Physicochemical, Rheology, and Mid-Infrared Spectroscopy Techniques for the Characterization of Artisanal and Industrial Maroilles Cheeses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Samples
2.2. Physicochemical Analyses
2.3. Color Measurements
2.4. Rheology Analysis
2.5. Mid-Infrared Spectroscopy Measurements
2.6. Statistical Analyses
3. Results and Discussion
3.1. Chemical and Color Characterization of Maroilles Cheese Samples
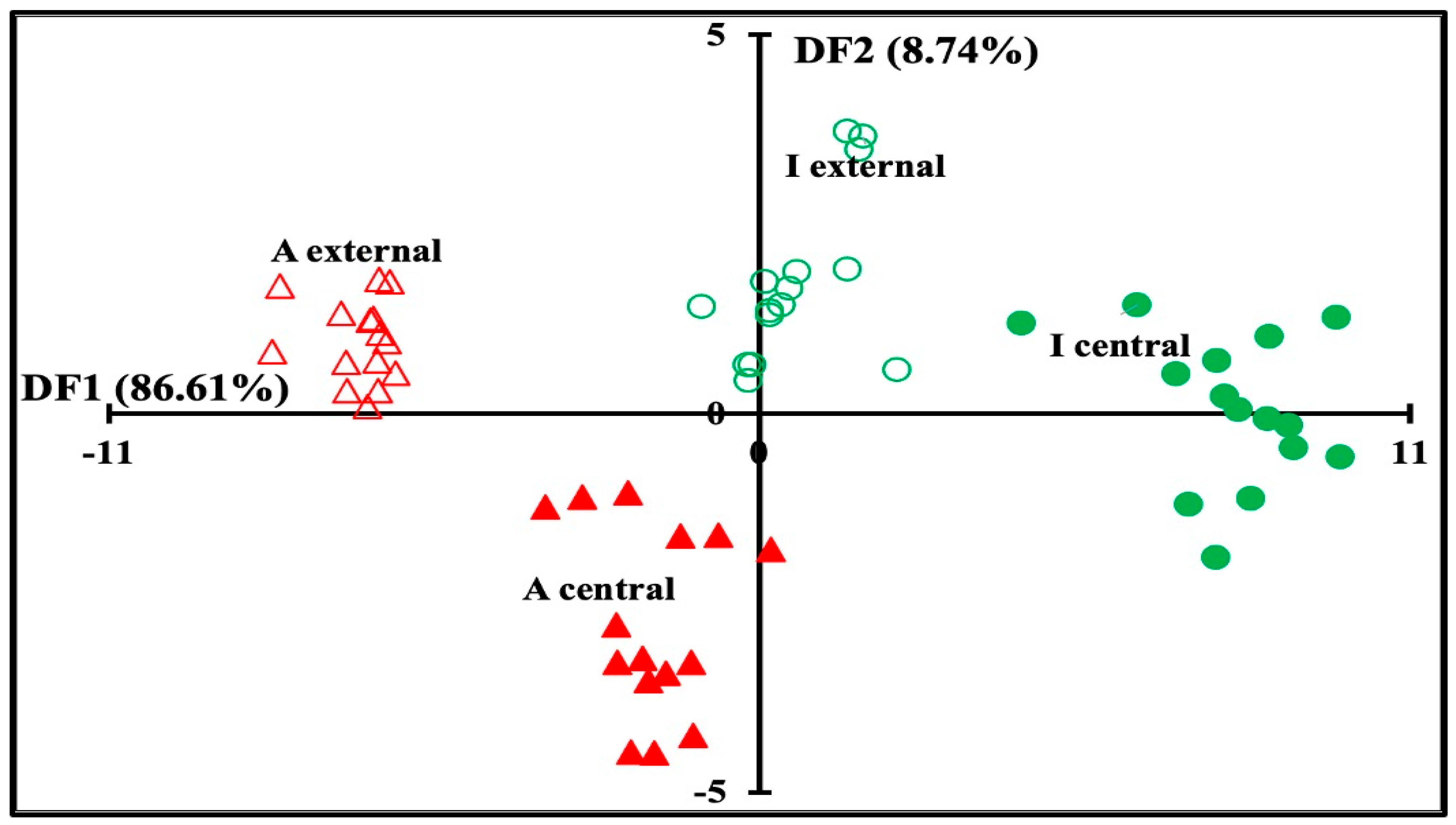
3.2. Global Analysis of Physicochemical and Color Data Sets
3.3. Viscoelastic Behavior of Maroilles Cheese Samples
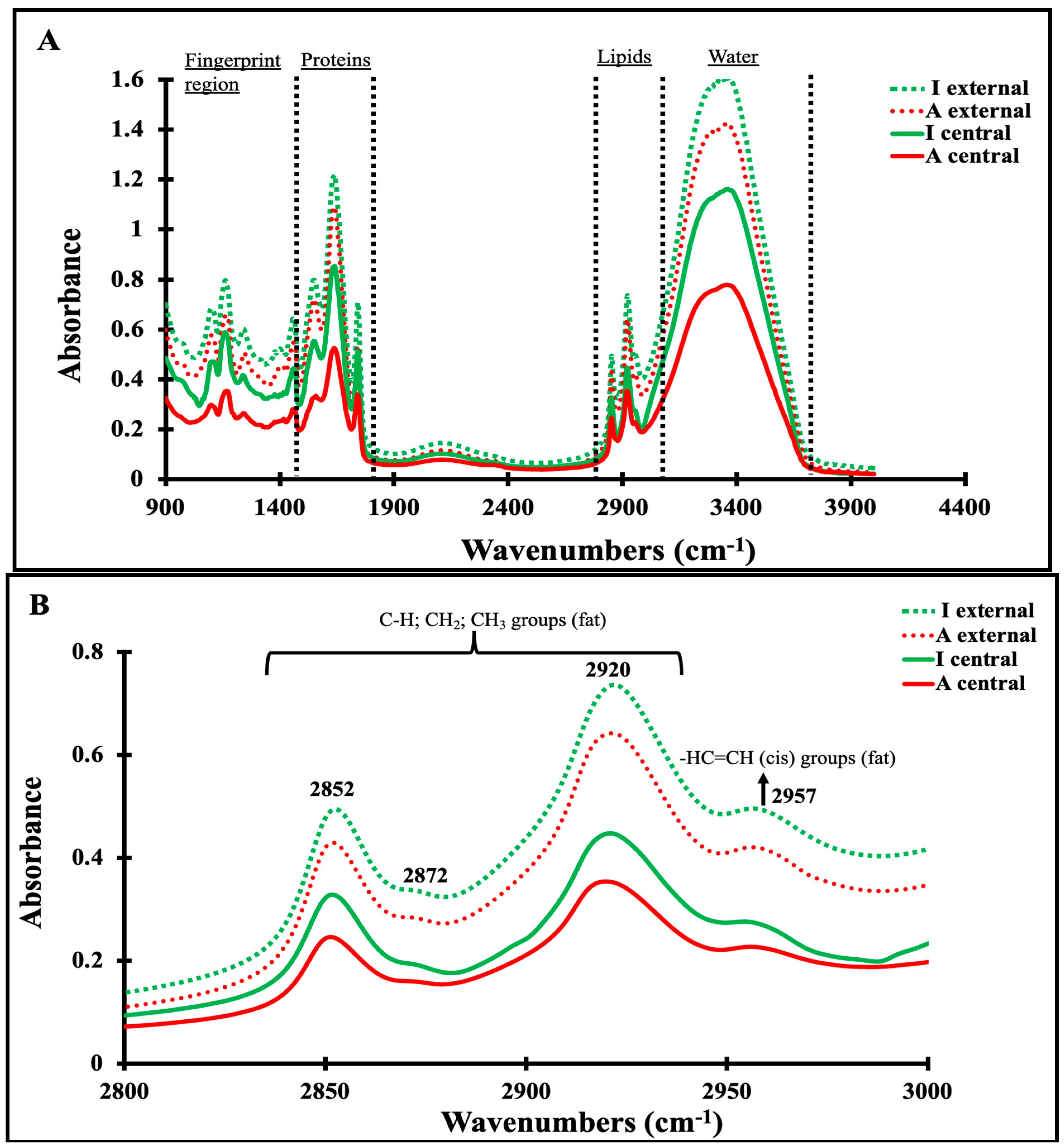
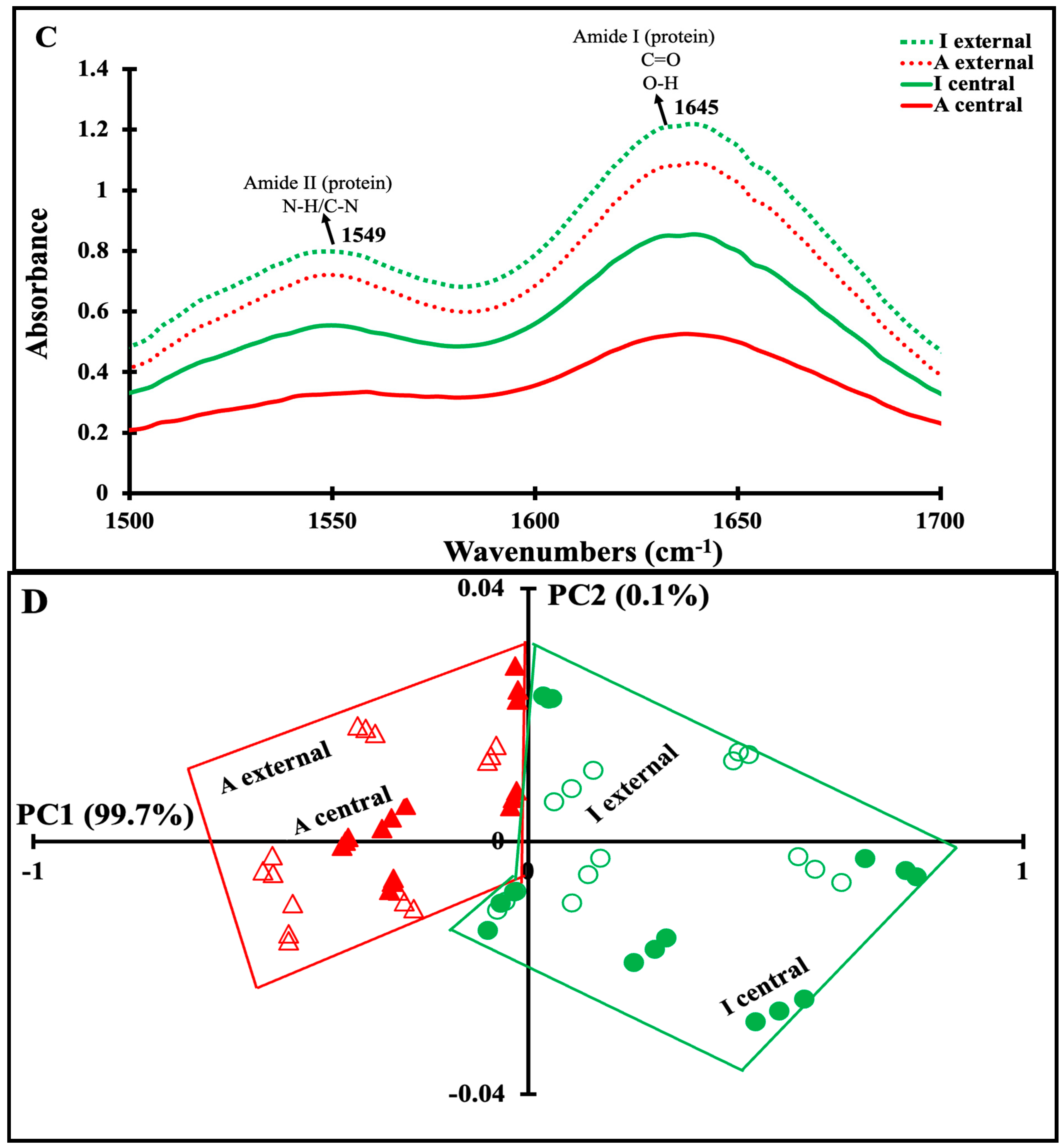
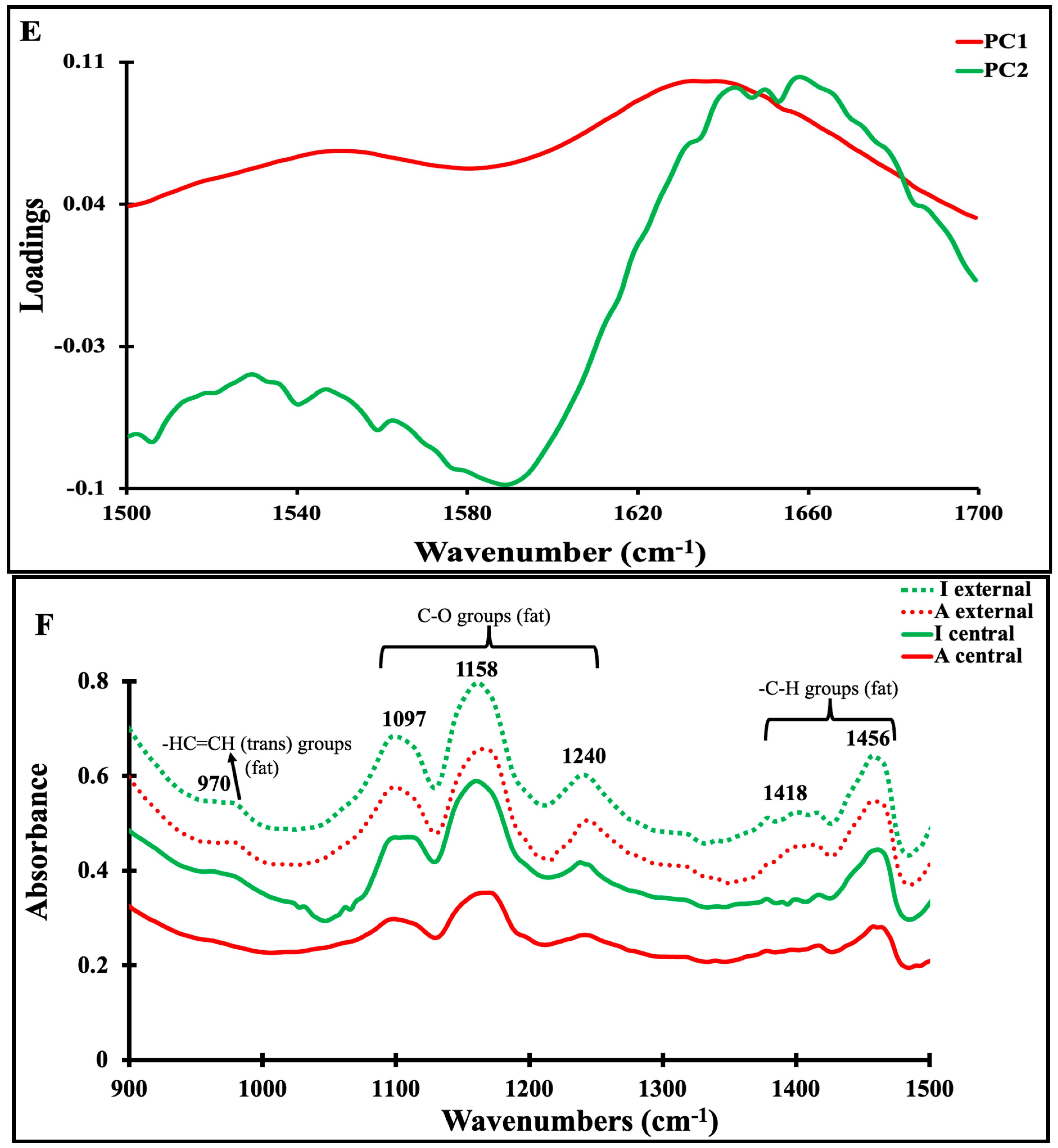
3.4. Mid-Infrared Spectra of Maroilles Cheese Samples
3.5. Correlation between Physicochemical and Secondary Structure of Maroilles Cheeses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muehlhoff, E.; Bennett, A.; McMahon, D. Milk and Dairy Products in Human Nutrition; Food and Agriculture Organization of the United Nation: Rome, Italy, 2013; pp. 11–35. [Google Scholar]
- Nacef, M.; Chevalier, M.; Chollet, S.; Drider, D.; Flahaut, C. MALDI-TOF mass spectrometry for the identification of lactic acid bacteria isolated from a French cheese: The Maroilles. Int. J. Food Microbiol. 2017, 247, 2–8. [Google Scholar] [CrossRef]
- CNIEL (Ed.) L’économie Laitière En Chiffres; Les Produits Laitiers: Paris, France, 2021; pp. 1–195. [Google Scholar]
- Bamba, T.; Hori, Y.; Umebayashi, K.; Soh, C.; Hakozaki, T.; Toyama, K.; Osumi, M.; Kondo, A.; Hasunuma, T. Comprehensive metabolic profiling of Geotrichum candidum and comparison with Saccharomyces cerevisiae. J. Biosci. Bioeng. 2024, 137, 9–15. [Google Scholar] [CrossRef]
- Gordillo-Andia, C.; Almirón, J.; Barreda-Del-Carpio, J.E.; Roudet, F.; Tupayachy-Quispe, D.; Vargas, M. Influence of Kluyveromyces lactis and Enterococcus faecalis on obtaining Lactic Acid by cheese whey fermentation. Appl. Sci. 2024, 14, 4649. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, É. Dynamic testing rheology and fluorescence spectroscopy investigations of surface to centre differences in ripened soft cheeses. Int. Dairy J. 2003, 13, 973–985. [Google Scholar] [CrossRef]
