Phenolic Compositions of Different Fractions from Coffee Silver Skin and Their Antioxidant Activities and Inhibition towards Carbohydrate-Digesting Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
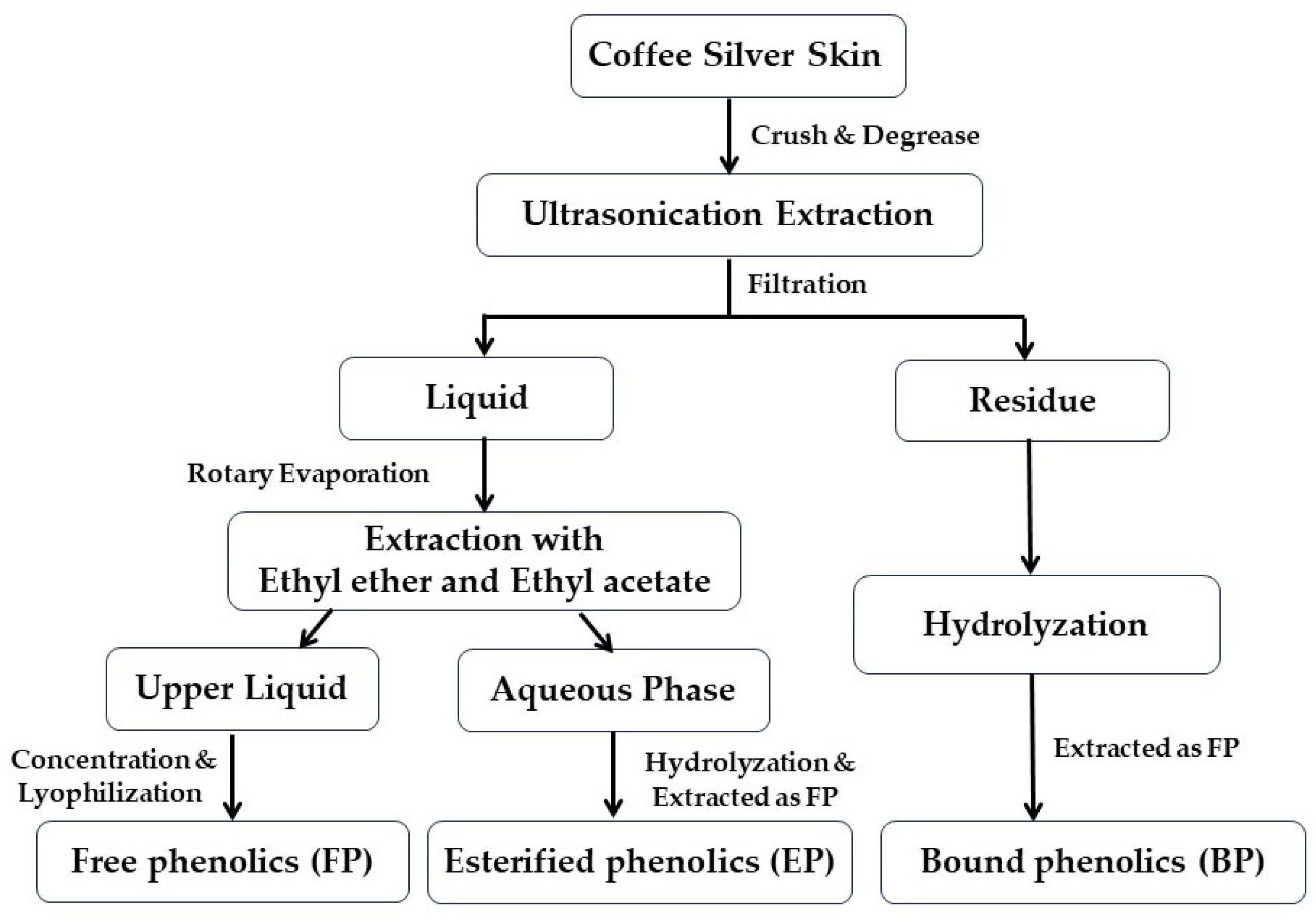
2.2. Extraction of Different Phenolic Fractions
2.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Analysis of Antioxidant Activity
2.5. Determination of Inhibitory Effect on α-Glucosidase and α-Amylase Activity
2.6. Characterization of Phenolics by UHPLC-ESI-HRMS/MS
2.7. Molecular Docking
2.8. Molecular Dynamics Simulation
2.9. Statistical Analysis
3. Results and Discussion
3.1. TPC and TFC of Different CSS Phenolic Fractions
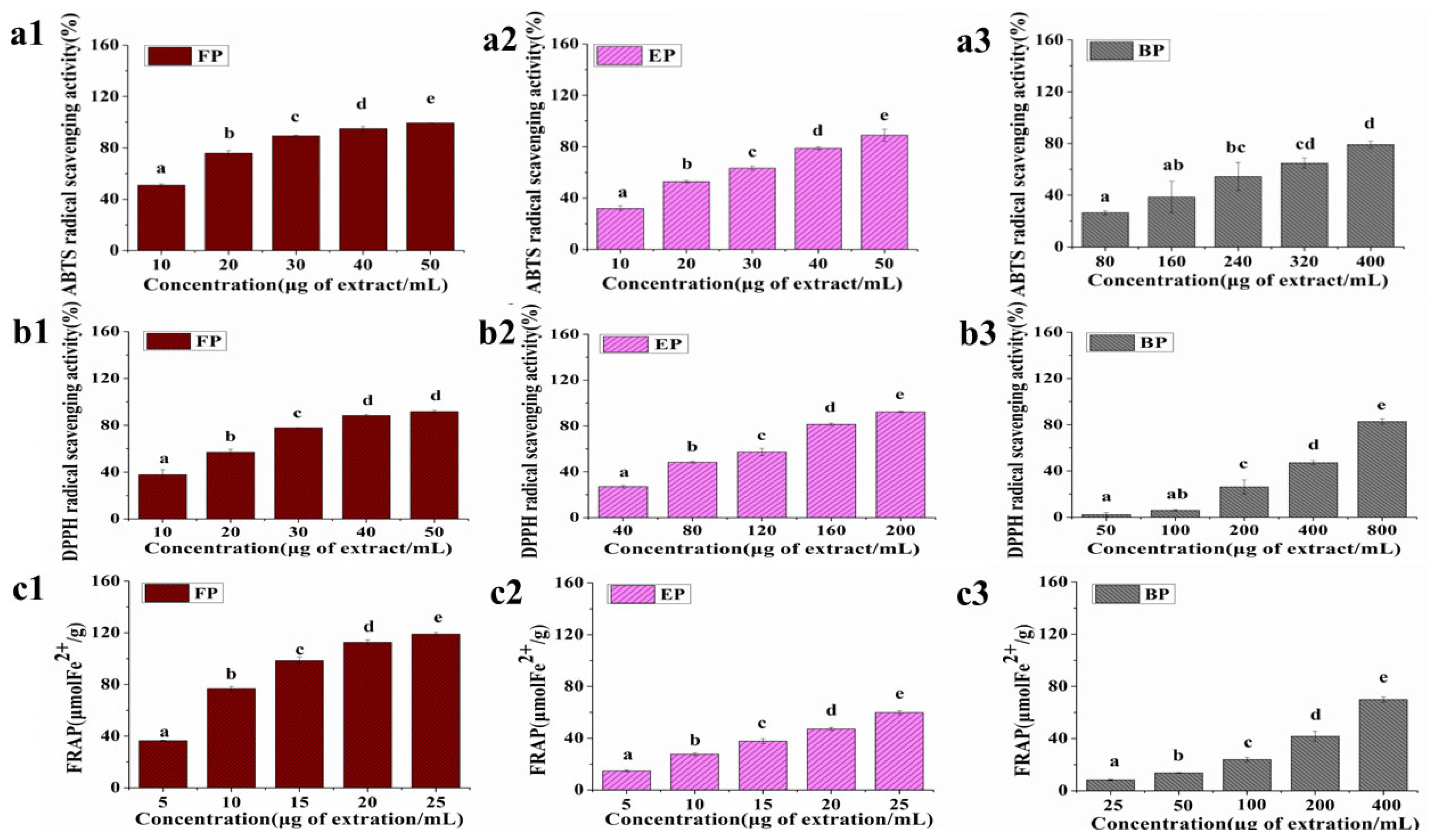
3.2. Antioxidant Capacity of Different CSS Phenolic Fractions
3.3. Inhibitory Effects of CSS Phenolic Fractions on the Activities of α-Glucosidase and α-Amylase
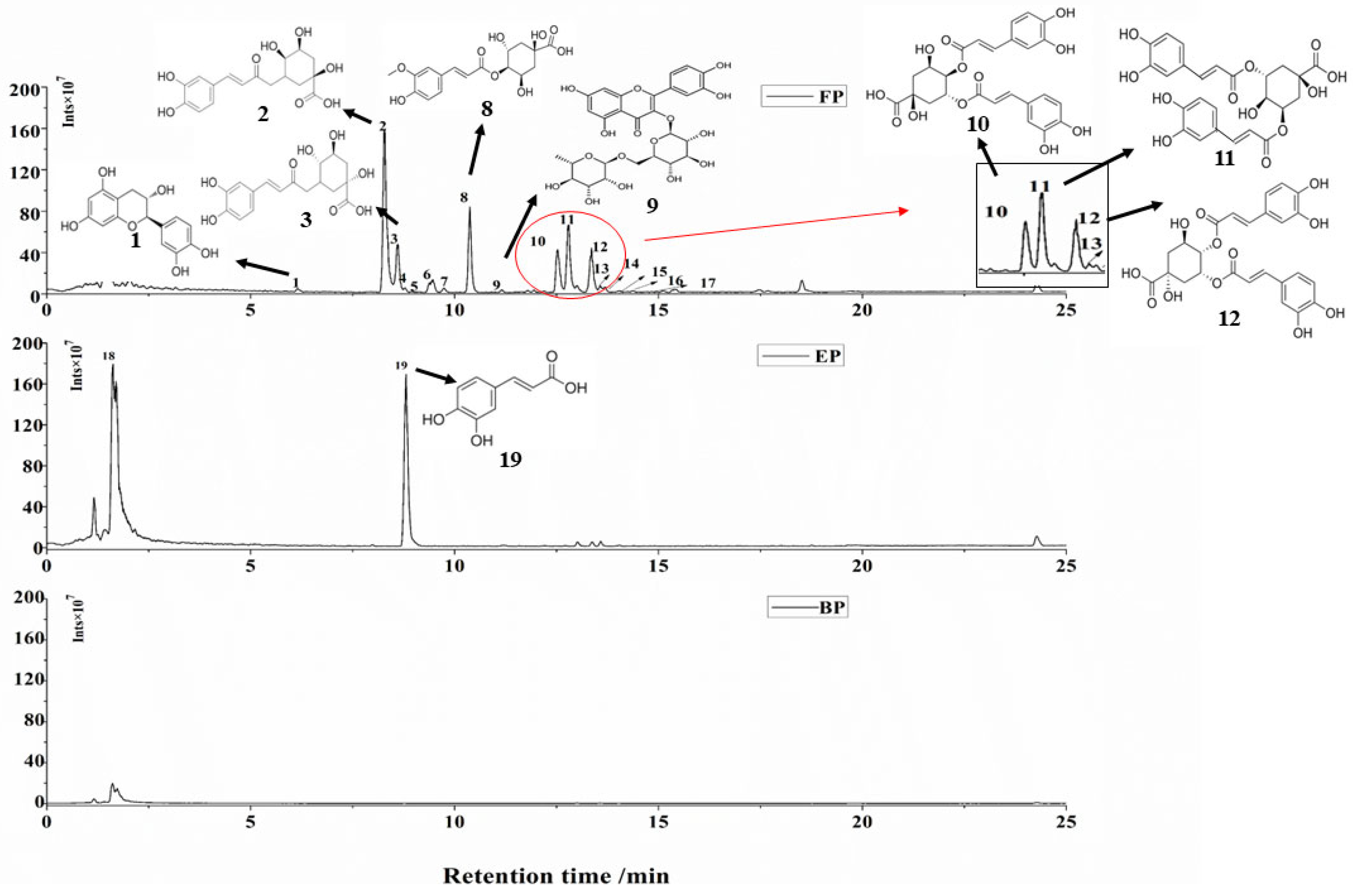
3.4. Identification and Quantitation of CSS Phenolic Compounds
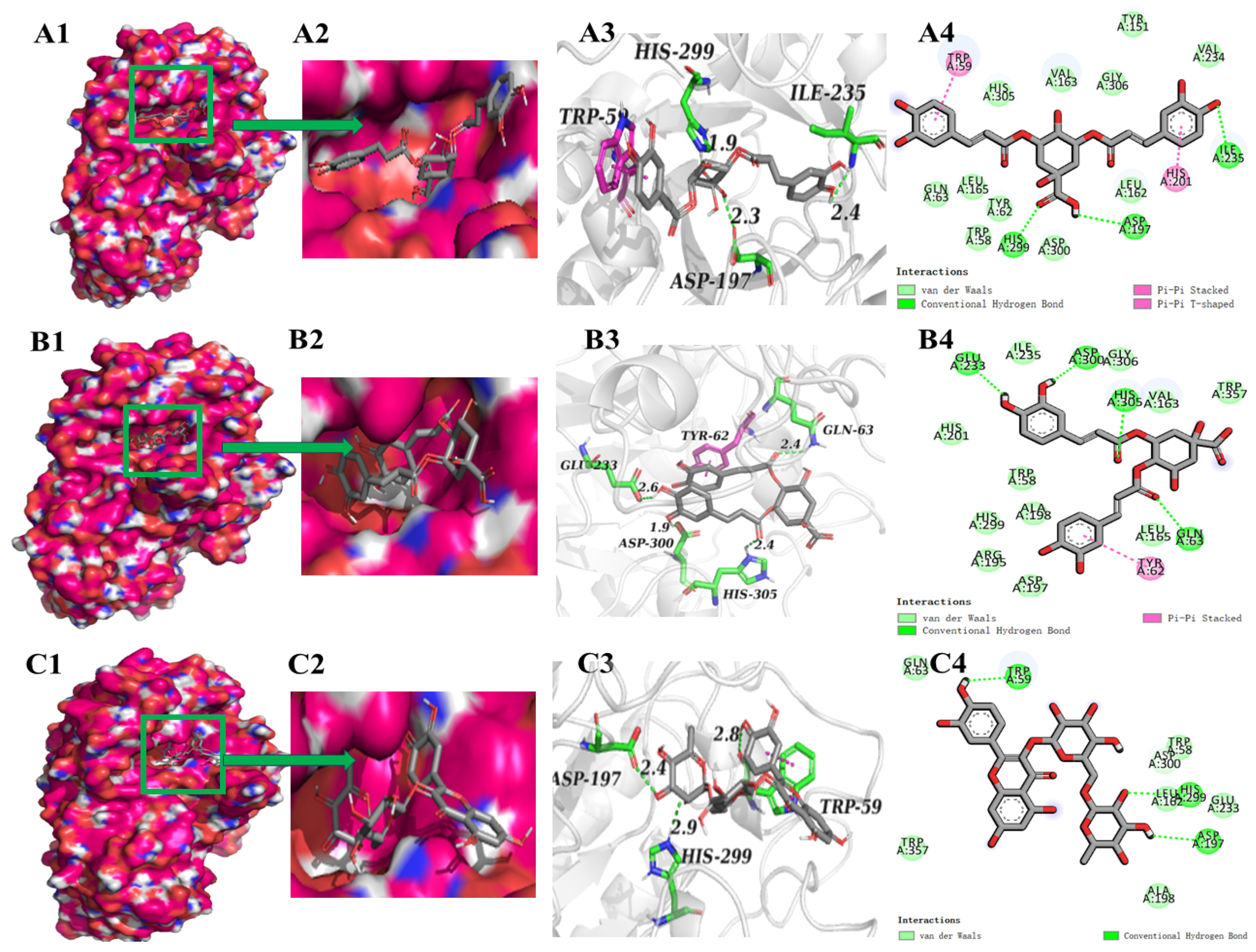
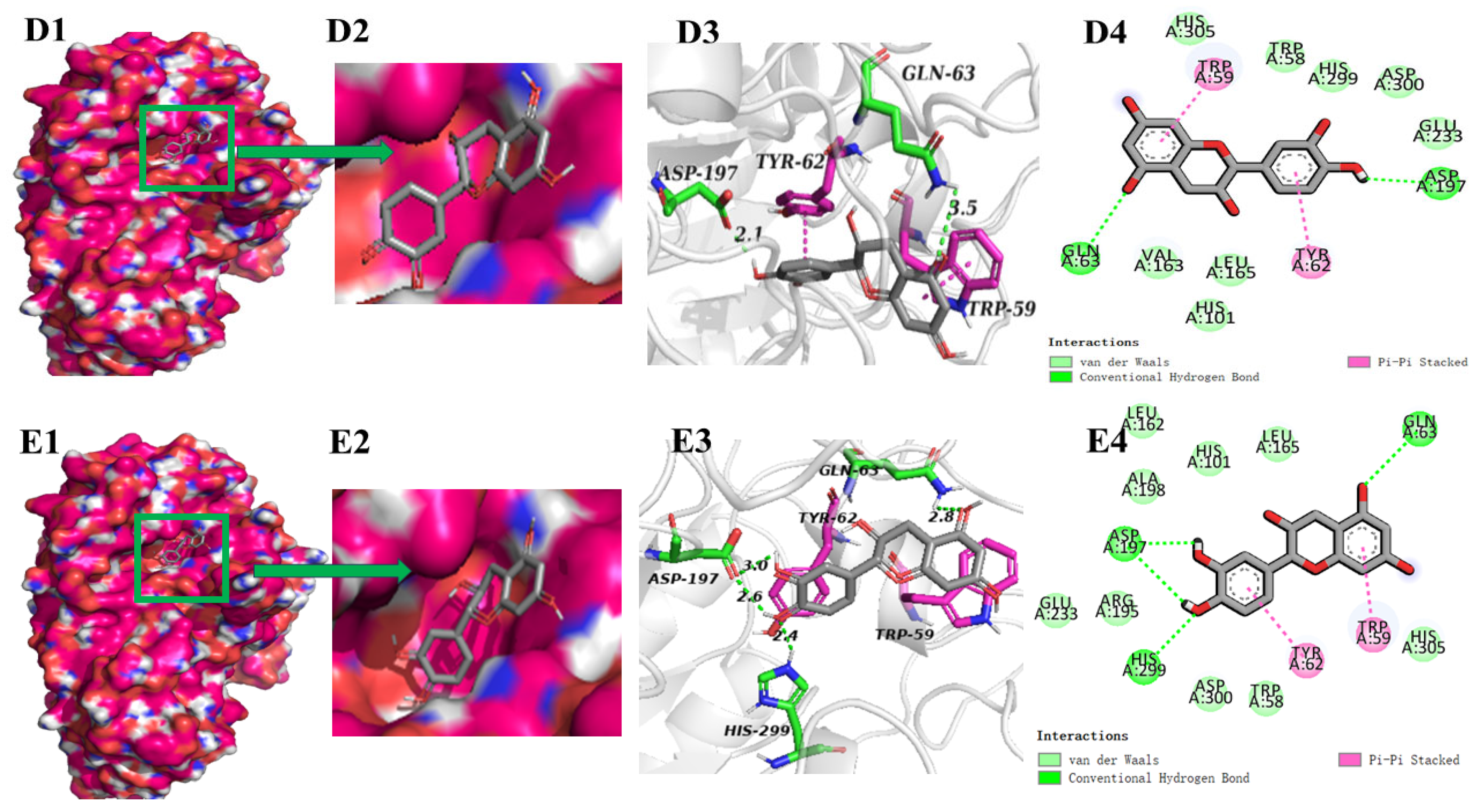
3.5. Screening of Main α-Glucosidase and α-Amylase Inhibitors from FP
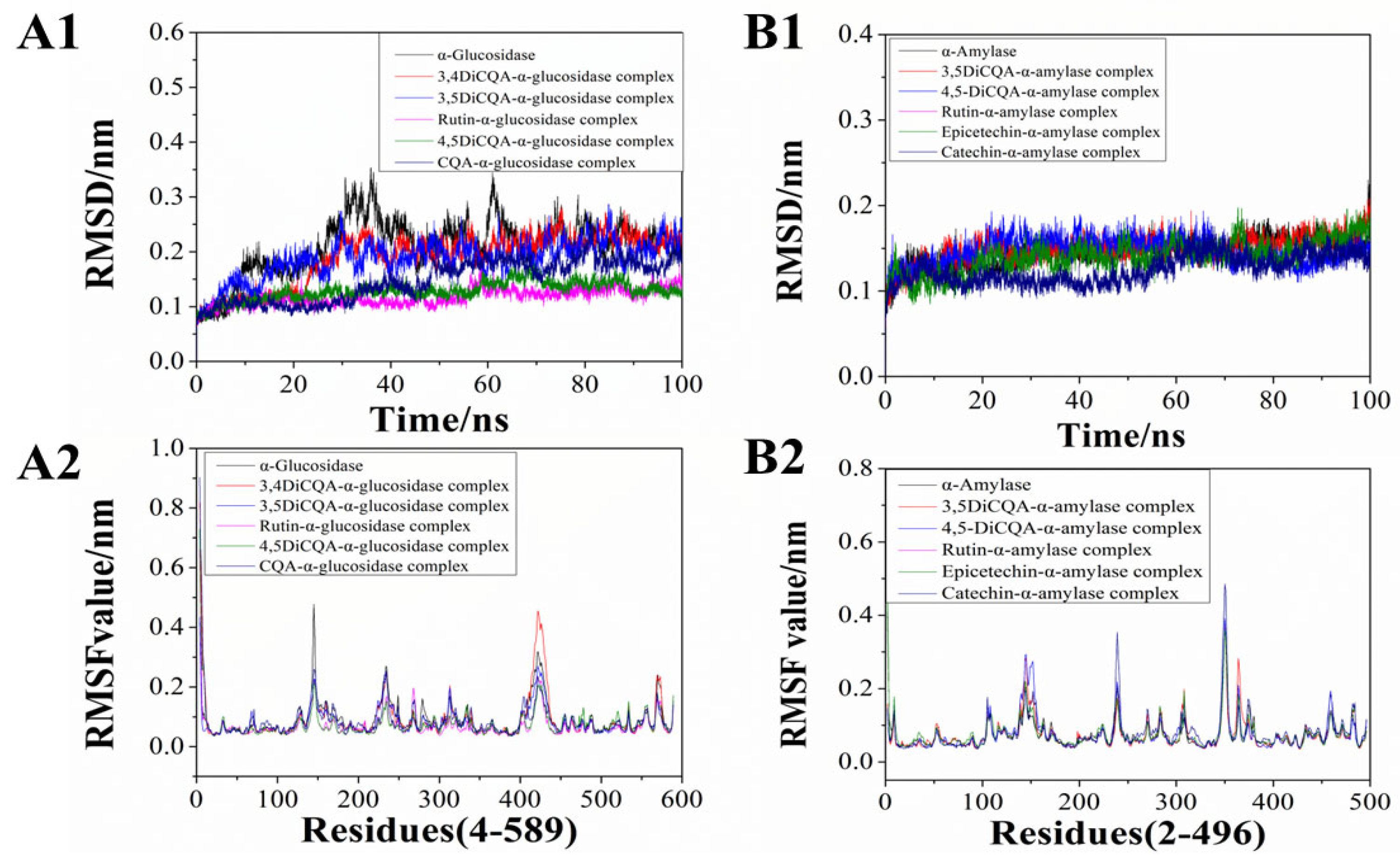
3.6. Molecular Dynamic Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, W.; Zhan, J.; Li, S.; Liu, Y.; Ho, C.-T. Hypoglycemic effects of naturally processed Polygonum multiflorum extract in KK CgAy/J mice and its mechanism of action. Food Sci. Hum. Wellness 2022, 11, 1177–1182. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Maratni, N.P.T.; Saraswati, M.R.; Dewi, N.N.A.; Yasa, I.; Eka Widyadharma, I.P.; Putra, I.B.K.; Suastika, K. Association of Apolipoprotein E Gene Polymorphism with Lipid Profile and Ischemic Stroke Risk in Type 2 Diabetes Mellitus Patients. J. Nutr. Metab. 2021, 2021, 5527736. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Han, J.X.; Wang, H.; Liang, H.H.; Guo, J.X. Correlation of the retinopathy degree with the change of ocular surface and corneal nerve in patients with type 2 diabetes mellitus. Int. J. Ophthalmol. 2021, 14, 750–758. [Google Scholar] [CrossRef]
