Characterization of the Composition of Bioactive Fractions from Dendrobium officinale Flowers That Protect against H2O2-Induced Oxidative Damage through the PI3K/AKT/Nrf2 Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Crude Extracts Preparation
2.3. Determination of Total Flavonoid Contents
2.4. UPLC-Q-Orbitrap HRMS Analysis
2.5. In Vitro Antioxidant Activity Analysis
2.5.1. DPPH Assay
2.5.2. Hydroxyl Radical Assay
2.5.3. ABTS Assay
2.5.4. FRAP Assay
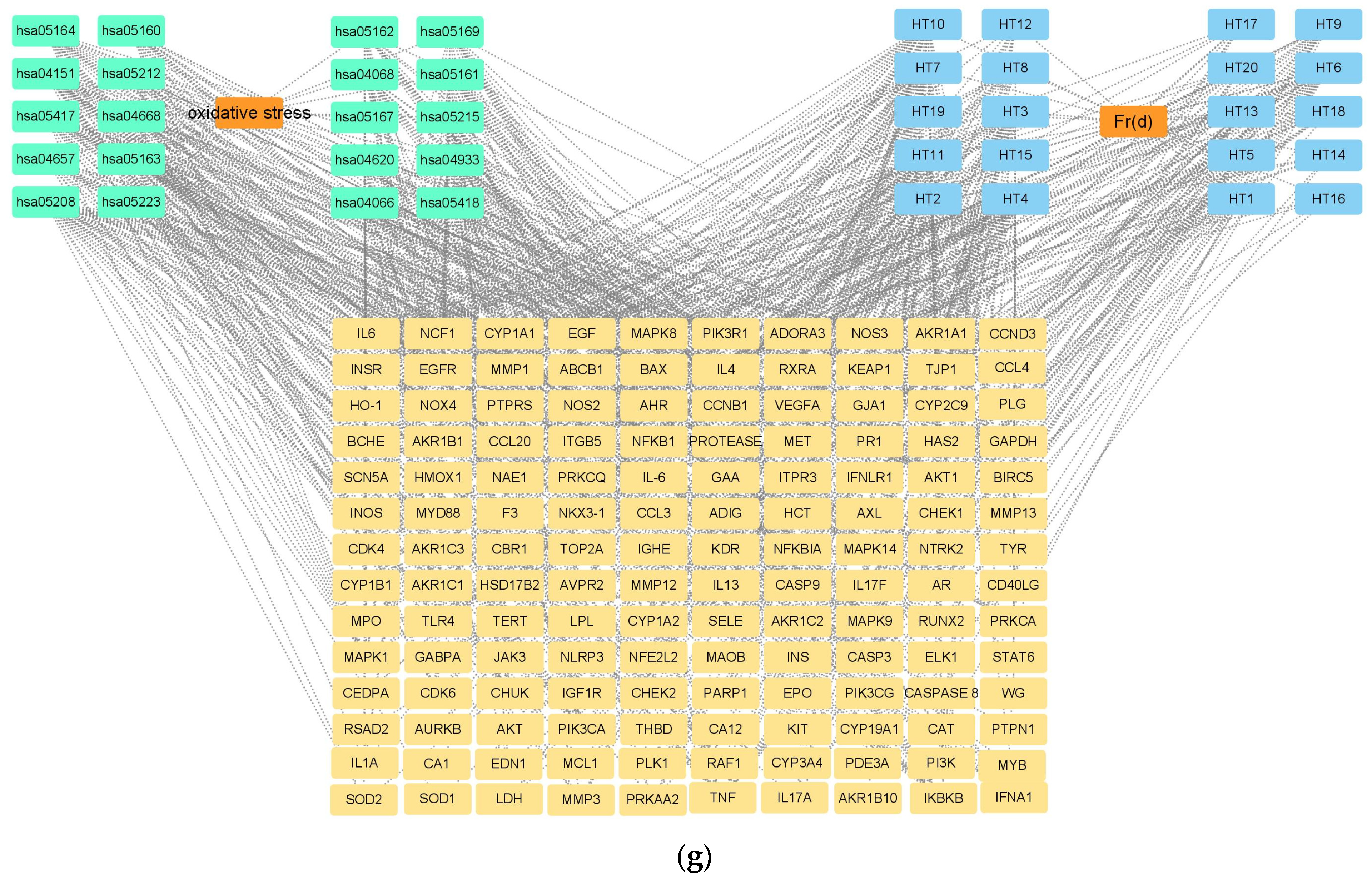
2.6. Network Pharmacology
2.7. Analyses of Fr. (d) Antioxidant and Anti-Inflammatory Activity
2.7.1. Cell Culture
2.7.2. MTT Assay
2.7.3. H2O2-Induced Modeling of Cell Injury
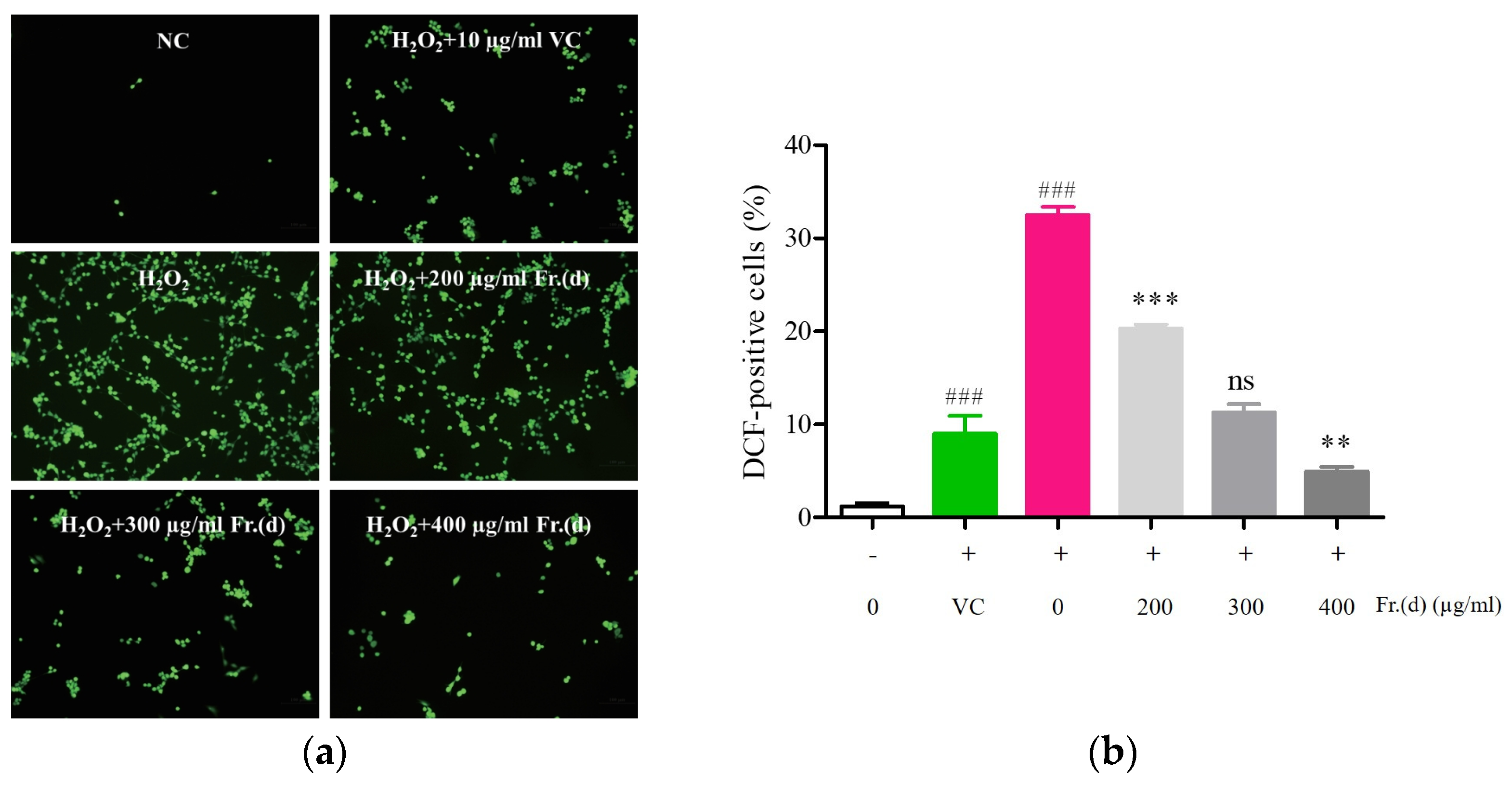
2.7.4. Intracellular ROS Detection
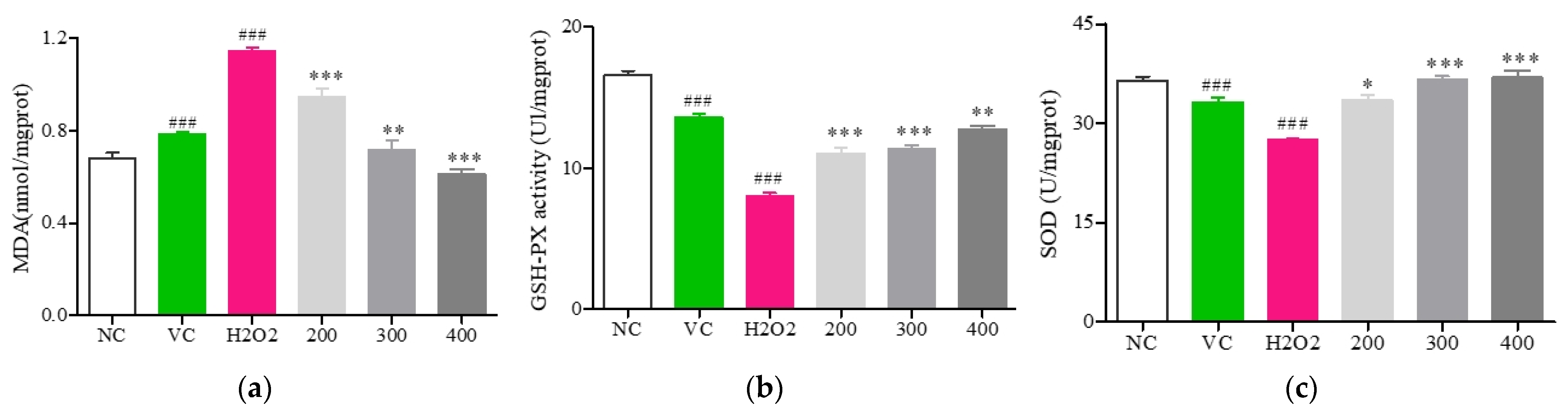
2.7.5. Analyses of MDA, SOD, and GSH-Px Activity
2.7.6. NO and Cytokine Analyses
2.7.7. Western Immunoblotting
2.8. Statistical Analyses
3. Results
3.1. Characterization of the Composition and Bioactivity of DOF Crude Extract and Fraction Samples
3.1.1. Total Flavonoid Levels in Crude Extract and Fraction Samples
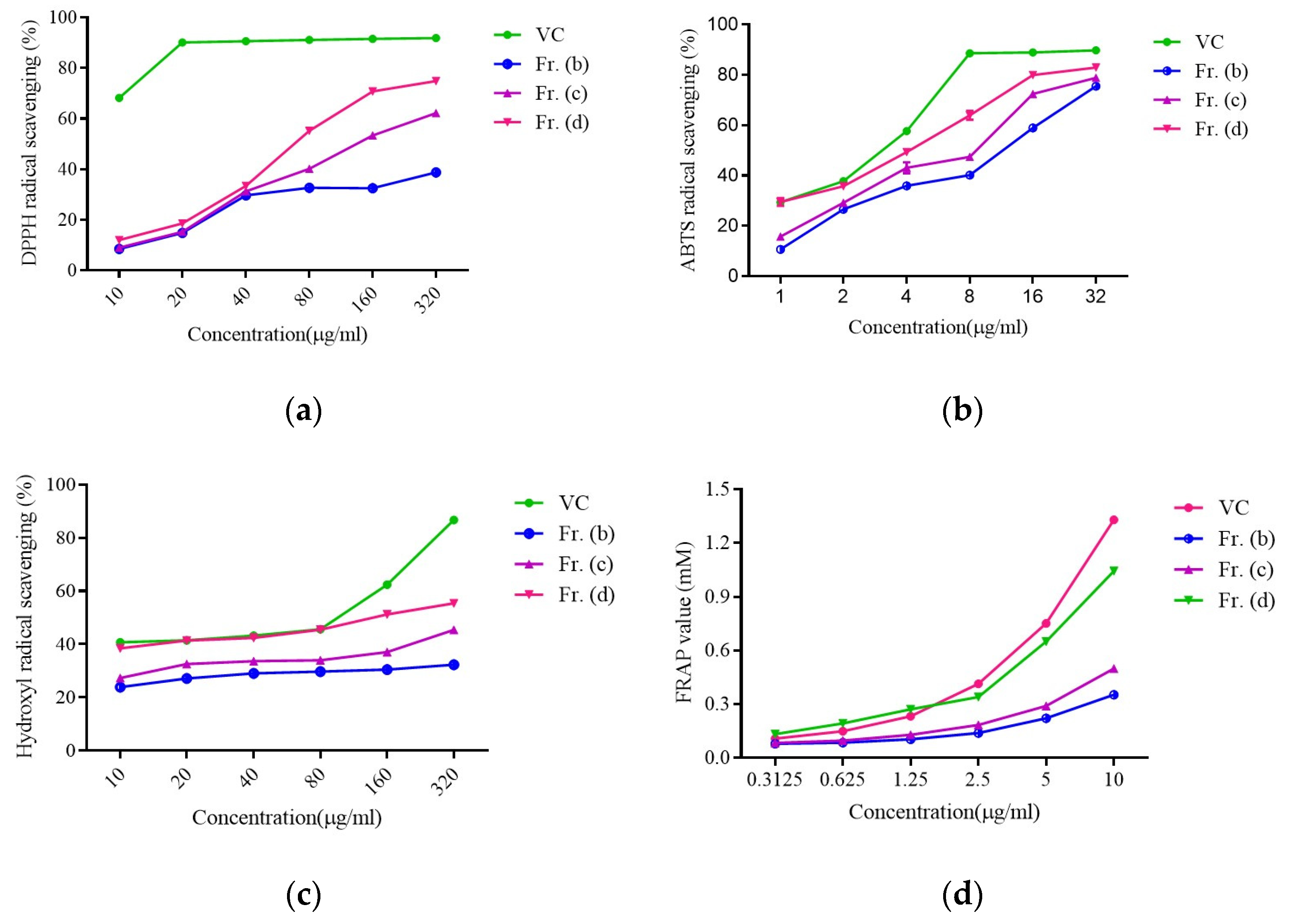
3.1.2. Evaluation of Fraction-Specific Antioxidant Activity
3.1.3. LC-MS Characterization of Flavonoids in DOF
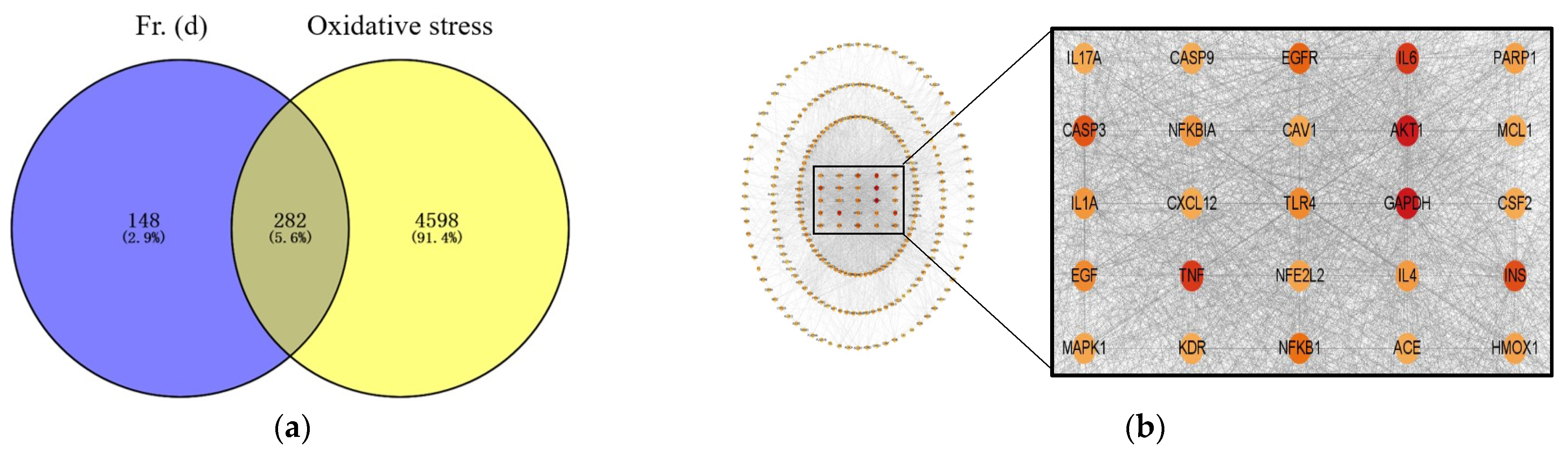
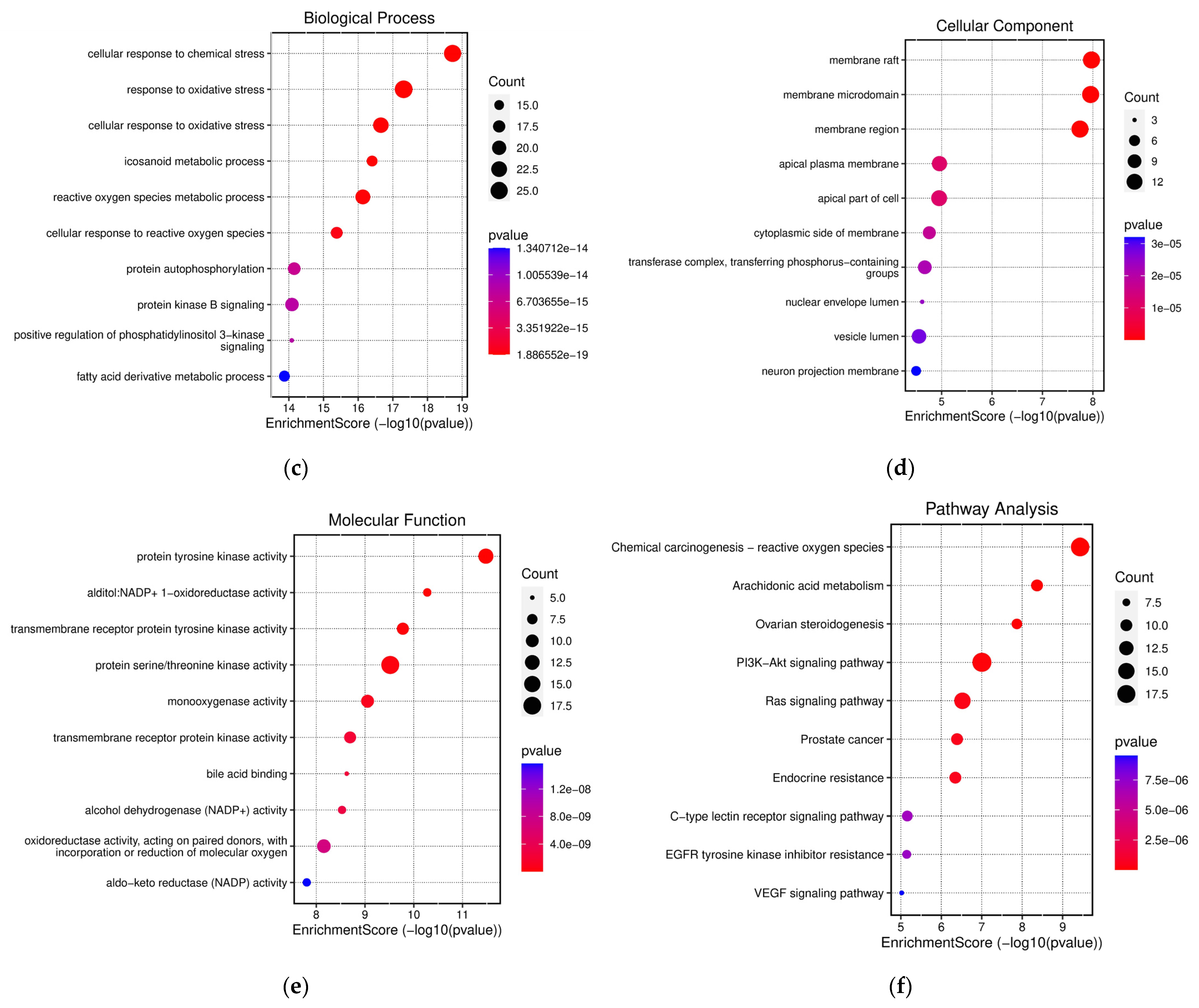
3.2. Network Pharmacology Analysis
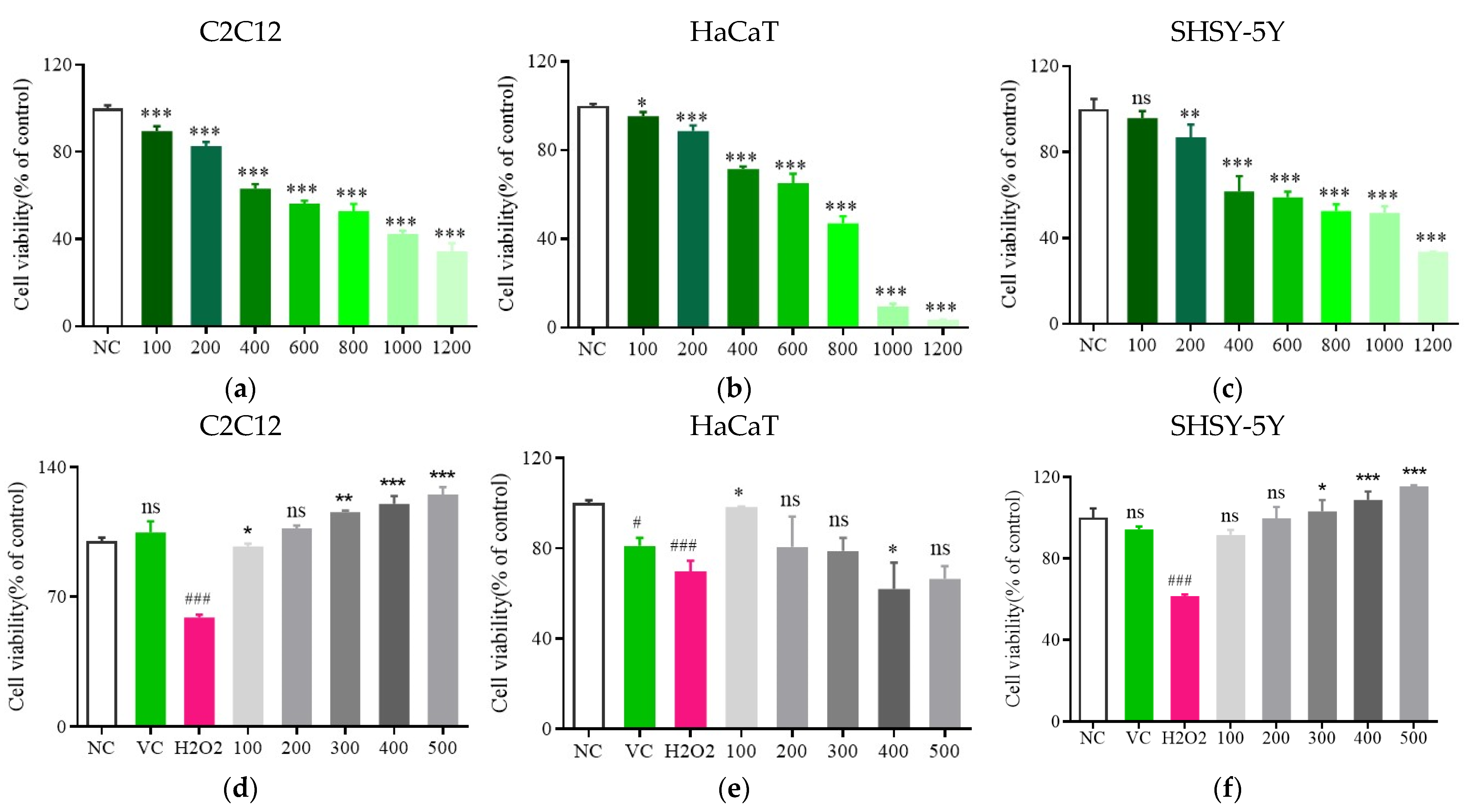
3.3. Analyses of Toxicity
3.4. Fr. (d) Protects C2C12 Cells against H2O2-Induced Injury
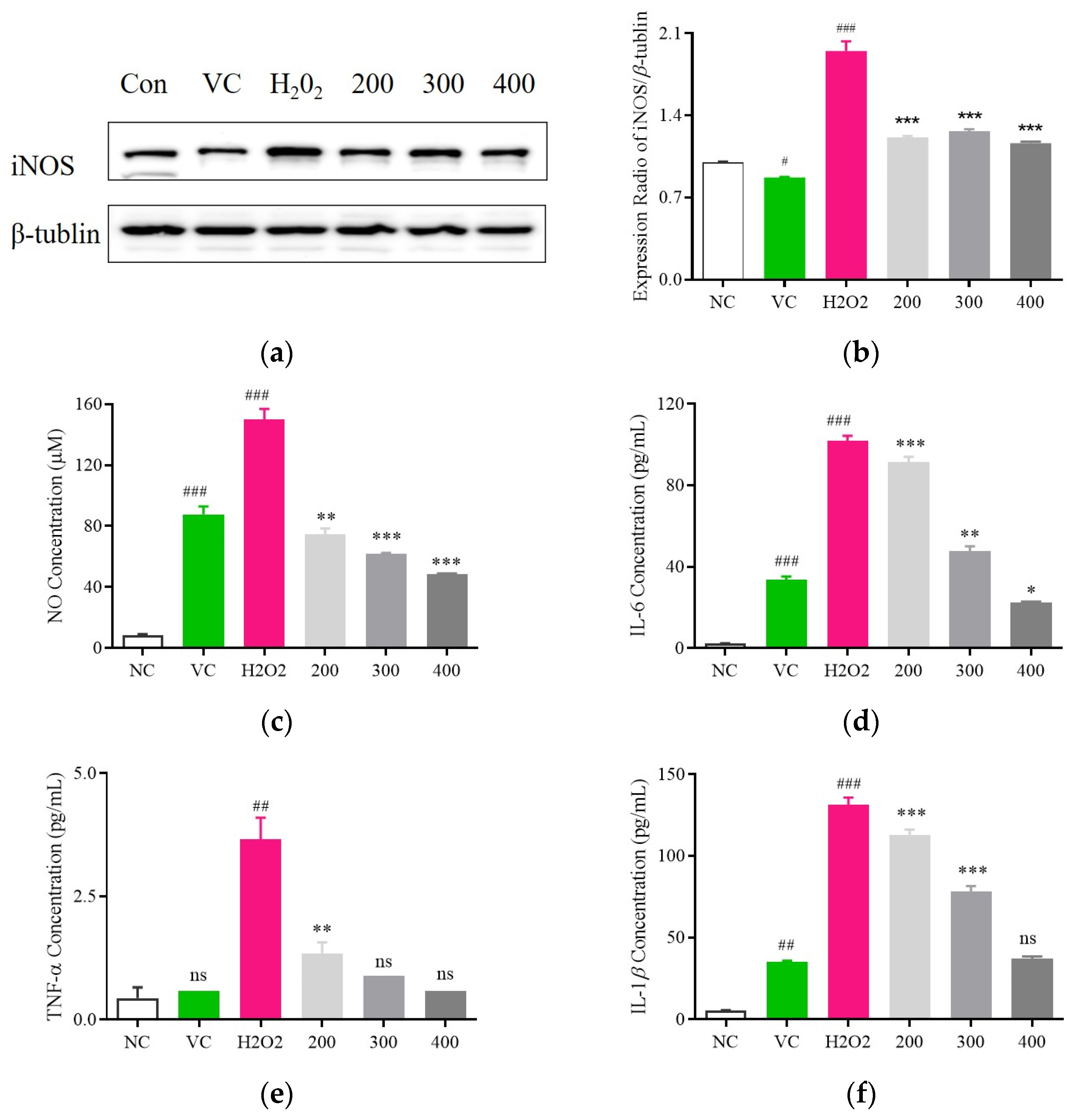
3.5. Examination of the Anti-Inflammatory Impact of Fr. (d) on H2O2-Exposed C2C12 Cells
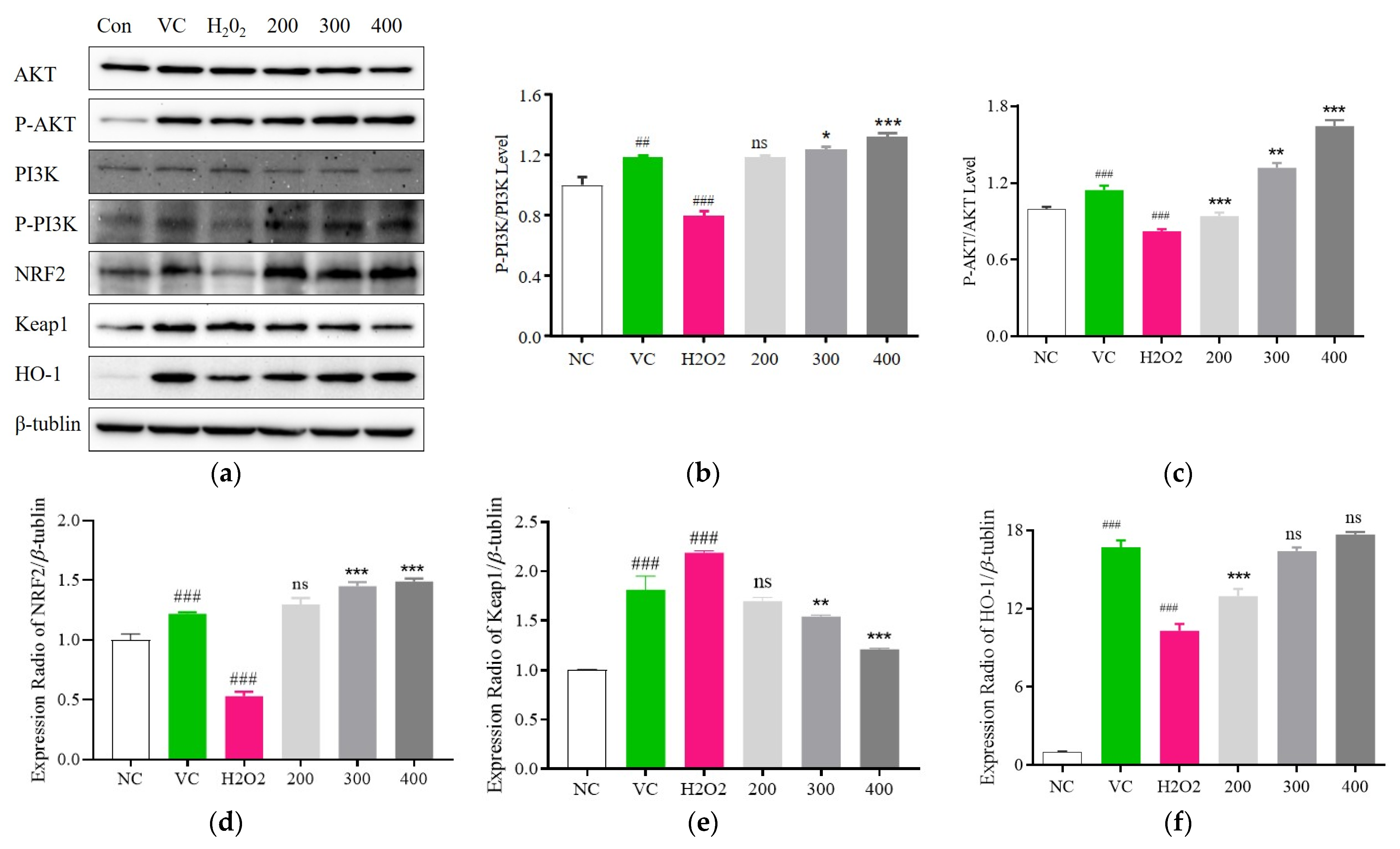
3.6. Fr. (d) Suppresses Oxidative Stress by Activating the Akt/Nrf2 Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, P.B.; Kaluç, N.; Aybastıer, Ö. SLX5 deletion confers tolerance to oxidative stress in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2022, 369, fnac077. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Hadanny, A.; Meir, O.; Bechor, Y. Seizures during hyperbaric oxygen therapy: Retrospective analysis of 62,614 treatment sessions. Undersea Hyperb. Med. 2016, 43, 21–28. [Google Scholar]