- Kulmyrzaev, A.; Dufour, É.; Noël, Y.; Hannafi, M.; Karoui, R.; Qannari, E.M.; Mazerolles, G. Investigation at the molecular level of soft cheese quality and ripening by infrared and fluorescence spectroscopies and chemometrics—Relations with rheology properties. Int. Dairy J. 2005, 15, 669–678. [Google Scholar] [CrossRef]
- Oulahal, N.; Adt, I.; Mariani, C.; Carnet-Pantiez, A.; Notz, E.; Degraeve, P. Examination of wooden shelves used in the ripening of a raw milk smear cheese by FTIR spectroscopy. Food Control 2009, 20, 658–663. [Google Scholar] [CrossRef]
- Merchán, A.V.; Ruiz-Moyano, S.; Hernández, M.V.; Martín, A.; Lorenzo, M.J.; Benito, M.J. Characterization of autochthonal Hafnia spp. strains isolated from Spanish soft raw ewe’s milk PDO cheeses to be used as adjunct culture. Int. J. Food Microbiol. 2022, 373, 109703. [Google Scholar] [CrossRef]
- Crippa, C.; Pasquali, F.; Lucchi, A.; Gambi, L.; De Cesare, A. Investigation on the microbiological hazards in an artisanal soft cheese produced in northern Italy and its production environment in different seasonal periods. Ital. J. Food Saf. 2022, 11, 9983. [Google Scholar] [CrossRef]
- Galaup, P.; Gautier, A.; Piriou, Y.; de Villeblanche, A.; Valla, A.; Dufossé, L. First pigment fingerprints from of the rinds of French PDO red-smear ripened soft cheeses Epoisses, Mont d’Or and Maroilles. Innov. Food Sci. Emerg. Technol. 2007, 8, 373–378. [Google Scholar] [CrossRef]
- Mounier, J.; Le Blay, G.; Vasseur, V.; Le Floch, G.; Jany, J.-L.; Barbier, G. Application of denaturing high-performance liquid chromatography (DHPLC) for yeasts identification in red smear cheese surfaces. Lett. Appl. Microbiol. 2010, 51, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nacef, M.; Lelièvre-Desmas, M.; Symoneaux, R.; Jombart, L.; Flahaut, C.; Chollet, S. Consumers’ expectation and liking for cheese: Can familiarity effects resulting from regional differences be highlighted within a country? Food Qual. Prefer. 2019, 72, 188–197. [Google Scholar] [CrossRef]
- Nacef, M.; Lelièvre-Desmas, M.; Drider, D.; Flahaut, C.; Chollet, S. Artisanal and industrial Maroilles cheeses: Are they different? Comparison using sensory. physico-chemical and microbiological approaches. Int. Dairy J. 2019, 89, 42–52. [Google Scholar] [CrossRef]
