Assessment of the Nutritional Benefits and Aflatoxin B1 Adsorption Properties of Blackberry Seed Cold-Pressed Oil By-Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Chemical Characterization of BBSOC
Minerals Content
2.3. Phenolic Compounds
HPLC-DAD
2.4. Determination of Antioxidant Activity
2.4.1. DPPH Radical Scavenging Assay
2.4.2. Reducing Power
2.4.3. ABTS Radical Scavenging Assay
2.5. Biosorbents Characterization of BBSOC
2.5.1. SEM and FTIR Analysis
2.5.2. Boehm Titration and pH of Zero Charge
2.6. AFB1 Adsorption Experiments
AFB1 HPLC Determination
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical and Mineral Components
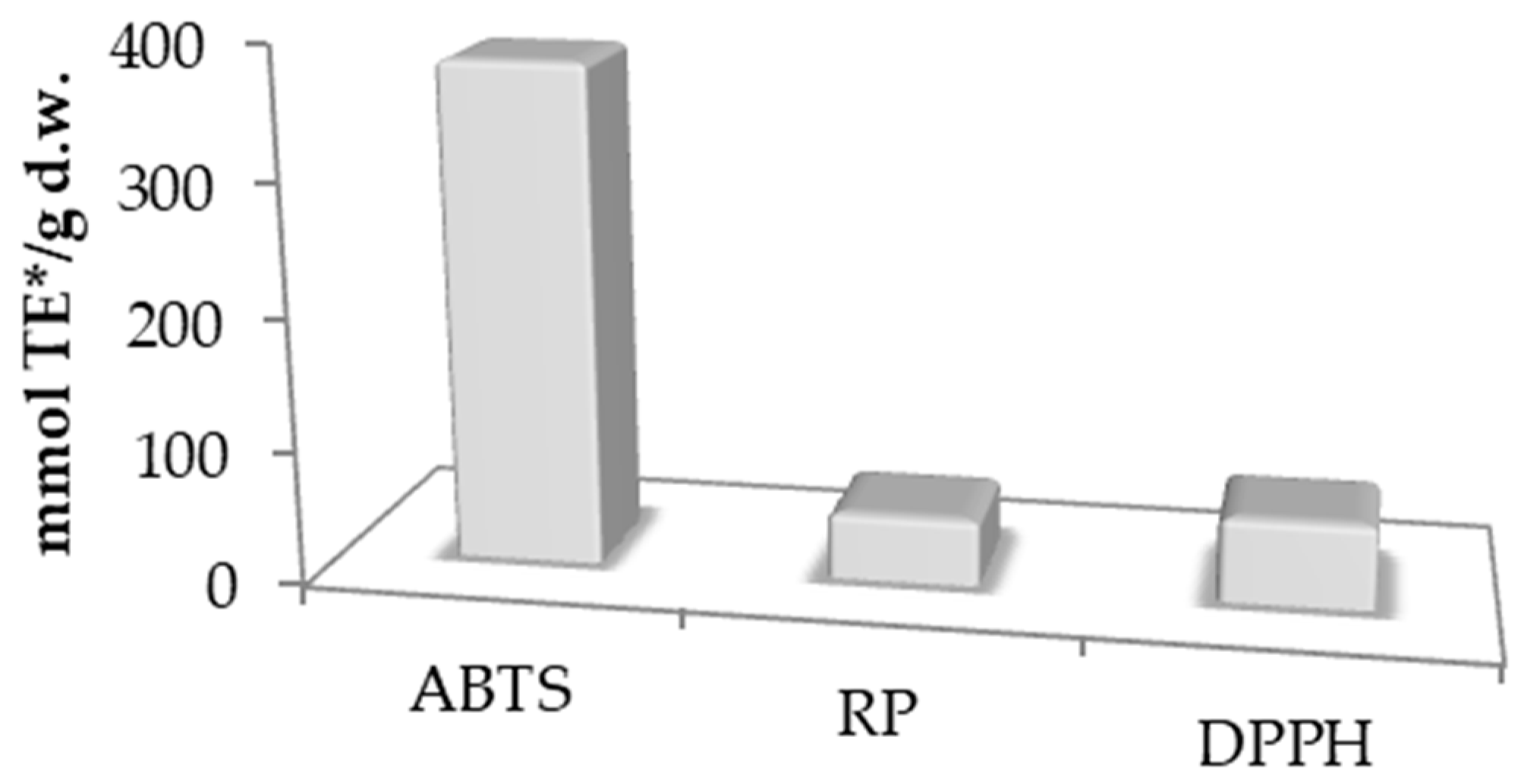
3.2. Polyphenol Compounds and Antioxidant Activity
3.3. Characterization of BBSOC as a Biosorbent for AFB1 Removal
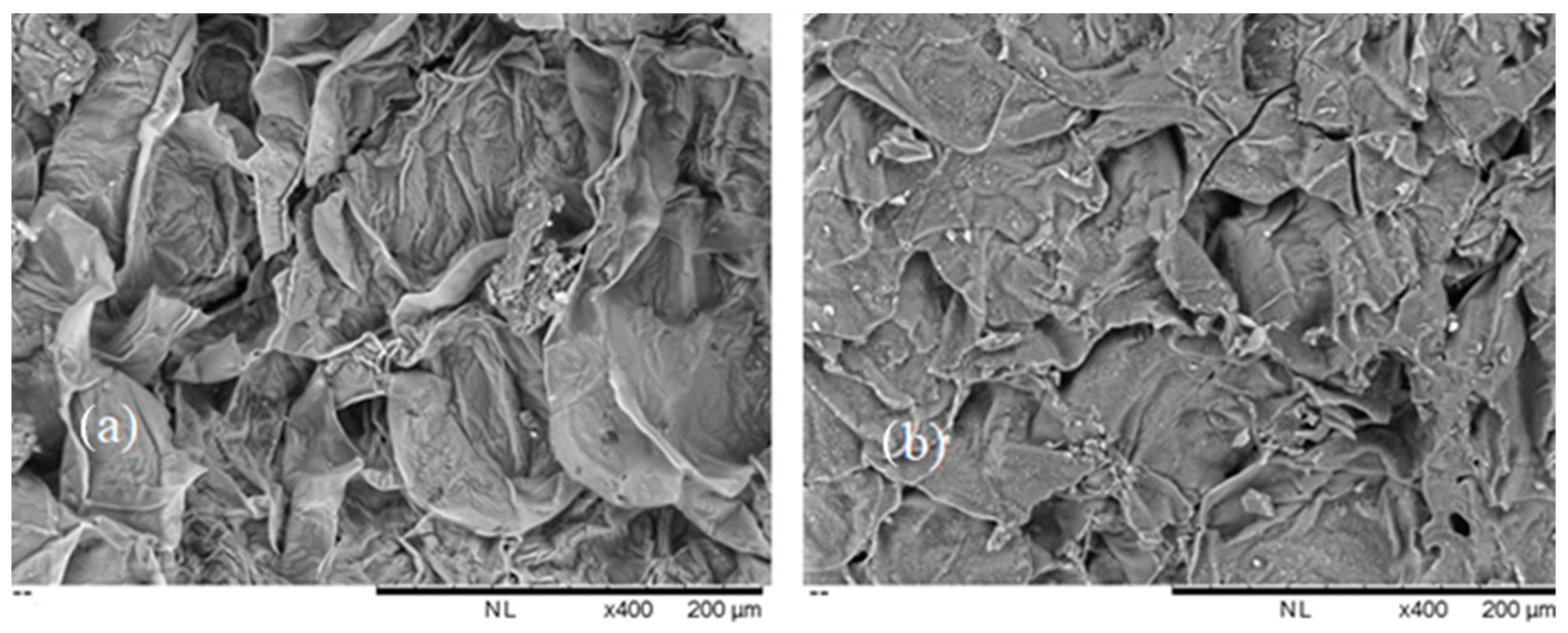
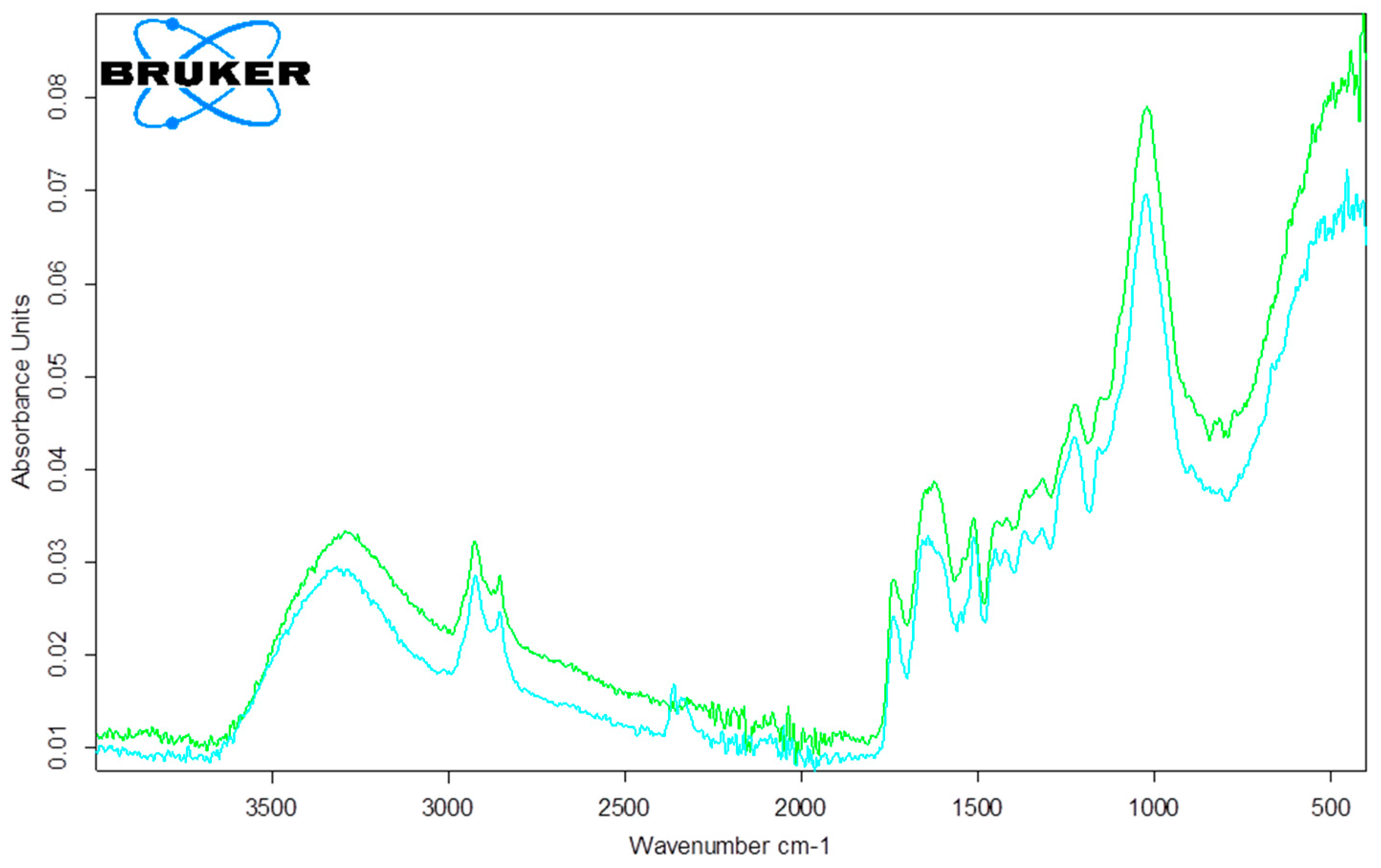
3.3.1. SEM and FTIR Characterization
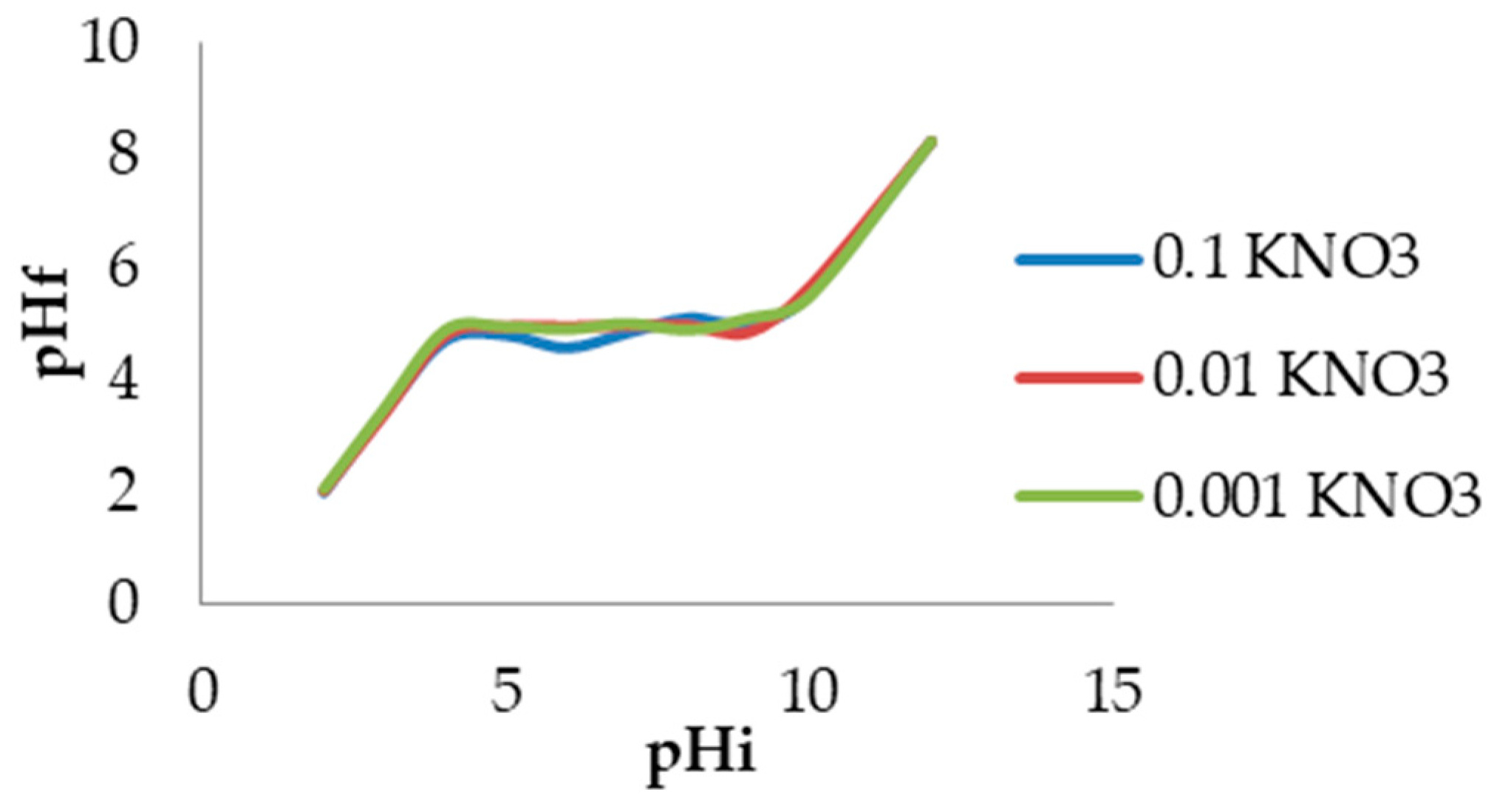
3.3.2. Boehm Titration and Zero Charge pH (pHpzc)
3.3.3. In Vitro Adsorption of AFB1 Using BBSOC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.; de Jiménez, M.M. Designer oil crops. In Breeding Oilseed Crops for Sustainable Production; Academic Press: San Diego, CA, USA, 2016; pp. 361–376. [Google Scholar]
- Abedini, A.; Alizadeh, A.M.; Mahdavi, A.; Golzan, S.A.; Salimi, M.; Tajdar-Oranj, B.; Hosseini, H. Oilseed cakes in the food industry; a review on applications, challenges, and future perspectives. Curr. Nutr. Food Sci. 2022, 18, 345–362. [Google Scholar] [CrossRef]
