Preparation and Characterization Study of Zein–Sodium Caseinate Nanoparticle Delivery Systems Loaded with Allicin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
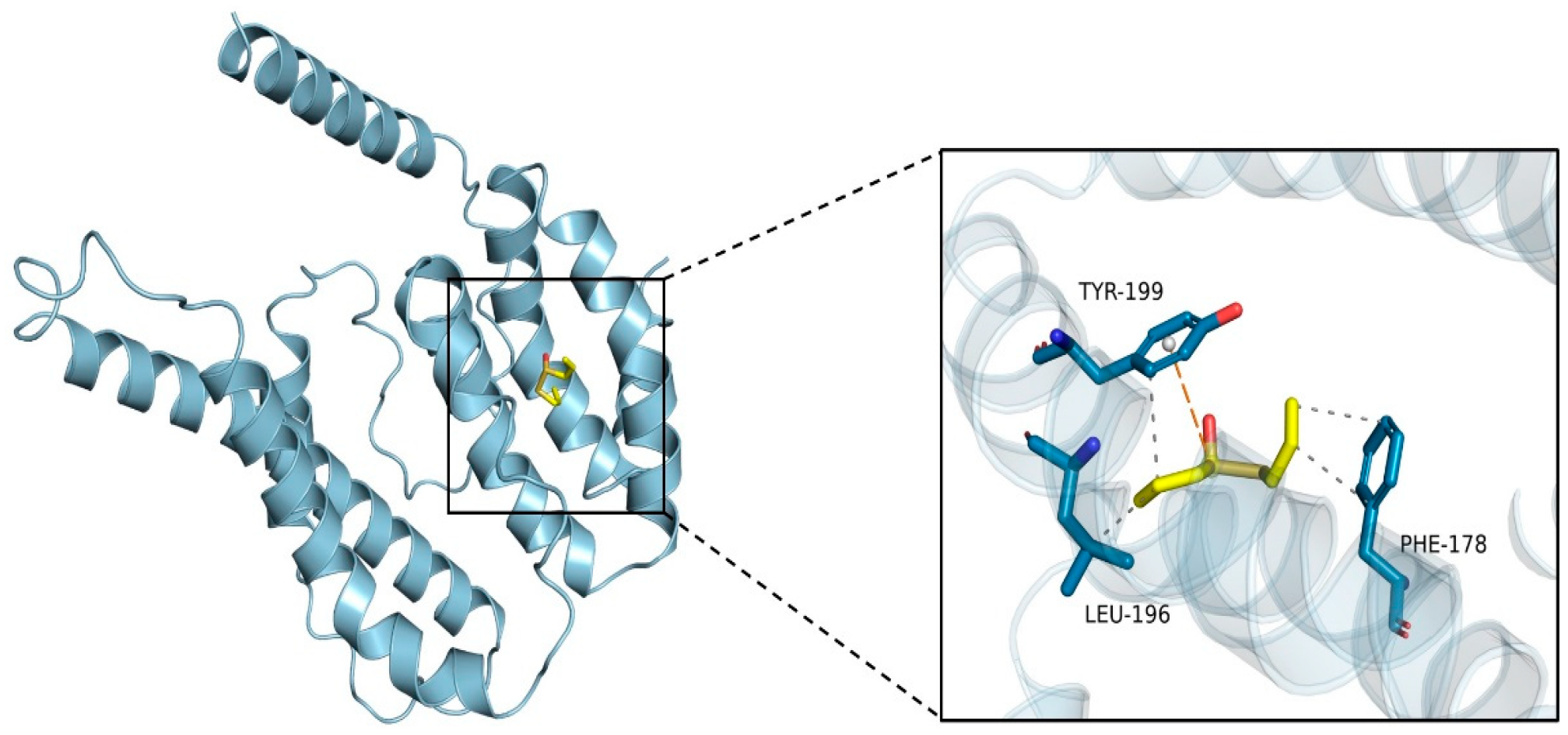
2.2. Molecular Docking
2.3. Sample Preparation
2.4. Particle Size, Polydispersity Index (PDI) and ζ-Potential
2.5. EE and LC
2.6. Microscopic Morphology Observation
2.7. Structural Characterization
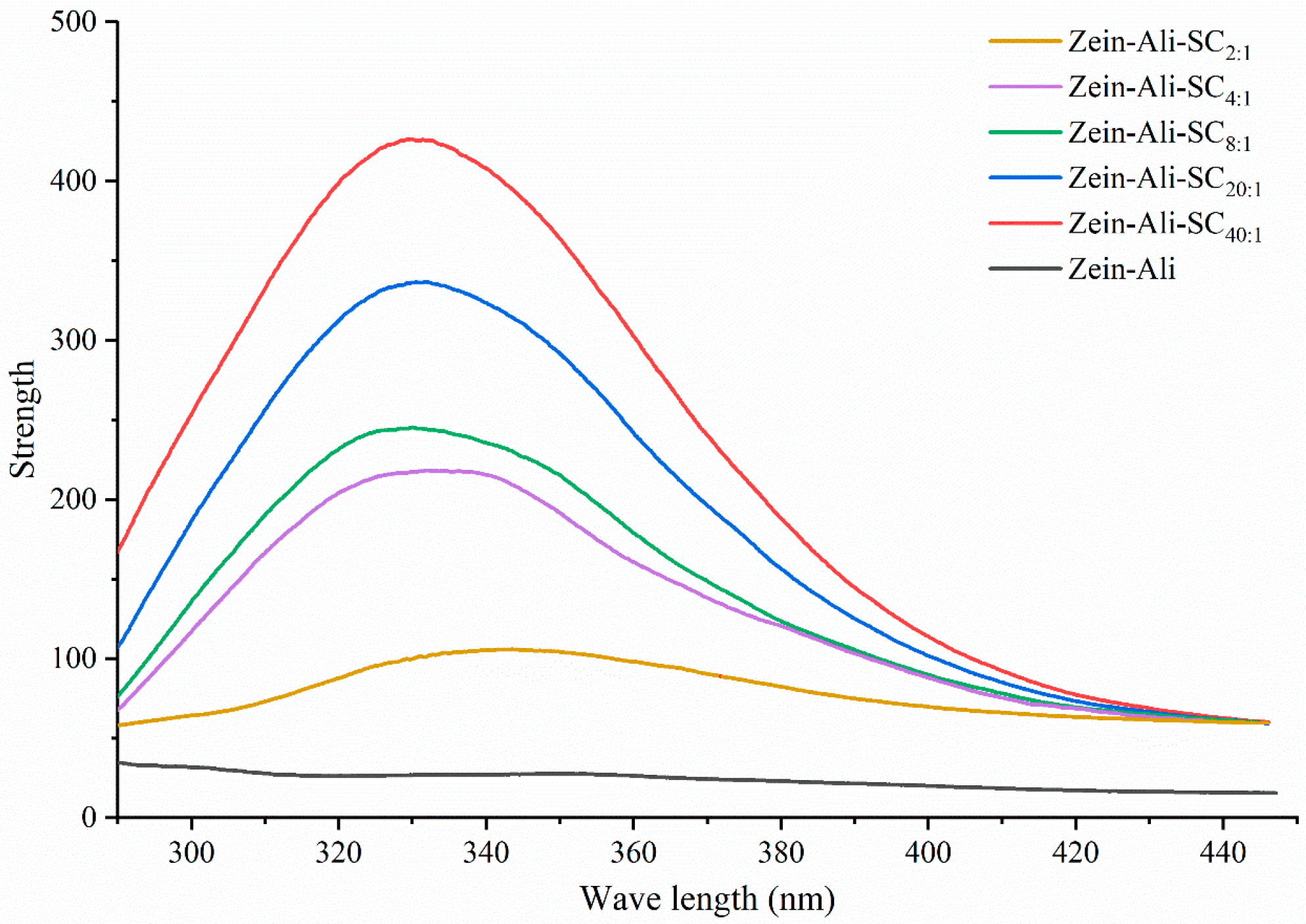
2.7.1. Fluorescence Spectrum
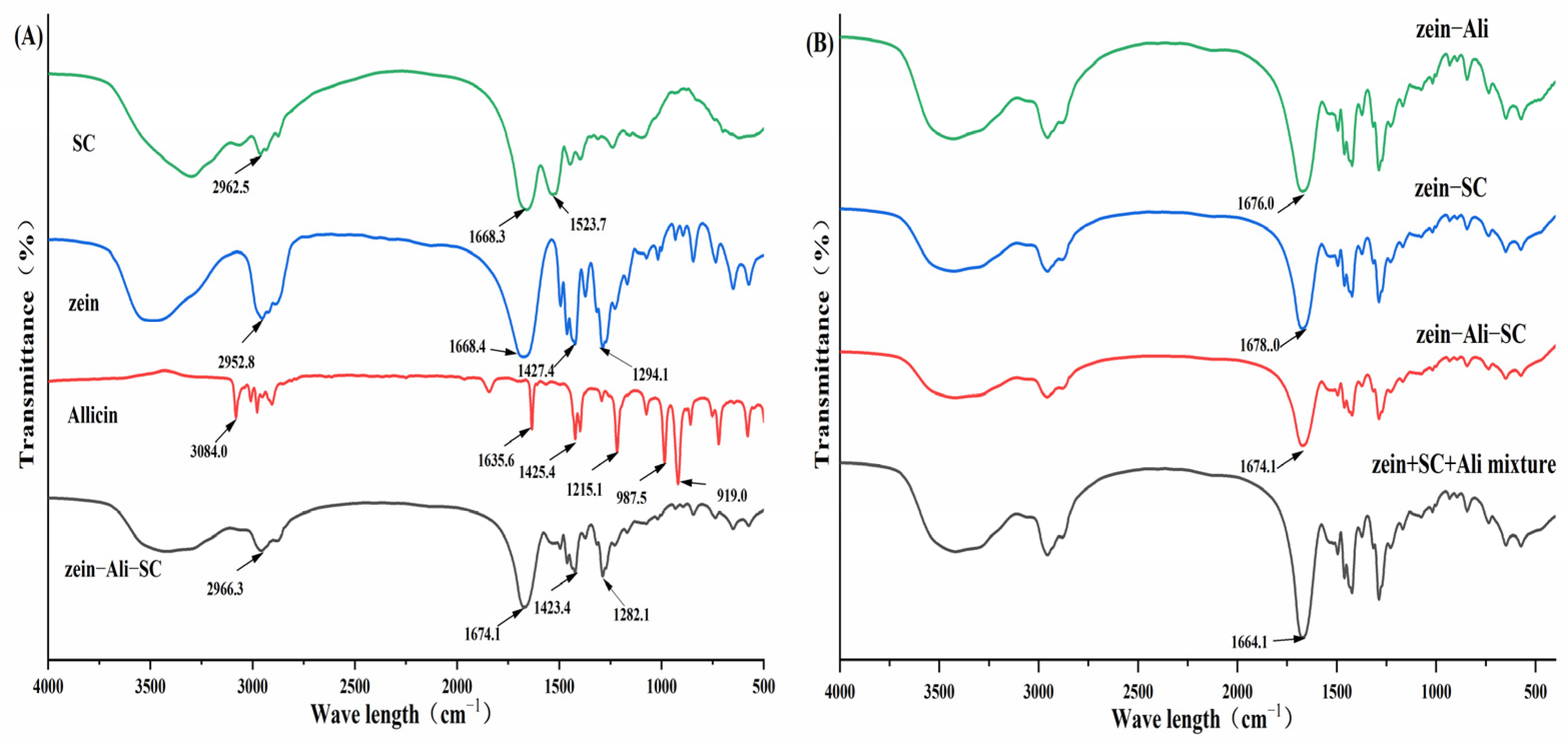
2.7.2. FTIR
2.7.3. XRD
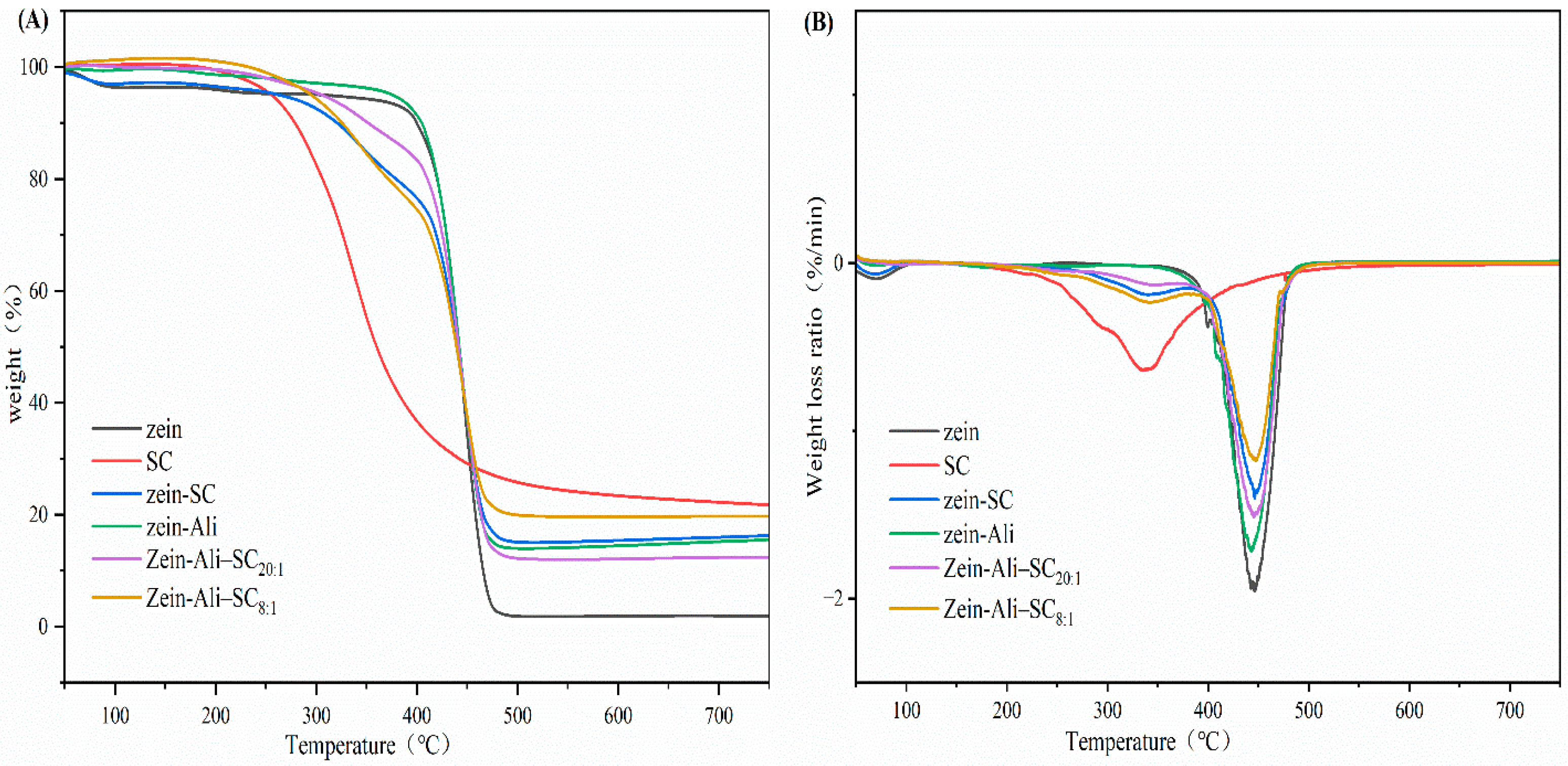
2.7.4. TGA
2.8. Stability under Different Conditions
2.8.1. pH
2.8.2. NaCl Concentration
2.8.3. Thermal Stability
2.8.4. Storage Stability
2.8.5. Redispersibility
2.9. Antioxidant Activity
2.9.1. DPPH Scavenging Activity
2.9.2. 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonic acid) Diammonium Salt (ABTS) Radical Scavenging Activity
2.10. Data Statistics and Analysis
3. Results and Discussion
3.1. Molecular Docking Analysis
3.2. Particle Size and ζ-Potential Analysis
3.3. Analysis of EE and LC
3.4. Microscopic Morphology Observation
3.5. Structural Characterization
3.5.1. Fluorescence Spectral Analysis
3.5.2. FTIR Analysis
3.5.3. XRD Analysis
3.5.4. TGA
3.6. Formation Mechanism of Nanoparticles
3.7. Stability Analysis
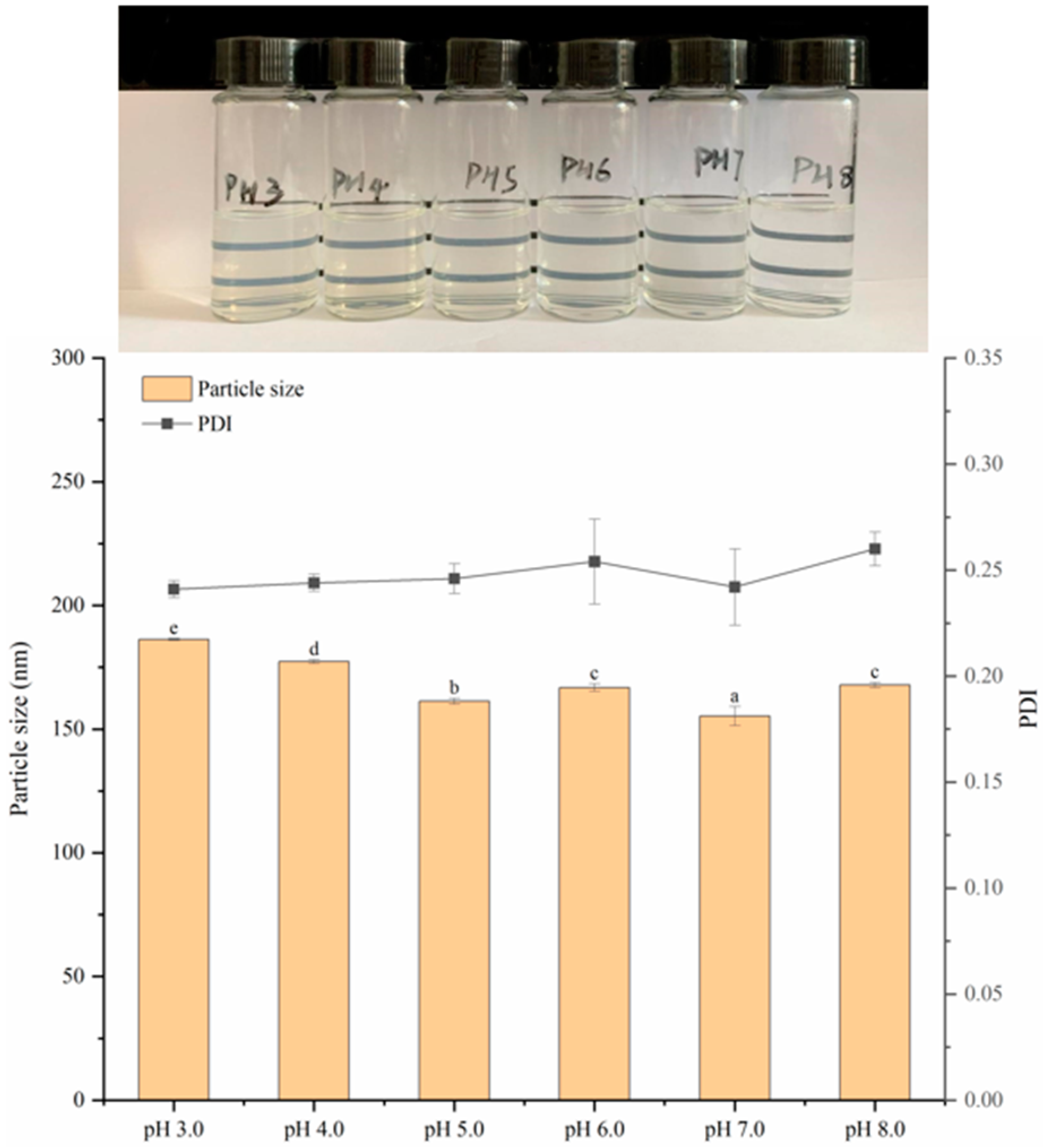
