Quality Variation of Pork Bellies by Cutting Manner and Quality Grade
Abstract
1. Introduction
2. Materials and Methods
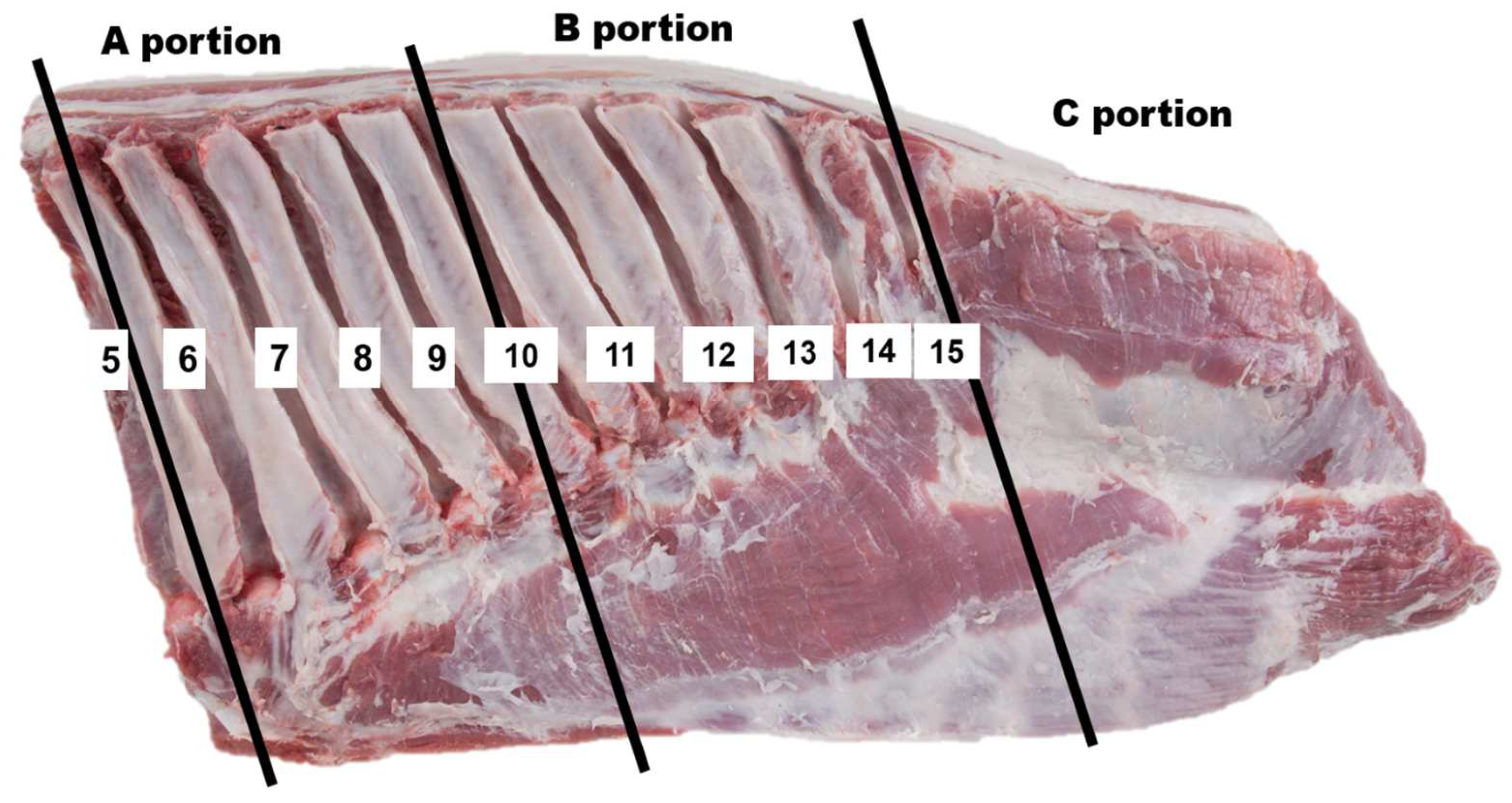
2.1. Sample Preparation
2.2. Meat Quality Analysis
2.3. Fatty Acid Profiles
2.4. Aroma Component Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect on Chemical Composition
3.2. Effect on Meat Quality Properties
3.3. Effect on Color of Lean and Fat
3.4. Effect on Fatty Acid Profiles
3.5. Effect on Aroma Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoa, V.B.; Min, Y.J.; Kim, H.W.; Moon, S.S.; Song, D.H.; Kim, Y.S.; Bae, I.S.; Kim, D.G.; Cho, S.H. Meat quality characteristics of Woori heukdon pigs as affected by dietary amino acids and chromium supplementation. J. Anim. Sci. Technol. 2023, in press. [Google Scholar] [CrossRef]
- Choe, J.H.; Yang, H.S.; Lee, S.H.; Go, G.W. Characteristics of pork belly consumption in South Korea and their health implication. J. Anim. Sci. Technol. 2015, 57, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.M. Breeding potential for pork belly to the novel economic trait. J. Anim. Sci. Technol. 2023, 65, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.P.; Prieto, N.; Lopez-Campos, O.; Aalhus, J.L.; Uttaro, B.; Roberts, J.C.; Juarez, M. Potential of near infrared (NIR) spectroscopy and dual energy X-ray absorptiometry (DXA) in predicting pork belly softness. Meat Sci. 2018, 142, 1–4. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture Foreign Agricultural Service. Pork Production Reports. Available online: https://www.fas.usda.gov (accessed on 15 June 2024).
