Evaluation of Functional Components of Lactobacillus plantarum AR495 on Ovariectomy-Induced Osteoporosis in Mice And RAW264.7 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Different Treatments of Strains
2.3. Different Treatments of AR495 Fermentation Broths
2.4. Experimental Animals and Design
2.5. Micro-Computed Tomography (μCT)
2.6. Measurements of Bone Turnover Markers
2.7. ELISA Assay
2.8. RANKL-Induced Cell Differentiation
2.9. Cell Viability, Counting, and Cell Morphology
2.10. Gene Expression
2.11. Statistical Analysis
3. Results
3.1. Effect of Different Treatments of AR495 on Body Weight and Gonadal Adiposity
3.2. Effect of Different Treatments of AR495 on Bone Morphology and Bone Conversion Markers
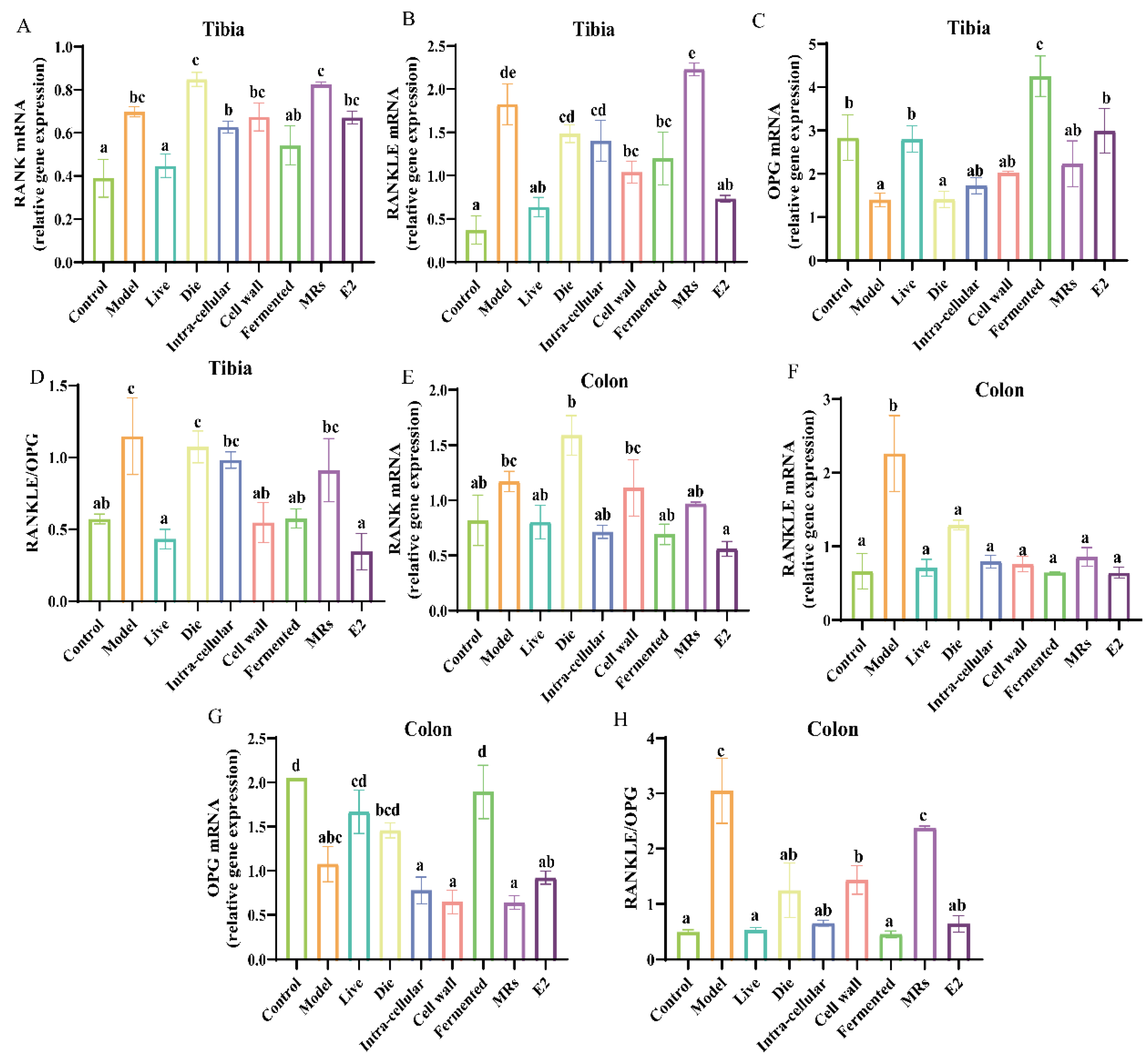
3.3. Effect of Different Treatments of AR495 on the Expression of Pro-Osteoclast-Related Factors
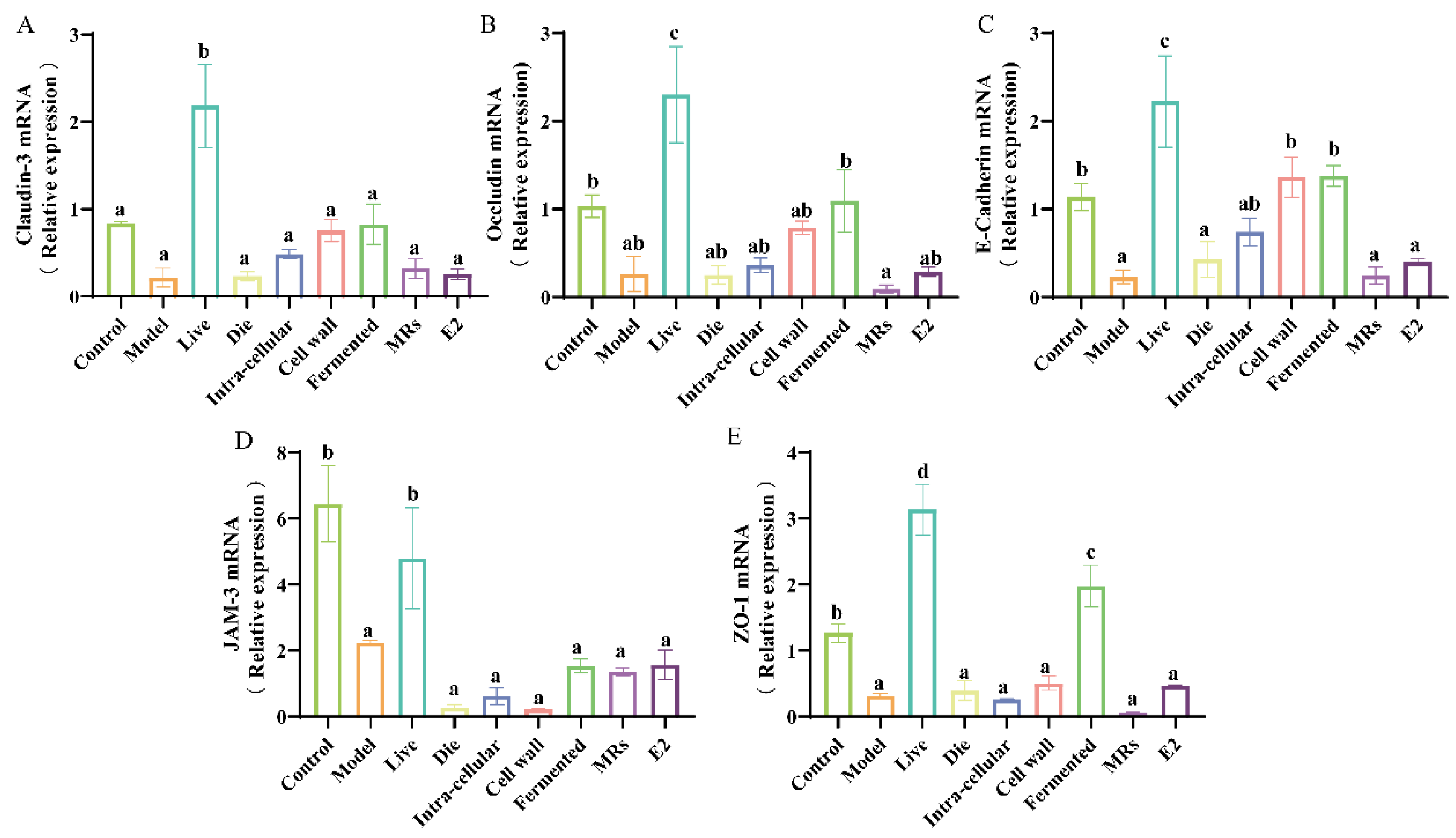
3.4. Effect of Different Treatments of AR495 on Intestinal Permeability in Mice
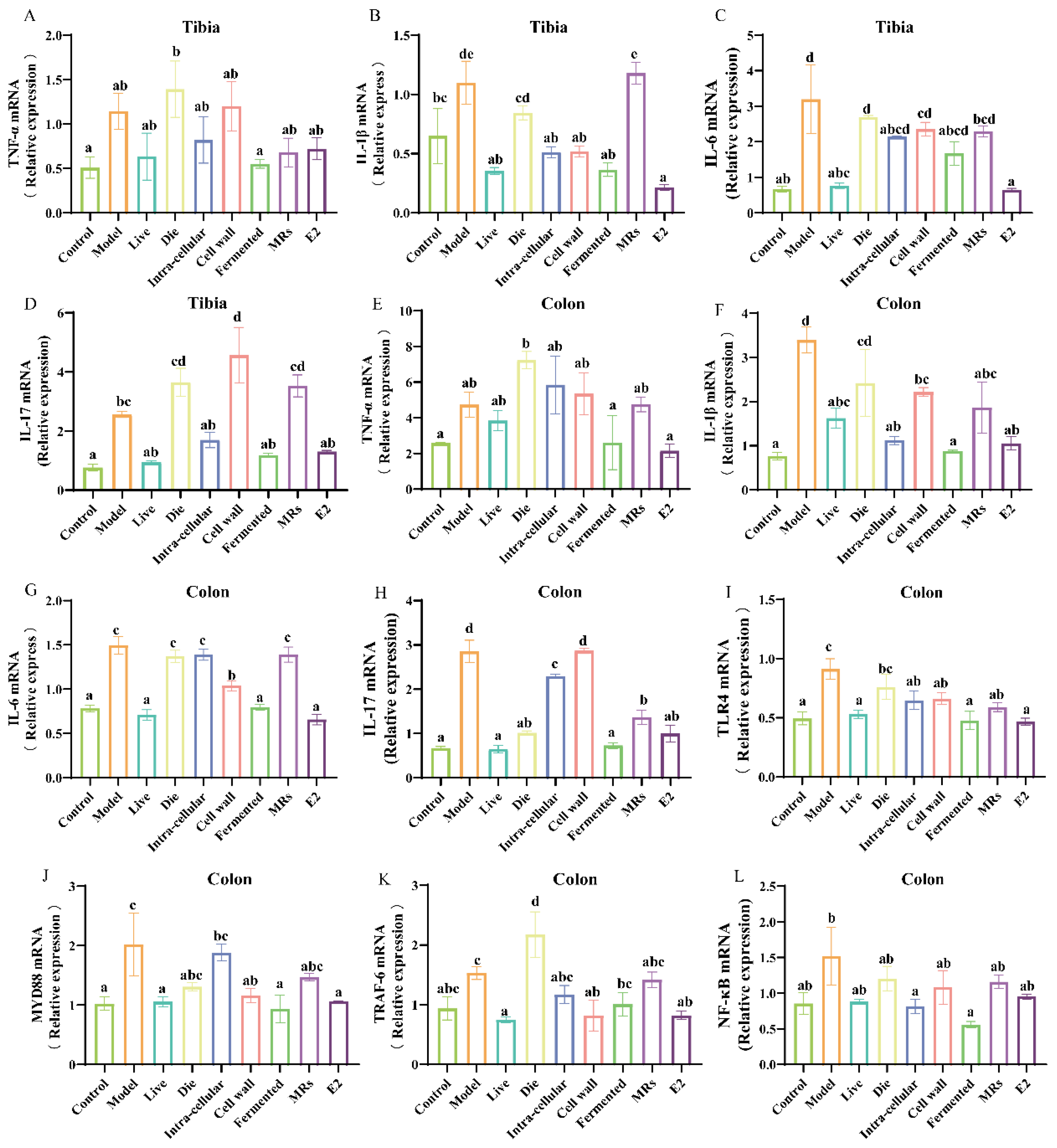
3.5. Effects of Different Treatments of AR495 on Inflammatory Factors and Osteoclast Differentiation Pathways
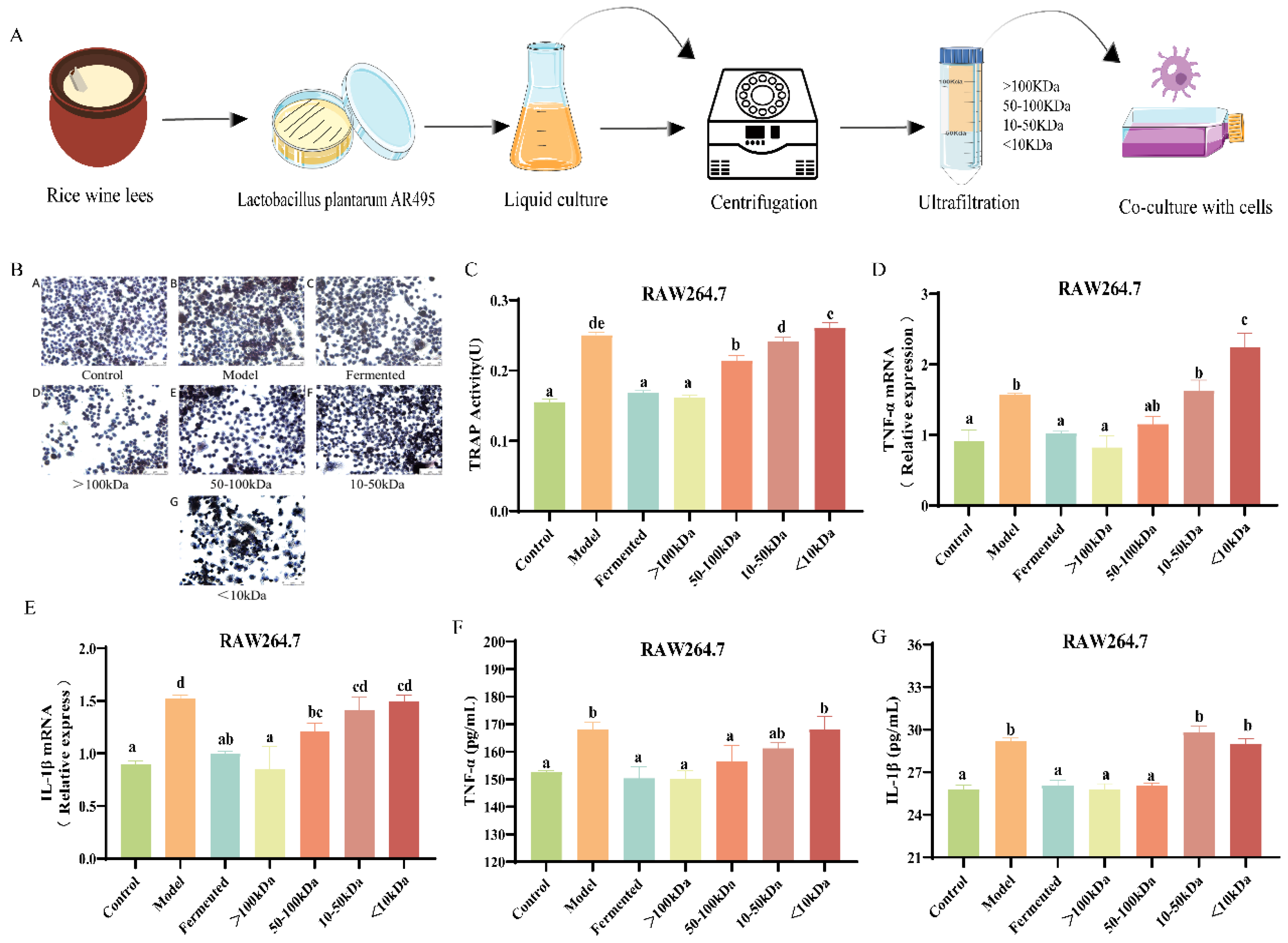
3.6. Effects of Different Molecular Weight Fractions of AR495 Fermentation Broth on Bone Metabolism and Inflammation in RAW264.7 Cells
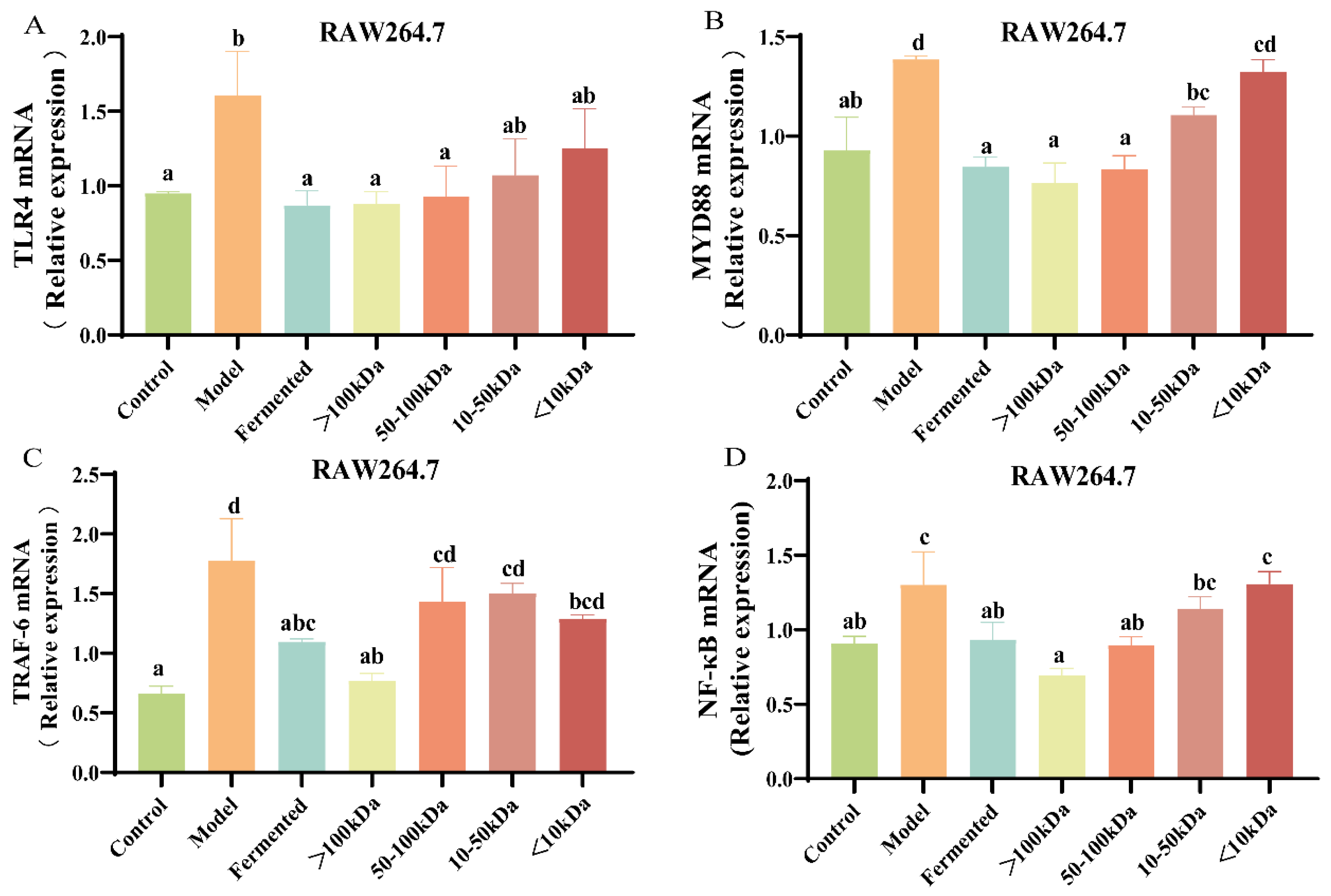