3.7.1. Stability Analysis at Different pH
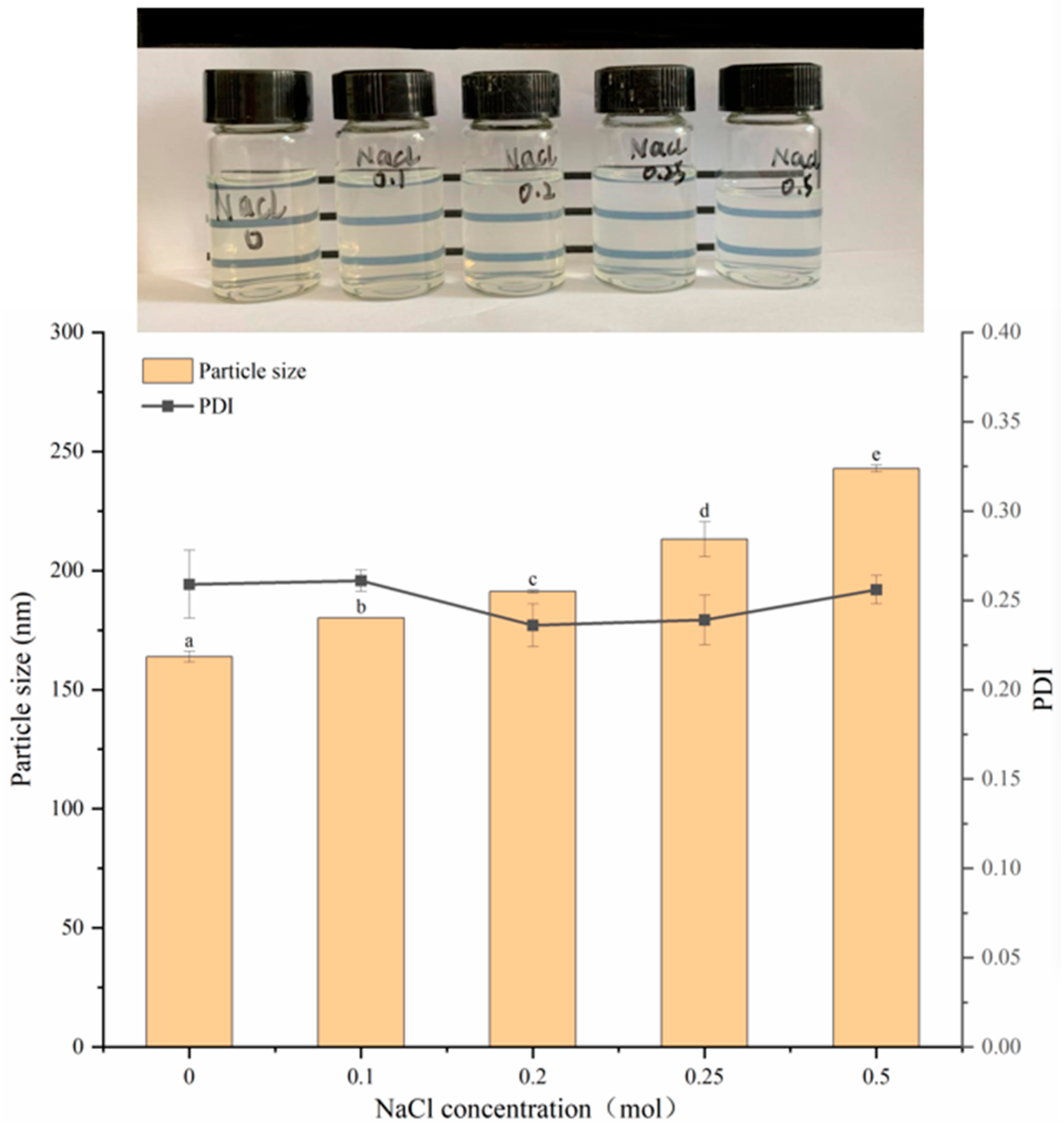
3.7.2. Stability Analysis at Different Concentrations of NaCl
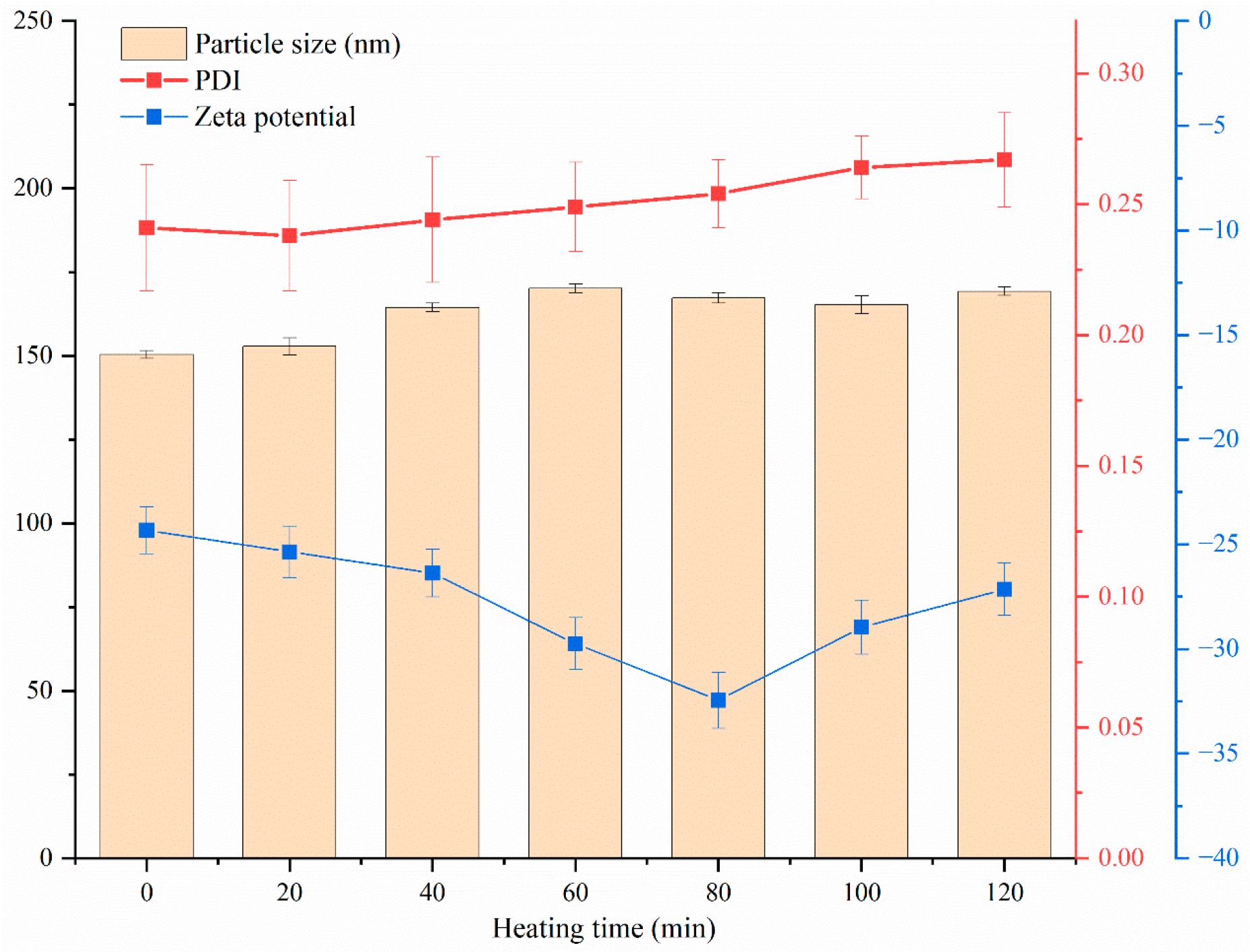
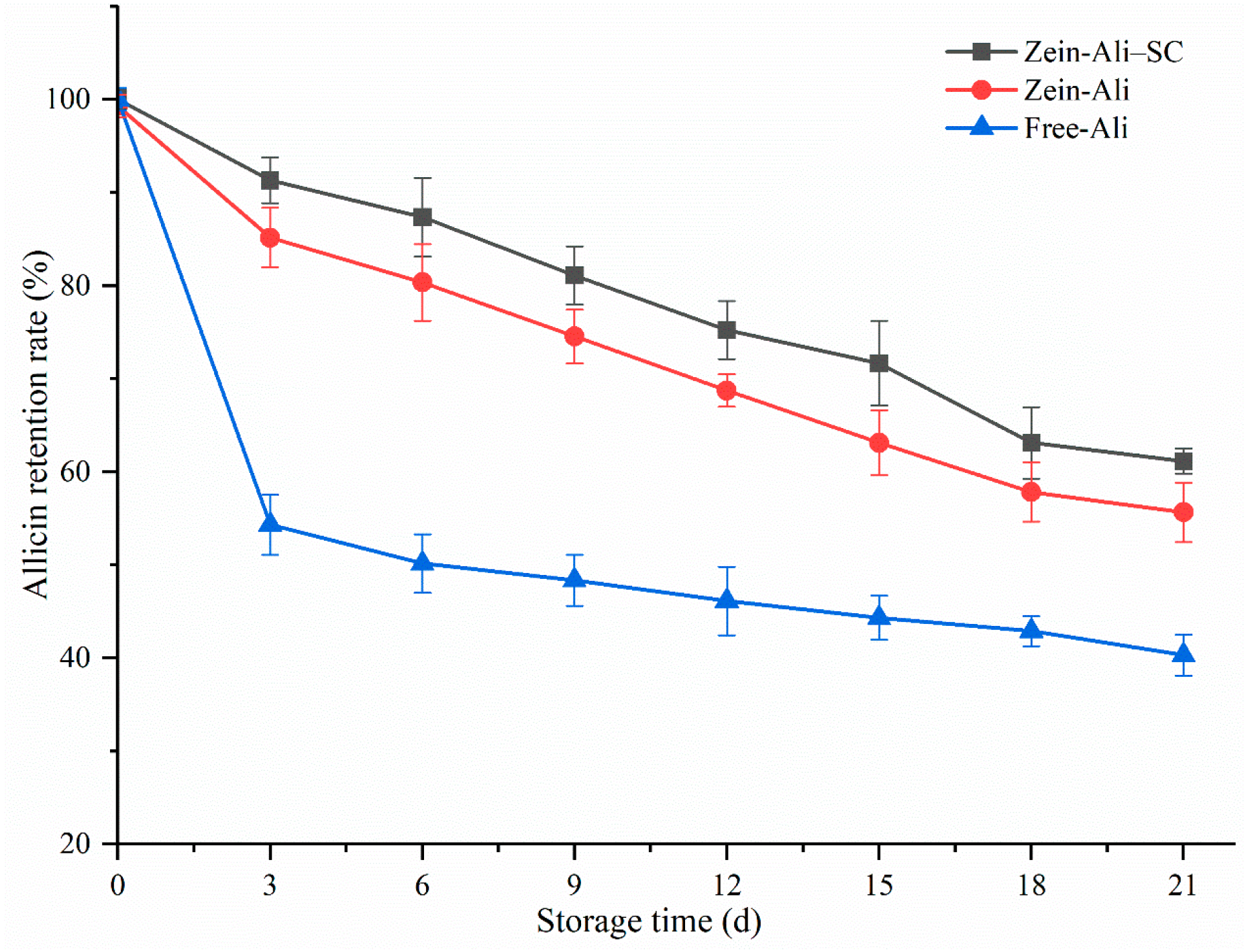
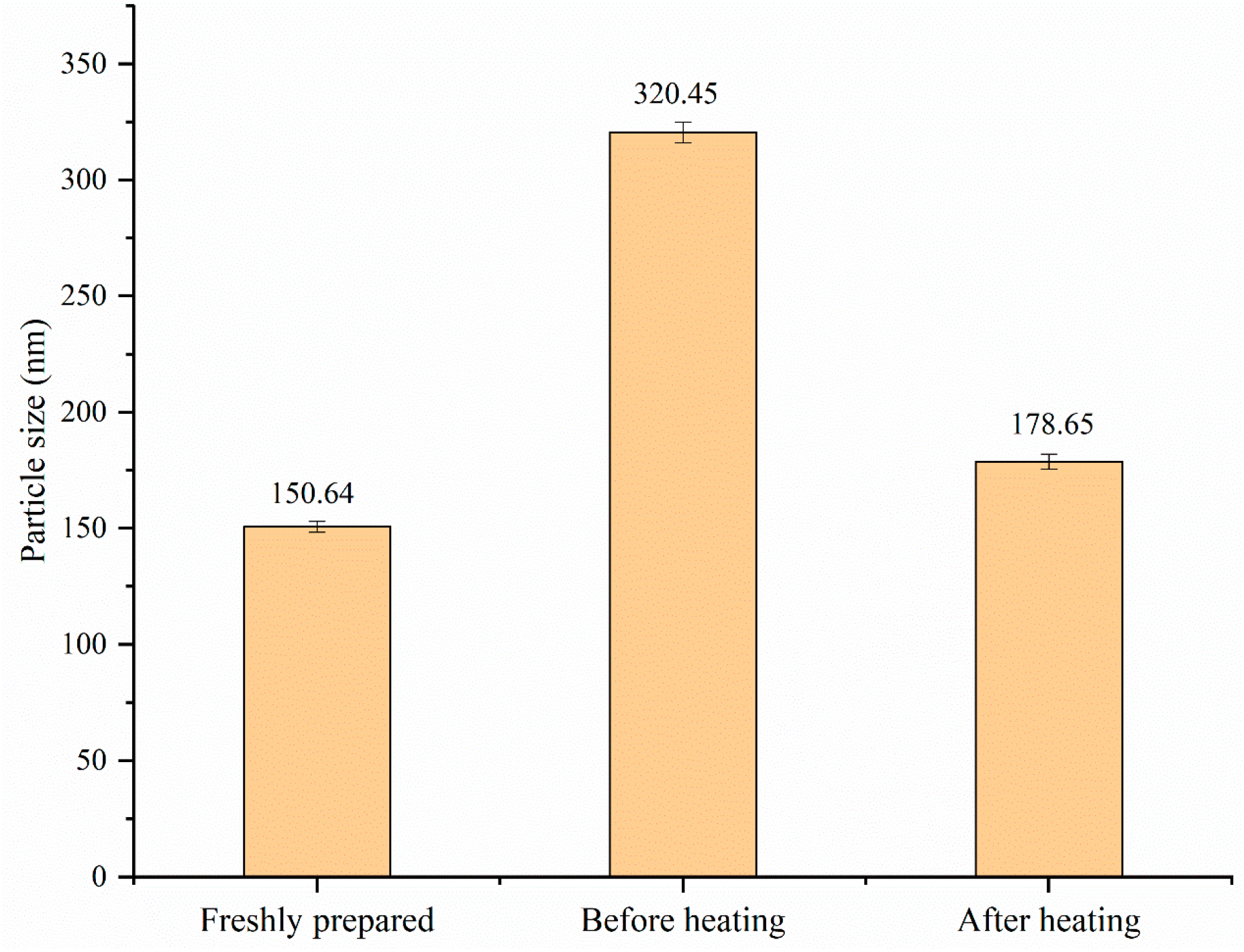
3.7.3. Thermal Stability and Storage Stability Analysis
3.7.4. Analysis of Redispersibility
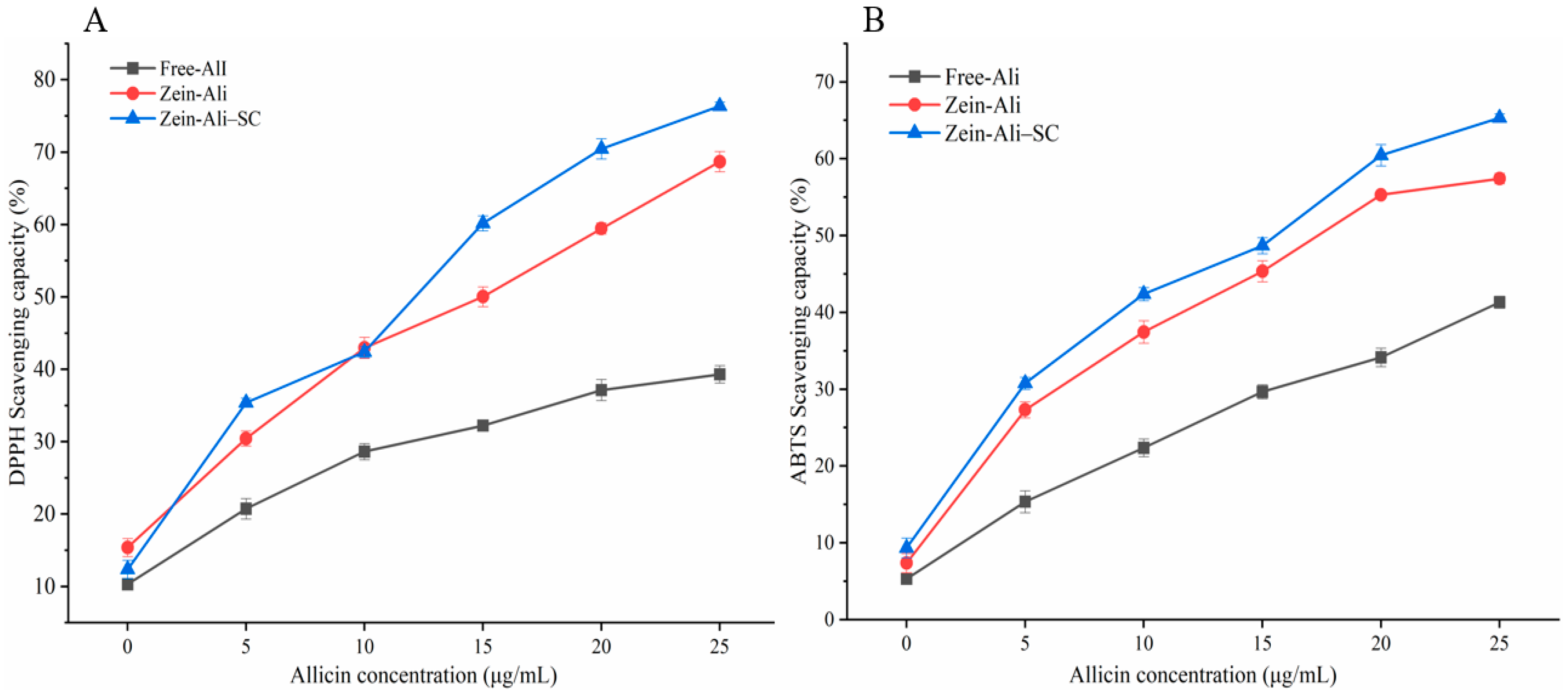
3.8. Analysis of Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.R.; Wilson, P. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 2004, 61, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.S.; Ben-Michael, T.; Kimhi, S.; Forer, I.; Rabinowitch, H.D.; Kamenetsky, R. Effects of different temperature regimes on flower development, microsporogenesis and fertility in bolting garlic (Allium sativum). Funct. Plant Biol. 2015, 42, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Bisharat, L.; AlKhatib, H.S.; Cespi, M. Zein as a Pharmaceutical Excipient in Oral Solid Dosage Forms: State of the Art and Future Perspectives. AAPS PharmSciTech 2018, 19, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Zein and zein-based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Yao, K.F.; Chen, W.J.; Song, F.L.; McClements, D.J.; Hu, K. Tailoring zein nanoparticle functionality using biopolymer coatings: Impact on curcumin bioaccessibility and antioxidant capacity under simulated gastrointestinal conditions. Food Hydrocoll. 