Intestinal-Targeted Digestion of Heme Chloride by Forming Inclusion Complexes In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Preparation of Inclusion Complexes of Hemin with CDs
2.3. The Preparation of Physical Mixture of Hemin with CDs
2.4. Determination of Encapsulation Efficiency (EE)
2.5. Determination of Solubility
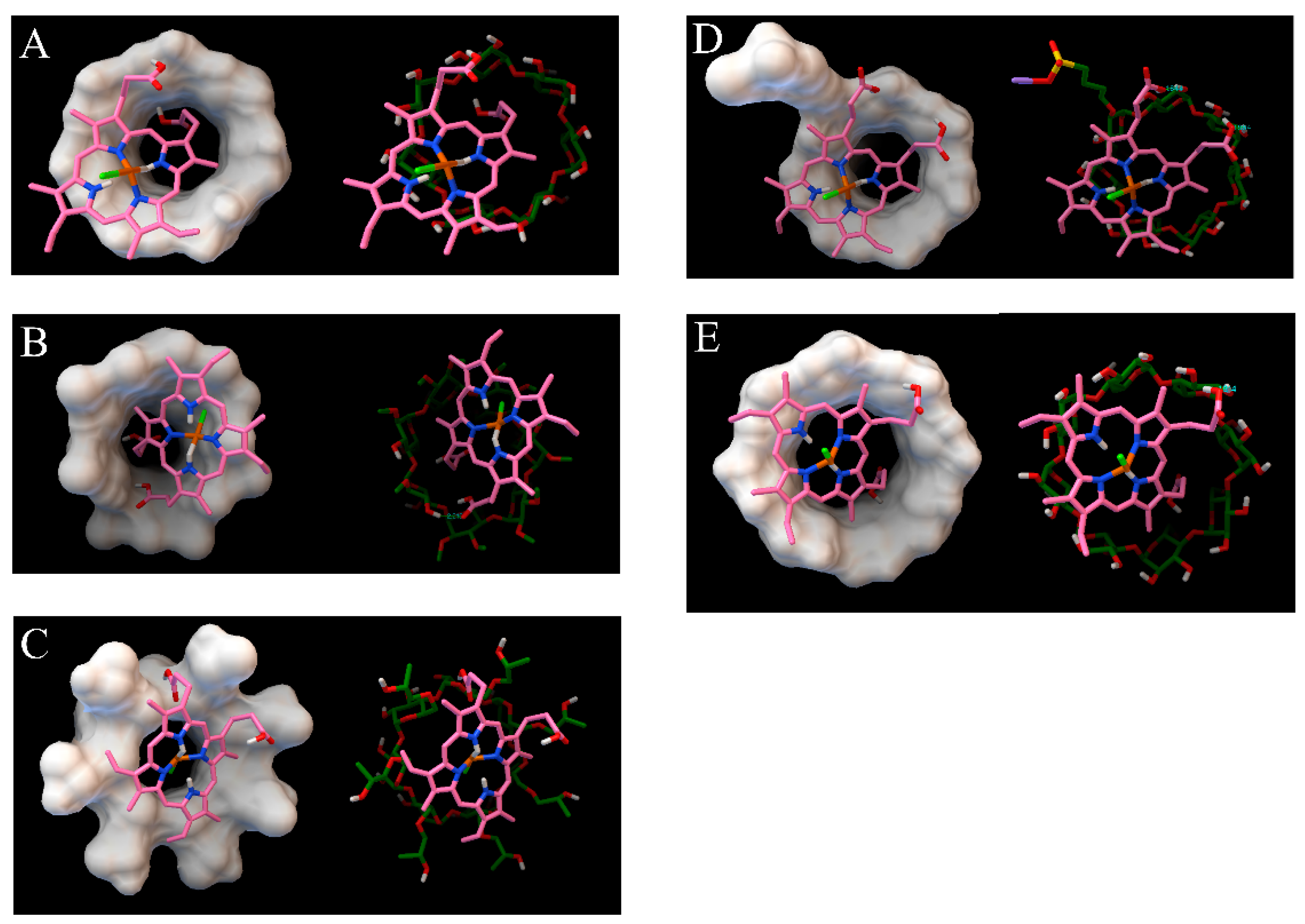
2.6. Molecular Docking
2.7. Characterization of the Inclusion Complexes
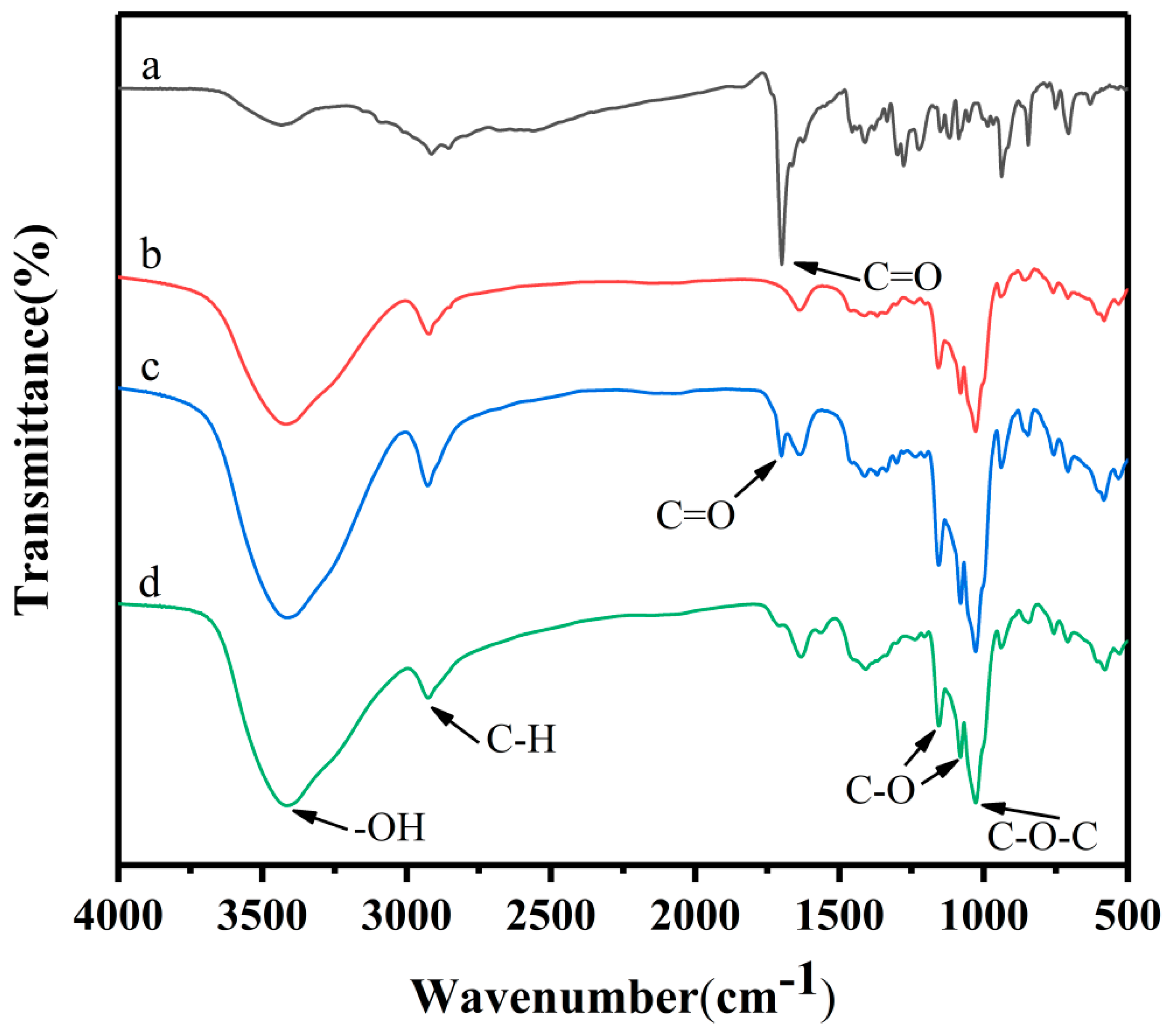
2.7.1. Fourier Transform-Infrared Spectroscopy (FTIR)
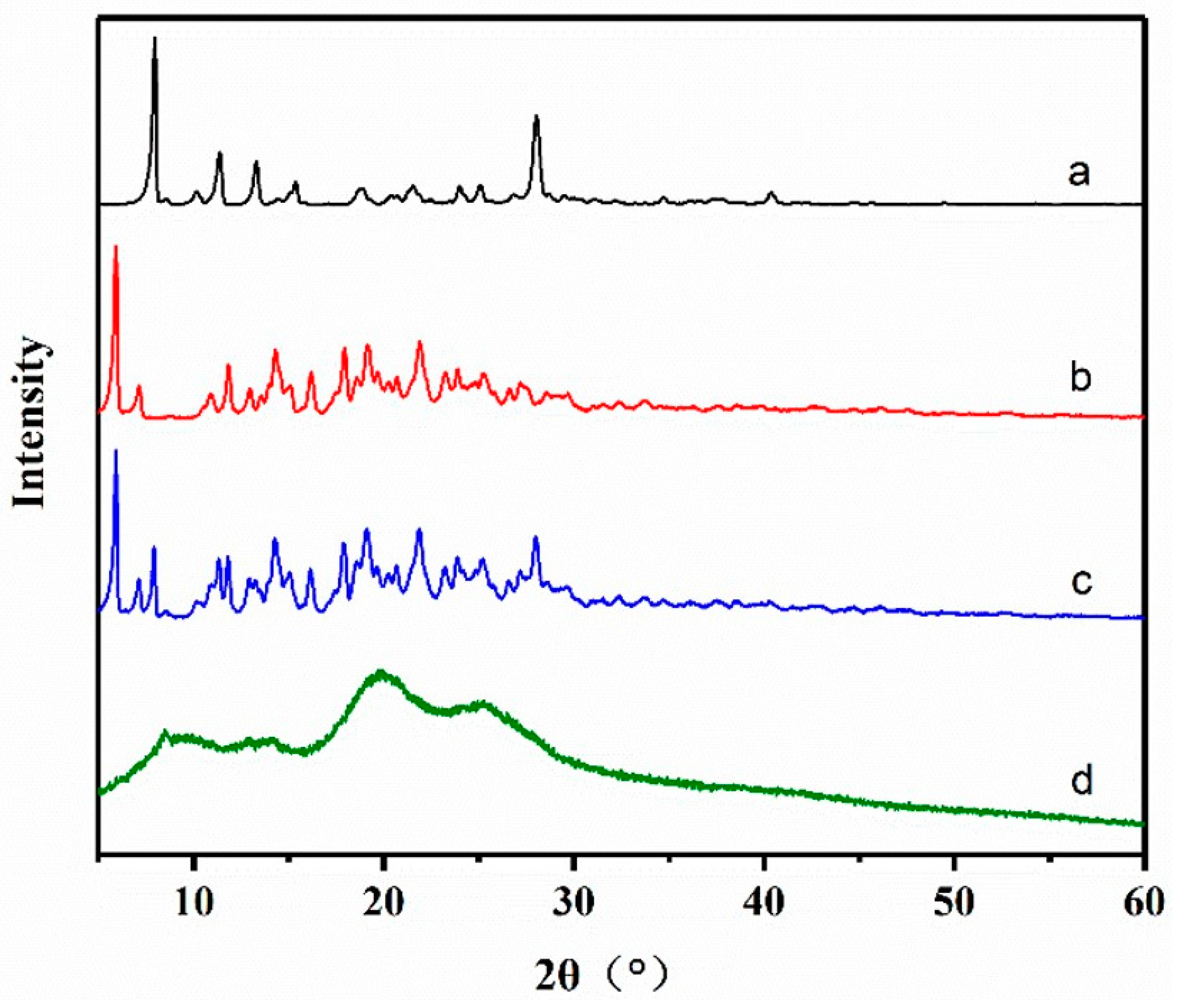
2.7.2. X-ray Diffractometry (XRD)
2.7.3. Scanning Electron Microscopy (SEM)
2.8. Cultures of IEC-6 Cells
2.9. Cytotoxicity Assay of IEC-6 Cells
2.10. Simulated Gastrointestinal Digestion In Vitro
2.10.1. Static Digestion Simulation
2.10.2. Dynamic Digestion Simulation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Cyclodextrin Selection by MD
3.2. Encapsulation Efficiency Analysis
3.3. Dispersibility Analysis
3.4. Characterization of the Inclusion Complexes
3.4.1. FTIR
3.4.2. XRD
3.4.3. SEM
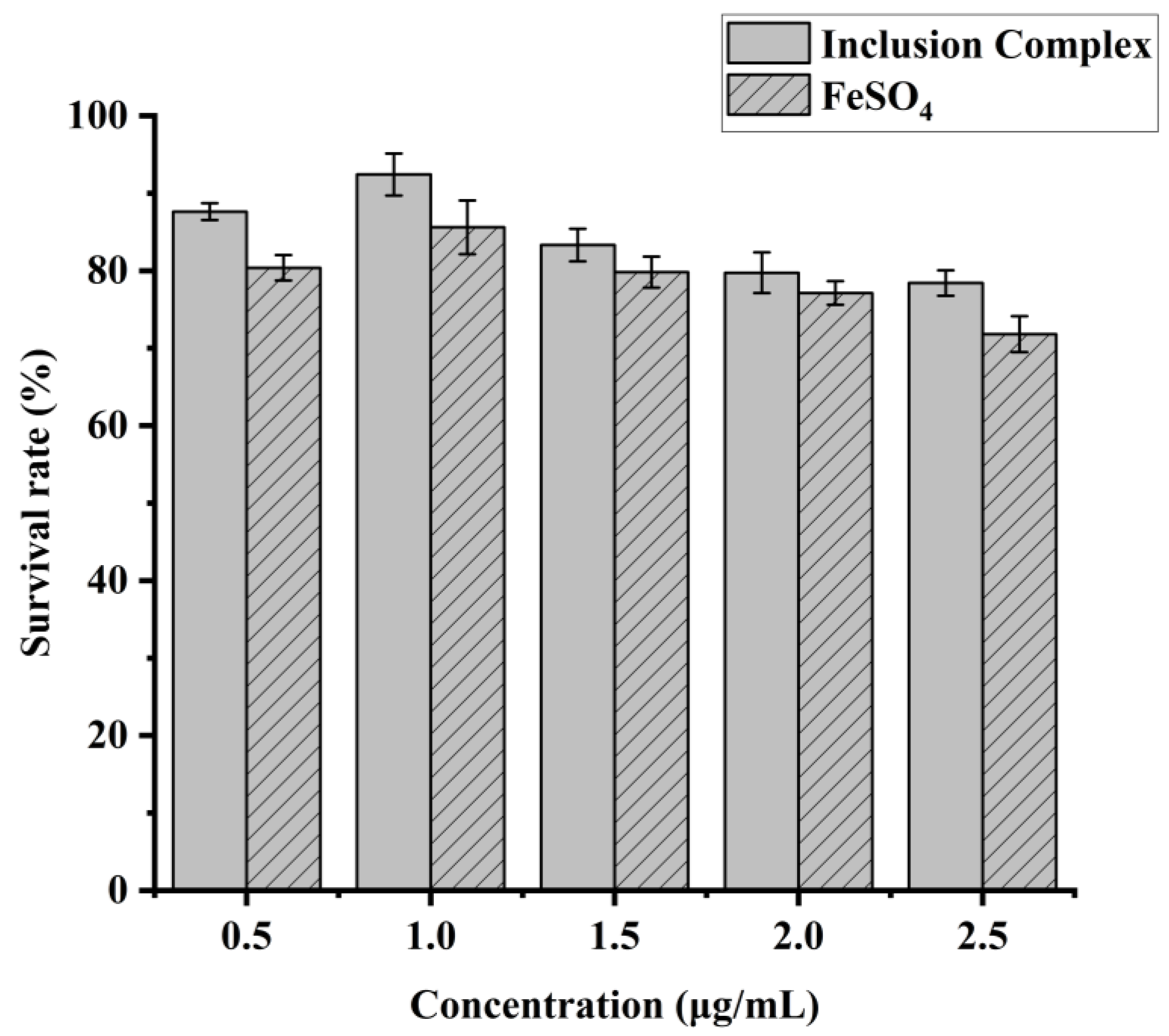
3.5. Cytotoxicity Assay of IEC-6 Cells
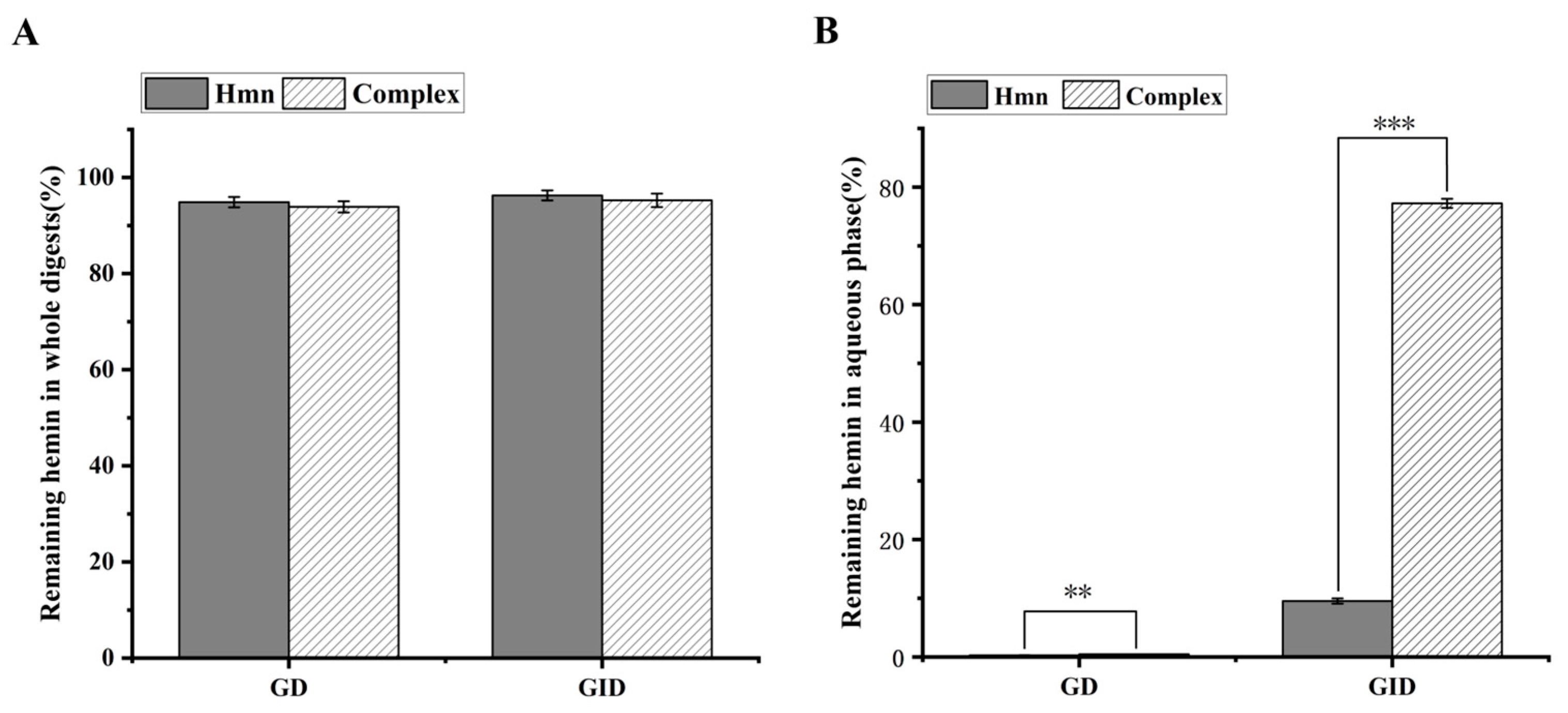
