Dry-Cured Sausages “Salchichón” Manufactured with a Valorized Ingredient from Red Grape Pomace (Var. Tempranillo)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacture of the Ingredient from Red Grape Pomace (RGP)
2.2. Dry-Cured Sausages Preparation
2.3. Analysis of the Ingredient from RGP
2.4. Analysis of Dry-Cured Sausage
2.5. Statistical Analysis
3. Results and Discussion
3.1. Valorization Process and Composition of the Red Grape Pomace
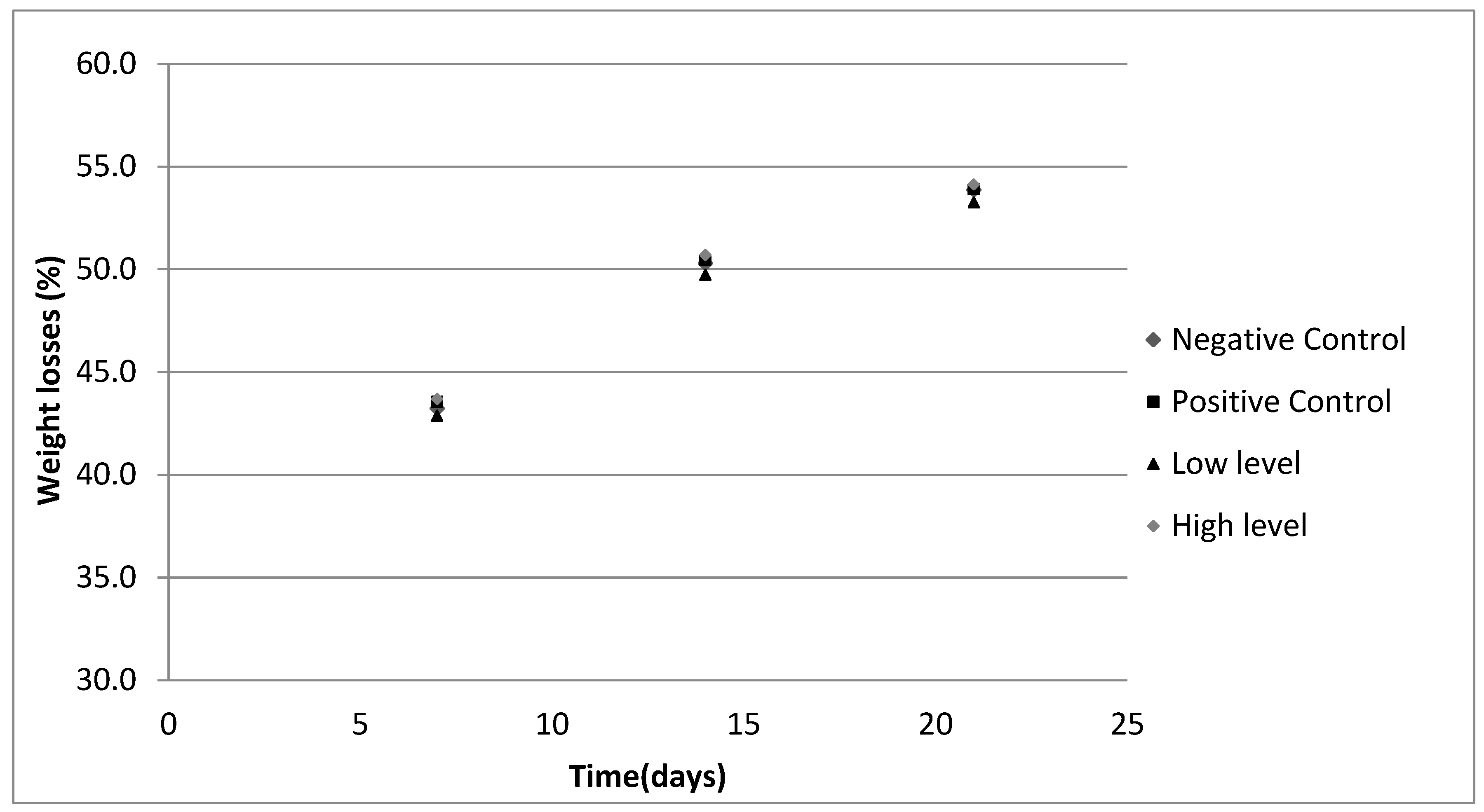
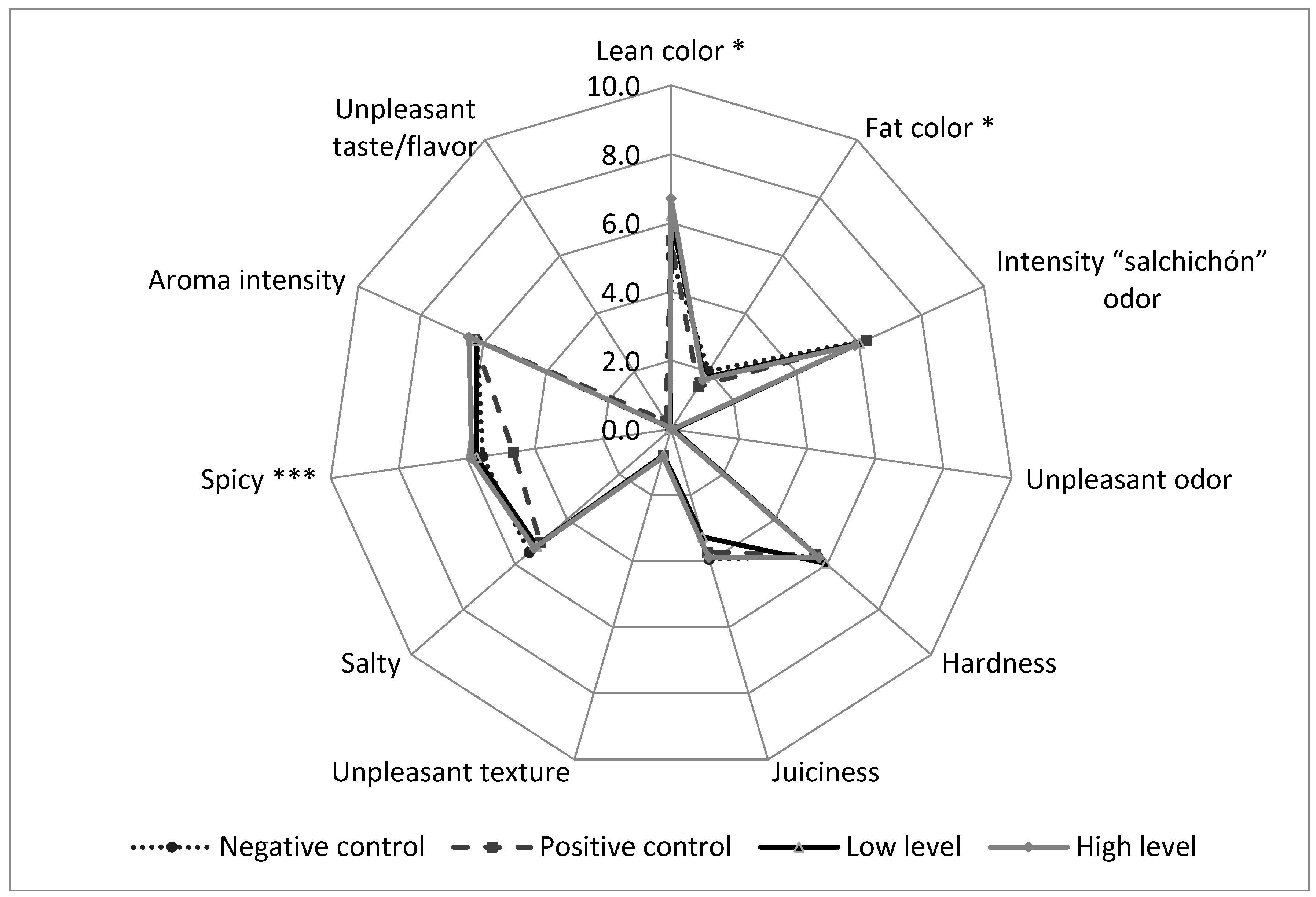
3.2. Effect of the Incorporation of the Valorized Pomace Ingredient in Dry-Cured Sausages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beriain, M.J.; Peña, M.P.; Bello, J. A Study of the Chemical Components Which Characterize Spanish Saucisson. Food Chem. 1993, 48, 31–37. [Google Scholar] [CrossRef]
- Lizaso, G.; Chasco, J.; Beriain, M.J. Microbiological and Biochemical Changes during Ripening of Salchichon, a Spanish Dry Cured Sausage. Food Microbiol. 1999, 16, 219–228. [Google Scholar] [CrossRef]
- Chasco, J.; Lizaso, G.; Beriain, M.J. Cured Colour Development during Sausage Processing. Meat Sci. 1996, 44, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.L.; Nadal, M.I.; Izquierdo, L.; Flores, J. Lipolysis in Dry Cured Sausages as Affected by Processing Conditions. Meat Sci. 1997, 45, 161–168. [Google Scholar] [CrossRef]
- Estévez, M. Protein Carbonyls in Meat Systems: A Review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Flores, M. Understanding the Implications of Current Health Trends on the Aroma of Wet and Dry Cured Meat Products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Franco, D. Fat Effect on Physico-Chemical, Microbial and Textural Changes through the Manufactured of Dry-Cured Foal Sausage Lipolysis, Proteolysis and Sensory Properties. Meat Sci. 2012, 92, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Berardo, A.; Claeys, E.; Vossen, E.; Leroy, F.; De Smet, S. Protein Oxidation Affects Proteolysis in a Meat Model System. Meat Sci. 2015, 106, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; González-Rodríguez, R.M.; Sánchez, M.; Amado, I.R.; Franco, D. Effects of Natural (Grape Seed and Chestnut Extract) and Synthetic Antioxidants (Buthylatedhydroxytoluene, BHT) on the Physical, Chemical, Microbiological and Sensory Characteristics of Dry Cured Sausage “Chorizo”. Food Res. Int. 2013, 54, 611–620. [Google Scholar] [CrossRef]
- Majou, D.; Christieans, S. Mechanisms of the Bactericidal Effects of Nitrate and Nitrite in Cured Meats. Meat Sci. 2018, 145, 273–284. [Google Scholar] [CrossRef]
- Higuero, N.; Moreno, I.; Lavado, G.; Vidal-Aragón, M.C.; Cava, R. Reduction of Nitrate and Nitrite in Iberian Dry Cured Loins and Its Effects during Drying Process. Meat Sci. 2020, 163, 108062. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shao, J.; Zhu, X.; Zhou, G.; Xu, X. Effect of Plant Polyphenols and Ascorbic Acid on Lipid Oxidation, Residual Nitrite and N-Nitrosamines Formation in Dry-Cured Sausage. Int. J. Food Sci. Technol. 2013, 48, 1157–1164. [Google Scholar] [CrossRef]