- Leite, A.I.N.; Pereira, C.G.; Andrade, J.; Vicentini, N.M.; Bell, M.J.V.; Anjos, V. FTIR-ATR spectroscopy as a tool for the rapid detection of adulterations in butter cheeses. LWT 2019, 109, 63–69. [Google Scholar] [CrossRef]
- Kirtil, H.E.; Cebi, N.; Yildirim, R.M.; Metin, B.; Arici, M. A rapid spectroscopic method for the identification of the filamentous fungi isolated from Turkish traditional mold-ripened cheeses. J. Microbiol. Methods 2024, 217–218, 106884. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, É.; Pillonel, L.; Schaller, E.; Picque, D.; Cattenoz, T.; Bosset, J.O. The potential of combined infrared and fluorescence spectroscopies as a method of determination of the geographic origin of Emmental cheeses. Int. Dairy J. 2005, 15, 287–298. [Google Scholar] [CrossRef]
- Rosa Silva, L.K.; de Jesus, J.C.; Onelli, R.R.V.; Conceição, D.G.; Santos, L.S.; Ferrão, S.P.B. Spectroscopy (MIR), chromatography (RP-HPLC) and chemometrics applied to soluble peptides to discriminate the geographic origin of coalho cheese. Biocatal. Agric. Biotechnol. 2023, 50, 102678. [Google Scholar] [CrossRef]
- Molle, A.; Cipolat-Gotet, C.; Stocco, G.; Ferragina, A.; Berzaghi, P.; Summer, A. The use of milk Fourier-transform infrared spectra for predicting cheesemaking traits in Grana Padano Protected Designation of Origin cheese. J. Dairy Sci. 2024, 107, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, L.E.E.; Koca, N.; Harper, W.J.J.; Alvarez, V.B.B. Rapid determination of Swiss cheese composition by Fourier transform infrared/attenuated total reflectance spectroscopy. J. Dairy Sci. 2006, 89, 1407–1412. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association Analytical Chemists, 17th ed.; Association of Official Analytical Chemists: Maryland, MD, USA, 2000. [Google Scholar]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Petrus, R.R.; Freire, M.T.D.A.; Setogute, L.D.C.; Higajo, V.M. Effect of pasteurization temperature and aseptic filling on the shelf-life of milk. Alim. Nutr. 2011, 22, 531–538. [Google Scholar]
- Ozbek, C.; Guzeler, N. Effects of stabilisers in brine on soft white cheese quality parameters. Int. Dairy J. 2022, 134, 105446. [Google Scholar] [CrossRef]