2018, 79, 262–272. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Niu, Y.G.; Luo, Y.C.; Ge, M.; Yang, T.; Yu, L.L.; Wang, Q. Fabrication, characterization and antimicrobial activities of thymol-loaded zein nanoparticles stabilized by sodium caseinate-chitosan hydrochloride double layers. Food Chem. 2014, 142, 269–275. [Google Scholar] [CrossRef]
- Liu, Q.G.; Jing, Y.Q.; Han, C.P.; Zhang, H.; Tian, Y.M. Encapsulation of curcumin in zein/caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Sun, L.P.; Bao, C.J.; Chang, W.D.; Zhuang, Y.L. Preparation, characterisation, antioxidant and antiglycation activities of the novel polysaccharides from the pileus of Dictyophora rubrovolvata. Int. J. Food Sci. Technol. 2017, 52, 161–170. [Google Scholar] [CrossRef]
- Xue, J.L.; Zhang, Y.Q.; Huang, G.R.; Liu, J.; Slavin, M.; Yu, L.L. Zein-caseinate composite nanoparticles for bioactive delivery using curcumin as a probe compound. Food Hydrocoll. 2018, 83, 25–35. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.H.; Wang, J.W.; Zhu, J.X.; Wang, D.F.; Zhao, L.L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Li, K.K.; Yin, S.W.; Yang, X.Q.; Tang, C.H.; Wei, Z.H. Fabrication and Characterization of Novel Antimicrobial Films Derived from Thymol-Loaded Zein-Sodium Caseinate (SC) Nanoparticles. J. Agric. Food Chem. 2012, 60, 11592–11600. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wang, T.R.; Hu, Q.B.; Zhou, M.Y.; Xue, J.Y.; Luo, Y.C. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for curcumin. Food Hydrocoll. 2017, 70, 143–151. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.C.M.; Velikov, K.P. Sodium Caseinate Stabilized Zein Colloidal Particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Islam, M.S.; Podaralla, S.; Kaushik, R.S.; Reineke, J.; Woyengo, T.; Perumal, O. Food Protein Based Core–Shell Nanocarriers for Oral Drug Delivery: Effect of Shell Composition on In Vitro and In Vivo Functional Performance of Zein Nanocarriers. Mol. Pharm. 2017, 14, 757–769. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, P.C.; Wei, Y.B.; Guo, X.; Deng, X.R.; Zhang, J. Properties of allicin-zein composite nanoparticles gelatin film and their effects on the quality of cold fresh beef during storage. Foods 2023, 12, 3713. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.G.; Ma, C.C.; McClements, D.J.; Gao, Y.X. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.Z.; Xie, Q.T.; Cheng, J.S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2021, 340, 127893. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.H.; Wang, Y.Q.; Yang, X.; Sun, C.X.; Mao, L.K.; Gao, Y.X. Zein-hyaluronic acid binary complex as a delivery vehicle of quercetagetin: Fabrication, structural characterization, physicochemical stability and in vitro release property. Food Chem. 2019, 276, 322–332. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Sun, C.X.; Xu, C.Q.; Mao, L.K.; Wang, D.; Yang, J.; Gao, Y.X. Preparation, characterization and stability of curcumin-loaded zein/shellac composite colloidal particles. Food Chem. 2017, 228, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, Y.K.; Zhu, J.X.; Wang, T.; Wang, D.F.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.X.; Wei, Y.; Zhan, X.Y.; Mao, L.K.; Gao, Y.X. Formation and characterization of zein-propylene glycol alginate-surfactant ternary complexes: Effect of surfactant type. Food Chem. 2018, 258, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yu, Z.P.; Lin, K.S.; Sun, C.X.; Dai, L.; Yang, S.F.; Mao, L.K.; Yuan, F.; Gao, Y.X. Fabrication and characterization of resveratrol loaded zein-propylene glycolalginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocoll. 2019, 95, 336–348. [Google Scholar] [CrossRef]
- Wang, Q.M.; Tang, Y.W.; Yang, Y.X.; Lei, L.; Lei, X.J.; Zhao, J.C.; Zhang, Y.H.; Li, L.; Wang, Q.; Ming, J. The interaction mechanisms, and structural changes of the interaction between zein and ferulic acid under different pH conditions. Food Hydrocoll. 2022, 124 Pt A, 107251. [Google Scholar] [CrossRef]