3.7. Effect of Different Molecular Weight Fractions of AR495 Fermentation Broth on RAW264.7 Pathway Proteins
4. Discussion
4.1. The Relationship between Osteoporosis and the Gut
4.2. AR495 Alleviates Osteoporosis by Regulating the RANK/RANKL/OPG Signaling Pathway
4.3. Effects of Different Molecular Weight Fractions of AR495 Fermentation Broth on Bone Metabolism and Inflammation and Protein Pathway in RAW264.7 Cells
4.4. Effect of Probiotics and Other Derivatives on Disease
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1940. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R.; Kaser, A.; Pines, A.; Dotan, I. Gut, inflammation and osteoporosis: Basic and clinical concepts. Gut 2008, 57, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Chen, C.-Y.; Liu, Z.-Z.; Luo, Z.-W.; Rao, S.-S.; Jin, L.; Wan, T.-F.; Yue, T.; Tan, Y.-J.; Yin, H.; et al. Extracellular Vesicles from Child Gut Microbiota Enter into Bone to Preserve Bone Mass and Strength. Adv. Sci. 2021, 8, 2004831. [Google Scholar] [CrossRef]
- Rizzoli, R. Atlas of Postmenopausal Osteoporosis; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Park, W.C.; Jordan, V.C. Selective estrogen receptor modulators (SERMS) and their roles in breast cancer prevention. Trends Mol. Med. 2002, 8, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Vannala, V.; Palaian, S.; Shankar, P.R. Therapeutic