A Lincomycin-Specific Antibody Was Developed Using Hapten Prediction, and an Immunoassay Was Established to Detect Lincomycin in Pork and Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Computational Chemistry Analysis of Haptens
2.3. Cells and Animals
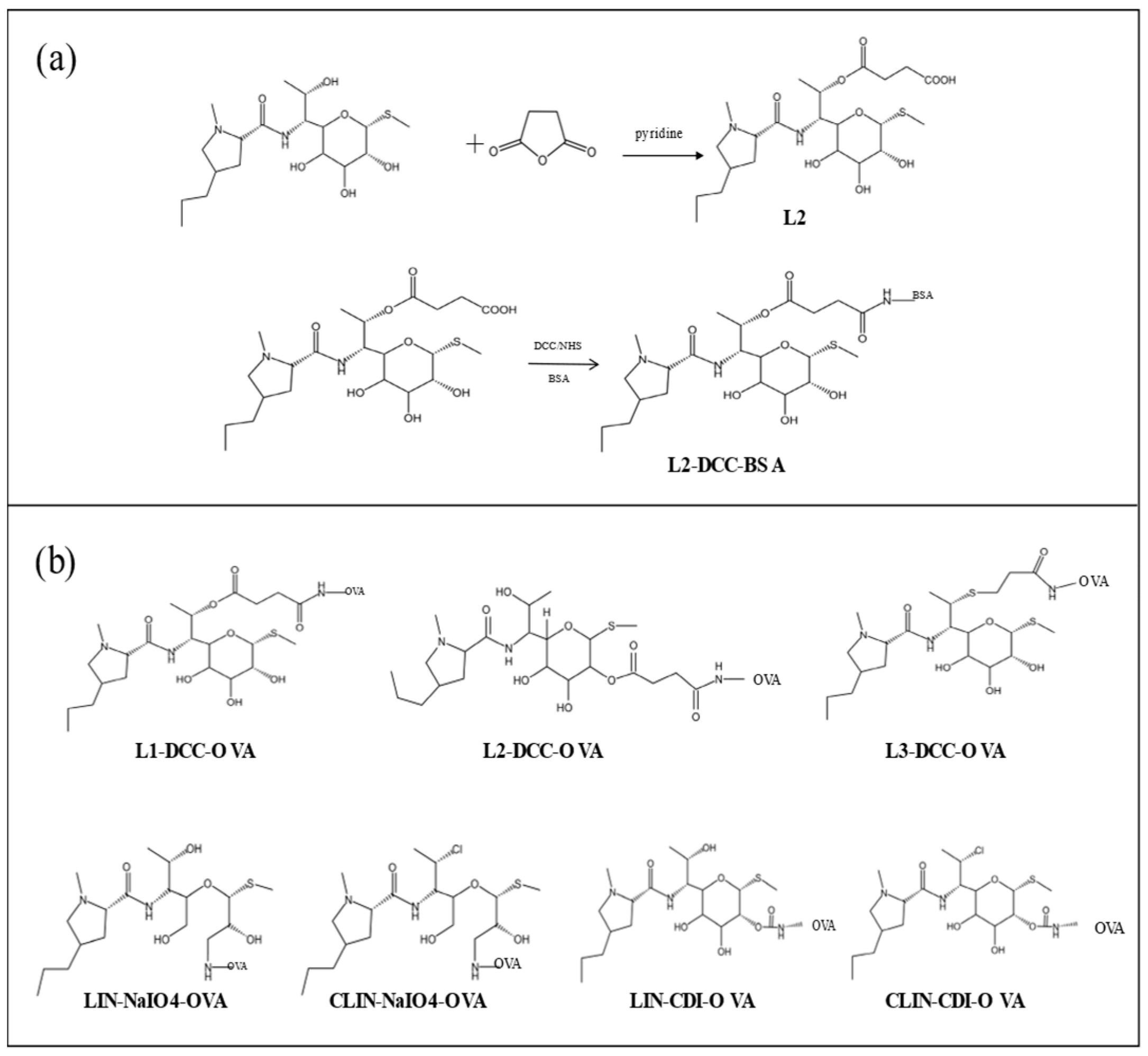
2.4. Synthesis of Haptens
2.5. Synthesis of Antigens
2.6. The Detection Mode of ELISA
2.7. Animal Immunization
2.8. Preparation of Monoclonal Antibodies
2.9. Standard Curve and Specificity Determination
2.10. Sample Preparation
2.11. Validation of the ic-ELISA
3. Results
3.1. Analysis and Prediction Hapten Structure by Computational Chemistry
3.2. Characterization of Haptens
3.3. Characterization of Conjugates
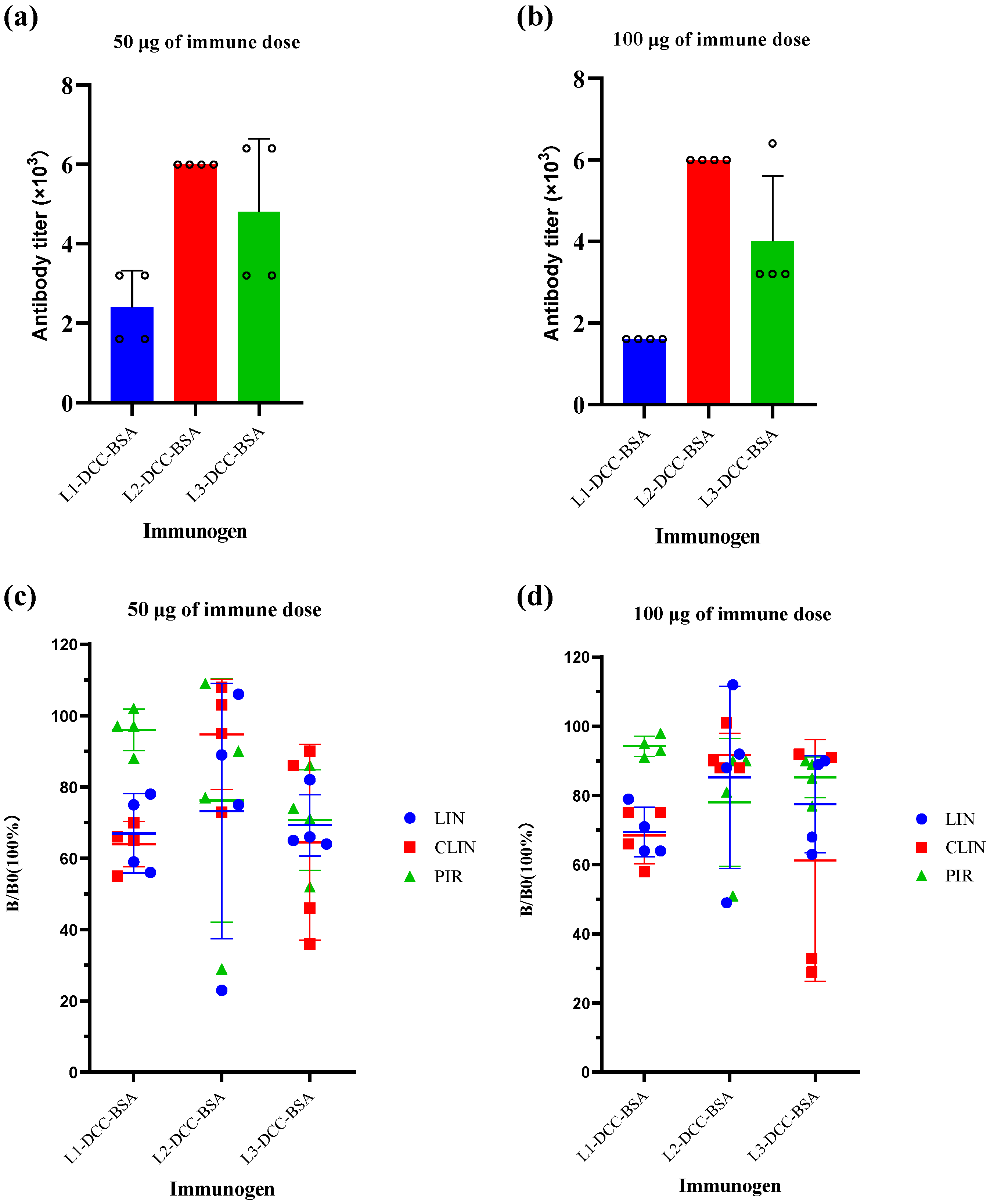
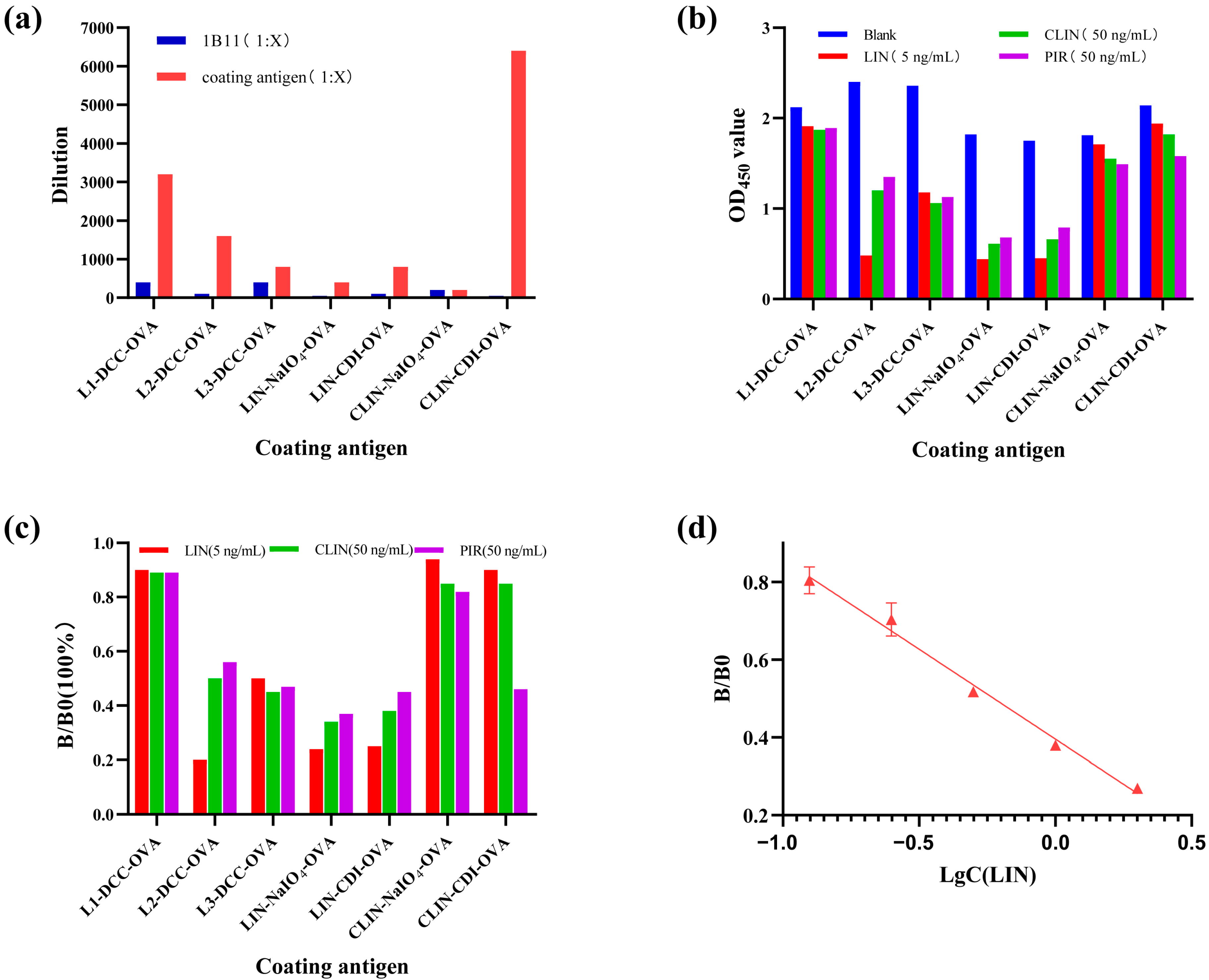
3.4. Characterization of the Antisera and the Monoclonal Antibody
3.5. Optimization and Standard Curve for the ic-ELISA
3.6. Elimination of Matrix Effect
3.7. Sample Preparation and Validation of the ic-ELISA Method