- Paula, E.M.; da Silva, L.G.; Brandao, V.L.N.; Dai, X.; Faciola, A.P. Feeding canola, camelina, and carinata meals to ruminants. Animals 2019, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Ermosh, L.G.; Prisuhina, N.V.; Koch, D.A.; Eremina, E.V. The use of oilseed cake for supplementation of bakery products. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Blicharz-Kania, A.; Starek-Wójcicka, A.; Andrejko, D. Assessment of the possibility of using poppy seed cake for the production of oat cookies. Appl. Sci. 2022, 12, 9966. [Google Scholar] [CrossRef]
- Purić, M.; Rabrenović, B.; Rac, V.; Pezo, L.; Tomašević, I.; Demin, M. Application of defatted apple seed cakes as a by-product for the enrichment of wheat bread. LWT 2020, 130, 109391. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G. Walnut oil and oilcake affect selected the physicochemical and antioxidant properties of wheat bread enriched with them. J. Food Process. Preserv. 2020, 44, e14573. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, F.; Wu, W.; Lyu, L.; Li, W. Proteins from Blackberry Seeds: Extraction, Osborne Isolate, Characteristics, Functional Properties, and Bioactivities. Int. J. Mol. Sci. 2023, 24, 15371. [Google Scholar] [CrossRef]