- Zhou, H.X.; Liu, Z.G.; Liu, X.J.; Chen, Q.X. Umbilical cord-derived mesenchymal stem cell transplantation combined with hyperbaric oxygen treatment for repair of traumatic brain injury. Neural Regen. Res. 2016, 11, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Zou, Y.P.; Zhang, Q.C.; Zhang, Q.Y.; Jiang, L.B.; Li, X.L. Procyanidin B2 alleviates oxidative stress-induced nucleus pulposus cells apoptosis through upregulating Nrf2 via PI3K-Akt pathway. J. Orthop. Res. 2023, 41, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhang, Z.; Zhao, Y.; Wang, D. Baicalin Reduces Immune Cell Infiltration by Inhibiting Inflammation and Protecting Tight Junctions in Ischemic Stroke Injury. Am. J. Chin. Med. 2023, 51, 355–372. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, L.; Nie, Q.; Huang, M.; Luo, C.; Sun, Y.; Ma, Y.; Yu, J.; Du, F. HPLC-based metabolomics of Dendrobium officinale revealing its antioxidant ability. Front. Plant Sci. 2023, 14, 1060242. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Wang, N.; Xu, Y.; Tang, G.; Xu, L.; Feng, Y. Bioactivities and Mechanism of Actions of Dendrobium officinale: A Comprehensive Review. Oxid. Med. Cell Longev. 2022, 2022, 6293355. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Chang, H.; Cai, B.; Gao, H.; Chen, G.; Hou, W.; Jappar, Z.; Yan, Y. Research progress on extraction, purification, structure and biological activity of Dendrobium officinale polysaccharides. Front. Nutr. 2022, 9, 965073. [Google Scholar] [CrossRef]
- Chen, W.H.; Wu, J.J.; Li, X.F.; Lu, J.M.; Wu, W.; Sun, Y.Q.; Zhu, B.; Qin, L.P. Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. Int. J. Biol. Macromol. 2021, 184, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Zhu, X.; Hua, Y. Chemical Constituents, Bioactivities, and Pharmacological Mechanisms of Dendrobium officinale: A Review of the Past Decade. J. Agric. Food Chem. 2023, 71, 14870–14889. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qi, J.; Du, D.; Liu, Y.; Jiang, X. Current advances of Dendrobium officinale polysaccharides in dermatology: A literature review. Pharm. Biol. 2020, 58, 664–673. [Google Scholar] [CrossRef]