Dimensions of Bisphosphonates: A Clinical Update. Int. J. Prev. Med. 2020, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.F.; Wang, F.X.; Wang, S.C.; Zhang, Y.Z.; Bai, L.; Su, J.C. Bone-organ axes: Bidirectional crosstalk. Mil. Med. Res. 2024, 11, 37. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef]
- Konieczna, P.; Groeger, D.; Ziegler, M.; Frei, R.; Ferstl, R.; Shanahan, F.; Quigley, E.M.; Kiely, B.; Akdis, C.A.; O’Mahony, L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: Potential role for myeloid and plasmacytoid dendritic cells. Gut 2012, 61, 354–366. [Google Scholar] [CrossRef]
- Shao, J.; Mu, Z.; Xia, Y.; Xiong, Z.; Song, X.; Yang, Y.; Zhang, H.; Ai, L.; Wang, G. bsh1 Gene of Lactobacillus plantarum AR113 Plays an Important Role in Ameliorating Western Diet-Aggravated Colitis. J. Agric. Food Chem. 2023, 71, 9337–9348. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.-W.; You, Y.S.; Kwon, H.-S.; Yang, E.H.; Ryu, J.-S.; Kang, B.H.; Kang, J.-H. Fermented milk of Lactobacillus helveticus IDCC3801 reduces beta-amyloid and attenuates memory deficit. J. Funct. Foods 2010, 2, 143–152. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, K.; Ebrahimi, M.; Sabran, M.R.; Karimi, G.; Hwei, A.N.; Abdul-Majeed, S.; Ahmad, Z.; Ibrahim, Z.; Jamaluddin, R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. BioMed Res. Int. 2015, 2015, 897639. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ji, B.; Luo, H.; Sundh, D.; Lorentzon, M.; Nielsen, J. One-year supplementation with Lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. NPJ Biofilms Microbiomes 2022, 8, 84. [Google Scholar] [CrossRef]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.K.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef]
- Sanz, Y.; Nadal, I.; Sánchez, E. Probiotics as drugs against human gastrointestinal infections. Recent Pat. Anti Infect. Drug Discov. 2007, 2, 148–156. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, Q.; Puthiyakunnon, S.; Zeng, Z.; Yang, W.; Qiu, J.; Du, L.; Boddu, S.; Wu, T.; Cai, D.; et al. Lactobacillus rhamnosus GG supernatant enhance neonatal resistance to systemic Escherichia coli K1 infection by accelerating development of intestinal defense. Sci. Rep. 2017, 7, 43305. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xia, Y.; Wang, G.; Xiong, Z.; Zhang, H.; Lai, P.F.H.; Song, X.; Ai, L. Anti-osteoporotic potential of Lactobacillus plantarum AR237 and AR495 in ovariectomized mice. J. Funct. Foods 2021, 87, 104762. [Google Scholar] [CrossRef]
- Sakka, S.D.; Cheung, M.S. Management of primary and secondary osteoporosis in children. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720x20969262. [Google Scholar] [CrossRef]