- Wu, B.; Niu, Z.; Hu, F. Study on Risk Factors of Peripheral Neuropathy in Type 2 Diabetes Mellitus and Establishment of Prediction Model. Diabetes Metab. J. 2021, 45, 526–538. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Tomé, S.M.; Oliveira, E.F.T.; Viegas, M.F.; Araújo, A.N.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; et al. Evaluation of a flavonoids library for inhibition of pancreatic α-amylase towards a structure-activity relationship. J. Enzym. Inhib. Med. Chem. 2019, 34, 577–588. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3137–3207. [Google Scholar] [CrossRef]
- Pradeep, P.M.; Sreerama, Y.N. Phenolic antioxidants of foxtail and little millet cultivars and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2018, 247, 46–55. [Google Scholar] [CrossRef]
- Bikkad, M.D.; Somwanshi, S.D.; Ghuge, S.H.; Nagane, N. Oxidative stress in type II diabetes mellitus. Biomed. Res. 2014, 25, 84–87. [Google Scholar]
- Kutan Fenercioglu, A.; Saler, T.; Genc, E.; Sabuncu, H.; Altuntas, Y. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J. Endocrinol. Investig. 2010, 33, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Antonelli, A.; Balercia, G.; Sabatelli, S.; Maggi, F.; Caprioli, G.; Giacchetti, G.; Micucci, M. Antioxidant, Anti-Inflammatory, Anti-Diabetic, and Pro-Osteogenic Activities of Polyphenols for the Treatment of Two Different Chronic Diseases: Type 2 Diabetes Mellitus and Osteoporosis. Biomolecules 2024, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef]
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 2016, 20, 472–485. [Google Scholar] [CrossRef]
- Jirarat, W.; Kaewsalud, T.; Yakul, K.; Rachtanapun, P.; Chaiyaso, T. Sustainable Valorization of Coffee Silverskin: Extraction of Phenolic Compounds and Proteins for Enzymatic Production of Bioactive Peptides. Foods 2024, 13, 1230. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, S.; Ma, S.; Zhao, S.; Yi, J.; Zhou, L. Water Caltrop (Trapa quadrispinosa Roxb.) Husk Improves Oxidative Stress and Postprandial Blood Glucose in Diabetes: Phenolic Profiles, Antioxidant Activities and α-Glycosidase Inhibition of Different Fractions with In Vitro and In Silico Analyses. Antioxidants 2022, 11, 1873. [Google Scholar] [CrossRef]