- Zhang, Y.; You, S.; Wang, D.; Zhao, D.; Zhang, J.; An, Q.; Li, M.; Wang, C. Fermented Dendrobium officinale polysaccharides protect UVA-induced photoaging of human skin fibroblasts. Food Sci. Nutr. 2022, 10, 1275–1288. [Google Scholar] [CrossRef]
- Huang, C.; Yu, J.; Da, J.; Dong, R.; Dai, L.; Yang, Y.; Deng, Y.; Yuan, J. Dendrobium officinale Kimura & Migo polysaccharide inhibits hyperglycaemia-induced kidney fibrosis via the miRNA-34a-5p/SIRT1 signalling pathway. J. Ethnopharmacol. 2023, 313, 116601. [Google Scholar] [PubMed]
- Yang, J.; Kuang, M.T.; Yang, L.; Huang, W.; Hu, J.M. Modern interpretation of the traditional application of Shihu—A comprehensive review on phytochemistry and pharmacology progress of Dendrobium officinale. J. Ethnopharmacol. 2023, 302 Pt A, 115912. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ge, J.C.; Zhang, F.Y.; Zha, X.Q.; Liu, J.; Li, Q.M.; Luo, J.P. Dendrobium officinale polysaccharide promotes M1 polarization of TAMs to inhibit tumor growth by targeting TLR2. Carbohydr. Polym. 2022, 292, 119683. [Google Scholar] [CrossRef]
- Wang, D.; Fan, B.; Wang, Y.; Zhang, L.; Wang, F. Optimum Extraction, Characterization, and Antioxidant Activities of Polysaccharides from Flowers of Dendrobium devonianum. Int. J. Anal. Chem. 2018, 2018, 3013497. [Google Scholar] [CrossRef]
- Hu, J.; Huang, W.; Zhang, F.; Luo, X.; Chen, Y.; Xie, J. Variability of Volatile Compounds in the Medicinal Plant Dendrobium officinale from Different Regions. Molecules 2020, 25, 5046. [Google Scholar] [CrossRef]
- Liu, G.; Yang, J.; Kang, C.Z. Determination of 10 flavonoids by UPLC-MS/MS and analysis of polysaccharide contents and compositions in dendrobii officinalis caulis from different habitats. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 47–52. [Google Scholar]
- Rao, D.; Hu, Y.; Zhao, R.; Li, H.; Chun, Z.; Zheng, S. Quantitative Identification of Antioxidant Basis for Dendrobium Nobile Flower by High Performance Liquid Chromatography-Tandem Mass Spectrometry. Int. J. Anal. Chem. 2022, 2022, 9510598. [Google Scholar] [CrossRef]
