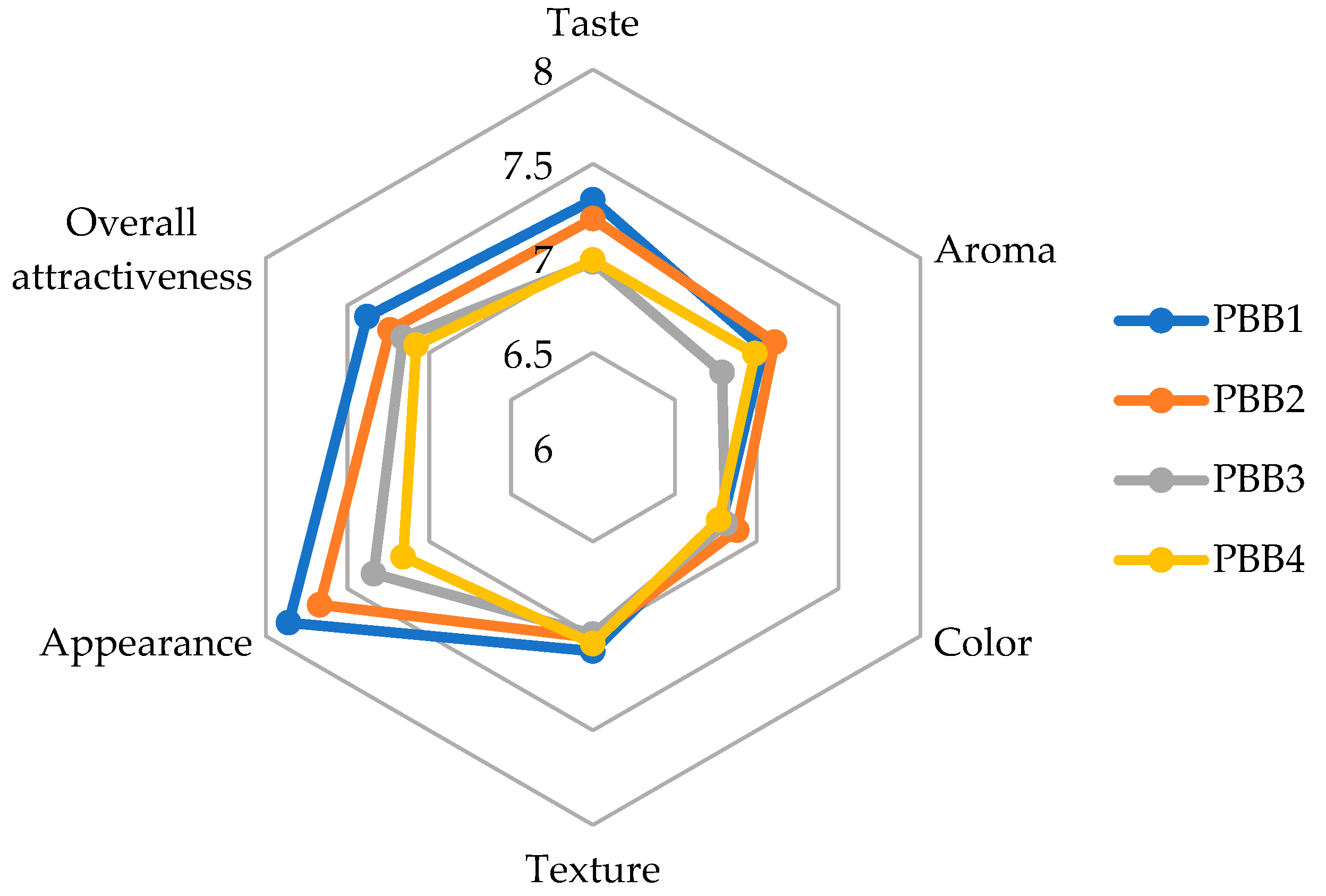
3.8. Consumer Acceptance
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rybicka, I.; Bohdan, K.; Kowalczewski, P.Ł. Meat alternatives—Market and cunsumption. In Sustainable Food. Production and Consumption Perspectives; Pawlak-Lemańska, K., Borusiak, B., Sikorska, E., Eds.; Wydawnictwo Uniwersytetu Ekonomicznego w Poznaniu: Poznań, Poland, 2024; pp. 118–131. [Google Scholar]
- Szenderák, J.; Fróna, D.; Rákos, M. Consumer Acceptance of Plant-Based Meat Substitutes: A Narrative Review. Foods 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Cardello, A.V.; Llobell, F.; Giacalone, D.; Chheang, S.L.; Jaeger, S.R. Consumer Preference Segments for Plant-Based Foods: The Role of Product Category. Foods 2022, 11, 3059. [Google Scholar] [CrossRef] [PubMed]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-Based Meat Alternatives: Technological, Nutritional, Environmental, Market, and Social Challenges and Opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Buzała, M.; Janicki, B.; Buzała, M.; Słomka, A. Heme iron in meat as the main source of iron in the human diet. J. Elem. 2015, 21, 303–314. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Laskowski, W.; Kulykovets, O.; Kudlińska-Chylak, A.; Czeczotko, M.; Rejman, K. Food Products as Sources of Protein and Amino Acids—The Case of Poland. Nutrients 2018, 10, 1977. [Google Scholar] [CrossRef]
- Orczewska-Dudek, S.; Bederska-Łojewska, D.; Pieszka, M.; Pietras, M. Cholesterol and Lipid Peroxides in Animal Products and Health Implications—A Review. Ann. Anim. Sci. 2012, 12, 25–52. [Google Scholar] [CrossRef]
- Valsta, L.M.; Tapanainen, H.; Männistö, S. Meat fats in nutrition. Meat Sci. 2005, 70, 525–530. [Google Scholar] [CrossRef]
- Moretti, D. Plant-Based Diets and Iron Status. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 715–727. [Google Scholar]
- De Marchi, M.; Costa, A.; Pozza, M.; Goi, A.; Manuelian, C.L. Detailed characterization of plant-based burgers. Sci. Rep. 2021, 11, 2049. [Google Scholar] [CrossRef]
- Costa-Catala, J.; Toro-Funes, N.; Comas-Basté, O.; Hernández-Macias, S.; Sánchez-Pérez, S.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Castell-Garralda, V.; Vidal-Carou, M.C. Comparative Assessment of the Nutritional Profile of Meat Products and Their Plant-Based Analogues. Nutrients 2023, 15, 2807. [Google Scholar] [CrossRef]
- Biesalski, H.-K. Meat as a component of a healthy diet—Are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005, 70, 509–524. [Google Scholar] [CrossRef] [PubMed]
- De Smet, S.; Vossen, E. Meat: The balance between nutrition and health. A review. Meat Sci. 2016, 120, 145–156. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef] [PubMed]
- Parlasca, M.C.; Qaim, M. Meat Consumption and Sustainability. Annu. Rev. Resour. Econ. 2022, 14, 17–41. [Google Scholar] [CrossRef]