- Riggs, B.L.; Wahner, H.W.; Seeman, E.; Offord, K.P.; Dunn, W.L.; Mazess, R.B.; Johnson, K.A.; Melton, L.J. Changes in bone mineral density of the proximal femur and spine with aging: Differences between the postmenopausal and senile osteoporosis syndromes. J. Clin. Investig. 1982, 70, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Soriano, R.; Herrera, S.; Nogués, X.; Diez-Perez, A. Current and future treatments of secondary osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 885–894. [Google Scholar] [CrossRef]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, J.; Pang, X.Y.; Zhao, L.P.; Tian, L.; Wang, X.P. Sex differences in colonization of gut microbiota from a man with short-term vegetarian and inulin-supplemented diet in germ-free mice. Sci. Rep. 2016, 6, 36137. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Du, J.; Liu, Y.; Wu, X.; Sun, J.; Shi, J.; Zhang, H.; Zheng, A.; Zhou, M.; Jiang, X. BRD9-mediated chromatin remodeling suppresses osteoclastogenesis through negative feedback mechanism. Nat. Commun. 2023, 14, 1413. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.P.; Tometsko, M.E.; Glaccum, M.; Sutherland, C.L.; Cosman, D.; Dougall, W.C. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J. Biol. Chem. 2002, 277, 44347–44356. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J.; Komadina, R.; Marc, J. The relationship between osteoclastogenic and anti-osteoclastogenic pro-inflammatory cytokines differs in human osteoporotic and osteoarthritic bone tissues. J. Biomed. Sci. 2012, 19, 28. [Google Scholar] [CrossRef]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Shanmugarajan, S.; Haycraft, C.J.; Reddy, S.V.; Ries, W.L. NIP45 negatively regulates RANK ligand induced osteoclast differentiation. J. Cell. Biochem. 2012, 113, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.S.; Hua, R.; Zhao, X.P.; Qin, X.; Sun, Z.Q.; Zhang, Y.; Wu, Y.Q.; Jia, M.X.; Cao, J.L.; Zhang, Y.M. Exogenous administration of PACAP alleviates traumatic brain injury in rats through a mechanism involving the TLR4/MyD88/NF-κB pathway. J. Neurotrauma 2012, 29, 1941–1959. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ren, D.; Zhao, Y.; Liu, L.; Yang, X. Fuzhuan Brick Tea Polysaccharide Improved Ulcerative Colitis in Association with Gut Microbiota-Derived Tryptophan Metabolism. J. Agric. Food Chem. 2021, 69, 8448–8459. [Google Scholar] [PubMed]
- Ren, M.; Ahmed, A.F.; Li, M.; Li, M.; Yan, Z.; Wang, J. A review: The mechanism of plant-derived polysaccharides on osteoblasts and osteoclasts. J. Future Foods 2024, 4, 183–192. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Miao, Z.; Yan, Y.; Chen, D.; Yang, Z.X.; Yue, L.; Hu, W.; Zhuo, L.; Wang, J.T.; et al. Proteome-wide profiling reveals dysregulated molecular features and accelerated aging in osteoporosis: A 9.8-year prospective study. Aging Cell 2024, 23, e14035. [Google Scholar] [CrossRef] [PubMed]
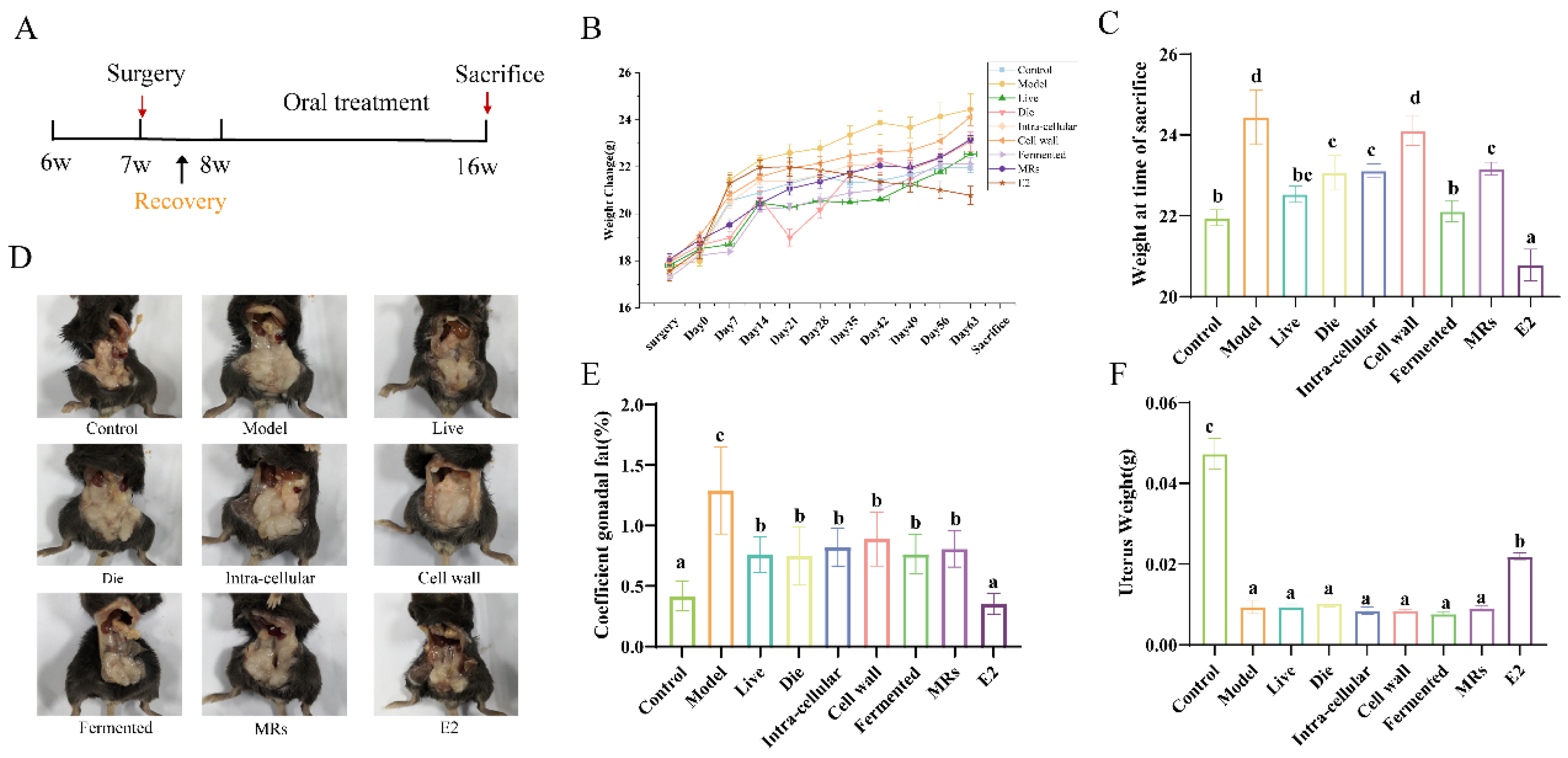

| Groups | Dosage (Gavage) |
| Control | sterilized water (10 mL/kg per day) |
| Model | sterilized water (10 mL/kg per day) |
| Live | 1 × 109 CFU/mL (10 mL/kg per day) |
| Die | 1 × 109 CFU/mL bacteria (10 mL/kg per day) |
| Intra-cellular | 10 mL/kg per day |
| Cell wall | 10 mL/kg per day |
| Fermented | 10 mL/kg per day |
| MRs | 10 mL/kg per day |
| E2 | 10 mg/kg per day |
| Gene | Forward Primer (5′-3′) Reverse Primer (5′-3′) |
|---|---|
| HPRT1 | GAAGGAGATGGGAGGCAATCACATT AATCCAGCAGGTCAGCAAAGAACTT |
| Claudin-3 | TCATCGGCAGCAGCATCATCAC CCAGCAGCGAGTCGTACATCTTG |
| E-cadherin 1 | CAGGTCTCCTCATGGCTTTGC CTTCCGAAAAGAAGGCTGTCC |
| Occludin | TTGAAAGTCCACCTCCTTACAGA CCGGATAAAAAGAGTACGCTGG |
| TLR4 | CCGCTCTGGCATCATCTTCA CCCACTCGAGGTAGGTGTTTCTG |
| TNF-α | AGGGTCTGGGCCATAGAACT CCACCACGCTCTTCTGTCTAC |
| IL-6 | GAGGATACCACTCCCAACAGACC AAGTGCATCATCGTTGTTCATACA |