3.6. In Vitro Digestion of Hemin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conrad, M.E.; Cortell, S.; Williams, H.L.; Foy, A.L. Polymerization and intraluminal factors in the absorption of hemoglobin-iron. J. Clin. Med. 1966, 68, 659–668. [Google Scholar]
- Vaghefi, N.; Nedjaoum, F.; Guillochon, D.; Bureau, F.; Arhan, P.; Bougle, D. Iron absorption from concentrated hemoglobin hydrolysate by rat. J. Nutr. Biochem. 2005, 16, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, D.; Xu, J.; Xu, C.; Dong, C.; Liu, Q.; Deng, S.; Zhao, J.; Zhang, W.; Chen, X. Effects of Dietary Factors on the Pharmacokinetics of 58Fe-labeled Hemin after Oral Administration in Normal Rats and the Iron-deficient Rats. Biol. Trace. Elem. Res. 2013, 153, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, X.; Chen, R.; He, L.; Zheng, L. Observation on the effect of hemin in improving human anaemia. J. Hyg. Res. 2000, 4, 242. [Google Scholar]
- Huang, P.; Yuan, X.; Hong, Q.; Lv, F. Development and efficacy observation of hemin oral solution. J. Chin. Pharm. Sci. 1995, 11, 664–666. [Google Scholar]
- Deng, X.; Zhang, N.; Tang, C. Soy protein isolate as a nanocarrier for enhanced water dispersibility, stability and bioaccessibility of -carotene. J. Sci. Food. Agric. 2017, 97, 2230–2237. [Google Scholar] [CrossRef]