- Collett, K.; O’Callaghan, B.; Mason, M.; Godfray, C.; Hepburn, C. The Climate Impact of Alternative Proteins; University of Oxford: Oxford, UK, 2021. [Google Scholar]
- Gupta, G.S. Land Degradation and Challenges of Food Security. Rev. Eur. Stud. 2019, 11, 63. [Google Scholar] [CrossRef]
- Schlink, A.C.; Nguyen, M.L.; Viljoen, G.J. Water requirements for livestock production: A global perspective. Rev. Sci. Tech. 2010, 29, 603–619. [Google Scholar] [CrossRef]
- Irfan Said, M. The Role of the Livestock Farming Industry in Supporting the Global Agricultural Industry. In Agricultural Development in Asia—Potential Use of Nano-Materials and Nano-Technology; IntechOpen: London, UK, 2022. [Google Scholar]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Świątek, M.; Antosik, A.; Kochanowska, D.; Jeżowski, P.; Smarzyński, K.; Tomczak, A.; Kowalczewski, P.Ł. The potential for the use of leghemoglobin and plant ferritin as sources of iron. Open Life Sci. 2023, 18, 20220805. [Google Scholar] [CrossRef]
- Zielińska-Dawidziak, M.; Białas, W.; Piasecka-Kwiatkowska, D.; Staniek, H.; Niedzielski, P. Digestibility of Protein and Iron Availability from Enriched Legume Sprouts. Plant Foods Hum. Nutr. 2023, 78, 270–278. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice. Nutrients 2019, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Olejnik, A.; Świtek, S.; Bzducha-Wróbel, A.; Kubiak, P.; Kujawska, M.; Lewandowicz, G. Bioactive compounds of potato (Solanum tuberosum L.) juice: From industry waste to food and medical applications. CRC Crit. Rev. Plant Sci. 2022, 41, 52–89. [Google Scholar] [CrossRef]
- Jeżowski, P.; Polcyn, K.; Tomkowiak, A.; Rybicka, I.; Radzikowska, D. Technological and antioxidant properties of proteins obtained from waste potato juice. Open Life Sci. 2020, 15, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Białas, W.; Kowalczewski, P.; Lewandowicz, G.; Olejnik, A.; Siger, A.; Dwiecki, K. Method for Obtaining a Pro-Healthy Protein Preparation. Patent No. PL240850, 13 June 2022. [Google Scholar]
- Zielińska-Dawidziak, M.; Twardowski, T. Method of Producing a Composition with an Increased Content of Plant Ferritin and Other Forms of Iron, Composition and Its Use in the Production of a Preparation for Supplementing the Human. Diet. Patent PL 218747, 30 January 2015. [Google Scholar]
- Zielińska-Dawidziak, M.; Staniek, H.; Król, E.; Piasecka-Kwiatkowska, D.; Twardowski, T. Legume seeds and cereal grains? capacity to accumulate iron while sprouting in order to obtain food fortificant. Acta Sci. Pol. Technol. Aliment. 2016, 15, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.; Lesiecki, M.; Smarzyński, K.; Kubiak, P. Plant-Based Burger Analogue and a Method of Its Preparation. Polish Patent Application No. P.445945, 30 August 2023. [Google Scholar]
- ISO 1871:2009; Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. ISO: Geneva, Switzerland, 2009.