- Helbig, D.; Bohmb, V.; Wagner, A.; Schubert, R.; Jahreis, G. Berry seed press residues and their valuable ingredients with special regard to black currant seed press residues. Food Chem. 2008, 111, 1043–1049. [Google Scholar] [CrossRef]
- Kosmala, M.; Jurgonski, A.; Juskiewicz, J.; Karlinska, E.; Macierzynski, J.; Rój, E.; Zdunczyk, Z. Chemical composition of blackberry press cake, polyphenolic extract, and defatted seeds, and their effects on cecal fermentation, bacterial metabolites, and blood lipid profile in rats. J. Agric. Food Chem. 2017, 65, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Kainth, S.; Sharma, P.; Pandey, O.P. Green sorbents from agricultural wastes: A review of sustainable adsorption materials. Appl. Surf. Sci. Adv. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.X.; Marchal, J.L.M.; Van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Zoghi, A.; Jazayeri, S.; Da Cruz, A.G. Decontamination of aflatoxins with a focus on aflatoxin B1 by probiotic bacteria and yeasts: A review. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 424–435. [Google Scholar] [CrossRef]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative efficacy of agricultural by-products in sequestering mycotoxins. J. Sci. Food Agric. 2019, 99, 1623–1634. [Google Scholar] [CrossRef]