- Liu, Q.; Han, C.; Tian, Y.; Liu, T. Fabrication of curcumin-loaded zein nanoparticles stabilized by sodium caseinate/sodium alginate: Curcumin solubility, thermal properties, rheology, and stability. Process Biochem. 2020, 94, 30–38. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, L.; Li, M.; Xiao, J.; Geng, X.; Wang, B.; Sun, Q. Preparation and Characterization of Tadpole- and Sphere-Shaped Hemin Nanoparticles for Enhanced Solubility. Nanoscale Res. Lett. 2019, 14, 47. [Google Scholar] [CrossRef]
- Churio, O.; Duran, E.; Guzman-Pino, S.A.; Valenzuela, C. Use of Encapsulation Technology to Improve the Efficiency of an Iron Oral Supplement to Prevent Anemia in Suckling Pigs. Animals 2019, 9, 1. [Google Scholar] [CrossRef]
- Fan, C.; Yuan, J.; Guo, J.; Kang, X. Soy protein isolate (SPI)-hemin complex nanoparticles as a novel water-soluble iron-fortifier: Fabrication, formation mechanism and in vitro bioavailability. Food Biosci. 2022, 49, 101889. [Google Scholar] [CrossRef]
- Span, K.; Verhoef, J.J.F.; Hunt, H.; van Nostrum, C.F.; Brinks, V.; Schellekens, H.; Hennink, W.E. A novel oral iron-complex formulation: Encapsulation of hemin in polymeric micelles and its in vitro absorption. Eur. J. Pharm. Biopharm. 2016, 108, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Churio, O.; Pizarro, F.; Valenzuela, C. Preparation and characterization of iron-alginate beads with some types of iron used in supplementation and fortification strategies. Food Hydrocoll. 2018, 74, 1–10. [Google Scholar] [CrossRef]
