Optimizing Lactic Acid Bacteria Fermentation for Enhanced Summer and Autumn Tea Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Drugs
2.2. Methods
2.2.1. Determination of LAB Fermentation Strains
2.2.2. Single-Factor Tests of the LAB-Fermented-Tea Process
2.2.3. Determination Method of Test Index
3. Results and Discussion
3.1. Results of LAB Screening
3.2. Determination of the Proportion of Bacteria in Mixed Fermentation
3.3. Determination of the Processing Technology of LAB Fermented Tea Samples
3.3.1. Single Factor Test Results of the Fermentation Process
3.3.2. LAB Fermented Tea Process Optimized by the RSM
3.4. Dynamic Changes in Quality of Mixed-Bacteria Fermented Tea
3.4.1. Changes of Soluble Solids before and after Fermentation
3.4.2. Changes of Tea Polyphenols before and after Fermentation
3.4.3. Changes of Total Amino Acids before and after Fermentation
3.4.4. Changes in Aroma Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Ruan, J. Tea: Analysis and Tasting. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 256–267. [Google Scholar] [CrossRef]
- Hessien, M.; Donia, T.; El-Gendy, S.; Sikkena, M.A. Unfractionated green tea and ginger polyphenols induce apoptotic, cytotoxic and antioxidant effects in hepatoma cells. J. Herb. Med. 2013, 3, 87–98. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Roach, P.D. Effects of aqueous brewing solution ph on the extraction of the major green tea constituents. Food Res. Int. 2013, 53, 713–719. [Google Scholar] [CrossRef]