- AOAC. AOAC Official Methods of Analysis, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- AACC. AACCI 32-07.01 Soluble, Insoluble, and Total Dietary Fiber in Foods and Food Products. In AACC International Approved Methods; AACC International: Eagan, MN, USA, 2009. [Google Scholar]
- AACC. 44-19.01 Moisture—Air-oven method, drying at 135 degrees. In AACC International Approved Methods; AACC International: Eagan, MN, USA, 2009. [Google Scholar]
- ISO 763:2003; Fruit and Vegetable Products—Determination of Ash Insoluble in Hydrochloric Acid. ISO: Geneva, Switzerland, 2003.
- Wang, X.-S.; Tang, C.-H.; Yang, X.-Q.; Gao, W.-R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- AOAC. Official Method 994.12 Amino Acids in Feeds; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Tomczak, A.; Zielińska-Dawidziak, M.; Piasecka-Kwiatkowska, D.; Lampart-Szczapa, E. Blue lupine seeds protein content and amino acids composition. Plant Soil Environ. 2018, 64, 147–155. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- AOCS Official Method Ce 1h-05. Determination of cis-, trans-, Saturated, Monounsaturated and Polyunsaturated Fatty Acids in Vegetable or Non-ruminant Animal Oils and Fats by Capillary GLC. Off. Methods Recomm. Pract. Am. Oil Chem. Soc. 2009, 1–29. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details/productId/111777 (accessed on 23 September 2024).
- Kowalczewski, P.Ł.; Gumienna, M.; Rybicka, I.; Górna, B.; Sarbak, P.; Dziedzic, K.; Kmiecik, D. Nutritional Value and Biological Activity of Gluten-Free Bread Enriched with Cricket Powder. Molecules 2021, 26, 1184. [Google Scholar] [CrossRef]
- Matusiewicz, H.; Ślachciński, M. A Comparison of ETV and LA for the Determination of Trace Elements in Solid Samples by MIP OES. Ecol. Chem. Eng. S 2019, 26, 429–441. [Google Scholar] [CrossRef]
- Olejnik, A.; Rychlik, J.; Kidoń, M.; Czapski, J.; Kowalska, K.; Juzwa, W.; Olkowicz, M.; Dembczyński, R.; Moyer, M.P. Antioxidant effects of gastrointestinal digested purple carrot extract on the human cells of colonic mucosa. Food Chem. 2016, 190, 1069–1077. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Wieczorek, M.N.; Zembrzuska, J.; Kowalska, K.; Lewandowicz, J.; Lewandowicz, G. Bioactive Substances of Potato Juice Reveal Synergy in Cytotoxic Activity against Cancer Cells of Digestive System Studied In Vitro. Nutrients 2022, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Zembrzuska, J.; Drożdżyńska, A.; Smarzyński, K.; Radzikowska, D.; Kieliszek, M.; Jeżowski, P.; Sawinska, Z. Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice. Open Chem. 2021, 19, 1225–1232. [Google Scholar] [CrossRef]
- Cybulska, I.; Zembrzuska, J.; Brudecki, G.; Hedegaard Thomsen, M. Optimizing methods to characterize caffeic, ferulic, and chlorogenic acids in Salicornia sinus-persica and Salicornia bigelovii extracts by tandem mass spectrometry (LC-MS/MS). BioResources 2021, 16, 5508–5523. [Google Scholar] [CrossRef]
- Chilón-Llico, R.; Siguas-Cruzado, L.; Apaza-Humerez, C.R.; Morales-García, W.C.; Silva-Paz, R.J. Protein Quality and Sensory Perception of Hamburgers Based on Quinoa, Lupin and Corn. Foods 2022, 11, 3405. [Google Scholar] [CrossRef]
- Joint FAO/WHO/UNU Expert Protein Digestibility and Absorption: Effects of Fibre, and the Extent of Individual Variation. Available online: https://www.fao.org/4/M2836e/M2836e00.htm (accessed on 4 April 2024).