- Valenzuela, C.; Hernandez, V.; Morales, M.S.; Neira-Carrillo, A.; Pizarro, F. Preparation and characterization of heme iron-alginate beads. Lwt-Food Sci. Technol. 2014, 59, 1283–1289. [Google Scholar] [CrossRef]
- Navas-Carretero, S.; Perez-Granados, A.M.; Sarria, B.; Vaquero, M.P. Iron absorption from meat pate fortified with ferric pyrophosphate in iron-deficient women. Nutrition 2009, 25, 20–24. [Google Scholar] [CrossRef]
- Valdes, F.; Carrillo, R.; Campos, F.; Saenz, L.; Valenzuela, C. Encapsulation of atomized erythrocytes in liposomes as source of heme iron for oral supplementation strategies. J. Food Process Eng. 2023, 46, e14393. [Google Scholar] [CrossRef]
- Churio, O.; Valenzuela, C. Development and characterization of maltodextrin microparticles to encapsulate heme and non-heme iron. Lwt-Food Sci. Technol. 2018, 96, 568–575. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, F.; Gao, S.; Ge, W.; Zhang, M. Double enzymatic hydrolysis preparation of heme from goose blood and microencapsulation to promote its stability and absorption. Food Chem. 2017, 217, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Li, T.; Guo, R.; Zong, Q.; Ling, G. Application of molecular docking in elaborating molecular mechanisms and interactions of supramolecular cyclodextrin. Carbohydr. Polym. 