- Hammes, W.P. Metabolism of Nitrate in Fermented Meats: The Characteristic Feature of a Specific Group of Fermented Foods. Food Microbiol. 2012, 29, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ferysiuk, K.; Wójciak, K.M. Reduction of Nitrite in Meat Products through the Application of Various Plant-Based Ingredients. Antioxidants 2020, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural Antioxidants against Lipid-Protein Oxidative Deterioration in Meat and Meat Products: A Review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Hospital, X.F.; Carballo, J.; Fernández, M.; Arnau, J.; Gratacós, M.; Hierro, E. Technological Implications of Reducing Nitrate and Nitrite Levels in Dry-Fermented Sausages: Typical Microbiota, Residual Nitrate and Nitrite and Volatile Profile. Food Control 2015, 57, 275–281. [Google Scholar] [CrossRef]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of Natural Antioxidants from Grape Seed and Chestnut in Combination with Hydroxytyrosol, as Sodium Nitrite Substitutes in Cinta Senese Dry-Fermented Sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant Extracts as Natural Antioxidants in Meat and Meat Products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, K.; Hosseinian, F.; Rod, M.R.M. The Market Potential of Grape Waste Alternatives. J. Field Robot. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable Green Processing of Grape Pomace for the Production of Value-Added Products: An Overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and Chemical Variability of Grape Pomaces from French Vineyard. Ind. Crops Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Makris, D.P. Green Extraction Processes for the Efficient Recovery of Bioactive Polyphenols from Wine Industry Solid Wastes—Recent Progress. Curr. Opin. Green. Sustain. Chem. 2018, 13, 50–55. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of High Hydrostatic Pressure and Pulsed Electric Fields for Energy Efficient and Environmentally Friendly Food Processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Culioli, J. Effects of High Pressure on Meat: A Review. Meat Sci. 1997, 46, 211–236. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of Anthocyanins from Grape Skins Assisted by High Hydrostatic Pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Ramírez, R.; Delgado, J.; Rocha-Pimienta, J.; Valdés, M.E.; Martín-Mateos, M.J.; Ayuso-Yuste, M.C. Preservation of White Wine Pomace by High Hydrostatic Pressure. Heliyon 2023, 9, e21199. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Delgado-Adámez, J.; Rocha-Pimienta, J.; Valdés-Sánchez, M.E.; Ramírez-Bernabé, M.R. Integral Use of Red Wine Pomace after Hydrostatic High Pressure: Application of Two Consecutive Cycles of Treatment. Foods 2024, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Martín-Mateos, M.J.; Delgado-Adámez, J.; Moreno-Cardona, D.; Valdés-Sánchezm, M.E.; Ramírez-Bernabé, M.R. Application of an Ingredient Made of White Wine Pomace for the Preservation of Fresh Pork Burgers. Foods 2023, 12, 4468. [Google Scholar] [CrossRef]