- Jin, H.-M.; Dang, B.; Zhang, W.-G.; Zheng, W.-C.; Yang, X.-J. Polyphenol and Anthocyanin Composition and Activity of Highland Barley with Different Colors. Molecules 2022, 27, 3411. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Gao, F.; Zhao, Y.; Cai, S.; Pang, M. The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis Mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. J. Funct. Foods 2018, 40, 729–735. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Jia, Y.; Pang, M.; Cheng, G.; Cai, S. Phenolic profiles, antioxidant activities and cytoprotective effects of different phenolic fractions from oil palm (Elaeis guineensis Jacq.) fruits treated by ultra-high pressure. Food Chem. 2019, 288, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cai, S.; Liu, X.; Shi, J.; Yi, J. Characterization of phytochemical components and identification of main antioxidants in Crateva unilocalaris Buch. shoots by UHPLC-Q-Orbitrap-MS (2) analysis. Food Res. Int. 2021, 143, 110264. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Pan, F.; Zhao, S.; Zhao, L.; Yi, J.; Cai, S. Phenolic characterization, antioxidant and α-glycosidase inhibitory activities of different fractions from Prinsepia utilis Royle seed shell using in vitro and in silico analyses. Eur. Food Res. Technol. 2023, 249, 375–386. [Google Scholar] [CrossRef]
- Yang, J.; Gu, D.; Wang, M.; Kou, D.; Guo, H.; Tian, J.; Yang, Y. In silico-assisted identification of α-amylase inhibitor from the needle oil of Pinus tabulaeformis Carr. Ind. Crop. Prod. 2018, 111, 360–363. [Google Scholar] [CrossRef]
- Lolok, N.; Sumiwi, S.A.; Ramadhan, D.S.F.; Levita, J.; Sahidin, I. Molecular dynamics study of stigmasterol and beta-sitosterol of Morinda citrifolia L. towards α-amylase and α-glucosidase. J. Biomol. Struct. Dyn. 2024, 42, 1952–1955. [Google Scholar] [CrossRef]