- Mukhtar, H.; Ahmad, N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000, 71 (Suppl. 6), 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Huangfu, L.T.; Dong, L.L.; Liu, S.L. Functional groups and antioxidant activities of polysaccharides from five categories of tea. Ind. Crops Prod. 2014, 58, 31–35. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.T.; Schwab, W.; Wan, X. Aroma profiles of green tea made with fresh tea leaves plucked in summer. Food Chem. 2021, 363, 130328. [Google Scholar] [CrossRef]
- Bi, C.H. The development and prospects of green tea in summer and autumn. J. Tea Bus. 2007, 29, 109–111. [Google Scholar]
- Shi, Z. Probe into mathematical model of chemical essence of bitterness and astringency in summer green tea. J. Tea Sci. 1987, 2, 7–12. [Google Scholar]
- Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Lin, Z. Nontargeted Analysis Using Ultraperformance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry Uncovers the Effects of Harvest Season on the Metabolites and Taste Quality of Tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef]
- Lee, L.S.; Choi, J.H.; Son, N.; Kim, S.H.; Park, J.D.; Jang, D.J.; Kim, H.J. Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. J. Agric. Food Chem. 2013, 61, 332–338. [Google Scholar] [CrossRef]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of shading intensity on morphological and color traits and on chemical components of new tea (Camellia sinensis L.) shoots under direct covering cultivation. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.K.; Bhattacharya, A.; Laxmikumaran, M.; Singh Ahuja, P. Recent advances of tea (Camellia sinensis) biotechnology. Plant Cell Tissue Organ Cult. 2004, 76, 195–254. [Google Scholar] [CrossRef]