- Buffa, M.N.; Trujillo, A.J.; Pavia, M.; Guamis, B. Changes in textural, microstructural, and colour characteristics during ripening of cheeses made from raw, pasteurized or high-pressure-treated goats’ milk. Int. Dairy J. 2001, 11, 927–934. [Google Scholar] [CrossRef]
- Le Graet, Y.; Lepienne, A.; Brule, G.; Ducruet, P. Migration du calcium et des phosphates inorganiques dans les fromages à pâte molle de type Camembert au cours de l’affinage. Le Lait 1983, 63, 317–332. [Google Scholar] [CrossRef]
- Le Graet, Y.; Brule, G.; Maubois, J.-L.; Oeuvrard, G. Répartition et évolution des éléments minéraux au cours de l’affinage des fromages à pâte cuite type Beaufort. Le Lait 1986, 66, 391–404. [Google Scholar] [CrossRef][Green Version]
- Kratochvílová, A.; Salek, R.N.; Vašina, M.; Lorencová, E.; Kurová, V.; Lazárková, Z.; Dostálová, J.; Šenkýrová, J. The impact of different hydrocolloids on the viscoelastic properties and microstructure of processed cheese manufactured without emulsifying salts in relation to storage time. Foods 2022, 11, 3605. [Google Scholar] [CrossRef]
- Pinho, O.; Mendes, E.; Alves, M.M.; Ferreira, I.M.P.L.V.O. Chemical, physical, and sensorial characteristics of “Terrincho” ewe cheese: Changes during ripening and intravarietal comparison. J. Dairy Sci. 2004, 87, 249–257. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L. Rheology and texture of commercial queso fresco cheeses made from raw and pasteurized milk. J. Food Qual. 2010, 33, 204–215. [Google Scholar] [CrossRef]
- Grewal, M.K.; Chandrapala, J.; Donkor, O.; Vasso Apostolopoulos, V.; Stojanovska, L.; Vasiljevic, T. Fourier transform infrared spectroscopy analysis of physicochemical changes in UHT milk during accelerated storage. Int. Dairy J. 2017, 66, 99–107. [Google Scholar] [CrossRef]
- Ozturka, M.; Dogan, M.A.; Menevseogluc, A.; Ayv, H. Infrared spectroscopy combined with chemometrics as a convenient method to detect adulterations in cooking/stretching process in commercial cheese. Int. Dairy J. 2022, 128, 105312. [Google Scholar] [CrossRef]
- Cevoli, C.; Gori, A.; Nocetti, M.; Cuibus, L.; Caboni, M.F.; Fabbri, A. FT-NIR and FT-MIR spectroscopy to discriminate competitors, non-compliance and compliance grated Parmigiano Reggiano cheese. Food Res. Int. 2013, 52, 214–220. [Google Scholar] [CrossRef]