- Wu, S.; Mo, R.; Wang, R.; Li, Q.; Shen, D.; Liu, Y. Identification of key antioxidants of free, esterified, and bound phenolics in walnut kernel and skin. Foods 2023, 12, 825. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, P.; Roopavathi, C.; Naidu, M.M. Profiling of phenolics in cashew nut (Anacardium occidentale L.) testa and evaluation of their antioxidant and antimicrobial properties. Food Biosci. 2023, 51, 102246. [Google Scholar] [CrossRef]
- Kang, O.-J. Distribution of free, esterified, and insoluble bound forms of phenolics in tea seeds and their antioxidant activity. Food Sci. Biotechnol. 2017, 26, 121–127. [Google Scholar] [CrossRef]
- Gemechu, F.G. Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH Methods as a Tool for Studying Antioxidant Capacity of Spring Barley and Malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Zhu, L.; Zhan, C.; Yu, X.; Hu, X.; Gao, S.; Zang, Y.; Yao, D.; Wang, C.; Xu, J. Extractions, Contents, Antioxidant Activities and Compositions of Free and Bound Phenols from Kidney Bean Seeds Represented by ‘Yikeshu’ Cultivar in Cold Region. Foods 2024, 13, 1704. [Google Scholar] [CrossRef]
- Aroufai, İ.A.; Sabuncu, M.; Dülger Altiner, D.; Sahan, Y. Antioxidant properties and bioaccessibility of coffee beans and their coffee silverskin grown in different countries. J. Food Meas. Charact. 2022, 16, 1873–1888. [Google Scholar] [CrossRef]
- Gadewar, M.M.; GK, P.; Mishra, P.C.; Ashraf, G.M.; Almashjary, M.N.; Harakeh, S.; Upadhye, V.; Dey, A.; Singh, P.; Jha, N.K.; et al. Evaluation of Antidiabetic, Antioxidant and Anti-Hyperlipidemic Effects of Solanum indicum Fruit Extract in Streptozotocin-Induced Diabetic Rats. Curr. Issues Mol. Biol. 2023, 45, 903–917. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Ng, Z.X.; Rosman, N.F. In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of selected edible mushrooms. J. Food Sci. Technol. 2019, 56, 865–877. [Google Scholar] [CrossRef]