- Oh, S.H.; See, M.T. Pork preference for consumers in China, Japan and South Korea. Asian-Australas. J. Anim. Sci. 2012, 25, 143–150. [Google Scholar] [CrossRef]
- Hoa, V.B.; Seol, K.H.; Seo, H.W.; Kang, S.M.; Kim, Y.S.; Seong, P.N.; Moon, S.S.; Kim, J.H.; Cho, S.H. Investigation of physicochemical and sensory quality differences in pork belly and shoulder butt cuts with different quality grades. Food Sci. Anim. Resour. 2021, 41, 224–236. [Google Scholar] [CrossRef]
- Animal Products Grading Statistical Yearbook, 16th ed.; Korea Institute for Animal Products Quality Evaluation: Sejong, Republic of Korea, 2021.
- Jin, S.K.; Yim, D.G. Influences of aging methods and temperature on meat quality of pork belly from purebred Berkshire and crossbred Landrace × Yorkshire × Duroc (LYD) pigs. Food Sci. Anim. Resour. 2022, 42, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Knecht, D.; Duziński, K.; Jankowska-Mąkosa, A. Pork ham and belly quality can be estimated from loin quality measurement? Meat Sci. 2018, 145, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Albano-Gaglio, M.; Zomeno, C.; Tejeda, J.F.; Brun, A.; Gispert, M.; Marcos, B.; Fonti-Furnols, M. Pork belly quality variation and its association with fatness level. Meat Sci. 2024, 213, 109482. [Google Scholar] [CrossRef]
- Hoa, V.B.; Seol, K.H.; Seo, H.W.; Seong, P.N.; Kang, S.M.; Kim, Y.S.; Cho, S.H. Meat quality characteristics of pork bellies in relation to fat level. Anim. Biosci. 2021, 34, 1663–1673. [Google Scholar] [CrossRef]
- Korea Institute of Animal Products Quality Evaluation [KAPE]. Korea Pork Grading Standards (Notification No. 2018–109). 2018. Available online: http://www.ekape.or.kr/index.do (accessed on 18 June 2023).
- Anderson, S. Determination of fat, moisture, and protein in meat and meat products by using the FOSS FoodScan™ near-infrared spectrophotometer with FOSS artificial neural network calibration model and associated database: Collaborative study. J. AOAC Int. 2007, 90, 1073–1083. [Google Scholar] [CrossRef]
- Fishcher, C.; Hofmann, K.; Hamm, R. Erfarungen mit der kapillarvolumeter-method nach hofmann zui bestimmung des wassebindungsvemogens von fleisch. Fleischwirtschaft 1976, 56, 91–95. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Hoa, V.B.; Oliveros, M.C.; Ryu, K.S.; Hwang, I. Development of analysis condition and detection of volatile compounds from cooked Hanwoo beef by SPME-GC/MS analysis. Korean J. Food Sci. Anim. Resour. 2010, 30, 73–86. [Google Scholar]
- Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat deposition and fat effects on meat quality—A Review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, P.O.; Shand, P.J.; Aalhus, J.L.; Gariepy, C.; Juarez, M. Review: Pork belly quality, bacon properties and recent consumer trends. Can. J. Anim. Sci. 2015, 95, 325–340. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Uttaro, B.; Zawadski, S.; Dugan, M.E.R.; Gariepy, C.; Aalhhus, J.L.; Shand, P.; Juarez, M. Compositional and dimensional factors influencing pork belly firmness. Meat Sci. 2017, 129, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Trusell, K.A.; Apple, J.K.; Yancey, J.W.S.; Johnson, T.M.; Galloway, D.L.; Stackhouse, R.J. Compositional and instrumental firmness variations within fresh pork bellies. Meat Sci. 2011, 88, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Albano-Gaglio, M.; Mishra, P.; Erasmus, S.W.; Tejeda, J.F.; Brun, A.; Marcos, B.; Zomeño, C.; Font-i-Furnols, M. Visible and near-infrared spectral imaging combined with robust regression for predicting firmness, fatness, and compositional properties of fresh pork bellies. Meat Sci. 2025, 219, 109645. [Google Scholar] [CrossRef] [PubMed]
- Vonada, M.L.; Bidner, B.S.; Belk, K.E.; McKeith, F.K.; Lloyd, W.R.; O’Connor, M.E.; Smith, G.C. Quantification of pork belly and boston butt quality attribute preferences of South Korean customers. J. Anim. Sci. 2000, 78, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Razmaite, V.; Pileckas, V.; Juskiene, V. Effect of muscle anatomical location on fatty acid composition of beaver (Castor fiber) females. Czech J. Food Sci. 2019, 37, 106–111. [Google Scholar] [CrossRef]
- Chauhan, S.S.; England, E.M. Postmortem glycolysis and glycogenolysis: Insights from species comparisons. Meat Sci. 2018, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Trout, G.R. Techniques for measuring water binding capacity in muscle foods: A review of methodology. Meat Sci. 1988, 23, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D.; Wheeler, T.L.; Ha, M.; Li, X.; Bekhit, A.E.D.; Morton, J.; Vaskoska, R.; Dunshea, F.R.; Liu, R.; Purslow, P.; et al. Meat tenderization: Advances in biology, biochemicstry, molecular mechanism and new technologies. Meat Sci. 2022, 185, 108657. [Google Scholar] [CrossRef]
- Lucherk, L.W.; O’Quinn, T.G.; Legako, J.F.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Consumer and trained panel evaluation of beef strip steaks of varying marbling and enhancement levels cooked to three degrees of doneness. Meat Sci. 2016, 122, 145–154. [Google Scholar] [CrossRef]
- Warner, R.; Miller, R.; Ha, M.; Wheeler, T.; Dunshea, F.; Li, X.; Vaskoska, R.; Purslow, P. Meat tenderness: Underlying mechanisms, instrumental measurement, and sensory assessment. Meat Muscle Biol. 2021, 4, 1–25. [Google Scholar]
- Beyer, E.S.; Prill, L.L.; Rice, E.A.; Drey, L.N.; Olson, B.A.; Gonzalez, J.M.; Chao, M.D.; Vipham, J.L.; Zumbaugh, M.D.; O’Quinn, T.G. Pork quality attributes and eating characteristics among different premium and commodity pork loin programs. Meat Muscle Biol. 2023, 7, 1–9. [Google Scholar] [CrossRef]
- Purslow, P.P.; Warner, R.D.; Clarke, F.M.; Hughes, J.M. Variations in meat color due to factors other than myoglobin chemistry; A synthesis of recent findings (invited review). Meat Sci. 2020, 159, 107941. [Google Scholar] [CrossRef]
- Seman, D.L.; Barron, W.N.G.; Matzinger, M. Evaluating the ability to measure pork fat quality for the production of commercial bacon. Meat Sci. 2013, 94, 262–266. [Google Scholar] [CrossRef]