- Karoui, R.; Mazerolles, G.; Bosset, J.O.; De Baerdemaeker, J.; Dufour, E. Utilisation of mid-infrared spectroscopy for determination of the geographic origin of Gruyère PDO and L’Etivaz PDO Swiss cheeses. Food Chem. 2007, 105, 847–854. [Google Scholar] [CrossRef]
- Nassar, K.S.; Yacoub, S.S.; Lv, J.; Ragab, E.S. Structural changes induced by pasteurisation and/or high-pressure treatment of skim caprine milk. Int. Dairy J. 2023, 138, 105528. [Google Scholar] [CrossRef]
- Ong, L.; Paxa, A.P.; Ong, A.; Vongsvivut, J.; Tobin, M.J.; Kentish, S.E.; Gras, S.L. The effect of pH on the fat and protein within cream cheese and their influence on textural and rheological properties. Food Chem. 2020, 332, 127327. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, Ö.; Kaya, A. Investigation of the compositional and structural changes in the proteins of cow milk when processed to cheese. LWT 2021, 151, 112102. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Gori, A.; Cerretani, L.; Simó-Alfonso, E.F.; Caboni, M.F. Classification of Pecorino cheeses produced in Italy according to their ripening time and manufacturing technique using Fourier transform infrared spectroscopy. J. Dairy Sci. 2010, 93, 4490–4496. [Google Scholar] [CrossRef]
- Pax, A.P.; Ong, L.; Kentish, S.E.; Gras, S.L. Effects of shredding on the functionality, microstructure and proteolysis of low-moisture mozzarella cheese. Int. Dairy J. 2021, 117, 104979. [Google Scholar] [CrossRef]



| Brand | Producer | Label | Characteristic |
|---|---|---|---|
| Bahardes | La ferme des Bahardes | A-Bah | Length: 8 cm Width: 8 cm Weight: 180 to 200 g |
| Maliécourt | La ferme de Maliécourt | A-Mal | |
| Fleur de l’helpe | La ferme du Château Courbet | A-Fhp | |
| Château Courbet | La ferme du Château Courbet | A-Cct | |
| Moulin | La ferme du Moulin | A-Mou | |
| Le p’tit fromager | SARL Laiterie des étangs de Sommeron | I-Lpf | |
| Lesire | Ets Lesire et Roger | I-Les | |
| Fauquet | Les fromagers de Thiéracle | I-Fau | |
| Auchan | Auchan | I-Auc | |
| Leclerc | Leclerc | I-Lec |
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cheeses | Protein (%) | Fat (%) | pH Central Zone | pH External Zone | Moisture % Central Zone | Moisture % External Zone | Ash % Central Zone | Ash % External Zone |