3.8. Comparison with Other Immunoassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarz, S.; Shen, J.; Kadlec, K.; Wang, Y.; Brenner Michael, G.; Feßler, A.T.; Vester, B. Lincosamides, Streptogramins, Phenicols, and Pleuromutilins: Mode of Action and Mechanisms of Resistance. Cold Spring Harb. Perspect Med. 2016, 6, a027037. [Google Scholar] [CrossRef] [PubMed]
- Bryskier, A. Lincosamines. In Antimicrobial Agents: Antibacterials and Antifungals; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Giguère, S. Lincosamides, Pleuromutilins, and Streptogramins. In Antimicrobial Therapy in Veterinary Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 199–210. [Google Scholar]
- Commission of Chinese Veterinary Pharmacopoeia. Chinese Veterinary Pharmacopoeia; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Dunkle, J.A.; Xiong, L.; Mankin, A.S.; Cate, J.H. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl. Acad. Sci. USA 2010, 107, 17152–17157. [Google Scholar] [CrossRef] [PubMed]
- Nissen, P.; Hansen, J.; Ban, N.; Moore, P.B.; Steitz, T.A. The structural basis of ribosome activity in peptide bond synthesis. Science 2000, 289, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Lin, X.; Jiaxin, Z.; Jing, X.; Jianping, X.; Rong, H.; Wenbiao, W. Underlying the inhibition mechanisms of sulfate and lincomycin on long-term anaerobic digestion: Microbial response and antibiotic resistance genes distribution. Sci. Total Environ. 2024, 915, 169837. [Google Scholar]
- Yueqiu, W.; Rongmei, W.; Junqing, W.; Hong, Y.; Juan, S.; Yuzhen, W.; Anping, D. A sensitive and specific enzyme-linked immunosorbent assay for the detection of lincomycin in food samples. J. Sci. Food Agric. 2010, 90, 2083–2089. [Google Scholar]
- Robertson, M.B.; Breen, K.J.; Desmond, P.V.; Mashford, M.L.; McHugh, A.M. Incidence of antibiotic-related diarrhoea and pseudomembranous colitis: A prospective study of lincomycin, clindamycin and ampicillin. Med. J. Aust. 1977, 1, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. 2007. Available online: https://books.google.com/books?id=P2zOGjHGoyIC%26printsec=frontcover#v=onepage%26q=lincomycin%26f=false (accessed on 10 April 2023).
- GB 31650-2019; National Food Safety Standards—Maximum Residue Limits for Pesticides in Food. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2019.
- Valese, A.C.; Molognoni, L.; de Souza, N.C.; de Sa Ploencio, L.A.; Costa, A.C.O.; Barreto, F.; Daguer, H. Development, validation and different approaches for the measurement uncertainty of a multi-class veterinary drugs residues LC-MS method for feeds. J. Chromatogr. B Anal. Technol Biomed. Life Sci. 2017, 1053, 48–59. [Google Scholar] [CrossRef]
- GB 29686-2013; National Standard for Food Safety—Determination of Avilamycin Residues in Edible Tissues of Pigs—Liquid Chromatography-Tandem Mass Spectrometry. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2013.
- GB/T 20762-2006; Determination of Lincomycin, Zhutao mycin, Erythromycin, Timicoxin, Tylosin, Clindamycin, Spiramycin, Kitamycin, and Jiaosha mycin Residues in Livestock and Poultry Meat by Liquid Chromatography-Tandem Mass Spectrometry. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2006.