- Pateiro, M.; Bermúdez, R.; Lorenzo, J.M.; Franco, D. Effect of Addition of Natural Antioxidants on the Shelf-Life of ‘Chorizo’, a Spanish Dry-Cured Sausage. Antioxidants 2015, 4, 42–67. [Google Scholar] [CrossRef]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the Microbial Community Composition in Italian Cinta Senese Sausages Dry-Fermented with Natural Extracts as Alternatives to Sodium Nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef]
- Kurt, S. The Effects of Grape Seed Flour on the Quality of Turkish Dry Fermented Sausage (Sucuk) during Ripening and Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2016, 36, 300–308. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; George, W.L., Jr., Ed.; Official Methods of Analysis of AOAC International: Rockville, MA, USA, 2016; ISBN 0935584870. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.J.; Barragán, R. Determinación Cuantitativa de La Fracción Hidrocarbonada En Alimentos. Anal. Bromatol. 1985, XXXVII, 61–77. [Google Scholar]
- Sørensen, G.; Jørgensen, S.S. A Critical Examination of Some Experimental Variables in the 2-Thiobarbituric Acid (TBA) Test for Lipid Oxidation in Meat Products. Z. Fur Lebensm. Unters. Und-Forsch. 1996, 202, 205–210. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.-W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-Related Changes in Oxidized Proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Qu, H.; Liu, C.; Yan, L.; You, J.; Shui, S.; Zheng, L. A Comparative Assess of High Hydrostatic Pressure and Superfine Grinding on Physicochemical and Antioxidant Properties of Grape Pomace. Int. J. Food Sci. Technol. 2017, 52, 2106–2114. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Javier, H.; Siles, J.A.; Aida, G.; del Carmen, G.M.; de los Ángeles, M.M. Revalorization of Grape Marc Waste from Liqueur Wine: Biomethanization. J. Chem. Technol. Biotechnol. 2019, 94, 1499–1508. [Google Scholar] [CrossRef]
- Hoz, L.; D’Arrigo, M.; Cambero, I.; Ordóñez, J.A. Development of an N-3 Fatty Acid and α-Tocopherol Enriched Dry Fermented Sausage. Meat Sci. 2004, 67, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Moretti, V.M.; Madonia, G.; Diaferia, C.; Mentasti, T.; Paleari, M.A.; Panseri, S.; Pirone, G.; Gandini, G. Chemical and Microbiological Parameters and Sensory Attributes of a Typical Sicilian Salami Ripened in Different Conditions. Meat Sci. 2004, 66, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Flores, M. Dry and Semidry. In Encyclopedia of Meat Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780123847317. [Google Scholar]
- Delgado Adámez, J.; Gamero Samino, E.; Valdés Sánchez, E.; González-Gómez, D. In Vitro Estimation of the Antibacterial Activity and Antioxidant Capacity of Aqueous Extracts from Grape-Seeds (Vitis vinifera L.). Food Control 2012, 24, 136–141. [Google Scholar] [CrossRef]