| A-Bah | 19.01 ± 0.17 a | 26.70 ± 0.34 cd | 4.96 ± 0.01 a | 5.68 ± 0.02 a | 46.63 ± 0.57 a | 48.81 ± 0.72 a | 3.72 ± 0.13 g | 3.68 ± 0.10 g |
| A-Mal | 20.43 ± 0.35 b | 31.36 ± 0.14 g | 6.25 ± 0.02 f | 6.58 ± 0.11 d | 48.78 ± 0.02 b | 50.67 ± 1.05 b | 2.39 ± 0.03 a | 2.69 ± 0.04 ab |
| A-Fhp | 20.94 ± 0.10 bc | 28.8 ± 0.32 e | 5.22 ± 0.04 b | 5.63 ± 0.00 e | 50.43 ± 0.88 c | 53.92 ± 0.09 c | 2.74 ± 0.12 bc | 2.94 ± 0.25 cd |
| A-Cct | 21.36 ± 0.09 cd | 29.84 ± 0.40 f | 5.19 ± 0.04 b | 6.90 ± 0.15 f | 53.59 ± 0.14 d | 53.91 ± 0.10 c | 2.66 ± 0.14 b | 2.73 ± 0.04 ab |
| A-Mou | 19.20 ± 0.13 a | 27.05 ± 0.24 d | 5.48 ± 0.17 c | 6.39 ± 0.01 bc | 48.43 ± 0.42 b | 49.73 ± 0.25 ab | 2.57 ± 0.02 ah | 2.61 ± 0.05 a |
| I-Lpf | 21.74 ± 0.11 d | 21.85 ± 0.27 a | 5.82 ± 0.04 e | 6.74 ± 0.05 e | 52.76 ± 0.25 d | 54.76 ± 0.58 cd | 2.89 ± 0.00 cd | 3.13 ± 0.00 de |
| I-Les | 19.23 ± 0.73 a | 25.88 ± 0.55 b | 5.78 ± 0.08 e | 6.36 ± 0.09 b | 54.49 ± 0.03 d | 55.51± 0.08 de | 3.17 ± 0.02 ef | 3.09 ± 0.07 de |
| I-Fau | 18.94 ± 0.79 a | 26.06 ± 0.38 bc | 5.67 ± 0.05 dej | 6.57 ± 0.00 d | 55.25 ± 0.03 c | 56.18± 0.97 e | 2.68 ± 0.12 b | 2.82 ± 0.04 bc |
| I-Auc | 20.39 ± 0.87 b | 26.14 ± 0.79 bc | 5.55 ± 0.03 cd | 6.53 ± 0.04 cd | 56.79 ± 0.02 f | 62.15 ± 0.02 f | 3.02 ± 0.17 de | 3.27 ± 0.06 ef |
| I-Lec | 24.09 ± 0.08 e | 25.74 ± 0.25 b | 5.45 ± 0.00 c | 6.38 ± 0.00 bc | 50.12 ± 0.22 c | 50.18 ± 0.32 b | 3.35 ± 0.01 f | 3.42 ± 0.02 f |
| Mean | ||||||||
| A cheeses | 20.19 ± 1.04 a | 28.75 ± 1.94 b | 5.42 ± 0.50 a | 6.24 ± 0.57 a | 49.57 ± 2.62 a | 51.41 ± 2.38 a | 2.81 ± 0.52 a | 2.93 ± 0.44 a |
| I cheeses | 20.88 ± 2.11 a | 25.13 ± 1.84 a | 5.65 ± 0.15 a | 6.52 ± 0.16 a | 53.88 ± 2.56 b | 55.76 ± 4.28 b | 3.02 ± 0.26 a | 3.15 ± 0.22 a |
| (B) | ||||||||
| Cheeses | Central Zone | External Zone | ||||||
| L* | a* | b* | L* | a* | b* | |||
| A-Bah | 85.52 ± 0.08 d | −0.63 ± 0.01 i | 25.66 ± 0.08 i | 83.93 ± 0.05 b | −0.23 ± 0.02 g | 25.45 ± 0.04 e | ||
| A-Mal | 87.66 ± 0.02 h | −0.85 ± 0.01 e | 17.32 ± 0.03 a | 85.00 ± 0.01 ef | −0.14 ± 0.01 h | 22.29 ± 0.01 a | ||
| A-Fhp | 84.48 ± 0.03 b | −1.77 ± 0.01 a | 22.37 ± 0.03 e | 84.04 ± 0.04 b | −0.76 ± 0.01 e | 28.75 ± 0.03 g | ||