- Liu, S.Q.; Zhi-Yuan, H.U.; Zhao, Y.L. Preliminary analysis of bacterial community structure during pile-fermentation process of fuzhuan brick tea by denaturing gradient gel electrophoresis(dgge). Food Sci. 2014, 35, 172–177. [Google Scholar]
- Wen, Z.J.; Zhang, L.Y.; Ping, W.U.; He, Y.Q. Research progress of microbiology mechanism of dark tea. Tea Commun. 2010, 37, 26–29. [Google Scholar]
- He, G.P.; Lin, Y.C.; Xu, F.X. Microscopic changes of cellular tissues and microbial analysis of Guangdong pu-erh tea in the pile. J. Tea Sci. 1987, 2, 56–59. [Google Scholar]
- Lin, F.J.; Wei, X.L.; Liu, H.Y.; Li, H.; Xia, Y.; Wu, D.T.; Gan, R.Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Febrisiantosa, A. The role of lactic acid bacteria in milk fermentation. Food Nutr. Sci. 2014, 5, 435–442. [Google Scholar] [CrossRef]
- Okada, S.; Takahashi, N.; Ohara, N.; Uchimura, T.; Kozaki, M. Microorganisms in Fermentation of Goishi-cha, Japanese Fermented Tea Leaves (Microorganisms Involving in the Fermentation of Japanese Fermented Tea Leaves Part II). Nippon. Shokuhin Kagaku Kogaku Kaishi 1996, 43, 12–20. [Google Scholar] [CrossRef]
- Liu, J.Q.; Xiong, T.; Li, J.B.; Liao, L.K.; Huang, T. Process of fermented tea beverage with lactic acid bacteria and analysis of its aroma components before and after fermentation. Food Ferment. Ind. 2016, 42, 109–114. [Google Scholar]