2022, 276, 118644. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef]
- Yuan, X.; Hong, Q.; Lin, G.; Lin, F. Development of hemin-βcyclodextrin inclusion complexes. Chin. Pharm. 2001, 6, 31–33. [Google Scholar]
- Bayat, F.; Homami, S.S.; Monzavi, A.; Olyai, M.R.T.B. A combined molecular docking and molecular dynamics simulation approach to probing the host-guest interactions of Ataluren with natural and modified cyclodextrins. Mol. Simul. 2022, 48, 108–119. [Google Scholar] [CrossRef]
- Gannimani, R.; Perumal, A.; Ramesh, M.; Pillay, K.; Soliman, M.E.; Govender, P. Antipyrine-gamma cyclodextrin inclusion complex: Molecular modeling, preparation, characterization and cytotoxicity studies. J. Mol. Struct. 2015, 1089, 38–47. [Google Scholar] [CrossRef]
- Yousaf, A.M.; Qadeer, A.; Raza, S.A.; Chohan, T.A.; Shahzad, Y.; Din, F.U.; Khan, I.U.; Hussain, T.; Alvi, M.N.; Mahmood, T. Influence of levodropropizine and hydroxypropyl-beta-cyclodextrin association on the physicochemical characteristics of levodropropizine loaded in hydroxypropyl-beta-cyclodextrin microcontainers: Formulation and in vitro characterization. Polim