- Toldrá, F.; Aristoy, M.-C.; Flores, M. Relevance of Nitrate and Nitrite in Dry-Cured Ham and Their Effects on Aroma Development. Grasas Y Aceites 2009, 60, 291–296. [Google Scholar] [CrossRef]
- Morita, H.; Yoshikawa, H.; Suzuki, T.; Hisamatsu, S.; Kato, Y.; Sakata, R.; Nagata, Y.; Yoshimura, T. Anti-Microbial Action against Verotoxigenic Escherichia Coli O157:H7 of Nitric Oxide Derived from Sodium Nitrite. Biosci. Biotechnol. Biochem. 2004, 68, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, C.; Jia, X.; Guo, Y.; Lei, N.; Hackman, R.M.; Chen, L.; Zhou, G. Influence of Sodium Nitrite on Protein Oxidation and Nitrosation of Sausages Subjected to Processing and Storage. Meat Sci. 2016, 116, 260–267. [Google Scholar] [CrossRef]
- Yu, H.; Qin, C.; Wu, X.; Ge, Q.; Wu, M.; Wu, J.; Wang, M.; Wang, Z. Effect of Grape Seed and Rosemary Phenolics on Protein Oxidation in Chinese-Style Sausage. J. Food Agric. Environ. 2013, 11, 231–236. [Google Scholar]
- Kilic-Buyukkurt, O.; Kelebek, H.; Bordiga, M.; Keskin, M.; Selli, S. Changes in the Aroma and Key Odorants from White Garlic to Black Garlic Using Approaches of Molecular Sensory Science: A Review. Heliyon 2023, 9, e19056. [Google Scholar] [CrossRef] [PubMed]
- Carrapiso, A.I.; Martín-Mateos, M.J.; D’Arrigo, M.; Delgado-Adámez, J.; Saraiva, J.A.; Ramírez-Bernabé, M.R. High-Hydrostatic-Pressure-Stabilized White Grape Pomace to Improve the Oxidative Stability of Dry-Cured Sausages (“Salchichón”). Foods 2024, 13, 687. [Google Scholar] [CrossRef]
- Sallan, S.; Kaban, G.; Kaya, M. The Effects of Nitrite, Sodium Ascorbate and Starter Culture on Volatile Compounds of a Semi-Dry Fermented Sausage. LWT 2022, 153, 112540. [Google Scholar] [CrossRef]
- Yalınkılıç, B.; Kaban, G.; Kaya, M. Effect of Sodium Replacement on the Quality Characteristics of Pastırma (a Dry-Cured Meat Product). Food Sci. Hum. Wellness 2022, 12, 266–274. [Google Scholar] [CrossRef]
| Initial (1) | TB (2) | TB + HHP (3) | p-Value (1–2) | p-Value (2–3) | p-Value (1–2–3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPC (mg 100 g−1) | 467.6 | ± | 3.3 | 882.0 | ± | 41.5 | 379.5 | ± | 43.7 | <0.001 | <0.001 | <0.001 |
| PPO (%) | 100.0 | ± | 4.9 | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | <0.001 | 0.341 | <0.001 |
| Valorized Red Grape Pomace | |||
|---|---|---|---|
| pH | 4.2 | ± | 0.1 |
| Aw | 0.963 | ± | 0.001 |
| Proximate composition (g 100 g−1) | |||
| Moisture | 39.6 | ± | 3.5 |
| Protein | 3.4 | ± | 0.1 |
| Fat | 5.1 | ± | 0.9 |
| Fiber | 50.3 | ± | 1.5 |
| Fatty acids profile (%) | |||
| C12:0 | 0.0 | ± | 0.1 |
| C14:0 | 0.0 | ± | 0.1 |
| C16:0 | 9.8 | ± | 0.2 |
| C16:1 | 0.5 | ± | 0.1 |
| C17:1 | 0.1 | ± | 0.0 |
| C17:1 | 0.1 | ± | 0.0 |
| C18:0 | 4.1 | ± | 0.1 |
| C18:1 | 18.1 | ± | 0.1 |
| C18:2 | 66.0 | ± | 0.5 |
| C18:3 | 1.2 | ± | 0.1 |
| C20:0 | 0.0 | ± | 0.0 |
| C20:1 | 0.0 | ± | 0.1 |
| Microbial counts (log CFU g−1) | |||
| Mesophilic | 1.5 | ± | 1.0 |