| IL-1β MYD88 NF-κB RANK RANKL OPG IL-17 ZO-1 JAM-3 TRAF-6 | GAAATGCCACCTTTTGACAGTG TGGATGCTCTCATCAGGACAG GCATGGTGGTGGTTGTTTCTG GAATCAGTCGCTTCTGTTGG ACACTGGAAGCACGGATGAC TGTCTGTGAGTTGCCGGTCT TGAGCCTCCGAGCAGAACTGAC TGCCTGTGTAGCCATCTGTTGAGT GATGGAAGGCTCATGGTTGGATGTG GGCAGCATTGATGGTGAGGTGTG GCAGAGACGCACCTAGCACTGA CGCAGCACAGCCACTTGTTCAT TTTAACTCCCTTGGCGCAAAA CTTTCCCTCCGCATTGACAC GCCGCTAAGAGCACAGCAA TCCCCACTCTGAAAATGAGGA CTGCGACTTCGACTGTACG TTCGGTTGCTGGATTTGAGATT AAAGCGAGAGATTCTTTCCCTG ACTGGGGACAATTCACTAGAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Shao, J.; Yang, Y.; Wang, G.; Xiong, Z.; Song, X.; Ai, L.; Xia, Y.; Zhu, B. Evaluation of Functional Components of Lactobacillus plantarum AR495 on Ovariectomy-Induced Osteoporosis in Mice And RAW264.7 Cells. Foods 2024, 13, 3115. https://doi.org/10.3390/foods13193115
Chen Z, Shao J, Yang Y, Wang G, Xiong Z, Song X, Ai L, Xia Y, Zhu B. Evaluation of Functional Components of Lactobacillus plantarum AR495 on Ovariectomy-Induced Osteoporosis in Mice And RAW264.7 Cells. Foods. 2024; 13(19):3115. https://doi.org/10.3390/foods13193115
Chicago/Turabian StyleChen, Zheng, Junlin Shao, Yijin Yang, Guangqiang Wang, Zhiqiang Xiong, Xin Song, Lianzhong Ai, Yongjun Xia, and Beiwei Zhu. 2024. "Evaluation of Functional Components of Lactobacillus plantarum AR495 on Ovariectomy-Induced Osteoporosis in Mice And RAW264.7 Cells" Foods 13, no. 19: 3115. https://doi.org/10.3390/foods13193115