- Ngapo, T.M.; Martin, J.F.; Dransfield, E. Consumer choices of pork chops: Results from three panels in France. Food Qual Pref. 2004, 15, 349–359. [Google Scholar] [CrossRef]
- Ngapo, T.M.; Fortin, J.; Aalhus, J.L.; Martin, J.F. Consumer choices of pork chops: Results from two Canadian sites. Food Res. Int. 2010, 43, 1559–1565. [Google Scholar] [CrossRef]
- Ngapo, T.M.; Rubio Lozano, M.S.; Brana-Varela, D. Mexican consumers at the point of meat purchase. Pork choice. Meat Sci. 2018, 135, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.T.; Guo, H.L.; Tseng, T.F.; Roan, S.W.; Ngapo, T.M. Consumer choice of pork chops in Taiwan. Meat Sci. 2010, 85, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Marcos, B.; Quinteiro, P.; Albano-Gaglio, M.; Muñoz, I.; Claret, A.; Guerrero, L.; Lloret, E.; Tejeda, J.F.; Font-i-Furnols, M. Tailor-made packaging strategies of fresh pork belly with different fat content: Enhancing shelf life while minimizing environmental impact. Meat Sci. 2024, 217, 109627. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.T.N.; To, K.V.; Schilling, M.W. Fatty acid composition of meat animals as flavor precursors. Meat Muscle Biol. 2021, 5, 34. [Google Scholar] [CrossRef]
- Malgwi, I.H.; Halas, V.; Grünvald, P.; Schiavon, S.; Jócsák, I. Genes related to fat metabolism in pigs and intramuscular fat content of pork: A focus on nutrigenetics and nutrigenomics. Animals 2022, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.M.; Ren, L.J.; Chen, L.; Zhang, X.; Cheng, M.L.; Li, W.Z.; Zhang, Y.Y.; Gao, S.Z. Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition. Lipids 2009, 44, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Tejerina, D.; García-Torres, S.; Cabeza de Vaca, M.; Vázquez, F.M.; Cava, R. Study of variability in antioxidant composition and fatty acids profile of Longissimus dorsi and Serratus ventralis muscles from Iberian pigs reared in two different Montanera seasons. Meat Sci. 2012, 90, 414–419. [Google Scholar] [CrossRef]
- Renaville, B.; Prandi, A.; Fan, B.; Sepulcri, A.; Rothschild, M.F.; Piasentier, E. Candidate gene marker associations with fatty acid profiles in heavy pigs. Meat Sci. 2013, 93, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Temme, E.H.; Mensink, R.P.; Hornstra, G. Comparison of the effects of diets enriched in lauric, palmitic or oleic acids on serum lipids and lipoproteins in healthy women and men. Am. J. Clin. Nutr. 1996, 63, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Artemis, S. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 5605–5695. [Google Scholar]
- Smith, S.B. Marbling and its nutritional impact on risk factors for cardiovascular disease. J. Food Sci. Anim. Resour. 2016, 36, 435–444. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors-A systemic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Review. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef] [PubMed]