| Molds and Yeasts | <1 | ± | 0.0 |
| Enterobacteriaceae | <1 | ± | 0.0 |
| Mean | SD | ||
|---|---|---|---|
| Moisture | 28.1 | ± | 1.5 |
| Protein | 40.5 | ± | 1.9 |
| Fat | 13.5 | ± | 2.1 |
| C12:0 | 0.1 | ± | 0.0 |
| C14:0 | 1.4 | ± | 0.0 |
| C16:0 | 23.2 | ± | 0.0 |
| C16:1 | 3.1 | ± | 0.0 |
| C17:0 | 0.4 | ± | 0.0 |
| C17:1 | 0.4 | ± | 0.0 |
| C18:0 | 10.8 | ± | 0.0 |
| C18:1 | 45.6 | ± | 0.1 |
| C18:2 | 13.6 | ± | 0.1 |
| C18:3 | 0.6 | ± | 0.0 |
| C20:0 | 0.0 | ± | 0.0 |
| C20:1 | 0.9 | ± | 0.0 |
| Negative Control | Positive Control | Low Level | High Level | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | 28.1 | ± | 1.5 | 28.3 | ± | 1.1 | 26.9 | ± | 1.1 | 27.7 | ± | 1.0 | 0.302 |
| Aw | 0.829 | ± | 0.011 | 0.833 | ± | 0.013 | 0.824 | ± | 0.009 | 0.832 | ± | 0.010 | 0.599 |
| pH | 5.8 a | ± | 0.1 | 5.5 c | ± | 0.0 | 5.7 b | ± | 0.0 | 5.7 b | ± | 0.0 | 0.000 |
| Microbial counts (log CFU g−1) | |||||||||||||
| Mesophilic | 8.1 | ± | 0.2 | 7.0 | ± | 3.9 | 8.4 | ± | 0.1 | 8.7 | ± | 0.2 | 0.556 |
| Psychrophilic | 8.1 | ± | 0.1 | 8.2 | ± | 0.2 | 8.0 | ± | 0.2 | 8.1 | ± | 0.2 | 0.323 |
| Lactic acid bacteria | 7.9 b | ± | 0.1 | 8.7 a | ± | 0.2 | 8.6 a | ± | 0.4 | 8.6 a | ± | 0.2 | 0.000 |
| Cl. perfringens | <1 | 0.9 | ± | 0.1 | <1 | <1 | 0.261 | ||||||
| S. aureus | <2 | 2.0 | ± | 0.2 | <2 | 1.9 | ± | 0.1 | 0.391 | ||||
| Total coliforms | <1 | <1 | <1 | <1 | - | ||||||||
| E. coli | <1 | <1 | <1 | <1 | - | ||||||||
| Molds and yeasts | 4.7 | ± | 1.1 | 5.2 | ± | 0.7 | 4.5 | ± | 0.2 | 3.9 | ± | 0.4 | 0.069 |
| Negative Control | Positive Control | Low Level | High Level | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrumental color | |||||||||||||
| L* | 45.0 | ± | 2.6 | 44.5 | ± | 1.7 | 44.4 | ± | 1.1 | 42.4 | ± | 2.1 | 0.197 |
| a* | 5.0 b | ± | 1.1 | 7.7 a | ± | 1.5 | 4.4 b | ± | 0.6 | 4.0 b | ± | 0.6 | 0.000 |
| b* | 12.1 a | ± | 1.7 | 9.0 b | ± | 1.7 | 10.3 ab | ± | 1.0 | 9.8 ab | ± | 1.1 | 0.019 |
| Chroma | 13.2 a | ± | 1.4 | 12.0 ab | ± | 1.0 | 11.2 ab | ± | 1.1 | 10.6 b | ± | 1.2 | 0.017 |
| Hue | 67.0 a | ± | 6.2 | 49.0 b | ± | 9.8 | 67.0 a | ± | 2.3 | 68.0 a | ± | 1.7 | 0.000 |
| Oxidative parameters | |||||||||||||
| TBA-RS | 0.5 a | ± | 0.2 | 0.2 b | ± | 0.0 | 0.3 ab | ± | 0.0 | 0.4 ab | ± | 0.1 | 0.013 |
| Carbonyls | 3.1 a | ± | 1.0 | 2.3 ab | ± | 0.4 | 1.8 b | ± | 0.3 | 2.1 b | ± | 0.3 | 0.016 |
| LRI | Negative Control | Positive Control | Low Level | High Level | p-Value | Descriptors a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfur compounds | |||||||||||||||
| Allyl methyl sulfide | 678.61 | 573.5 ab | ± | 370.1 | 879.4 a | ± | 376.4 | 212.9 b | ± | 113.7 | 527.5 ab | ± | 310.3 | 0.030 | Garlic |
| Dimethyldisulfide | 720.40 | 123.9 ab | ± | 71.6 | 163.4 a | ± | 71.2 | 29.1 c | ± | 16.7 | 61.6 bc | ± | 32.1 | 0.005 | Garlic |
| Allylsulfide | 856.13 | 1021.5 | ± | 782.6 | 1318.9 | ± | 556.1 | 470.8 | ± | 167.8 | 1144.0 | ± | 645.2 | 0.160 | Cabbage |