Med. 2019, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Oates, P.S. IEC-6 cells are an appropriate model of intestinal iron absorption in rats. J. Nutr. 2002, 132, 680–687. [Google Scholar] [CrossRef]
- Ding, B.; Yu, Y.; Geng, S.; Liu, B.; Hao, Y.; Liang, G. Computational Methods for the Interaction between Cyclodextrins and Natural Compounds: Technology, Benefits, Limitations, and Trends. J. Agric. Food. Chem. 2022, 70, 2466–2482. [Google Scholar] [CrossRef]
- Gatiatulin, A.K.; Oselskaya, V.Y.; Klimovitskii, A.E.; Ziganshin, M.A.; Gorbachuk, V.V. Influence of hydration and the size of the macrocycle of natice cyclodextrins on the solid phase inclusion of ritonavir. J. Struct. Chem. 2023, 64, 1702–1714. [Google Scholar] [CrossRef]
- Yang, B.; Yang, L.; Lin, J.; Chen, Y.; Liu, Y. Binding behaviors of scutellarin with α, β, γ-cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 149–155. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Chen, Y.; Liao, X.; Gao, C.; Xiao, D.; Qin, Q.; Yi, D.; Yang, B. Host–guest inclusion system of mangiferin with β-cyclodextrin and its derivatives. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Loh, G.O.K.; Tan, Y.T.F.; Peh, K. Enhancement of norfloxacin solubility via inclusion complexation with β-cyclodextrin and its derivative hydroxypropyl-β-cyclodextrin. Asian J. Pharm. Sci. 2016, 11, 536–546. [Google Scholar] [CrossRef]