- Avdeef, A.; Berger, C.M.; Brownell, C. pH-metric solubility. 2: Correlation between the acid-base titration and the saturation shake-flask solubility-pH methods. Pharm. Res. 2000, 17, 85–89. [Google Scholar] [CrossRef]
- Guo, X.; Song, C.; Ho, C.T.; Wan, X. Contribution of l-theanine to the formation of 2, 5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chem. 2018, 263, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.Z.; Zheng, Y.T.; Wang, M.X.; Bo, X.P.; Cui, L.; Han, B.Y. Taste and Delicious Amino Acid Composition of Huoshanhuangya Tea with Different Grades Determined by an Electronic Tongue. Guizhou Agric. Sci. 2017, 45, 105–109. [Google Scholar]
- Teng, J.; Zeng, Z.; Huang, Y. Composition characteristics of purine alkaloids and biochemical components of Camellia gymnogyna Chang. China Guihaia 2018, 38, 568–576. [Google Scholar]
- Guo, X.; Schwab, W.; Ho, C.T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Z.J. Optimization of the Processing Technique of Fermented Lactic Acid Bacteria Beverage Containing the Extract from Tea. J. Anhui Agric. Sci. 2011, 39, 7703–7704+7707. [Google Scholar]
- Huang, H.; Huang, J.A.; Li, S.; Liu, Z.H. Study on the variation of polyphenol, carbohydrate and the number of eurotium cristatum during the processing of Fu Tea withFungus growing on the loose tea. Chin. Agric. Sci. Bull. 2012, 28, 227–232. [Google Scholar]
- Sanderson, G.W.; Ranadive, A.S.; Eisenberg, L.S.; Farrell, F.J.; Simons, R.; Manley, C.H.; Coggon, P. Contribution of polyphenolic compounds to the taste of tea. ACS Symp. Ser. 1976, 26, 14–46. [Google Scholar]