- Jang, S.; Seo, H.; Kim, H.; Kim, H.; Ahn, J.; Cho, H.; Hong, S.; Lee, S.; Na, T. Development of a Quantitative Method for Detection of Multiclass Veterinary Drugs in Feed Using Modified QuPPe Extraction and LC-MS/MS. Molecules 2022, 27, 4483. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, L.; Liu, L.; Kuang, H.; Xu, C. Colloidal gold-based immunochromatographic strip assay for the rapid detection of three natural estrogens in milk. Food Chem. 2018, 259, 122–129. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Biolatti, B.; Cannizzo, F.T.; Pavlovic, R.; Arioli, F. A Liquid Chromatography-Tandem Mass Spectrometry Method for the Detection of Antimicrobial Agents from Seven Classes in Calf Milk Replacers: Validation and Application. J. Agric. Food Chem. 2016, 64, 2635–2640. [Google Scholar] [CrossRef]
- Zhao, H.; Zulkoski, J.; Mastovska, K. Development and Validation of a Multiclass, Multiresidue Method for Veterinary Drug Analysis in Infant Formula and Related Ingredients Using UHPLC-MS/MS. J. Agric. Food Chem. 2017, 65, 7268–7287. [Google Scholar] [CrossRef] [PubMed]
- Chiaochan, C.; Koesukwiwat, U.; Yudthavorasit, S.; Leepipatpiboon, N. Efficient hydrophilic interaction liquid chromatography-tandem mass spectrometry for the multiclass analysis of veterinary drugs in chicken muscle. Anal. Chim. Acta 2010, 682, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Abd El-Aty, A.M.; Kim, S.K.; Choi, J.M.; Park, D.H.; Yoo, K.H.; Kang, Y.S.; Jeon, J.S.; Hacimuftuoglu, A.; Shim, J.H.; et al. Development and validation of a solid-phase extraction method coupled with LC-MS/MS for the simultaneous determination of 16 antibiotic residues in duck meat. Biomed. Chromatogr. 2019, 33, e4501. [Google Scholar] [CrossRef]
- Xiaoming, Z.; Zuguang, L.; Huizhen, W.; Jianmei, W.; Huiyu, Z.; Xiaofeng, J.; Yan, X.; Rui, L.; Hu, Z.; Hua, Y.; et al. High-throughput method based on a novel thin-film microextraction coating for determining macrolides and lincosamides in honey. Food Chem. 2021, 346, 128920. [Google Scholar]
- Jank, L.; Martins, M.T.; Arsand, J.B.; Campos Motta, T.M.; Hoff, R.B.; Barreto, F.; Pizzolato, T.M. High-throughput method for macrolides and lincosamides antibiotics residues analysis in milk and muscle using a simple liquid-liquid extraction technique and liquid chromatography-electrospray-tandem mass spectrometry analysis (LC-MS/MS). Talanta 2015, 144, 686–695. [Google Scholar] [CrossRef]
- Lopez, M.I.; Pettis, J.S.; Smith, I.B.; Chu, P.-S. Multiclass determination and confirmation of antibiotic residues in honey using LC-MS/MS. J. Agric. Food Chem. 2008, 56, 1553–1559. [Google Scholar] [CrossRef]
- Carretero, V.; Blasco, C.; Pico, Y. Multi-class determination of antimicrobials in meat by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2008, 1209, 162–173. [Google Scholar] [CrossRef]
- Spisso, B.F.; Ferreira, R.G.; Pereira, M.U.; Monteiro, M.A.; Cruz, T.A.; da Costa, R.P.; Belem Lima, A.M.; da Nobrega, A.W. Simultaneous determination of polyether ionophores, macrolides and lincosamides in hen eggs by liquid chromatography-electrospray ionization tandem mass spectrometry using a simple solvent extraction. Anal. Chim. Acta 2010, 682, 82–92. [Google Scholar] [CrossRef]