- Singh, S.; Negi, J.S.; Bisht, R.; Negi, V.; Kasliwal, N.; Thakur, V.; Upadhyay, A. Development and evaluation of orodispersible sustained release formulation of amisulpride–γ-cyclodextrin inclusion complex. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 239–247. [Google Scholar] [CrossRef]
- Muankaew, C.; Jansook, P.; Stefansson, E.; Loftsson, T. Effect of γ-cyclodextrin on solubilization and complexation of irbesartan: Influence of pH and excipients. Int. J. Pharm. 2014, 474, 80–90. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Moriwaki, M.; Ozeki, M.; Timm, D. Structural elucidation of novel isoquercitrin-γ-cyclodextrin (IQC-γCD) molecular inclusion complexes of potential health benefits. Carbohydr. Polym. Technol. Appl. 2021, 2, 100046. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, M.; Zeng, H.; Zhao, H.; Zhang, M.; Yang, Z. Preparation, Characterization and Iron Absorption by Caco-2 Cells of the Casein Peptides-Iron Chelate. Int. J. Pept. Res. Ther. 2022, 28, 116. [Google Scholar] [CrossRef]


| Cyclodextrins | Encapsulation Efficiency (% ± SD, n = 3) |
|---|---|
| β-CD | 70.91 ± 0.86 a |
| Me-β-CD | 70.24 ± 0.61 ab |
| HP-β-CD | 71.67 ± 1.23 a |