- Gallaher, D.; Schneeman, B. Effect of Dietary Fiber on Protein Digestibility and Utilization. In CRC Handbook of Dietary Fiber in Human Nutrition, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 133–160. [Google Scholar]
- Eggum, B.O. The Influence of Dietary Fibre on Protein Digestion and Utilisation. In Dietary Fibre—A Component of Food. Nutritional Function in Health and Disease; Schweizer, T.F., Edwards, C.A., Eds.; Springer: London, UK, 1992; pp. 153–165. [Google Scholar]
- Vellinga, R.E.; Rippin, H.L.; Gonzales, G.B.; Temme, E.H.M.; Farrand, C.; Halloran, A.; Clough, B.; Wickramasinghe, K.; Santos, M.; Fontes, T.; et al. Nutritional composition of ultra-processed plant-based foods in the out-of-home environment: A multi-country survey with plant-based burgers. Br. J. Nutr. 2024, 131, 1691–1698. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, H.J.; Lim, J.-Y. Effects of leucine-rich protein supplements in older adults with sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Arch. Gerontol. Geriatr. 2022, 102, 104758. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924 (accessed on 12 July 2024).
- Seljak, B.K.; Valenčič, E.; Hristov, H.; Hribar, M.; Lavriša, Ž.; Kušar, A.; Žmitek, K.; Krušič, S.; Gregorič, M.; Blaznik, U.; et al. Inadequate Intake of Dietary Fibre in Adolescents, Adults, and Elderlies: Results of Slovenian Representative SI. Menu Study. Nutrients 2021, 13, 3826. [Google Scholar] [CrossRef]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tian, Y.; Wang, R.; Zhang, T.; Shen, S.; Zeng, P.; Zou, T. Sodium, potassium intake, and all-cause mortality: Confusion and new findings. BMC Public Health 2024, 24, 180. [Google Scholar] [CrossRef]
- Cole, E.; Goeler-Slough, N.; Cox, A.; Nolden, A. Examination of the nutritional composition of alternative beef burgers available in the United States. Int. J. Food Sci. Nutr. 2022, 73, 425–432. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O.; Kajarabille, N.; Rose, S.; Arafsha, S.M.; Kose, T.; Aslam, M.F.; Hall, W.L.; Sharp, P.A. Content and Availability of Minerals in Plant-Based Burgers Compared with a Meat Burger. Nutrients 2023, 15, 2732. [Google Scholar] [CrossRef]
- Mitrevski, J.; Pantelić, N.Đ.; Dodevska, M.S.; Kojić, J.S.; Vulić, J.J.; Zlatanović, S.; Gorjanović, S.; Laličić-Petronijević, J.; Marjanović, S.; Antić, V.V. Effect of Beetroot Powder Incorporation on Functional Properties and Shelf Life of Biscuits. Foods 2023, 12, 322. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Celka, K.; Białas, W.; Lewandowicz, G. Antioxidant activity of potato juice. Acta Sci. Pol. Technol. Aliment. 2012, 11, 175–181. [Google Scholar]
- dos, S.; Baião, D.; da Silva, D.V.T.; Paschoalin, V.M.F. Beetroot, A Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef]
- Zielinski, H.; Kozlowska, H.; Lewczuk, B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov. Food Sci. Emerg. Technol. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Antoine, C.; Peyron, S.; Lullien-Pellerin, V.; Abecassis, J.; Rouau, X. Wheat bran tissue fractionation using biochemical markers. J. Cereal Sci. 2004, 39, 387–393. [Google Scholar] [CrossRef]