- Hoa, V.B.; Hwang, I.; Jeong, D.; Touseef, A. Principle of meat aroma flavors and future prospect. In Latest Research into Quality Control; Isin, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 145–176. [Google Scholar]
- Hoa, V.B.; Amna, T.; Hwang, I.H. Significant influence of particular unsaturated fatty acids and pH on the volatile compounds in meat-like model systems. Meat Sci. 2013, 94, 480–488. [Google Scholar]
- Hoa, V.B.; Oliveros, C.M.; Park, K.M.; Dashdorj, D.; Hwang, I. Effect of marbling and chilled ageing on meat-quality traits, volatile compounds and sensory characteristics of beef Longissimus dorsi muscle. Anim. Prod. Sci. 2017, 57, 981–992. [Google Scholar]
- Elmore, J.S.; Campo, M.M.; Enser, M.; Mottram, D.S. Effect of lipid composition on meat-like model systems containing cystein, ribose and polyunsaturated fatty acids. Food Chem. 2002, 50, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
| Traits | Retail Cut | Quality Grade | |||
|---|---|---|---|---|---|
| 1+ | 1 | 2 | Off-Grade | ||
| Protein (%) | A | 12.89 ± 1.27 Bc | 13.25 ± 0.88 Bc | 12.87 ± 1.76 Bb | 14.60 ± 1.94 Ac |
| B | 14.30 ± 1.54 Bb | 14.16 ± 1.56 BCb | 13.10 ± 1.94 Cb | 15.77 ± 2.33 Ab | |
| C | 16.64 ± 0.84 Ba | 16.44 ± 1.03 Ba | 16.33 ± 1.60 Ba | 17.12 ± 1.61 ABa | |
| Fat (%) | A | 41.50 ± 6.60 Aa | 39.96 ± 5.40 Aa | 40.37 ± 8.48 Aa | 31.22 ± 10.90 Ba |
| B | 35.90 ± 8.62 Ab | 36.27 ± 6.40 Ab | 40.14 ± 9.38 Aa | 26.97 ± 11.76 Ba | |
| C | 25.76 ± 5.02 Ac | 21.37 ± 5.61 Bc | 23.21 ± 5.15 Bb | 20.70 ± 8.22 Bb | |
| Moisture (%) | A | 45.72 ± 5.46 Bc | 47.13 ± 4.50 Bc | 46.81 ± 6.47 Bb | 53.85 ± 8.58 Ab |
| B | 49.71 ± 6.92 Bb | 49.58 ± 4.82 Bb | 46.39 ± 7.56 Bb | 56.84 ± 9.15 Ab | |
| C | 57.01 ± 4.93 Ca | 60.23 ± 2.77 ABa | 57.94 ± 5.44 BCa | 61.62 ± 6.43 Aa | |
| Collagen (%) | A | 1.93 ± 0.15 | 1.93 ± 0.17 | 1.67 ± 0.53 | 1.94 ± 0.16 |
| B | 2.02 ± 0.30 | 2.09 ± 0.14 | 1.73 ± 0.63 | 2.00 ± 0.23 | |
| C | 2.00 ± 0.24 | 1.85 ± 0.19 | 1.67 ± 0.68 | 1.93 ± 0.23 | |
| Traits | Retail Cut | Quality Grade | |||
|---|---|---|---|---|---|
| 1+ | 1 | 2 | Off-Grade | ||
| Cooking loss (%) | A | 31.51 ± 3.37 | 31.60 ± 3.13 | 32.06 ± 2.33 | 30.78 ± 3.7 |
| B | 29.17 ± 2.76 | 29.32 ± 4.63 | 29.58 ± 3.08 | 30.01 ± 4.42 | |
| C | 27.67 ± 4.44 | 30.98 ± 3.82 | 29.45 ± 5.51 | 29.62 ± 4.25 | |
| Water-holding capacity (%) | A | 73.85 ± 6.33 | 70.07 ± 5.64 | 70.13 ± 3.77 | 69.92 ± 12.36 |
| B | 73.01 ± 7.01 | 72.41 ± 8.05 | 66.24 ± 6.25 | 71.07 ± 11.77 | |
| C | 77.41 ± 7.22 | 74.95 ± 4.86 | 73.03 ± 6.27 | 73.92 ± 11.17 | |
| Shear force (kg/cm2) | A | 2.48 ± 0.65 Bb | 2.66 ± 0.73 ABc | 2.56 ± 0.54 Bb | 2.79 ± 0.54 Ac |
| B | 2.30 ± 0.76 Cb | 3.01 ± 0.63 ABb | 2.77 ± 1.12 Bb | 3.21 ± 0.98 Ab | |
| C | 3.24 ± 1.03 Ba | 3.67 ± 0.62 Aa | 3.46 ± 0.58 ABa | 3.51 ± 0.64 Aa | |
| pH | A | 6.20 ± 0.26 A | 6.05 ± 0.24 AB | 5.98 ± 0.28 B | 5.96 ± 0.30 Ba |
| B | 6.04 ± 0.19 A | 5.90 ± 0.28 AB | 5.90 ± 0.34 AB | 5.77 ± 0.23 Bb | |