- Bočarov-Stančić, A.; Lopičić, Z.; Krstović, S.; Miljanić, J.; Milojković, J.; Maslovarić, M.; Bodroza-Solarov, M. Agents of different origins for reduction of mycotoxins’ level in feed. Ann. Anim. Sci. 2024, 24, 707–729. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of multi-mycotoxin adsorption efficacy of grape pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Lin, L.J.; Shih, H.D.; Shy, Y.M.; Chang, S.C.; Lee, T.T. The potential utilization of high-fiber agricultural by-products as monogastric animal feed and feed additives: A review. Animals 2021, 11, 2098. [Google Scholar] [CrossRef]
- Gutzwiller, A.; Czegledi, L.; Stoll, P.; Bruckner, L. Effects of Fusarium toxins on growth, humoral immune response and internal organs in weaner pigs, and the efficacy of apple pomace as an antidote. J. Anim. Physiol. Anim. Nutr. 2007, 91, 432–438. [Google Scholar] [CrossRef]
- Wang, C.C.; Lin, L.J.; Chao, Y.P.; Chiang, C.J.; Lee, M.T.; Chang, S.C.; Yu, B.; Lee, T.T. Anti-oxidant molecular targets of wheat bran fermented by white rot fungi and its potential modulation of antioxidative status in broiler chickens. Br. Poult. Sci. 2017, 58, 262–271. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin B1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon 2019, 162, 24–31. [Google Scholar] [CrossRef]
- Popescu, R.G.; Bulgaru, C.; Untea, A.; Vlassa, M.; Filip, M.; Hermenean, A.; Marin, D.; Țăranu, I.; Georgescu, S.E.; Dinischiotu, A. The Effectiveness of Dietary Byproduct Antioxidants on Induced CYP Genes Expression and Histological Alteration in Piglets Liver and