- Uzun, S.; Ilavsky, J.; Padua, G.W. Characterization of zein assemblies by ultra-small-angle X-ray scattering. Soft Matter. 2017, 13, 3053–3060. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y. Heat-induced self-assembly of zein nanoparticles: Fabrication, stabilization and potential application as oral drug delivery. Food Hydrocoll. 2019, 90, 403–412. [Google Scholar] [CrossRef]
- Yuan, Y.K.; Li, H.; Zhu, J.X.; Liu, C.Z.; Sun, X.; Wang, D.F.; Xu, Y. Fabrication and characterization of zein nanoparticles by dextran sulfate coating as vehicles for delivery of curcumin. Int. J. Biol. Macromol. 2020, 151, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.K.; Li, H.; Liu, C.Z.; Zhu, J.X.; Xu, Y.; Zhang, S.Z.; Fan, M.H.; Zhang, D.D.; Zhang, Y.N.; Zhang, Z.J.; et al. Fabrication of stable zein nanoparticles by chondroitin sulfate deposition based on antisolvent precipitation method. Int. J. Biol. Macromol. 2019, 139, 30–39. [Google Scholar] [CrossRef]
- Goncalves, R.F.S.; Madalena, D.A.; Fernandes, J.M.; Marques, M.; Vicente, A.A.; Pinheiro, A.C. Application of nanostructured delivery systems in food: From incorporation to detection and characterization. Trends Food Sci. Technol. 2022, 129, 111–125. [Google Scholar] [CrossRef]