- Maga, J.A.; Fitzpatrick, T.J. Potato glycoalkaloids. CRC Crit. Rev. Food Sci. Nutr. 1980, 12, 371–405. [Google Scholar] [CrossRef] [PubMed]
- Benkeblia, N. Potato Glycoalkaloids: Occurrence, biological activities and extraction for biovalorisation—A review. Int. J. Food Sci. Technol. 2020, 55, 2305–2313. [Google Scholar] [CrossRef]
- Singh, B.; Dutt, S.; Raigond, P. Potato Glycoalkaloids. In Potato; Springer: Singapore, 2020; pp. 191–211. [Google Scholar]
- Friedman, M. Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Pęksa, A.; Miedzianka, J. Potato Industry By-Products as a Source of Protein with Beneficial Nutritional, Functional, Health-Promoting and Antimicrobial Properties. Appl. Sci. 2021, 11, 3497. [Google Scholar] [CrossRef]
- Friedman, M.; McDonald, G.M.; Filadelfi-Keszi, M. Potato Glycoalkaloids: Chemistry, Analysis, Safety, and Plant Physiology. CRC Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Hassan, S.H.; Gul, S.; Zahra, H.S.; Maryam, A.; Shakir, H.A.; Khan, M.; Irfan, M. Alpha Solanine: A Novel Natural Bioactive Molecule with Anticancer Effects in Multiple Human Malignancies. Nutr. Cancer 2021, 73, 1541–1552. [Google Scholar] [CrossRef]
- Lee, K.-R.; Kozukue, N.; Han, J.-S.; Park, J.-H.; Chang, E.; Baek, E.-J.; Chang, J.-S.; Friedman, M. Glycoalkaloids and Metabolites Inhibit the Growth of Human Colon (HT29) and Liver (HepG2) Cancer Cells. J. Agric. Food Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef]
- Friedman, M.; Lee, K.-R.; Kim, H.-J.; Lee, I.-S.; Kozukue, N. Anticarcinogenic Effects of Glycoalkaloids from Potatoes against Human Cervical, Liver, Lymphoma, and Stomach Cancer Cells. J. Agric. Food Chem. 2005, 53, 6162–6169. [Google Scholar] [CrossRef]
- Lu, M.-K.; Shih, Y.-W.; Chang Chien, T.-T.; Fang, L.-H.; Huang, H.-C.; Chen, P.-S. ALPHA.-Solanine Inhibits Human Melanoma Cell Migration and Invasion by Reducing Matrix Metalloproteinase-2/9 Activities. Biol. Pharm. Bull. 2010, 33, 1685–1691. [Google Scholar] [CrossRef]
- Mensinga, T.T.; Sips, A.J.A.M.; Rompelberg, C.J.M.; van Twillert, K.; Meulenbelt, J.; van den Top, H.J.; van Egmond, H.P. Potato glycoalkaloids and adverse effects in humans: An ascending dose study. Regul. Toxicol. Pharmacol. 2005, 41, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Szymandera-Buszka, K.; Zielińska-Dawidziak, M.; Makowska, A.; Majcher, M.; Jędrusek-Golińska, A.; Kaczmarek, A.; Niedzielski, P. Quality assessment of corn snacks enriched with soybean ferritin among young healthy people and patient with Crohn’s disease: The effect of extrusion conditions. Int. J. Food Sci. Technol. 2021, 56, 6463–6473. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wadhwa, S.S. Industry-Relevant Approaches for Minimising the Bitterness of Bioactive Compounds in Functional Foods: A Review. Food Bioprocess Technol. 2013, 6, 607–627. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Waliat, S.; Arshad, M.S.; Hanif, H.; Ejaz, A.; Khalid, W.; Kauser, S.; Al-Farga, A. A review on bioactive compounds in sprouts: Extraction techniques, food application and health functionality. Int. J. Food Prop. 2023, 26, 647–665. [Google Scholar] [CrossRef]