- Liew, S.S.; Ho, W.Y.; Yeap SKSharifudin, S.A.B. Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts. Peer J. 2018, 6, e5331. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef]
- Mei, X.; Yang, W.; Huang, G.; Huang, H. The antioxidant activities of balsam pear polysaccharide. Int. J. Biol. Macromol. 2020, 142, 232–236. [Google Scholar] [CrossRef]
- Li, G.; Yan, N.; Li, G. The Effect of In Vitro Gastrointestinal Digestion on the Antioxidants, Antioxidant Activity, and Hypolipidemic Activity of Green Jujube Vinegar. Foods 2022, 11, 1647. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Zhao, M.; Fan, J.; Liu, Q.; Luo, H.; Tang, Q.; Li, C.; Zhao, J.; Zhang, X. Phytochemical profiles of edible flowers of medicinal plants of Dendrobium officinale and Dendrobium devonianum. Food Sci. Nutr. 2021, 9, 6575–6586. [Google Scholar] [CrossRef]
- Neto, F.C.; Raftery, D. Expanding Urinary Metabolite Annotation through Integrated Mass Spectral Similarity Networking. Anal. Chem. 2021, 93, 12001–12010. [Google Scholar] [CrossRef]
- Calderone, V.; Chericoni, S.; Martinelli, C.; Testai, L.; Nardi, A.; Morelli, I.; Breschi, M.C.; Martinotti, E. Vasorelaxing effects of flavonoids: Investigation on the possible involvement of potassium channels. Naunyn. Schmiedebergs. Arch. Pharmacol. 2004, 370, 290–298. [Google Scholar] [CrossRef]
- Lindahl, M.; Tagesson, C. Flavonoids as phospholipase A2 inhibitors: Importance of their structure for selective inhibition of group II phospholipase A2. Inflammation 1997, 21, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.M.; Endo, E.H.; Cortez, D.A.; Nakamura, T.U.; Nakamura, C.V.; Dias Filho, B.P. Antimicrobial effects of Piper hispidum extract, fractions and chalcones against Candida albicans and Staphylococcus aureus. J. Mycol. Med. 2016, 26, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yannai, S. (Ed.) Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A comprehensive computational tool for small molecule metabolism prediction and metabolite identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Yue, J.Z.; Zhou, W.; Li, L. An investigation into the preparation, characterization and antioxidant activity of puerarin/cyclodextrin inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 453–460. [Google Scholar] [CrossRef]