- Song, J.R.; Sun, C.X.; Gul, K.; Mata, A.; Fang, Y.P. Prolamin-based complexes: Structure design and food-related applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1120–1149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xing, Z.; Wang, Y.; Zhang, Z.L.; Mintah, B.K.; Dabbour, M.; Li, Y.H.; He, R.H.; Huang, L.R.; Ma, H.L. Preparation of allicin-whey protein isolate conjugates: Allicin extraction by water, conjugates’ ultrasound-assisted binding and its stability, solubility and emulsibility analysis. Ultrason. Sonochemistry 2020, 63, 104981. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Chen, L.Y.; Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. 2021, 113, 106477. [Google Scholar] [CrossRef]
- Li, D.D.; Wei, Z.H.; Sun, J.L.; Xue, C.H. Tremella polysaccharides-coated zein nanoparticles for enhancing stability and bioaccessibility of curcumin. Curr. Res. Food Sci. 2022, 5, 611–618. [Google Scholar] [CrossRef] [PubMed]



| EE (%) | LC (%) | Particle Size (nm) | PDI | ζ-Potential (mV) | |
|---|---|---|---|---|---|
| Zein | / | / | 121.3 ± 3.8 a | 0.220 ± 0.015 a | 29.34 ± 1.06 d |
| Zein–Ali–SC40:1 | 71.54 ± 2.31 a | 5.56 ± 0.06 a | 132.8 ± 6.5 b | 0.222 ± 0.002 a | −30.27 ± 0.61 c |
| Zein–Ali–SC20:1 | 77.32 ± 3.82 b | 5.62 ± 0.02 a | 146.0 ± 1.4 c | 0.228 ± 0.178 a | −31.79 ± 0.89 bc |