- Zhang, Y.N.; Yin, J.F.; Chen, J.X.; Wang, F.; Du, Q.Z.; Jiang, Y.W.; Xu, Y.Q. Improving the sweet aftertaste of green tea infusion with tannase. Food Chem. 2016, 192, 470–476. [Google Scholar] [CrossRef]
- İbanoglu, Ş.; Ainsworth, P.; Özer, E.A.; Plunkett, A. Physical and sensory evaluation of a nutritionally balanced gluten-free extruded snack. J. Food Eng. 2006, 75, 469–472. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of oral gamma-aminobutyric acid (GABA) administration on stress and sleep in humans: A systematic review. Front. Neurosci. 2020, 14, 559962. [Google Scholar] [CrossRef] [PubMed]
- Sanchart, C.; Rattanaporn, O.; Haltrich, D.; Phukpattaranont, P.; Maneerat, S. Technological and safety properties of newly isolated GABA-producing Lactobacillus futsaii strains. J. Appl. Microbiol. 2016, 121, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 2, 585–599. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma characterization of chinese rice wine by gas chromatography-olfactometry, chemical quantitative analysis, and aroma reconstitution. J. Agric. Food Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef]
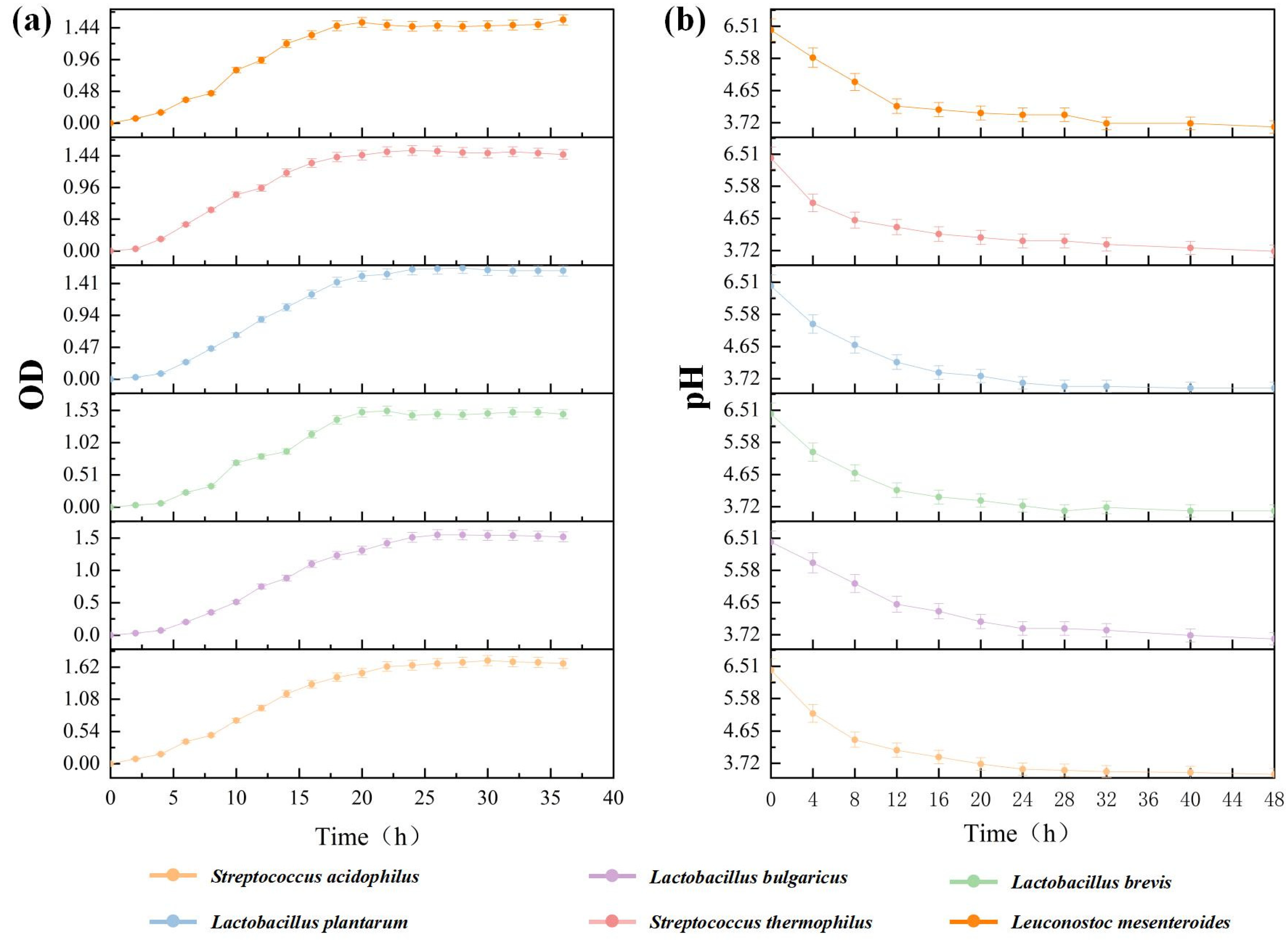
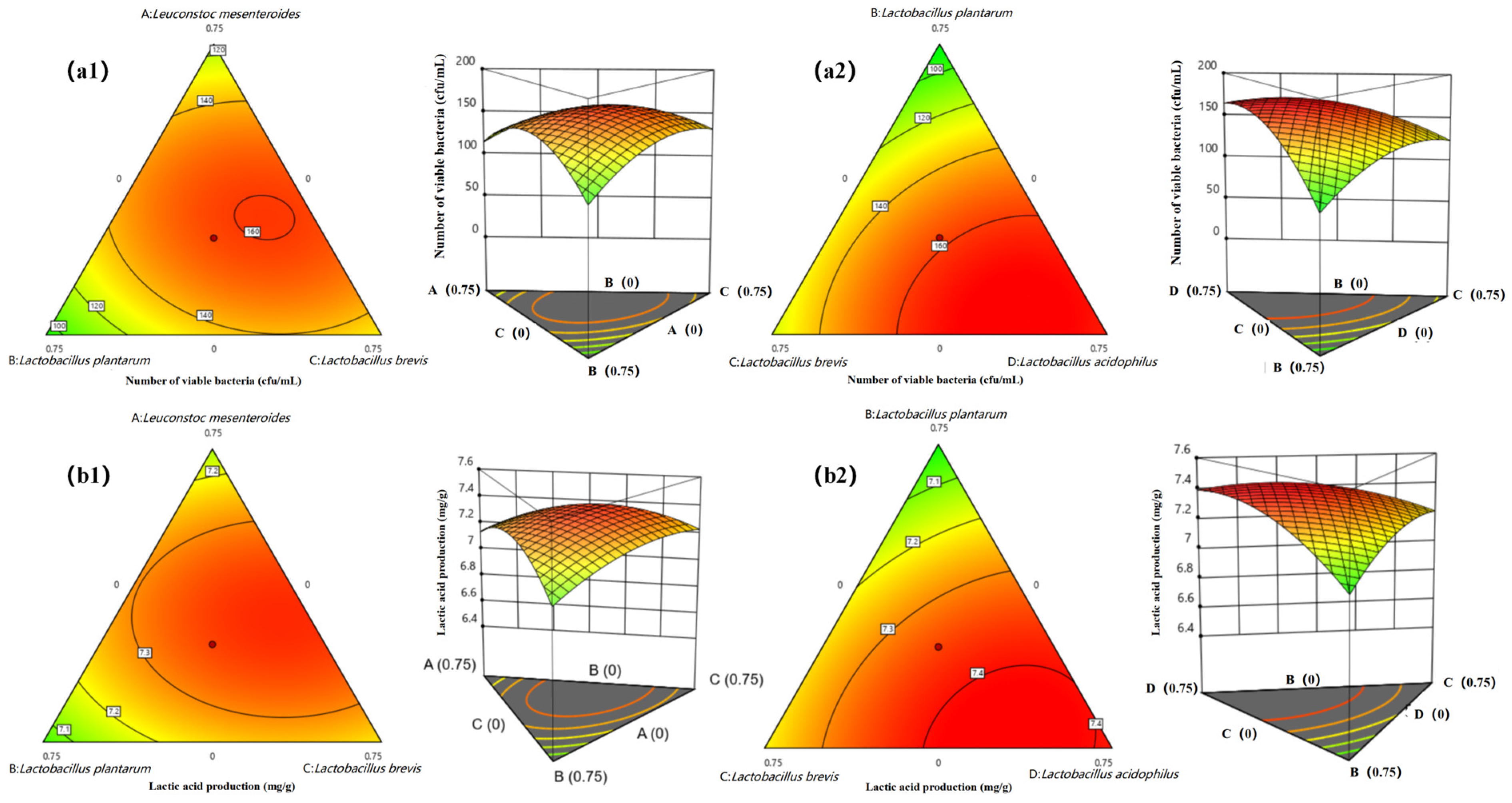

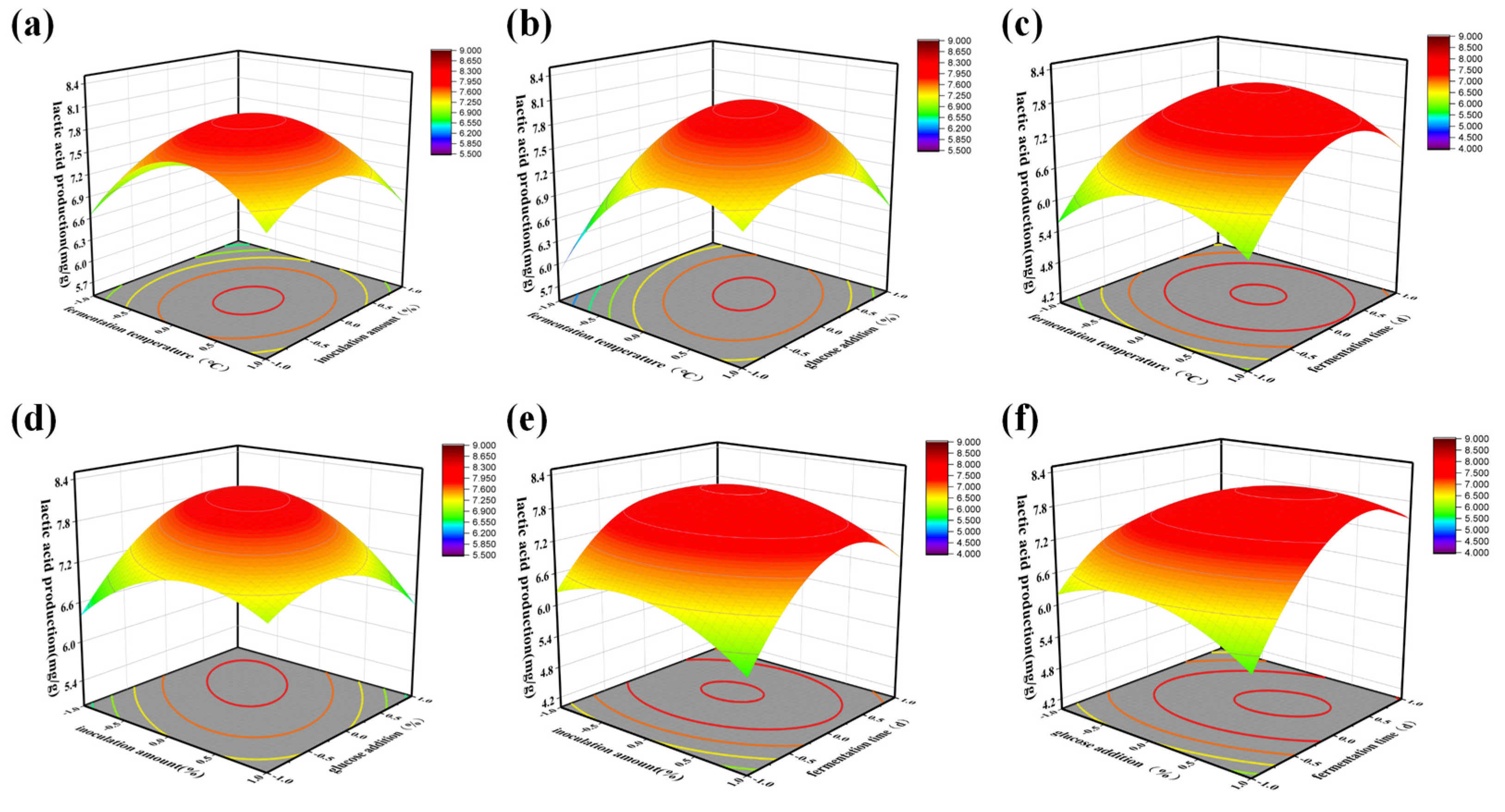
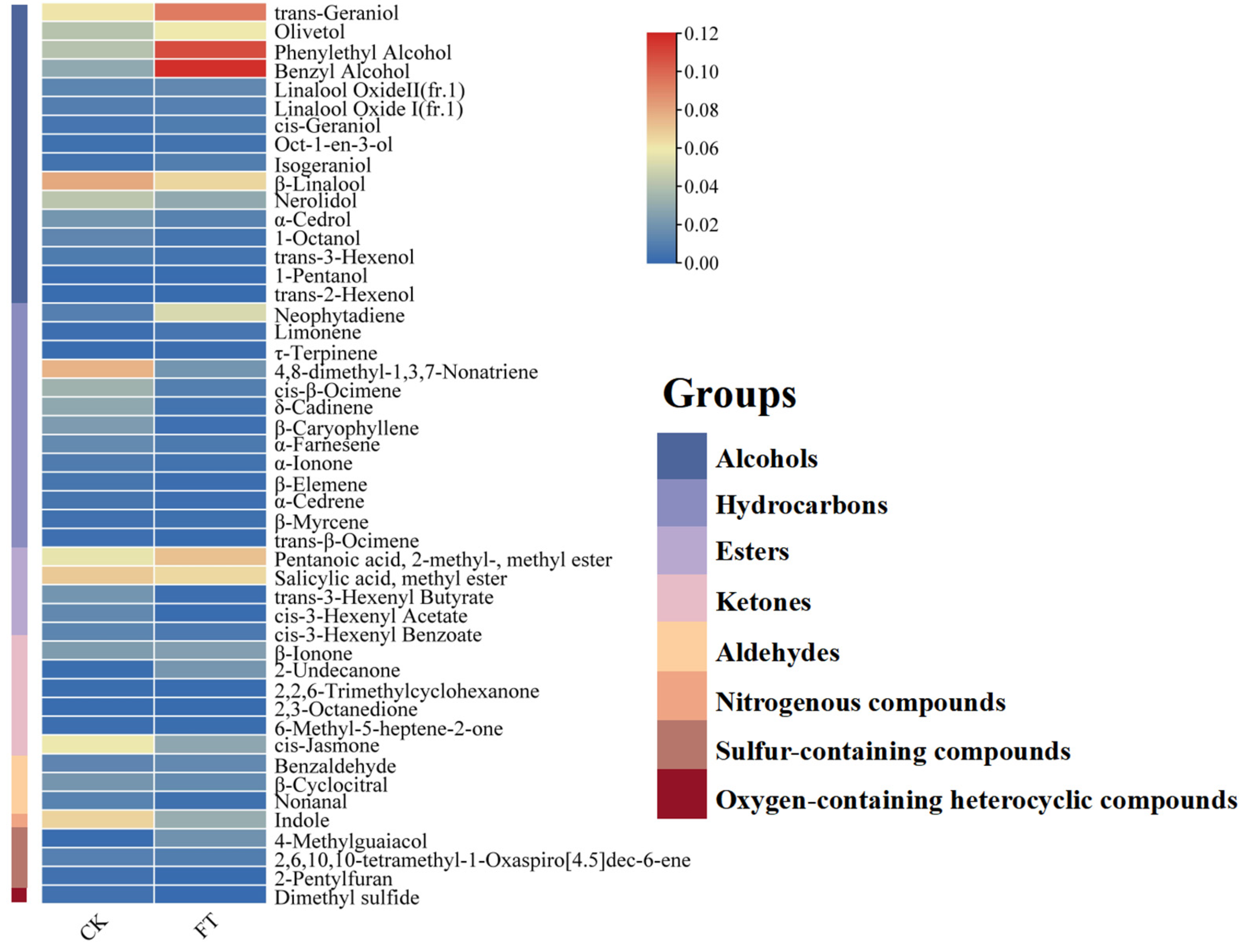
| Strains | Active Bacteria Count(cfu/mL) | Lactic Acid Yield(mg/g) |
|---|---|---|
| Lactobacillus bulgaricus | 8.61 × 107 e | 6.09 ± 0.06 d |