- Nisar, J.; Shah, S.M.A.; Akram, M.; Ayaz, S.; Rashid, A. Phytochemical screening, antioxidant, and inhibition activity of Picrorhiza kurroa against α-amylase and α-glucosidase. Dose-Response 2022, 20, 15593258221095960. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Abshiru, N. Quantification of major bioactive constituents, antioxidant activity, and enzyme inhibitory effects of whole coffee cherries (Coffea arabica) and their extracts. Molecules 2021, 26, 4306. [Google Scholar] [CrossRef] [PubMed]
- Foss, K.; Przybyłowicz, K.E.; Sawicki, T. Antioxidant Activity and Profile of Phenolic Compounds in Selected Herbal Plants. Plant Foods Hum. Nutr. 2022, 77, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lim, J.M.; Kim, Y.J.; Kim, W. Alterations in pH of Coffee Bean Extract and Properties of Chlorogenic Acid Based on the Roasting Degree. Foods 2024, 13, 1757. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Edwards, J.; Kalita, D. Matrix-Specific Effects on Caffeine and Chlorogenic Acid Complexation in a Novel Extract of Whole Coffea arabica Coffee Cherry by NMR Spectroscopy. Molecules 2022, 27, 7803. [Google Scholar] [CrossRef]
- Xing, M.; Xie, F.; Zeng, J.; Zhu, Z.; Wang, G.; Xia, Y.; Zhang, H.; Song, Z.; Ai, L. Inhibitory Activities and Mechanisms of Free and Bound Phenolics on α-Glucosidase in Fresh Fruits of Phyllanthus emblica Linn. Using Spectroscopy and Molecular Docking. Food Funct. 2024, 15, 6028–6041. [Google Scholar] [CrossRef]
- Limanto, A.; Simamora, A.; Santoso, A.W.; Timotius, K.H. Antioxidant, α-glucosidase inhibitory activity and molecular docking study of gallic acid, quercetin and rutin: A comparative study. Mol. Cell. Biomed. Sci. 2019, 3, 67–74. [Google Scholar] [CrossRef]
- Zheng, X.; Chi, H.; Ma, S.; Zhao, L.; Cai, S. Identification of Novel α-Glucosidase Inhibitory Peptides in Rice Wine and Their Antioxidant Activities Using in Silico and in Vitro Analyses. LWT 2023, 178, 114629. [Google Scholar] [CrossRef]