| C | 6.03 ± 0.26 A | 5.85 ± 0.25 AB | 5.81 ± 0.32 B | 5.70 ± 0.21 Bb | |
| Traits | Retail Cut | Quality Grade | ||||
|---|---|---|---|---|---|---|
| 1+ | 1 | 2 | Off-Grade | |||
| Meat | CIE L* | A | 50.91 ± 2.65 | 52.10 ± 4.05 | 51.54 ± 3.72 | 52.42 ± 4.01 |
| B | 51.37 ± 4.80 | 52.26 ± 4.33 | 51.40 ± 3.21 | 53.24 ± 3.47 | ||
| C | 50.09 ± 6.51 AB | 50.66 ± 5.60 AB | 49.75 ± 6.71 B | 52.96 ± 6.31 A | ||
| CIE a* | A | 11.43 ± 1.97 b | 12.55 ± 2.35 | 12.54 ± 2.10 | 12.18 ± 2.32 | |
| B | 12.50 ± 2.04 a | 12.66 ± 1.52 | 13.12 ± 2.09 | 12.85 ± 2.21 | ||
| C | 12.63 ± 2.31 a | 12.93 ± 3.97 | 11.92 ± 3.00 | 13.11 ± 3.39 | ||
| CIE b* | A | 6.06 ± 1.24 B | 6.60 ± 1.09 ABab | 6.85 ± 1.43 Aa | 6.91 ± 1.35 Aa | |
| B | 6.15 ± 1.18 B | 7.01 ± 1.79 Aa | 6.67 ± 1.34 ABa | 6.82 ± 1.27 ABa | ||
| C | 5.93 ± 1.52 AB | 5.94 ± 1.87 ABb | 5.37 ± 1.30 Bb | 6.17 ± 1.50 Ab | ||
| Fat | CIE L* | A | 79.98 ± 1.56 Ab | 80.93 ± 2.74 A | 80.06 ± 2.01 Ab | 78.54 ± 2.16 B |
| B | 80.19 ± 2.37 b | 81.27 ± 1.69 | 80.22 ± 2.34 ab | 80.41 ± 2.15 | ||
| C | 81.15 ± 1.83 Aa | 81.39 ± 2.47 A | 81.18 ± 1.68 Aa | 79.79 ± 3.35 B | ||
| CIE a* | A | 3.31 ± 0.96 b | 3.03 ± 1.92 | 3.44 ± 1.41 | 3.23 ± 1.44 | |
| B | 4.00 ± 0.95 a | 3.07 ± 1.12 | 3.93 ± 1.70 | 3.80 ± 1.14 | ||
| C | 3.83 ± 1.11 a | 3.63 ± 1.19 | 3.20 ± 1.53 | 3.62 ± 1.58 | ||
| CIE b* | A | 7.32 ± 0.98 b | 6.88 ± 1.60 | 6.80 ± 1.55 | 6.73 ± 1.73 | |
| B | 7.96 ± 1.03 a | 7.11 ± 1.31 | 7.51 ± 1.94 | 7.64 ± 1.61 | ||
| C | 7.79 ± 1.25 ab | 7.70 ± 1.29 | 6.98 ± 1.39 | 7.00 ± 1.87 | ||
| Items | Retail Cut | Quality Grade | |||
|---|---|---|---|---|---|
| 1+ | 1 | 2 | Off-Grade | ||
| C10:0 (Capric acid) | A | 0.06 ± 0.01 aA | 0.05 ± 0.01 aA | 0.05 ± 0.01 aA | 0.04 ± 0.00 aB |
| B | 0.03 ± 0.00 bC | 0.05 ± 0.01 aA | 0.04 ± 0.01 bB | 0.03 ± 0.00 bC | |
| C | 0.05 ± 0.01 aA | 0.02 ± 0.02 bC | 0.03 ± 0.00 cBC | 0.04 ± 0.01 aAB | |
| C12:0 (Lauric acid) | A | 0.09 ± 0.01 aA | 0.06 ± 0.01 bB | 0.05 ± 0.01 bB | 0.04 ± 0.01 bC |
| B | 0.05 ± 0.01 bA | 0.07 ± 0.00 aA | 0.07 ± 0.01 aA | 0.04 ± 0.01 bB | |
| C | 0.05 ± 0.01 b | 0.05 ± 0.01 b | 0.04 ± 0.00 c | 0.05 ± 0.01 a | |
| C14:0 (Myristic acid) | A | 0.72 ± 0.13 AB | 0.78 ± 0.10 A | 0.76 ± 0.09 aA | 0.58 ± 0.10 bB |
| B | 0.71 ± 0.11 A | 0.73 ± 0.06 A | 0.84 ± 0.13 aA | 0.51 ± 0.10 bB | |
| C | 0.80 ± 0.18 A | 0.67 ± 0.17 AB | 0.59 ± 0.07 bB | 0.76 ± 0.12 aAB | |
| C14:1 (Myristoleic acid) | A | 0.02 ± 0.01 | 0.03 ± 0.00 A | 0.03 ± 0.00 | 0.02 ± 0.00 |
| B | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.01 | |
| C | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.01 | |
| C15:0 (Pentadecanoic acid) | A | 0.03 ± 0.01 b | 0.04 ± 0.00 | 0.03 ± 0.01 b | 0.04 ± 0.01 |
| B | 0.06 ± 0.01 aA | 0.03 ± 0.00 B | 0.03 ± 0.00 bB | 0.03 ± 0.01 B | |
| C | 0.04 ± 0.01 b | 0.04 ± 0.01 | 0.06 ± 0.01 a | 0.04 ± 0.01 | |
| C16:0 (Palmitic acid) | A | 10.85 ± 2.01 A | 11.17 ± 1.71 A | 11.02 ± 1.43 A | 8.42 ± 1.40 B |
| B | 10.38 ± 1.80 A | 9.29 ± 0.95 A | 12.51 ± 2.10 A | 7.38 ± 1.31 B | |