| Zein–Ali–SC8:1 | 86.62 ± 1.25 d | 7.86 ± 0.13 c | 151.5 ± 1.1 c | 0.250 ± 0.015 b | −35.53 ± 1.06 a |
| Zein–Ali–SC4:1 | 85.17 ± 2.49 d | 6.75 ± 0.25 b | 176.6 ± 0.5 d | 0.272 ± 0.012 c | −33.18 ± 0.66 b |
| Zein–Ali–SC2:1 | 81.38 ± 2.12 c | 6.84 ± 0.05 b | 196.5 ± 2.5 e | 0.291 ± 0.010 d | −32.65 ± 1.57 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Zhao, P.; Wei, Y.; Lei, Y.; Guo, X.; Deng, X.; Zhang, J. Preparation and Characterization Study of Zein–Sodium Caseinate Nanoparticle Delivery Systems Loaded with Allicin. Foods 2024, 13, 3111. https://doi.org/10.3390/foods13193111
Hu L, Zhao P, Wei Y, Lei Y, Guo X, Deng X, Zhang J. Preparation and Characterization Study of Zein–Sodium Caseinate Nanoparticle Delivery Systems Loaded with Allicin. Foods. 2024; 13(19):3111. https://doi.org/10.3390/foods13193111
Chicago/Turabian StyleHu, Ling, Pengcheng Zhao, Yabo Wei, Yongdong Lei, Xin Guo, Xiaorong Deng, and Jian Zhang. 2024. "Preparation and Characterization Study of Zein–Sodium Caseinate Nanoparticle Delivery Systems Loaded with Allicin" Foods 13, no. 19: 3111. https://doi.org/10.3390/foods13193111
APA StyleHu, L., Zhao, P., Wei, Y., Lei, Y., Guo, X., Deng, X., & Zhang, J. (2024). Preparation and Characterization Study of Zein–Sodium Caseinate Nanoparticle Delivery Systems Loaded with Allicin. Foods, 13(19), 3111. https://doi.org/10.3390/foods13193111