- Ooi, K.L.; Muhammad, T.S.T.; Tan, M.L.; Sulaiman, S.F. Cytotoxic, Apoptotic and Anti-α-Glucosidase Activities of 3,4-Di-O-Caffeoyl Quinic Acid, an Antioxidant Isolated from the Polyphenolic-Rich Extract of Elephantopus mollis Kunth. J. Ethnopharmacol. 2011, 135, 685–695. [Google Scholar] [CrossRef]
- Le, D.T.; Kumar, G.; Williamson, G.; Devkota, L.; Dhital, S. Molecular Interactions between Polyphenols and Porcine α-Amylase: An Inhibition Study on Starch Granules Probed by Kinetic, Spectroscopic, Calorimetric and in Silico Techniques. Food Hydrocoll. 2024, 151, 109821. [Google Scholar] [CrossRef]
- Huang, F.; Pan, F.; Wang, L.; Xiao, Z.; He, J.; Yan, M.; Wang, J.; Qiu, W.; Liu, M.; Dong, H. The interaction between citronellol and bovine serum albumin: Spectroscopic, computational and thermal imaging studies. J. Mol. Struct. 2022, 1251, 131986. [Google Scholar] [CrossRef]
- Rajabi, M.; Shareghi, B.; Farhadian, S.; Momeni, L. Evaluation of maltose on conformation and activity parameters of trypsin. J. Biomol. Struct. Dyn. 2019, 37, 4557–4562. [Google Scholar] [CrossRef]
- Bhatt, P.; Joshi, T.; Bhatt, K.; Zhang, W.; Huang, Y.; Chen, S. Binding interaction of glyphosate with glyphosate oxidoreductase and C-P lyase: Molecular docking and molecular dynamics simulation studies. J. Hazard. Mater. 2021, 409, 124927. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dong, S.; Wu, Y.; Zhao, H.; Li, X.; Gao, W. Inhibitor discovery from pomegranate rind for targeting human salivary α-amylase. Med. Chem. Res. 2018, 27, 1559–1577. [Google Scholar] [CrossRef]


| TPC (mg GAE/g Dry Extract) | TFC (mg RE/g Dry Extract) | |
|---|---|---|
| FP | 474.64 ± 22.76 a | 102.95 ± 8.13 a |
| EP | 112.85 ± 8.38 b | 52.43 ± 1.21 b |
| BP | 33.96 ± 0.74 c | 14.83 ± 0.99 c |
| IC50 of α-Glucosidase Inhibition (μg/mL) | IC50 of α-Amylase Inhibition (μg/mL) | |
|---|---|---|
| FP | 40.28 ± 1.40 a | 114.52 ± 3.62 |
| EP | 180.53 ± 4.23 b | >200 |
| BP | >200 | >200 |
| Acarbose | 0.63 ± 0.08 | 0.86 ± 0.07 |