| Methyl 2-propenyl disulfide (methyl allyl disulfide) | 911.37 | 2595.8 ab | ± | 1748.9 | 4226.2 a | ± | 2006.8 | 1130.8 b | ± | 441.1 | 2598.8 ab | ± | 1706.5 | 0.049 | |
| Diallyl disulfide | 1084.33 | 11,691.2 | ± | 5637.8 | 10,850.7 | ± | 4436.5 | 3971.4 | ± | 1505.3 | 8689.8 | ± | 5081.0 | 0.061 | Garlic |
| Allyl disulfide | 1105.74 | 865.5 | ± | 532.1 | 1369.1 | ± | 978.8 | 356.0 | ± | 151.7 | 693.6 | ± | 494.1 | 0.110 | |
| 1,3,5-Trithiane | 1160.24 | 12.1 | ± | 10.0 | 17.6 | ± | 5.1 | 7.2 | ± | 3.5 | 12.1 | ± | 7.1 | 0.165 | |
| Allyl trisulfide | 1315.72 | 98.1 | ± | 51.8 | 98.9 | ± | 38.9 | 28.5 | ± | 13.7 | 65.2 | ± | 46.4 | 0.043 | Pungent sulfur, garlic |
| Diallyl tetrasulfide | 1566.49 | 63.0 | ± | 34.7 | 57.2 | ± | 17.7 | 19.4 | ± | 9.3 | 43.8 | ± | 28.6 | 0.057 | |
| Terpenes | |||||||||||||||
| 1R-α-Pinene * | 933.06 | 804.4 | ± | 1665.1 | 328.6 | ± | 135.1 | 825.8 | ± | 294.0 | 495.0 | ± | 705.3 | 0.790 | Pine, turpentine |
| L-β-Pinene * | 977.07 | 3424.8 ab | ± | 1763.5 | 4360.0 a | ± | 2100.3 | 1230.1 b | ± | 415.2 | 2759.0 ab | ± | 1845.4 | 0.049 | pine, resin, turpentine |
| β-Myrcene | 994.66 | 177.5 | ± | 387.7 | 0.0 | ± | 0.0 | 303.3 | ± | 81.1 | 673.3 | ± | 495.7 | 0.208 | balsamic, must, spice |
| α-Phellandrene | 1004.95 | 1157.7 | ± | 747.6 | 1425.6 | ± | 897.4 | 337.8 | ± | 117.6 | 709.8 | ± | 403.7 | 0.061 | dill |
| α- Terpinene * | 1019.19 | 9485.1 | ± | 4989.5 | 10,656.7 | ± | 5530.4 | 3215.3 | ± | 822.7 | 6977.9 | ± | 458.6 | 0.075 | lemon |
| Limonene * | 1033.14 | 6443.7 | ± | 3893.3 | 6682.2 | ± | 3533.9 | 1994.7 | ± | 548.6 | 4374.3 | ± | 3067.5 | 0.092 | lemon, orange |
| γ- Terpinene | 1064.63 | 626.8 a | ± | 269.9 | 685.1 a | ± | 226.3 | 192.2 b | ± | 111.2 | 492.4 ab | ± | 279.1 | 0.018 | |
| Terpinolene | 1093.76 | 226.9 | ± | 130.1 | 226.1 | ± | 210.9 | 113.1 | ± | 46.2 | 160.8 | ± | 83.2 | 0.479 | Pine, wood, mint |
| β-Terpineol | 1186.98 | 1033.6 a | ± | 529.4 | 968.3 a | ± | 272.2 | 315.9 b | ± | 93.9 | 713.0 ab | ± | 366.0 | 0.022 | must |
| α-Terpineol | 1200.39 | 94.5 a | ± | 53.1 | 83.4 ab | ± | 22.3 | 31.0 b | ± | 9.6 | 63.4 ab | ± | 32.0 | 0.040 | oil, anise, mint |
| δ-Elemene | 1355.56 | 176.0 | ± | 123.3 | 155.2 | ± | 69.0 | 45.3 | ± | 14.8 | 104.1 | ± | 63.1 | 0.072 | wood |
| α-Copaene | 1394.98 | 240.1 | ± | 163.0 | 206.4 | ± | 80.7 | 54.8 | ± | 35.4 | 150.9 | ± | 88.9 | 0.056 | wood, spice |
| L-Caryophyllene | 1429.80 | 46.3 | ± | 36.4 | 38.0 | ± | 16.2 | 11.9 | ± | 4.7 | 27.8 | ± | 17.5 | 0.117 | wood, spice |
| β-Caryophyllene | 1443.58 | 87.3 | ± | 58.9 | 72.2 | ± | 24.7 | 22.3 | ± | 8.8 | 54.4 | ± | 33.2 | 0.064 | wood, spice |
| trans-α-Bergamotene | 1456.64 | 12.6 a | ± | 8.0 | 10.1 ab | ± | 3.7 | 3.3 b | ± | 1.2 | 8.1 ab | ± | 4.4 | 0.052 | wood, warm, tea |
| α-Caryophyllene | 1478.68 | 1112.6 | ± | 834.1 | 955.9 | ± | 418.4 | 300.1 | ± | 107.5 | 688.7 | ± | 421.4 | 0.107 | earth |
| β-Bisabolene | 1530.86 | 7.3 b | ± | 2.7 | 12.7 a | ± | 3.6 | 0 c | 5.0 b | ± | 3.6 | 0.000 | balsamic | ||