- Bousova, K.; Senyuva, H.; Mittendorf, K. Quantitative multi-residue method for determination antibiotics in chicken meat using turbulent flow chromatography coupled to liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1274, 19–27. [Google Scholar] [CrossRef]
- Suo, D.; Wang, P.; Li, Y.; Wang, R.; Wei, S.; Xiao, Z.; Zhang, S. Simultaneous determination of antibiotics and amantadines in animal-derived feedstuffs by ultraperformance liquid chromatographic-tandem mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2018, 1095, 183–190. [Google Scholar]
- Xie, W.; Han, C.; Hou, J.; Wang, F.; Qian, Y.; Xi, J. Simultaneous determination of multiveterinary drug residues in pork meat by liquid chromatography-tandem mass spectrometry combined with solid phase extraction. J. Sep. Sci. 2012, 35, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Bohm, D.A.; Stachel, C.S.; Gowik, P. Validation of a multi-residue method for the determination of several antibiotic groups in honey by LC-MS/MS. Anal. Bioanal. Chem. 2012, 403, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Bohm, D.A.; Stachel, C.S.; Gowik, P. Validated Determination of Eight Antibiotic Substance Groups in Cattle and Pig Muscle by HPLC/MS/MS. J. Aoac Int. 2011, 94, 407–419. [Google Scholar] [CrossRef]
- Pratiwi, R.; Ramadhanti, S.P.; Amatulloh, A.; Megantara, S.; Subra, L. Recent Advances in the Determination of Veterinary Drug Residues in Food. Foods 2023, 12, 3422. [Google Scholar] [CrossRef]
- Kai, W.; Yuchen, B.; Yujie, W.; Chenglong, L.; Jianzhong, S.; Zhanhui, W. Influence of Small Molecular Property on Antibody Response. J. Agric. Food Chem. 2020, 68, 10944–10950. [Google Scholar]
- Peipei, L.; Yuchen, B.; Haiyang, J.; Yingjie, Z.; Yuan, L.; Changfei, D.; Kai, W.; Xuezhi, Y.; Zhanhui, W. Broad-specificity antibody profiled by hapten prediction and its application in immunoassay for fipronil and major metabolites. J. Hazard. Mater. 2023, 441, 129931. [Google Scholar]
- Burkin, M.A.; Galvidis, I.A. Development of a competitive indirect ELISA for the determination of lincomycin in milk, eggs, and honey. J. Agric. Food Chem. 2010, 58, 9893–9898. [Google Scholar] [CrossRef]
- He, J.; Wu, N.; Luo, P.; Guo, P.; Qu, J.; Zhang, S.; Zou, X.; Wu, F.; Xie, H.; Wang, C.; et al. Development of a heterologous enzyme-linked immunosorbent assay for the detection of clindamycin and lincomycin residues in edible animal tissues. Meat Sci. 2017, 125, 137–142. [Google Scholar] [CrossRef]
- Jiang, W.; Beier, R.C.; Luo, P.; Zhai, P.; Wu, N.; Lin, G.; Wang, X.; Xu, G. Analysis of Pirlimycin Residues in Beef Muscle, Milk, and Honey by a Biotin-Streptavidin-Amplified Enzyme-Linked Immunosorbent Assay. J. Agric. Food Chem. 2016, 64, 364–370. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, K.; Xu, F.; Wang, W.; Jiang, H.; Wang, Z.; Ding, S. Development of a microsphere-based fluorescence immunochromatographic assay for monitoring lincomycin in milk, honey, beef, and swine urine. J. Agric. Food Chem. 2014, 62, 12061–12066. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, P.; Chen, J.; Huang, Y.; Jiang, W. Development of a quantitative immuno-affinity test column assay for on-site screening of clindamycin residues in milk. Int. Dairy J. 2016, 55, 59–63. [Google Scholar] [CrossRef]