| C | 11.41 ± 2.31 A | 9.67 ± 2.33 AB | 11.60 ± 1.99 A | 8.27 ± 1.14 B | |
| C16:1 (Palmitoleic acid) | A | 0.94 ± 0.18 AB | 1.12 ± 0.15 A | 1.08 ± 0.15 aA | 0.86 ± 0.16 B |
| B | 0.81 ± 0.58 B | 1.02 ± 0.19 aAB | 1.26 ± 0.21 aA | ND | |
| C | 1.25 ± 0.29 A | ND | 0.35 ± 0.50 bB | 1.36 ± 0.20 A | |
| C17:0 (Margaric acid) | A | 0.14 ± 0.03 aB | 0.17 ± 0.02 aB | 0.16 ± 0.02 B | 0.17 ± 0.05 A |
| B | 0.20 ± 0.14 aA | 0.15 ± 0.02 aB | 0.17 ± 0.02 AB | ND | |
| C | 0.01 ± 0.00 bB | 0.01 ± 0.00 bB | 0.09 ± 0.13 AB | 0.16 ± 0.07 A | |
| C17:1 (Heptadecenoic acid) | A | 0.11 ± 0.03 b | 0.16 ± 0.02 ab | 0.13 ± 0.02 b | 0.16 ± 0.04 ab |
| B | 0.20 ± 0.03 a | 0.13 ± 0.02 b | 0.15 ± 0.02 b | 0.13 ± 0.04 b | |
| C | 0.18 ± 0.05 a | 0.19 ± 0.05 a | 0.20 ± 0.03 a | 0.20 ± 0.04 a | |
| C18:0 (Stearic acid) | A | 5.75 ± 1.05 A | 5.70 ± 0.91 aA | 5.81 ± 0.84 aA | 4.49 ± 0.64 aB |
| B | 5.34 ± 1.07 A | 4.31 ± 0.53 bA | 6.27 ± 1.10 aA | 3.99 ± 0.66 bB | |
| C | 5.49 ± 1.02 A | 4.78 ± 1.07 bAB | 3.94 ± 0.59 bB | 3.40 ± 1.00 bA | |
| C18:1 n9 (Oleic acid) | A | 17.59 ± 0.37 | 20.09 ± 0.41 a | 19.29 ± 0.42 ab | 16.58 ± 0.19 ab |
| B | 16.52 ± 1.31 B | 14.90 ± 1.36 bB | 22.17 ± 1.67 aA | 13.72 ± 0.18 bB | |
| C | 20.83 ± 1.43 | 18.53 ± 1.58 ab | 15.77 ± 2.17 b | 20.96 ± 1.63 a | |
| C18:2 n6 (Linoleic acid) | A | 8.29 ± 0.49 A | 6.67 ± 0.92 bAB | 6.12 ± 0.76 bB | 6.34 ± 0.58 bB |
| B | 7.42 ± 0.43 AB | 6.38 ± 0.73 bB | 8.44 ± 1.04 aA | 6.27 ± 0.84 bB | |
| C | 7.92 ± 0.12 | 8.32 ± 0.13 a | 8.41 ± 0.08 a | 6.86 ± 0.37 a | |
| C18:3 n-3 (Linolenic acid) | A | 0.36 ± 0.06 b | 0.39 ± 0.05 | 0.36 ± 0.04 b | 0.31 ± 0.08 b |
| B | 0.36 ± 0.07 b | 0.38 ± 0.01 | 0.49 ± 0.06 a | 0.29 ± 0.07 b | |
| C | 0.42 ± 0.23 aAB | 0.38 ± 0.02 B | 0.41 ± 0.01 abB | 0.49 ± 0.03 aA | |
| C20:0 (Arachidic acid) | A | 0.10 ± 0.02 aB | 0.08 ± 0.01 B | 0.13 ± 0.02 aA | 0.08 ± 0.01 B |
| B | 0.05 ± 0.04 abB | 0.07 ± 0.01 B | 0.14 ± 0.07 aA | ND | |
| C | 0.05 ± 0.05 b | ND | 0.02 ± 0.03 b | 0.08 ± 0.02 | |
| C20:1 (Eicocenoic acid) | A | 0.30 ± 0.04 B | 0.41 ± 0.07 aAB | 0.45 ± 0.06 aA | 0.41 ± 0.08 aAB |
| B | 0.38 ± 0.08 B | 0.32 ± 0.04 bB | 0.54 ± 0.09 aA | 0.30 ± 0.09 bB | |
| C | ND | 0.01 ± 0.00 c | 0.01 ± 0.01 b | 0.01 ± 0.00 c | |
| C20:3 n3 (Eicosatrienoic acid) | A | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 a | 0.05 ± 0.01 ab |
| B | 0.06 ± 0.01 AB | 0.05 ± 0.01 BC | 0.07 ± 0.01 aA | 0.04 ± 0.02 bC | |
| C | 0.07 ± 0.02 A | ND | 0.02 ± 0.03 bB | 0.06 ± 0.01 aA | |
| C20:5 n3 (Eicosapentaenoic acid) | A | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| B | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| C | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| C21:0 (Heneicocanoic acid) | A | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| B | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| C | ND | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| C22:0 (Behenic acid) | A | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.01 |