| δ-Cadinene | 1548.32 | 23.3 | ± | 22.0 | 19.4 | ± | 13.4 | 3.6 | ± | 5.0 | 17.6 | ± | 10.4 | 0.178 | thyme, medicine, wood |
| Caryophyllene oxide | 1613.59 | 15.2 | ± | 11.9 | 13.1 | ± | 4.3 | 5.0 | ± | 1.8 | 12.5 | ± | 7.3 | 0.189 | herb, sweet, spice |
| Alcohols | |||||||||||||||
| Ethanol | - | 897.7 a | ± | 494.2 | 386.7 ab | ± | 290.3 | 47.7 b | ± | 65.7 | 398.2 ab | ± | 218.2 | 0.005 | sweet |
| 3-Methyl-1-butanol * | 714.29 | 84.3 | ± | 55.0 | 114.6 | ± | 37.7 | 56.1 | ± | 11.1 | 113.3 | ± | 75.5 | 0.253 | whiskey, malt, burnt |
| 2,3-Butanediol * | 767.92 | 3641.1 a | ± | 1791.2 | 988.6 b | ± | 702.6 | 343.8 b | ± | 417.5 | 2649.0 ab | ± | 2121.9 | 0.009 | fruit, onion |
| 3,4-Dimethyl-2-hexanol | 789.78 | 98.8 | ± | 148.0 | 367.4 | ± | 127.0 | 112.9 | ± | 103.8 | 433.0 | ± | 497.8 | 0.155 | |
| 1-Octen-3-ol | 985.90 | 59.3 a | ± | 31.1 | 27.5 ab | ± | 8.9 | 13.8 b | ± | 2.6 | 31.6 ab | ± | 27.7 | 0.027 | Herb, earth, molds |
| Benzyl alcohol | 1040.49 | 299.5 | ± | 108.0 | 276.4 | ± | 78.3 | 118.8 | ± | 53.9 | 239.0 | ± | 145.3 | 0.057 | sweet, flower |
| Phenylethyl alcohol * | 1120.61 | 324.4 a | ± | 203.5 | 136.2 ab | ± | 64.1 | 81.8 b | ± | 26.3 | 253.1 ab | ± | 110.4 | 0.024 | honey, spice, rose, lilac |
| d-Mannose | 1206.52 | 80.6 a | ± | 47.6 | 67.5 ab | ± | 12.7 | 20.9 b | ± | 7.0 | 41.6 ab | ± | 25.9 | 0.018 | |
| Aldehydes | |||||||||||||||
| 3-Methylbutanal * | 639.07 | 229.8 | ± | 162.3 | 39.3 | ± | 38.9 | 62.5 | ± | 38.7 | 149.4 | ± | 150.0 | 0.066 | cocoa, almond, malt |
| Benzaldehyde * | 962.63 | 184.4 | ± | 151.1 | 84.0 | ± | 54.5 | 86.2 | ± | 43.3 | 170.3 | ± | 228.5 | 0.555 | almond, burnt sugar |
| Benzeneacetaldehyde | 1048.85 | 914.7 | ± | 698.7 | 530.6 | ± | 118.9 | 311.8 | ± | 152.7 | 748.4 | ± | 363.0 | 0.142 | Benzeneacetaldehyde |
| Nonanal * | 1111.01 | 77.2 | ± | 33.5 | 261.1 | ± | 328.6 | 40.9 | ± | 18.0 | 72.8 | ± | 39.5 | 0.187 | fat, citrus, green |
| Ketones | |||||||||||||||
| 3-Hydroxybutanone | 686.92 | 848.9 | ± | 568.8 | 662.1 | ± | 210.3 | 258.3 | ± | 145.8 | 659.1 | ± | 407.9 | 0.124 | |
| 3-Octanone * | 1028.26 | 1570.6 | ± | 1132.3 | 1382.1 | ± | 686.3 | 345.4 | ± | 205.5 | 890.2 | ± | 604.5 | 0.074 | herb, butter, resin |
| 1-Phenylethan-1-one | 1073.10 | 248.1 | ± | 121.6 | 243.3 | ± | 75.8 | 100.8 | ± | 41.1 | 167.4 | ± | 89.9 | 0.050 | |
| Lineal hydrocarbons | |||||||||||||||
| 2-Methylheptane | 743.57 | 31.1 | ± | 43.0 | 36.3 | ± | 21.9 | 107.6 | ± | 63.7 | 161.1 | ± | 185.6 | 0.172 | |
| 3-Ethylhexane | 752.17 | 33.4 | ± | 60.9 | 34.4 | ± | 31.0 | 136.8 | ± | 90.1 | 205.3 | ± | 242.4 | 0.159 | |
| 2,2,4,6,6-Pentamethylheptane | 991.59 | 1117.6 | ± | 954.4 | 1085.8 | ± | 523.2 | 1896.0 | ± | 1141.7 | 2561.3 | ± | 2259.1 | 0.306 | |
| Aromatic hydrocarbons | |||||||||||||||
| O-Methyleugenol | 1421.26 | 332.3 a | ± | 205.7 | 289.2 ab | ± | 82.2 | 86.5 b | ± | 50.3 | 226.3 ab | ± | 119.2 | 0.040 | clove, honey |
| Myristicin | 1544.97 | 250.8 | ± | 154.9 | 214.6 | ± | 62.0 | 77.1 | ± | 24.2 | 170.0 | ± | 93.6 | 0.060 | spice, warm, balsamic |