| Ingredient [%] | PBB1 | PBB2 | PBB3 | PBB4 |
|---|---|---|---|---|
| Protein base | 40 | 40 | 40 | 40 |
| Coconut oil | 5 | 5 | 5 | 5 |
| Oil blend | 6 | 6 | 6 | 6 |
| Potato starch | 4 | 4 | 4 | 4 |
| Corn starch | 2 | 2 | 2 | 2 |
| Yeast flakes with vitamin B12 | 4 | 4 | 4 | 4 |
| Oat flakes | 4 | 4 | 4 | 4 |
| Methylcellulose | 2 | 2 | 2 | 2 |
| Carrageenan | 2 | 2 | 2 | 2 |
| Aroma | 2 | 2 | 2 | 2 |
| Dried beetroot juice | 0.75 | 0.75 | 0.75 | 0.75 |
| Salt | 0.5 | 0.5 | 0.5 | 0.5 |
| Vinegar | 0.5 | 0.5 | 0.5 | 0.5 |
| Potato fiber | 2 | 0 | 2 | 0 |
| Oat fiber | 0 | 2 | 0 | 2 |
| Powdered sprouts containing ferritin | 1.5 | 1.5 | 0 | 0 |
| Iron (II) sulfate | 0 | 0 | 0.007 | 0.007 |
| Water | 23.75 | 23.75 | 25.243 | 25.243 |
| Parameter | Unit | Sample | |||
|---|---|---|---|---|---|
| PBB1 | PBB2 | PBB3 | PBB4 | ||
| Protein content | g/100 g | 21.59 ± 0.35 a | 21.66 ± 0.86 a | 22.16 ± 0.95 a | 20.80 ± 1.06 a |
| Fat content | g/100 g | 9.63 ± 0.77 a | 9.65 ± 0.60 a | 9.62 ± 0.63 a | 10.03 ± 0.92 a |
| Fiber content | g/100 g | 8.35 ± 0.18 b | 9.20 ± 0.03 a | 8.42 ± 0.37 b | 9.08 ± 0.11 ab |
| including: | |||||
| IDF | g/100 g | 7.45 ± 0.20 b | 8.08 ± 0.03 a | 7.54 ± 0.39 b | 8.05 ± 0.13 a |
| SDF | g/100 g | 0.90 ± 0.03 b | 1.12 ± 0.02 a | 0.88 ± 0.03 b | 1.03 ± 0.05 ab |
| Carbohydrate content | g/100 g | 10.13 ± 0.09 b | 7.65 ± 1.52 c | 12.34 ± 0.37 a | 11.22 ± 1.31 ab |
| Mineral content | g/100 g | 7.10 ± 0.08 b | 6.96 ± 0.10 b | 7.56 ± 0.07 a | 6.50 ± 0.06 c |
| Energy value | kcal/100 g | 248.0 ± 7.5 | 237.9 ± 2.9 | 248.9 ± 4.4 | 245.0 ± 7.2 |
| Protein digestibility | % | 95.14 ± 1.66 a | 85.51 ± 1.27 b | 95.84 ± 1.39 a | 84.21 ± 2.01 b |
| Amino Acid | Unit | Sample | |||
|---|---|---|---|---|---|
| PBB1 | PBB2 | PBB3 | PBB4 | ||
| His | g/16 gN | 3.03 ± 0.12 b | 3.48 ± 0.42 ab | 4.15 ± 0.70 a | 3.97 ± 0.10 a |
| Ile | g/16 gN | 4.93 ± 0.61 b | 4.72 ± 0.10 c | 5.92 ± 0.69 a | 5.38 ± 0.14 b |
| Leu | g/16 gN | 10.15 ± 0.99 b | 9.89 ± 0.01 c | 12.11 ± 0.48 a | 10.98 ± 0.21 b |
| Lys | g/16 gN | 5.67 ± 0.51 b | 5.50 ± 0.11 b | 6.88 ± 0.37 a | 6.35 ± 0.43 a |
| Met+Cys | g/16 gN | 2.99 ± 0.23 a | 2.55 ± 0.09 b | 2.05 ± 0.42 b | 2.67 ± 0.08 a |
| Phe+Tyr | g/16 gN | 7.84 ± 1.11 ab | 7.23 ± 0.32 b | 8.57 ± 0.24 a | 6.72 ± 0.22 c |
| Thr | g/16 gN | 6.11 ± 0.43 b | 6.13 ± 0.44 b | 6.99 ± 0.28 a | 6.54 ± 0.13 a |
| Val | g/16 gN | 5.66 ± 0.58 b | 5.56 ± 0.02 b | 6.91 ± 0.71 a | 6.36 ± 0.18 ab |
| Ala | g/16 gN | 6.75 ± 0.04 b | 6.73 ± 0.01 b | 8.12 ± 0.60 a | 7.76 ± 0.15 a |
| Arg | g/16 gN | 7.41 ± 0.43 a | 6.18 ± 0.04 b | 7.41 ± 0.53 a | 6.12 ± 0.16 b |
| Asp+Asn | g/16 gN | 12.47 ± 0.42 b | 12.22 ± 0.09 b | 14.52 ± 1.05 a | 13.73 ± 1.06 a |
| Glu+Gln | g/16 gN | 19.88 ± 0.20 b | 18.92 ± 0.54 b | 23.24 ± 1.77 a | 21.63 ± 0.91 a |
| Gly | g/16 gN | 8.13 ± 0.73 a | 4.21 ± 0.09 c | 4.90 ± 0.31 b | 4.45 ± 0.05 c |
| Pro | g/16 gN | 5.43 ± 0.23 b | 5.23 ± 0.11 b | 6.55 ± 0.38 a | 5.66 ± 0.15 b |
| Ser | g/16 gN | 6.59 ± 1.41 a | 5.66 ± 0.58 a | 6.62 ± 0.10 a | 6.04 ± 0.21 a |
| Fatty Acid | PBB1 | PBB2 | PBB3 | PBB4 |
|---|---|---|---|---|