- Cao, S.; Song, S.; Liu, L.; Kong, N.; Kuang, H.; Xu, C. Comparison of an Enzyme-Linked Immunosorbent Assay with an Immunochromatographic Assay for Detection of Lincomycin in Milk and Honey. Immunol. Investig. 2015, 44, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold Immunochromatographic Assay for Rapid On-Site Detection of Lincosamide Residues in Milk, Egg, Beef, and Honey Samples. Biotechnol. J. 2020, 15, e1900174. [Google Scholar] [CrossRef]
- Jianxin, H.; Chan-Yuan, Y.; Jin-Yi, Y.; Zhenfeng, L.; Fan, H.; Yuanxin, T.; Hong, W.; Zhen-Lin, X.; Yu-Dong, S. Design of Novel Haptens and Development of Monoclonal Antibody-Based Immunoassays for the Simultaneous Detection of Tylosin and Tilmicosin in Milk and Water Samples. Biomolecules 2019, 9, 770. [Google Scholar] [CrossRef]
- Fuyuan, Z.; Bing, L.; Guozhen, L.; Yan, Z.; Junping, W.; Shuo, W. Substructure-activity relationship studies on antibody recognition for phenylurea compounds using competitive immunoassay and computational chemistry. Sci. Rep. 2018, 8, 3131. [Google Scholar]
- Jin-Yi, Y.; Mei-Chan, X.; Xuecai, T.; Yuanxin, T.; Hong, W.; Zhen-Lin, X.; Tingting, Y.; Yi-Mei, X.; Yu-Dong, S. Improved molecular softness of tadalafil hapten enhancing antibody performance in immunoassay: Evidence from computational chemistry. J. Food Sci. 2022, 87, 1342–1354. [Google Scholar]
- Georges, K.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar]
- Zijian, C.; Huiling, W.; Zhengguo, X.; Hui-Jun, F.; Yu-Dong, S.; Lin, L.; Hong, W.; Hongtao, L.; Surat, H.; Zhen-Lin, X. Rational hapten design to produce high-quality antibodies against carbamate pesticides and development of immunochromatographic assays for simultaneous pesticide screening. J. Hazard. Mater. 2021, 412, 125241. [Google Scholar]
- Hongtao, L.; Yu-Dong, S.; Lijun, S.; Jin-Yi, Y.; Olivier, P.C.; Simon, A.H.; Hong, W.; Yuan, S.; Christopher, T.E. Hapten synthesis and antibody production for the development of a melamine immunoassay. Anal. Chim. Acta 2010, 665, 84–90. [Google Scholar]
- Zhen-Lin, X.; Yu-Dong, S.; Yuanming, S.; Katrina, C.; Yuanxin, T.; Shiwei, Z.; Hongtao, L.; Yueming, J. Novel hapten synthesis for antibody production and development of an enzyme-linked immunosorbent assay for determination of furaltadone metabolite 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ). Talanta 2013, 103, 306–313. [Google Scholar]
- Ghulam Mujtaba, M.; Weilin, W.; Aisha, T.; Jan Muhammad, S.; Saqib, A.; Faiz Muhamad, K.; Bughio, E.; Abdul, S.; Zhanhui, W.; Sergei, A.E. Hapten design to prepare monoclonal antibodies and establishment of immunoassay for direct screening of oxamichydrazide in chicken, the metabolite of nifuraldezone. One Health Adv. 2023, 1, 6–18. [Google Scholar]