| SBE-β-CD | 68.17 ± 0.58 b |
| γ-CD | 72.11 ± 0.08 a |
| Cyclodextrins | Hemin Solubility (μg/mL ± SD, n = 3) | Solubilization Ratio |
|---|---|---|
| β-CD | 21.01 ± 1.82 e | 30.90 |
| Me-β-CD | 75.63 ± 4.49 d | 111.22 |
| HP-β-CD | 136.39 ± 0.80 c | 200.57 |
| SBE-β-CD | 151.84 ± 1.59 b | 223.29 |
| γ-CD | 163.61 ± 1.78 a | 240.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Huang, L.; Zhang, Y.; Teng, W.; Wang, Y.; Cao, J.; Wang, J. Intestinal-Targeted Digestion of Heme Chloride by Forming Inclusion Complexes In Vitro. Foods 2024, 13, 3078. https://doi.org/10.3390/foods13193078
Yu Q, Huang L, Zhang Y, Teng W, Wang Y, Cao J, Wang J. Intestinal-Targeted Digestion of Heme Chloride by Forming Inclusion Complexes In Vitro. Foods. 2024; 13(19):3078. https://doi.org/10.3390/foods13193078
Chicago/Turabian StyleYu, Qianfan, Li Huang, Yuemei Zhang, Wendi Teng, Ying Wang, Jinxuan Cao, and Jinpeng Wang. 2024. "Intestinal-Targeted Digestion of Heme Chloride by Forming Inclusion Complexes In Vitro" Foods 13, no. 19: 3078. https://doi.org/10.3390/foods13193078
APA StyleYu, Q., Huang, L., Zhang, Y., Teng, W., Wang, Y., Cao, J., & Wang, J. (2024). Intestinal-Targeted Digestion of Heme Chloride by Forming Inclusion Complexes In Vitro. Foods, 13(19), 3078. https://doi.org/10.3390/foods13193078