- Hu, Y.; Fang, X.; Wang, J.; Ren, T.T.; Zhao, Y.Y.; Dai, J.F.; Qin, X.Y.; Lan, R. Astragalin attenuates AlCl3/D-galactose-induced aging-like disorders by inhibiting oxidative stress and neuroinflammation. Neurotoxicology 2022, 91, 60–68. [Google Scholar] [CrossRef]
- Barupal, D.K.; Fiehn, O. Generating the Blood Exposome Database Using a Comprehensive Text Mining and Database Fusion Approach. Environ. Health Perspect. 2019, 127, 97008. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Zhu, L.; Eckert, R.L. Apigenin inhibition of involucrin gene expression is associated with a specific reduction in phosphorylation of protein kinase Cdelta Tyr311. J. Biol. Chem. 2006, 281, 36162–36172. [Google Scholar] [CrossRef]
- Jiang, B.; Song, J.; Jin, Y. A flavonoid monomer tricin in Gramineous plants: Metabolism, bio/chemosynthesis, biological properties, and toxicology. Food Chem. 2020, 320, 126617. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Waters, W.R.; Woods, K.M.; Upton, S.J.; Keithly, J.S.; Kiderlen, A.F. E valuation of in vitro activity of aurones and related compounds against Cryptosporidium parvum. Planta Med. 2001, 67, 722–725. [Google Scholar] [CrossRef]
- Cha, B.Y.; Shi, W.L.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. An inhibitory effect of chrysoeriol on platelet-derived growth factor (PDGF)-induced proliferation and PDGF receptor signaling in human aortic smooth muscle cells. J. Pharmacol. Sci. 2009, 110, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Goedert, J.J.; Sampson, J.N.; Moore, S.C.; Xiao, Q.; Xiong, X.; Hayes, R.B.; Ahn, J.; Shi, J.; Sinha, R. Fecal metabolomics: Assay performance and association with colorectal cancer. Carcinogenesis 2014, 35, 2089–2096. [Google Scholar] [CrossRef]