| C6:0 | 0.259 ± 0.011 | 0.261 ± 0.024 | 0.263 ± 0.009 | 0.258 ± 0.010 |
| C8:0 | 3.346 ± 0.037 | 3.350 ± 0.021 | 3.347 ± 0.013 | 3.345 ± 0.011 |
| C10:0 | 2.655 ± 0.008 | 2.653 ± 0.006 | 2.648 ± 0.010 | 2.651 ± 0.009 |
| C12:0 | 21.043 ± 0.011 | 21.033 ± 0.007 | 21.029 ± 0.006 | 21.052 ± 0.010 |
| C14:0 | 8.409 ± 0.006 | 8.422 ± 0.004 | 8.401 ± 0.007 | 8.416 ± 0.009 |
| C16:0 | 11.137 ± 0.012 | 11.133 ± 0.022 | 11.127 ± 0.016 | 11.140 ± 0.014 |
| C16:1 | 0.109 ± 0.003 | 0.106 ± 0.004 | 0.110 ± 0.003 | 0.102 ± 0.006 |
| C18:0 | 2.335 ± 0.006 | 2.341 ± 0.008 | 2.329 ± 0.006 | 2.334 ± 0.005 |
| C18:1 (n9) | 30.583 ± 0.018 | 30.591 ± 0.026 | 30.576 ± 0.012 | 30.587 ± 0.010 |
| C18:2 (n6) | 14.991 ± 0.012 | 14.983 ± 0.020 | 14.996 ± 0.019 | 14.988 ± 0.011 |
| C18:3 (n3) | 2.286 ± 0.012 | 2.281 ± 0.023 | 2.279 ± 0.018 | 2.292 ± 0.014 |
| C18:3 (n6) | 0.116 ± 0.004 | 0.112 ± 0.008 | 0.120 ± 0.008 | 0.111 ± 0.009 |
| C20:0 | 0.381 ± 0.006 | 0.383 ± 0.004 | 0.377 ± 0.009 | 0.392 ± 0.010 |
| C20:1 | 0.445 ± 0.008 | 0.451 ± 0.019 | 0.416 ± 0.032 | 0.444 ± 0.022 |
| C22:0 | 0.146 ± 0.002 | 0.144 ± 0.006 | 0.144 ± 0.008 | 0.150 ± 0.008 |
| C22:1 | 0.056 ± 0.002 | 0.061 ± 0.002 | 0.058 ± 0.003 | 0.052 ± 0.001 |
| ∑SFA | 49.71 | 49.72 | 49.66 | 49.74 |
| ∑MUFA | 31.19 | 31.21 | 31.16 | 31.18 |
| ∑PUFA | 19.10 | 19.07 | 19.18 | 19.08 |
| Sample | PUFA/SFA | AI | TI | HH |
|---|---|---|---|---|
| PBB1 | 0.35 | 1.35 | 0.73 | 1.18 |
| PBB2 | 0.35 | 1.35 | 0.73 | 1.18 |
| PBB3 | 0.35 | 1.35 | 0.73 | 1.18 |
| PBB4 | 0.35 | 1.35 | 0.73 | 1.18 |
| Parameter | Unit | Sample | |||
|---|---|---|---|---|---|
| PBB1 | PBB2 | PBB3 | PBB4 | ||
| Ca | µg/g dm | 1240 ± 100 a | 1210 ± 100 a | 1250 ± 100 a | 1150 ± 90 a |
| Mg | µg/g dm | 508 ± 41 a | 566 ± 45 a | 490 ± 39 a | 492 ± 39 a |
| Na | µg/g dm | 14,500 ± 1100 a | 14,500 ± 1100 a | 14,800 ± 1200 a | 14,900 ± 1200 a |
| K | µg/g dm | 13,400 ± 1100 a | 13,600 ± 1100 a | 13,200 ± 1100 a | 13,800 ± 1100 a |
| Fe | µg/g dm | 385 ± 31 b | 429 ± 34 a | 58.7 ± 4.7 c | 52.8 ± 4.2 c |
| Zn | µg/g dm | 54.8 ± 4.4 a | 55.6 ± 4.4 a | 54.9 ± 4.4 a | 52.2 ± 4.2 a |
| Cu | µg/g dm | 54.4 ± 4.4 a | 49.7 ± 4.0 a | 40.3 ± 3.2 b | 40.2 ± 3.2 b |
| Mn | µg/g dm | 47.3 ± 1.4 b | 47.9 ± 3.8 b | 49.4 ± 4.0 a | 49.6 ± 4.0 a |
| Pb | µg/g dm | 94.4 ± 7.6 a | 81.8 ± 6.5 a | 56.7 ± 4.5 b | 56.6 ± 4.5 b |
| Cd | µg/g dm | 0.957 ± 0.068 b | 1.112 ± 0.143 ab | 1.061 ± 0.101 b | 1.296 ± 0.300 a |
| Parameter | Unit | Sample | |||
|---|---|---|---|---|---|
| PBB1 | PBB2 | PBB3 | PBB4 | ||
| Before digestion process | |||||
| TPC | mg/g dm | 0.380 ± 0.082 a | 0.343 ± 0.024 a | 0.167 ± 0.020 b | 0.380 ± 0.249 a |
| TEACABTS | µmol/g dm | 3.305 ± 0.653 b | 4.726 ± 0.046 a | 3.408 ± 0.455 b | 4.621 ± 0.446 a |
| TEACFRAP | µmol/g dm | 1.230 ± 0.043 b | 1.466 ± 0.059 a | 0.823 ± 0.040 c | 1.275 ± 0.048 b |
| Gallic acid | µg/g dm | 2.464 ± 0.068 b | 2.658 ± 0.386 a | 2.501 ± 0.001 b | 2.548 ± 0.033 ab |
| Caffeic acid | µg/g dm | 0.952 ± 0.063 a | 0.402 ± 0.021 b | 0.964 ± 0.044 a | 0.438 ± 0.019 b |
| Ferulic acid | µg/g dm | 1.210 ± 0.044 a | 1.085 ± 0.071 b | 1.175 ± 0.100 a | 1.025 ± 0.054 b |