| Peaks | Compounds | TR (min) | [M-H]− (m/z) | Molecular Formula | MS/MS Fragment Ions | Content (μg/g) | Extract |
|---|---|---|---|---|---|---|---|
| 1 | Catechin | 7.91 | 289.0717 | C15H14O6 | 123.0439, 109.0282 | 70.54 ± 2.89 | FP |
| 2 | 3-Caffeoylquinic acid | 8.28 | 353.0875 | C16H18O9 | 135.0440, 179.0345 | 24,163.04 ± 761.13 | FP |
| 3 | 5-Caffeoylquinic acid | 8.65 | 353.0876 | C16H18O9 | 191.0564, 151.0249 | 2663.01 ± 61.24 | FP |
| 4 | Lactones of caffeoylquinic acid isomeride 1 | 9.55 | 335.0772 | C16H16O8 | 135.0440, 161.0234 | 23.56 ± 0.95 | FP |
| 5 | Epicatechin | 9.56 | 289.0717 | C15H14O6 | 109.0282, 123.0438, | 729.21 ± 23.27 | FP |
| 6 | Para-coumaroyl-caffeoylquinic | 9.70 | 337.0929 | C16H18O8 | 173.0461, 63.0729 | 63.60 ± 1.83 | FP |
| 7 | Lactones of caffeoylquinic acid isomeride 2 | 9.90 | 335.0773 | C16H16O8 | 135.0439, 161.0233 | 32.98 ± 0.65 | FP |
| 8 | 4-Feruloylquinic acid | 10.37 | 367.1021 | C17H20O9 | 173.0459, 134.0283 | 12,529.73 ± 325.77 | FP |
| 9 | Rutin | 11.39 | 609.1470 | C27H30O16 | 300.0273, 301.0345 | 39.25 ± 0.423 | FP |
| 10 | 3,4-Dicaffeoylquinic acid | 12.53 | 515.1176 | C25H24O12 | 179.0274, 173.0450 | 6515.49 ± 134.21 | FP |
| 11 | 3,5-Dicaffeoylquinic acid | 12.73 | 515.1173 | C25H24O12 | 191.0915, 179.0353 | 9822.35 ± 214.13 | FP |
| 12 | 4,5-Dicaffeoylquinic acid | 13.36 | 515.1176 | C25H24O12 | 173.0777, 179.0352, | 6076.10 ± 123.89 | FP |
| 13 | 3-p-Coumaroyl-caffeoylquinic acid | 13.83 | 337.0928 | C16H18O8 | 119.0489, 163.0390 | 159.23 ± 3.80 | FP |
| 14 | Feruloyl-caffeoylquinic acid isomeride 1 | 13.91 | 529.1351 | C26H26O12 | 173.0445, 193.0500 | 257.28 ± 7.87 | FP |
| 15 | Feruloyl-caffeoylquinic acid isomeride 2 | 14.06 | 529.1351 | C26H26O12 | 173.0445, 193.0498 | 497.70 ± 10.44 | FP |
| 16 | Feruloyl-caffeoylquinic acid isomeride 3 | 14.37 | 529.1351 | C26H26O12 | 191.0552, 179.0340 | 573.81 ± 6.13 | FP |
| 17 | Feruloyl-caffeoylquinic acid isomeride 4 | 14.85 | 529.1351 | C26H26O12 | 173.0445, 191.0552 | 428.87 ± 12.92 | FP |