- Kaattari, S.L.; Zhang, H.L.; Khor, I.W.; Kaattari, I.M.; Shapiro, D.A. Affinity maturation in trout: Clonal dominance of high affinity antibodies late in the immune response. Dev. Comp. Immunol. 2002, 26, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Nichkova, M.; Galve, R.; Marco, M.P. Biological monitoring of 2,4,5-trichlorophenol (I): Preparation of antibodies and development of an immunoassay using theoretical models. Chem. Res. Toxicol. 2002, 15, 1360–1370. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cho, Y.A.; Lee, H.-S.; Lee, Y.T.; Gee, S.J.; Hammock, B.D. Synthesis of haptens for immunoassay of organophosphorus pesticides and effect of heterology in hapten spacer arm length on immunoassay sensitivity. Anal. Chim. Acta 2003, 475, 85–96. [Google Scholar] [CrossRef]


| Detection Method | Hapten | Target Analytes | IC50 (µg/kg) | LOD (µg/kg) | Source |
|---|---|---|---|---|---|
| ELISA | LIN derivative | LIN, CLIN | LIN: 29.1 CLIN:153.8 | 0.15–0.98 | (Wang et al. 2010) [9] |
| ELISA | LIN | LIN, CLIN | LIN: 9.15 CLIN: 18.3 | 0.43–0.65 | (Burkin et al. 2010) [35] |
| ELISA | CLIN-HS | LIN, CLIN | CLIN: 0.3 LIN: 1.2 | 1.8–6.8 | (He et al. 2017) [36] |
| ELISA | PIR | PIR | 1.6 | 1.65–4.45 | (Jiang et al. 2016) [37] |
| FICA | LIN | LIN | 2.2 | 0.69 | (Zhou et al. 2014) [38] |
| ICA | CLIN-HS | CLIN | 2.4 | 1.0 | (Wang et al. 2016) [39] |
| GICA | LIN-HS | LIN, CLIN | LIN: 0.3 CLIN: 50 | 2.1 | (Cao et al. 2015) [40] |
| GICA | CLIN chlorine substituents | LIN, CLIN, PIR | CLIN: 0.42 LIN: 3.27 PIR: 12.8 | 0.04–20.91 | (Guo et al. 2019) [41] |
| Recovery (%) for Samples Spiked at Concentrations | ||||
|---|---|---|---|---|
| Matrix | Sample Dilution | 2.5 | 5 | 10 |
| Pork | 1:6 | 99.8 ± 7.8 a | 106.5 ± 2.3 | 102.5 ± 2.8 |
| milk | 1:10 | 94.9 ± 3.8 | 92.6 ± 10.6 | 92.6 ± 10.6 |
| Targets | Samples | LOD | LOQ | Spiked Level (µg/kg) | Recovery (%) | CV (%) |
|---|---|---|---|---|---|---|
| (µg/kg) | (µg/kg) | |||||
| LIN | pork | 1.2 | 2.29 | 2.5, 5, 10 | 92.0–108.8 | 2.2–7.9 |
| milk | 0.81 | 2.09 | 2.5, 5, 10 | 81.9–103.2 | 3.4–11.4 | |
| CLIN | pork | 30.37 | 52.87 | 50, 100, 200 | 80.4–97.0 | 3.9–6.2 |
| milk | 33.74 | 75.63 | 75, 150, 300 | 81.9–112.0 | 0.8–5.8 | |
| PIR | pork | 28.03 | 55.92 | 60, 120, 240 | 69.7–100.0 | 11.3–16.6 |
| milk | 52.96 | 106.62 | 100, 200, 400 | 72.2–104.0 | 11.0–14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Y.; Zhang, D.; Shen, Y.; Pan, Y.; Wang, J.; Wang, Y. A Lincomycin-Specific Antibody Was Developed Using Hapten Prediction, and an Immunoassay Was Established to Detect Lincomycin in Pork and Milk. Foods 2024, 13, 3118. https://doi.org/10.3390/foods13193118
Shang Y, Zhang D, Shen Y, Pan Y, Wang J, Wang Y. A Lincomycin-Specific Antibody Was Developed Using Hapten Prediction, and an Immunoassay Was Established to Detect Lincomycin in Pork and Milk. Foods. 2024; 13(19):3118. https://doi.org/10.3390/foods13193118
Chicago/Turabian StyleShang, Yuhan, Dandan Zhang, Yun Shen, Yuanhu Pan, Jing Wang, and Yulian Wang. 2024. "A Lincomycin-Specific Antibody Was Developed Using Hapten Prediction, and an Immunoassay Was Established to Detect Lincomycin in Pork and Milk" Foods 13, no. 19: 3118. https://doi.org/10.3390/foods13193118
APA StyleShang, Y., Zhang, D., Shen, Y., Pan, Y., Wang, J., & Wang, Y. (2024). A Lincomycin-Specific Antibody Was Developed Using Hapten Prediction, and an Immunoassay Was Established to Detect Lincomycin in Pork and Milk. Foods, 13(19), 3118. https://doi.org/10.3390/foods13193118