| Chlorogenic acid | µg/g dm | 3.315 ± 0.064 a | 2.781 ± 0.154 b | 3.216 ± 0.150 a | 3.107 ± 0.105 ab |
| After digestion process | |||||
| TPC | mg/g dm | 4.016 ± 0.092 a | 3.616 ± 0.177 b | 3.687 ± 0.369 ab | 4.141 ± 0.202 a |
| TEACABTS | µmol/g dm | 50.56 ± 6.78 b | 75.56 ± 9.16 a | 51.39 ± 10.17 b | 78.58 ± 7.26 a |
| TEACFRAP | µmol/g dm | 2.296 ± 0.301 a | 1.875 ± 0.342 b | 1.751 ± 0.098 b | 2.293 ± 0.204 a |
| Gallic acid | µg/g dm | 1.541 ± 0.161 a | 1.601 ± 0.082 a | 1.653 ± 0.019 a | 1.640 ± 0.063 a |
| Caffeic acid | µg/g dm | 0.116 ± 0.017 a | 0.123 ± 0.011 a | 0.118 ± 0.007 a | 0.123 ± 0.003 a |
| Ferulic acid | µg/g dm | 0.938 ± 0.031 a | 0.811 ± 0.010 b | 0.894 ± 0.131 a | 0.816 ± 0.010 b |
| Chlorogenic acid | µg/g dm | 0.697 ± 0.093 a | 0.804 ± 0.043 a | 0.744 ± 0.001 a | 0.749 ± 0.035 a |
| Parameter | Unit | Sample | |||
|---|---|---|---|---|---|
| PBB1 | PBB2 | PBB3 | PBB4 | ||
| Before digestion process | |||||
| α-solanine content | µg/g dm | 40.69 ± 2.68 a | 28.74 ± 3.29 b | 39.57 ± 0.22 a | 22.28 ± 11.56 c |
| α-chaconine content | µg/g dm | 15.11 ± 2.49 a | 5.09 ± 0.57 b | 15.78 ± 0.43 a | 3.48 ± 0.96 c |
| After digestion process | |||||
| α-solanine content | µg/g dm | 3.43 ± 0.57 bc | 2.59 ± 0.17 c | 5.97 ± 0.44 a | 3.66 ± 0.77 b |
| α-chaconine content | µg/g dm | 1.13 ± 0.15 b | 0.37 ± 0.06 c | 2.10 ± 0.06 a | 0.53 ± 0.18 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczewski, P.Ł.; Wróbel, M.M.; Smarzyński, K.; Zembrzuska, J.; Ślachciński, M.; Jeżowski, P.; Tomczak, A.; Kulczyński, B.; Zielińska-Dawidziak, M.; Sałek, K.; et al. Potato Protein-Based Vegan Burgers Enriched with Different Sources of Iron and Fiber: Nutrition, Sensory Characteristics, and Antioxidants before and after In Vitro Digestion. Foods 2024, 13, 3060. https://doi.org/10.3390/foods13193060
Kowalczewski PŁ, Wróbel MM, Smarzyński K, Zembrzuska J, Ślachciński M, Jeżowski P, Tomczak A, Kulczyński B, Zielińska-Dawidziak M, Sałek K, et al. Potato Protein-Based Vegan Burgers Enriched with Different Sources of Iron and Fiber: Nutrition, Sensory Characteristics, and Antioxidants before and after In Vitro Digestion. Foods. 2024; 13(19):3060. https://doi.org/10.3390/foods13193060
Chicago/Turabian StyleKowalczewski, Przemysław Łukasz, Martyna Maria Wróbel, Krzysztof Smarzyński, Joanna Zembrzuska, Mariusz Ślachciński, Paweł Jeżowski, Aneta Tomczak, Bartosz Kulczyński, Magdalena Zielińska-Dawidziak, Karina Sałek, and et al. 2024. "Potato Protein-Based Vegan Burgers Enriched with Different Sources of Iron and Fiber: Nutrition, Sensory Characteristics, and Antioxidants before and after In Vitro Digestion" Foods 13, no. 19: 3060. https://doi.org/10.3390/foods13193060
APA StyleKowalczewski, P. Ł., Wróbel, M. M., Smarzyński, K., Zembrzuska, J., Ślachciński, M., Jeżowski, P., Tomczak, A., Kulczyński, B., Zielińska-Dawidziak, M., Sałek, K., & Kmiecik, D. (2024). Potato Protein-Based Vegan Burgers Enriched with Different Sources of Iron and Fiber: Nutrition, Sensory Characteristics, and Antioxidants before and after In Vitro Digestion. Foods, 13(19), 3060. https://doi.org/10.3390/foods13193060