- Fernando, P.D.S.M.; Ko, D.; Piao, M.J.; Kang, K.A.; Herath, H.M.U.L.; Hyun, J.W. Protective effect of luteolin against oxidative stress mediated cell injury via enhancing antioxidant systems. Mol. Med. Rep. 2024, 30, 121. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.H.; Wang, Z.P.; Fan, H.; Yang, K. Adsorption and isolation of macroporous resin for three isoflavonoids from puerariae lobatae radix. Zhong Yao Cai 2013, 36, 650–653. [Google Scholar] [PubMed]
- Saha, S.; Panieri, E.; Suzen, S.; Saso, L. The Interaction of Flavonols with Membrane Components: Potential Effect on Antioxidant Activity. J. Membr. Biol. 2020, 253, 57–71. [Google Scholar] [CrossRef]
- Bhatia, G.; Dhuna, V.; Dhuna, K.; Kaur, M.; Singh, J. Bacopa monnieri extracts prevent hydrogen peroxide-induced oxidative damage in a cellular model of neuroblastoma IMR32 cells. Chin. J. Nat. Med. 2017, 15, 834–846. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Lu, X.; Jin, S.; Min, Y.; Yang, J. Combined effects of microcystin and nitrite on the growth, lipid peroxidation, and antioxidant responses of the freshwater rotifer Brachionus calyciflorus. Aquat. Toxicol. 2017, 192, 78–88. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, D.; Song, Z.L.; Liu, T.; Hou, Y.; Fang, J. Small molecules regulating reactive oxygen species homeostasis for cancer therapy. Med. Res. Rev. 2021, 41, 342–394. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, Q.; Ma, W.; Li, J.; Qu, L.; Wang, M. Protocatechuic Acid Ameliorated Palmitic-Acid-Induced Oxidative Damage in Endothelial Cells through Activating Endogenous Antioxidant Enzymes via an Adenosine-Monophosphate-Activated-Protein-Kinase-Dependent Pathway. J. Agric. Food Chem. 2018, 66, 10400–10409. [Google Scholar] [CrossRef]
- Lv, A.; Ge, M.; Hu, X.; Liu, W.; Li, G.; Zhang, R. Effects of Agaricus blazei Murill Polysaccharide on Cadmium Poisoning on the MDA5 Signaling Pathway and Antioxidant Function of Chicken Peripheral Blood Lymphocytes. Biol. Trace Elem. Res. 2018, 181, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yuan, S.; Wang, X.; Lei, Y.; Zhang, X.; Huang, M.; Ouyang, H. Attenuation of pristimerin on TNF-α-induced endothelial inflammation. Int. Immunopharmacol. 