| 18 | Unknown | 0.83 | - | - | - | - | EP |
| 19 | Caffeic acid | 7.69 | 179.0034 | C9H8O | 135.0021, 134.1002 | 16,325.27 ± 359.16 | EP |
| Peaks | Pubchem ID | Phenolics | Affinity of α-Glucosidase (kcal/mol) | Affinity of α-Amylase (kcal/mol) |
|---|---|---|---|---|
| 1 | 9064 | Catechin | −8.5 | −9.0 |
| 2 | 1794427 | 3-Caffeoylquinic acid | −9.5 | −8.1 |
| 3 | 12310830 | 5-Ccaffeoylquinic acid | −9.1 | −8.2 |
| 4 | 73160 | Epicatechin | −8.5 | −9.0 |
| 5 | 5281766 | Para-coumaroyl-caffeoylquinic | −8.9 | −8.1 |
| 6 | 10177048 | 4-Feruloylquinic acid | −8.4 | −8.1 |
| 7 | 5280805 | Rutin | −10.3 | −9.1 |
| 8 | 5281780 | 3,4-Dicaffeoylquinic acid | −10.6 | −8.5 |
| 9 | 6274310 | 3,5-Dicaffeoylquinic acid | −10.5 | −9.1 |
| 10 | 6474309 | 4,5-Dicaffeoylquinic acid | −10.1 | −9.1 |
| 11 | 9945785 | 3-p-Coumaroyl-caffeoylquinic acid | −8.5 | −8.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Ding, L.; Zheng, X.; Wang, O.; Cai, S. Phenolic Compositions of Different Fractions from Coffee Silver Skin and Their Antioxidant Activities and Inhibition towards Carbohydrate-Digesting Enzymes. Foods 2024, 13, 3083. https://doi.org/10.3390/foods13193083
Dong S, Ding L, Zheng X, Wang O, Cai S. Phenolic Compositions of Different Fractions from Coffee Silver Skin and Their Antioxidant Activities and Inhibition towards Carbohydrate-Digesting Enzymes. Foods. 2024; 13(19):3083. https://doi.org/10.3390/foods13193083
Chicago/Turabian StyleDong, Shiyu, Lixin Ding, Xiuqing Zheng, Ou Wang, and Shengbao Cai. 2024. "Phenolic Compositions of Different Fractions from Coffee Silver Skin and Their Antioxidant Activities and Inhibition towards Carbohydrate-Digesting Enzymes" Foods 13, no. 19: 3083. https://doi.org/10.3390/foods13193083
APA StyleDong, S., Ding, L., Zheng, X., Wang, O., & Cai, S. (2024). Phenolic Compositions of Different Fractions from Coffee Silver Skin and Their Antioxidant Activities and Inhibition towards Carbohydrate-Digesting Enzymes. Foods, 13(19), 3083. https://doi.org/10.3390/foods13193083