| A-Cct | 84.23 ± 0.07 a | −0.56 ± 0.01 g | 23.37 ± 0.06 g | 79.20 ± 0.00 a | −1.46 ± 0.00 c | 30.49 ± 0.01 h | ||
| A-Mou | 85.80 ± 0.08 e | −0.33 ± 0.00 h | 23.52 ± 0.14 g | 84.00 ± 0.06 ef | −0.55 ± 0.02 f | 25.69 ± 0.13 f | ||
| I-Lpf | 84.58 ± 0.02 b | −1.37 ± 0.01 c | 24.69 ± 0.03 h | 84.32 ± 0.01 c | 1.06 ± 0.01 d | 25.00 ± 0.08 d | ||
| I-Les | 86.62 ± 0.02 f | −0.71 ± 0.00 f | 21.34 ± 0.01 d | 84.70 ± 0.17 d | 2.35 ± 0.01 a | 22.56 ± 0.04 b | ||
| I-Fau | 87.39 ± 0.02 g | −0.95 ± 0.02 d | 23.10 ± 0.06 f | 86.52 ± 0.02 g | 1.71 ± 0.01 b | 22.36 ± 0.02 a | ||
| I-Auc | 84.97 ± 0.04 c | −1.72 ± 0.00 b | 19.68 ± 0.09 c | 84.87 ± 0.02 e | 1.07 ± 0.02 d | 24.11 ± 0.07 c | ||
| I-Lec | 88.47 ± 0.05 i | −0.83± 0.00 e | 19.00 ± 0.01 b | 85.10 ± 0.03 f | 1.43 ± 0.01 c | 24.06 ± 0.03 c | ||
| Mean | ||||||||
| A cheeses | 85.54 ± 1.36 a | −0.83 ± 0.56 a | 22.45 ± 3.11 a | 83.43 ± 2.42 a | −0.63 ± 0.53 b | 26.53 ± 3.18 b | ||
| I cheeses | 86.40 ± 1.63 a | −1.12 ± 0.42 a | 21.56 ± 2.36 a | 85.10 ± 0.84 a | −1.52 ± 0.54 a | 23.62 ± 1.12 a | ||
| Cheeses | Central Zone | External Zone | ||||||
|---|---|---|---|---|---|---|---|---|
| β Sheet | Random Coil Structure | α Helix | β Turn | β Sheet | Random Coil Structure | α Helix | β Turn | |
| A-Bah | 28.64 ± 0.21 cd | 11.53 ± 0.48 bcd | 14.25 ± 0.05 abc | 45.67 ± 0.11 d | 27.63 ± 0.40 c | 11.00 ± 0.22 b | 14.73 ± 0.08 de | 46.63 ± 0.21 e |
| A-Mal | 27.45 ± 0.26 b | 11.63 ± 0.50 bcd | 14.47 ± 0.02 bcd | 45.38 ± 0.13 c | 26.87 ± 0.15 a | 10.53 ± 0.17 a | 14.63 ± 0.04 d | 47.93 ± 0.14 g |
| A-Fhp | 27.49 ± 0.40 b | 12.11 ± 0.73 d | 14.73 ± 0.09 d | 44.86 ± 0.20 a | 30.00 ± 0.12 f | 12.20 ± 0.06 d | 13.93 ± 0.09 ab | 43.8 ± 0.07 a |
| A-Cct | 28.40 ± 0.28 c | 11.63 ± 0.04 bcd | 14.57 ± 0.06 cd | 45.40 ± 0.06 c | 29.33 ± 0.06 e | 11.90 ± 0.16 cd | 14.03 ± 0.15 b | 44.70 ± 0.14 c |
| A-Mou | 24.47 ± 0.43 a | 11.80 ± 0.18 cd | 15.61 ± 0.19 e | 45.29 ± 0.13 c | 30.00 ± 0.17 f | 12.10 ± 0.16 d | 14.00 ± 0.09 b | 43.90 ± 0.08 a |
| I-Lpf | 28.57 ± 0.29 cd | 11.60 ± 0.23 bcd | 14.10 ± 0.22 ab | 45.03 ± 0.05 ab | 28.83 ± 0.15 d | 11.60 ± 0.03 c | 14.33 ± 0.16 c | 45.23 ± 0.04 d |