| Lactobacillus brevis | 4.63 × 108 d | 6.50 ± 0.03 c |
| Lactobacillus plantarum | 6.11 × 108 c | 6.54 ± 0.05 c |
| Streptococcus thermophilus | 7.24 × 107 e | 5.99 ± 0.06 e |
| Leuconostoc mesenteroides | 6.82 × 107 b | 6.78 ± 0.06 b |
| Fermentation Time (day) | Main Biochemical Components (%) | |||
|---|---|---|---|---|
| Soluble Solids | Tea Polyphenols | Total Free Amino Acids | Soluble Sugar | |
| 0 | 36.53 ± 0.16 a | 24.08 ± 0.13 a | 1.95 ± 0.03 a | 4.23 ± 0.03 a |
| 1 | 34.46 ± 0.16 d | 21.52 ± 0.18 b | 1.78 ± 0.03 c | 3.11 ± 0.10 b |
| 2 | 35.20 ± 0.13 c | 19.54 ± 0.09 c | 1.72 ± 0.03 d | 2.47 ± 0.03 c |
| 3 | 36.09 ± 0.11 b | 17.75 ± 0.14 d | 1.80 ± 0.03 c | 1.81 ± 0.02 d |
| 4 | 37.42 ± 0.05 a | 17.01 ± 0.03 e | 1.84 ± 0.02 bc | 1.29 ± 0.03 |
| 5 | 37.40 ± 0.16 a | 16.54 ± 0.19 f | 1.89 ± 0.03 ab | 1.22 ± 0.02 e |
| Groups | Groups | CK | FT |
|---|---|---|---|
| Catechins (mg/g) | Galloylated catechins | 56.84 | 79.88 |
| Non-Galloylated catechins | 59.24 | 24.82 | |
| Total catechins | 116.08 | 104.7 | |
| Amino acids (mg/g) | Theanine | 5.327 | 2.835 |
| Leu | 0.073 | 0.105 | |
| Iie | 0.042 | 0.064 | |
| Phe | 0.085 | 0.152 | |
| Lys | 0.038 | 0.186 | |
| Met | 0.139 | 0.178 | |
| Val | 0.147 | 0.163 | |
| Thr | 0.087 | 0.102 | |
| Asn | 0.008 | 0.019 | |
| Glu | 0.342 | 0.298 | |
| GABA | 0.051 | 0.126 | |
| Ser | 0.418 | 0.334 | |
| Gly | 0.469 | 0.425 | |
| His | 0.126 | 0.112 | |
| Arg | 0.063 | 0.149 | |
| Ala | 0.088 | 0.132 | |
| Pro | 0.315 | 0.478 | |
| Cys | 0.847 | 1.157 | |
| Tyr | 0.051 | 0.134 | |
| Total | 8.716 | 8.278 |