| Elemicin | 1576.72 | 54.8 a | ± | 34.9 | 46.9 ab | ± | 13.1 | 16.0 b | ± | 5.0 | 36.9 ab | ± | 19.8 | 0.055 | spice, flower |
| LRI | Ingredient Red Grape Pomace | Descriptors a | ||||
|---|---|---|---|---|---|---|
| Alcohols | Ethanol * | - | 1014.4 | ± | 416.6 | sweet |
| 3-Methyl-1-butanol * | 715.16 | 2173.3 | ± | 1185.8 | whiskey, malt, burnt | |
| 2,3-Butanediol * | 810.65 | 462.5 | ± | 409.3 | fruit, onion | |
| 1-Hexanol * | 873.64 | 524.3 | ± | 30.5 | resin, flower, green | |
| Benzyl Alcohol * | 1034.79 | 328.3 | ± | 64.6 | sweet, flower | |
| Phenylethyl alcohol * | 1113.88 | 3043.5 | ± | 100.3 | honey, spice, rose, lilac | |
| Aldehydes | Hexanal | 790.34 | 316.0 | ± | 7.5 | grass, tallow, fat |
| Benzaldehyde * | 958.98 | 70.7 | ± | 26.2 | almond, burnt sugar | |
| Phenyl acetaldehyde | 1043.62 | 48.7 | ± | 4.2 | Hawthorne, honey, sweet | |
| Nonanal * | 1103.98 | 76.8 | ± | 39.2 | fat, citrus, green | |
| Esters | 3-Methylbutyl acetate * | 880.34 | 122.6 | ± | 16.7 | banana |
| 2-Methylbutyl acetate | 882.62 | 13.5 | ± | 23.4 | fruit | |
| Ethyl hexanoate * | 999.81 | 552.6 | ± | 47.7 | Pineapple | |
| Hexyl acetate * | 1015.21 | 24.9 | ± | 2.5 | Fruit, herb | |
| Ethyl octanoate * | 1198.16 | 3686.7 | ± | 428.6 | fruit, fat | |
| 2-Phenylethyl acetate | 1260.84 | 23.4 | ± | 3.3 | rose, honey, tobacco | |
| Ethyl decanoate * | 1399.01 | 5706.5 | ± | 629.4 | grape | |
| Dibutyl hexanedioate | 1772.74 | 43.9 | ± | 7.6 | ||
| Hydrocarbons | 2-Methyl heptane | 743.57 | 875.2 | ± | 902.3 | |
| 3-Ethyl hexane | 751.71 | 424.6 | ± | 85.9 | ||
| 3-Ethyl octane | 972.65 | 61.1 | ± | 26.1 | ||
| 2,2,4,6,6-Pentamethyl heptane | 989.05 | 7740.3 | ± | 2245.9 | ||
| 2,2,4,4-Tetramethyl octane | 1025.93 | 595.0 | ± | 178.8 | ||
| Dodecane, 2,6,10-trimethyl | 1049.14 | 75.3 | ± | 23.1 | ||
| Others | Acetic acid | 692.27 | 2343.8 | ± | 610.4 | sour |
| Methoxy-phenyl-oxime | 928.85 | 1094.5 | ± | 360.5 | ||
| Eucalyptol | 1029.41 | 15.8 | ± | 27.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Arrigo, M.; Petrón, M.J.; Delgado-Adámez, J.; García-Parra, J.J.; Martín-Mateos, M.J.; Ramírez-Bernabé, M.R. Dry-Cured Sausages “Salchichón” Manufactured with a Valorized Ingredient from Red Grape Pomace (Var. Tempranillo). Foods 2024, 13, 3133. https://doi.org/10.3390/foods13193133
D’Arrigo M, Petrón MJ, Delgado-Adámez J, García-Parra JJ, Martín-Mateos MJ, Ramírez-Bernabé MR. Dry-Cured Sausages “Salchichón” Manufactured with a Valorized Ingredient from Red Grape Pomace (Var. Tempranillo). Foods. 2024; 13(19):3133. https://doi.org/10.3390/foods13193133
Chicago/Turabian StyleD’Arrigo, Matilde, María Jesús Petrón, Jonathan Delgado-Adámez, Jesús Javier García-Parra, María Jesús Martín-Mateos, and María Rosario Ramírez-Bernabé. 2024. "Dry-Cured Sausages “Salchichón” Manufactured with a Valorized Ingredient from Red Grape Pomace (Var. Tempranillo)" Foods 13, no. 19: 3133. https://doi.org/10.3390/foods13193133