| B | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | |
| C | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | |
| C22:1 n9 (Erucic acid) | A | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 b | 0.02 ± 0.00 |
| B | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 a | 0.01 ± 0.01 | |
| C | 0.01 ± 0.00 | ND | 0.01 ± 0.00 c | 0.02 ± 0.00 | |
| C24:0 (Lignoceric acid) | A | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | ND |
| B | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| C | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | |
| C24:1 n9 (Nervonic acid) | A | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| B | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| C | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | |
| Aroma Class | Retail Cut | Quality Grade | |||
|---|---|---|---|---|---|
| 1+ | 1 | 2 | Off-Grade | ||
| Ʃ Aldehyde | A | 2.21 ± 0.25 A | 1.90 ± 0.05 B | 1.56 ± 0.13 aC | 1.30 ± 0.10 aD |
| B | 2.15 ± 0.13 A | 1.84 ± 0.08 B | 1.29 ± 0.09 bCD | 1.15 ± 0.04 bD | |
| C | 2.38 ± 0.08 A | 1.86 ± 0.06 B | 1.26 ± 0.07 bC | 0.90 ± 0.03 cD | |
| Ʃ Alcohols | A | 0.08 ± 0.01 A | 0.07 ± 0.00 cB | 0.06 ± 0.00 C | 0.07 ± 0.00 aB |
| B | 0.08 ± 0.01 B | 0.08 ± 0.00 bB | 0.13 ± 0.06 A | 0.07 ± 0.00 aB | |
| C | 0.09 ± 0.01 A | 0.09 ± 0.00 aA | 0.05 ± 0.00 C | 0.06 ± 0.00 bB | |
| Ʃ Hydrocarbons | A | 0.05 ± 0.01 c | 0.10 ± 0.02 ab | 0.04 ± 0.00 | 0.05 ± 0.00 b |
| B | 0.07 ± 0.01 b | 0.11 ± 0.01 a | 0.04 ± 0.00 | 0.06 ± 0.00 a | |
| C | 0.12 ± 0.01 a | 0.08 ± 0.01 b | 0.05 ± 0.00 | 0.06 ± 0.00 a | |
| Ʃ Sulfur and nitrogen compounds | A | ND | ND | ND | 0.01 ± 0.00 b |
| B | ND | 0.01 ± 0.00 b | 0.01 ± 0.00 | 0.01 ± 0.00 b | |
| C | 0.02 ± 0.00 B | 0.03 ± 0.00 aA | 0.03 ± 0.00 A | 0.03 ± 0.00 aA | |
| Ʃ Amount of all aroma classes | A | 2.33 ± 0.26 A | 2.17 ± 0.06 B | 1.83 ± 0.01 C | 1.42 ± 0.11 aD |
| B | 2.30 ± 0.25 A | 2.03 ± 0.09 B | 1.47 ± 0.14 C | 1.28 ± 0.04 bD | |
| C | 2.61 ± 0.08 A | 2.07 ± 0.12 B | 1.38 ± 0.07 C | 1.05 ± 0.03 cD | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, P.-N.; Lee, J.-A.; Song, D.-H.; Kim, H.-W.; Kim, D.-G.; Jung, S.; Hoa, V.-B. Quality Variation of Pork Bellies by Cutting Manner and Quality Grade. Foods 2024, 13, 3129. https://doi.org/10.3390/foods13193129
Seong P-N, Lee J-A, Song D-H, Kim H-W, Kim D-G, Jung S, Hoa V-B. Quality Variation of Pork Bellies by Cutting Manner and Quality Grade. Foods. 2024; 13(19):3129. https://doi.org/10.3390/foods13193129
Chicago/Turabian StyleSeong, Pil-Nam, Jeong-Ah Lee, Dong-Heon Song, Hyun-Wook Kim, Dong-Gun Kim, Samooel Jung, and Van-Ba Hoa. 2024. "Quality Variation of Pork Bellies by Cutting Manner and Quality Grade" Foods 13, no. 19: 3129. https://doi.org/10.3390/foods13193129
APA StyleSeong, P.-N., Lee, J.-A., Song, D.-H., Kim, H.-W., Kim, D.-G., Jung, S., & Hoa, V.-B. (2024). Quality Variation of Pork Bellies by Cutting Manner and Quality Grade. Foods, 13(19), 3129. https://doi.org/10.3390/foods13193129