| Fermentation Time (day) | GA | C | EC | EGC | ECG | EGCG | Total Catechins |
|---|---|---|---|---|---|---|---|
| 0 | 1.67 ± 0.04 f | 7.86 ± 0.04 e | 9.56 ± 0.06 e | 39.26 ± 0.06 f | 8.68 ± 0.05 a | 49.05 ± 0.05 a | 116.08 ± 0.16 a |
| 1 | 2.47 ± 0.07 e | 7.17 ± 0.04 f | 10.15 ± 0.10 d | 40.21 ± 0.08 e | 8.36 ± 0.05 a | 46.23 ± 0.05 b | 114.58 ± 0.09 b |
| 2 | 4.02 ± 0.04 d | 8.83 ± 0.07 d | 13.72 ± 0.10 c | 42.66 ± 0.25 d | 7.89 ± 0.04 b | 37.57 ± 0.04 c | 114.70 ± 0.31 b |
| 3 | 4.85 ± 0.05 c | 10.17 ± 0.07 c | 15.36 ± 0.08 b | 44.75 ± 0.05 c | 7.29 ± 0.20 c | 26.12 ± 0.08 d | 108.54 ± 0.15 c |
| 4 | 5.35 ± 0.09 b | 11.74 ± 0.05 b | 18.43 ± 0.27 a | 47.41 ± 0.05 b | 7.00 ± 0.23 c | 18.38 ± 0.05 e | 108.41 ± 0.20 c |
| 5 | 6.15 ± 0.07 a | 13.10 ± 0.10 a | 18.17 ± 0.10 a | 48.45 ± 0.05 a | 6.50 ± 0.30 d | 12.33 ± 0.05 | 104.70 ± 0.33 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, X.; Chen, Y.; Zeng, Z.; Xiao, S.; Huang, Y. Optimizing Lactic Acid Bacteria Fermentation for Enhanced Summer and Autumn Tea Quality. Foods 2024, 13, 3126. https://doi.org/10.3390/foods13193126
Mo X, Chen Y, Zeng Z, Xiao S, Huang Y. Optimizing Lactic Acid Bacteria Fermentation for Enhanced Summer and Autumn Tea Quality. Foods. 2024; 13(19):3126. https://doi.org/10.3390/foods13193126
Chicago/Turabian StyleMo, Xiaoli, Yingyu Chen, Zhen Zeng, Sui Xiao, and Yahui Huang. 2024. "Optimizing Lactic Acid Bacteria Fermentation for Enhanced Summer and Autumn Tea Quality" Foods 13, no. 19: 3126. https://doi.org/10.3390/foods13193126