2020, 82, 106326. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, E.M.; Kim, D.H.; Jung, K.; Jung, J.S.; Lee, E.J.; Hyun, J.W.; Kang, J.L.; Kim, H.S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J. Neuroimmunol. 2009, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Huang, D.; Yu, Q.; Yang, J.; Yao, J. Neuroligin-3 protects retinal cells from H2O2-induced cell death via activation of Nrf2 signaling. Biochem. Biophys. Res. Commun. 2018, 502, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, J.; Zhang, L.; Sun, Y.; Jiang, J.; Huang, Y.; Xu, H.; Jiang, H.; Hu, R. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic. Biol. Med. 2018, 121, 78–85. [Google Scholar] [CrossRef] [PubMed]

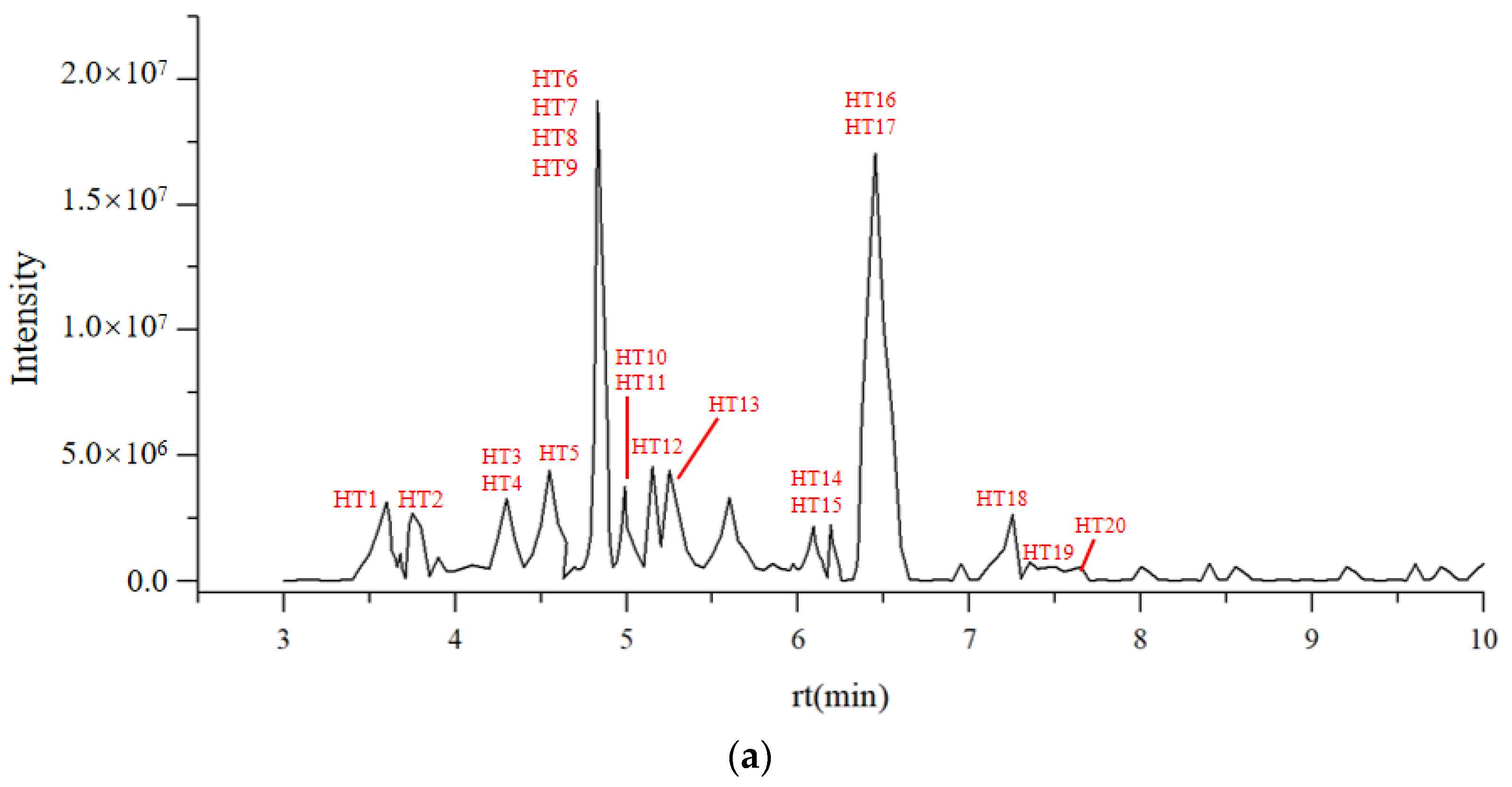

| NO | ID | Compounds | RT (min) | Measured Value Exptl (m/z) | Theoretical Value Exptl (m/z) | Fragmentation (m/z) | References |
|---|---|---|---|---|---|---|---|
| 1 | HT 1 | 5,7-Dimethoxyflavanone | 3.686 | 285.1218 | 285.1082 | 267.1123/195.0891 | [28] |
| 2 | HT 2 | 5-Methoxyflavone | 3.742 | 253.0943 | 253.0820 | 134.0943/207.0894/162.0914/117.0700/235.0896 | [29] |
| 3 | HT 3 | Rutin | 4.300 | 610.151 | 610.1534 | 593.1523/503.1192/473.1105/399.0742/369.0615/301.0372/293.0483 | [30] |
| 4 | HT 4 | 2′,3-Dihydroxy-4,4′,6′-Trimethoxychalcon | 4.494 | 331.1113 | 331.1137 | 331.2293/331.2449/331.1526/331.1780/331.1316/285.1210/177.0579/178.0557/107.041 | [31] |
| 5 | HT 5 | Delphinidin 3-glucoside | 4.565 | 465.093 | 465.1028 | 301.0369/300.0322 | [32] |
| 6 | HT 6 | Maysin | 4.853 | 576.1438 | 576.1479 | 455.0999/341.0695 | [32] |
| 7 | HT 7 | Biorobin | 4.853 | 594.1536 | 594.1585 | 285.0419/284.0330 | [32] |
| 8 | HT 8 | Cyanidin 3-O-glucoside | 4.858 | 449.2615 | 449.1079 | 287.0548 | [33] |
| 9 | HT 9 | Puerarin | 4.993 | 416.1056 | 416.1107 | 415.1983/463.1313/323.0758/295.0614/267.0638/161.0625/179.034 0/117.0759 | [34] |
| 10 | HT 10 | Astragalin | 4.999 | 449.1617 | 449.1039 | 449.2500/287.0515 | [32] |
| 11 | HT 11 | Kaempferol-3-O-glucoside | 5.257 | 448.1006 | 448.0960 | 284.0333/285.0406/255.0302/161.025 | [35] |