| I-Les | 28.90 ± 0.56 cde | 11.43 ± 0.24 bc | 14.22 ± 0.37 abc | 44.87 ± 0.06 a | 27.30 ± 0.22 b | 10.60 ± 0.05 a | 14.80 ± 0.20 e | 47.23 ± 0.05 f |
| I-Fau | 29.47 ± 0. 35 f | 11.11 ± 0.28 b | 13.41 ± 0.36 a | 46.01 ± 0.22 e | 27.60 ± 0.09 bc | 11.07 ± 0.34 b | 14.77 ± 0.04 de | 46.53 ± 0.10 e |
| I-Auc | 29.10 ± 0.40 def | 11.30 ± 0.26 bc | 14.17 ± 0.09 abc | 45.42 ± 0.21 c | 29.63 ± 0.04 e | 12.07 ± 0.34 d | 13.80 ± 0.10 a | 44.47 ± 0.08 b |
| I-Lec | 29.33 ± 0.61 ef | 9.31 ± 0.11 a | 14.00 ± 0.03 a | 47.36 ± 0.15 f | 28.90 ± 0.15 d | 11.60 ± 0.06 c | 14.30 ± 0.15 c | 45.20 ± 0.05 d |
| Mean | ||||||||
| A cheeses | 27.29 ± 1.66 a | 11.74 ± 0.23 a | 14.73 ± 0.52 b | 45.32 ± 0.29 a | 28.77 ± 1.44 a | 11.55 ± 0.74 a | 14.26 ± 0.38 a | 45.39 ± 1.82 a |
| I cheeses | 29.07 ± 0.36 b | 10.95 ± 0.93 a | 13.98 ± 0.33 a | 45.74 ± 1.01 a | 28.45 ± 0.97 a | 11.39 ± 0.57 a | 14.40 ± 0.41 a | 45.73 ± 1.12 a |
| Predicted/Observed | A Central | A External | I Central | I External | Total | % of Correct Classification |
|---|---|---|---|---|---|---|
| A central | 14 | 1 | 0 | 0 | 15 | 93.33% |
| A external | 0 | 15 | 0 | 0 | 15 | 100.00% |
| I central | 0 | 0 | 14 | 1 | 15 | 93.33% |
| I external | 0 | 0 | 0 | 15 | 15 | 100.00% |
| Total | 14 | 16 | 14 | 16 | 60 | 96.67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamoko, G.; Karoui, R. Physicochemical, Rheology, and Mid-Infrared Spectroscopy Techniques for the Characterization of Artisanal and Industrial Maroilles Cheeses. Foods 2024, 13, 3086. https://doi.org/10.3390/foods13193086
Karamoko G, Karoui R. Physicochemical, Rheology, and Mid-Infrared Spectroscopy Techniques for the Characterization of Artisanal and Industrial Maroilles Cheeses. Foods. 2024; 13(19):3086. https://doi.org/10.3390/foods13193086
Chicago/Turabian StyleKaramoko, Gaoussou, and Romdhane Karoui. 2024. "Physicochemical, Rheology, and Mid-Infrared Spectroscopy Techniques for the Characterization of Artisanal and Industrial Maroilles Cheeses" Foods 13, no. 19: 3086. https://doi.org/10.3390/foods13193086
APA StyleKaramoko, G., & Karoui, R. (2024). Physicochemical, Rheology, and Mid-Infrared Spectroscopy Techniques for the Characterization of Artisanal and Industrial Maroilles Cheeses. Foods, 13(19), 3086. https://doi.org/10.3390/foods13193086