| 12 | HT 12 | Quercitrin | 5.262 | 448.0955 | 448.1006 | 300.0291/301.0360/323.0776/271.0248/161.0253 | [32] |
| 13 | HT 13 | Isovitexin | 5.567 | 432.0991 | 432.1056 | 431.1771/431.2180/431.0645/431.2556/431.1956/311.0560/251.1276/161.0249/89.0243 | [36] |
| 14 | HT 14 | Apigenin | 6.091 | 270.0469 | 270.0528 | 269.1814/181.1223/72.9931 | [37] |
| 15 | HT 15 | Tricin | 6.157 | 331.0805 | 331.0773 | 302.0384/331.2030/316.0507/315.453/331.1346/315.0570/270.0533/331.0191/165.0539/153.0077 | [38] |
| 16 | HT 16 | (2Z)-4,6-Dihydroxy-2-[(4-hydroxy-3,5-dimethoxyphenyl)methylidene]-1-benzofuran-3-one | 6.184 | 330.0682 | 330.0740 | 313.0375/314.0444/299.0207/271.0251 | [39] |
| 17 | HT 17 | Chrysoeriol | 6.506 | 301.0683 | 301.0667 | 286.0466/301.0298/258.0513/301.1205/301.0090/283.0991/86 | [40] |
| 18 | HT 18 | Chrysin | 7.236 | 254.0517 | 254.0579 | 235.1676/209.0580 | [32] |
| 19 | HT 19 | Galangin | 7.485 | 270.0456 | 270.0528 | - | [32] |
| 20 | HT 20 | Kaempferol | 7.526 | 286.0444 | 286.0477 | 229.0522/107.0153 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Wang, X.; Liu, X.; Shen, X.; Li, A.; Zheng, X.; Sheng, J.; Yuan, W. Characterization of the Composition of Bioactive Fractions from Dendrobium officinale Flowers That Protect against H2O2-Induced Oxidative Damage through the PI3K/AKT/Nrf2 Pathway. Foods 2024, 13, 3116. https://doi.org/10.3390/foods13193116
Zhu P, Wang X, Liu X, Shen X, Li A, Zheng X, Sheng J, Yuan W. Characterization of the Composition of Bioactive Fractions from Dendrobium officinale Flowers That Protect against H2O2-Induced Oxidative Damage through the PI3K/AKT/Nrf2 Pathway. Foods. 2024; 13(19):3116. https://doi.org/10.3390/foods13193116
Chicago/Turabian StyleZhu, Pengyan, Xinting Wang, XinLan Liu, Xiaojing Shen, Ai Li, Xiaohong Zheng, Jun Sheng, and Wenjuan Yuan. 2024. "Characterization of the Composition of Bioactive Fractions from Dendrobium officinale Flowers That Protect against H2O2-Induced Oxidative Damage through the PI3K/AKT/Nrf2 Pathway" Foods 13, no. 19: 3116. https://doi.org/10.3390/foods13193116