Kidney Fed with Aflatoxin B1 and Ochratoxin A. Toxins 2021, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ngabura, M.; Choong, T.S.; Masood, H.; Chuah, L.A. Biosorption and desorption of nickel on oil cake: Batch and column studies. Bioresour. Technol. 2012, 103, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaprakash, M.; Sivakumar, V. Batch and fixed-bed column studies for biosorption of Zn (II) ions onto pongamia oil cake (Pongamia pinnata) from biodiesel oil extraction. J. Environ. Manag. 2015, 164, 161–170. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Khan, M.A. Biosorption of bivalent metal ions from aqueous solution by an agricultural waste: Kinetics, thermodynamics and environmental effects. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 121–128. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- SRPS EN ISO 6869; Animal Feeding Stuffs-Determination of the Contents of Calcium, Copper, Iron, Magnesium, Manganese, Sodium and Zinc-Method Using Atomic Absorption Spectrometry. Institute for Standardization of Serbia: Belgrade, Serbia, 2008.
- Tumbas Šaponjac, V.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.; Čanadanović-Brunet, J.; Stajčić, S.; Krunić, M. Anthocyanin profiles and biological properties of cranberry (Rubus spp.) press residues. J. Sci. Food Agric. 2014, 94, 2393–2400. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Ćetković, G.; Čanadanović-Brunet, J.; Pajin, B.; Đilas, S.; Petrović, J.; Lončarević, I.; Stajčić, S.; Vulić, J. Sour cherry pomace extract encapsulated in whey and soy proteins: Incorporation in cookies. Food Chem. 2016, 207, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Krulj, J.; Pezo, L.; Kojić, J.; Solarov, M.B.; Teslić, N. Quality evaluation of cold-pressed oils and semi-defatted cake flours obtained on semi-industrial scale. J. Food Nutr. Res. 2021, 60, 217–228. [Google Scholar]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. OJ L 304/18. 22 November 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 29 August 2024).
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Gowd, V.; Xie, L.; Zheng, X.; Chen, W. Dietary fibers as emerging nutritional factors against diabetes: Focus on the involvement of gut microbiota. Crit. Rev. Biotechnol. 2019, 39, 524–540. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th Revised Edition; Subcommittee on Dairy Cattle Nutrition, Committee on Animal Nutrition, Board on Agriculture and Natural Resources, National Research Council, National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Jung, H.G.; Allen, M.S. Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J. Anim. Sci. 1995, 73, 2774–2790. [Google Scholar] [CrossRef] [PubMed]
- Krstić, Đ.; Vukojević, V.; Mutić, J.; Fotirić Akšić, M.; Ličina, V.; Milojković-Opsenica, D.; Trifković, J. Distribution of elements in seeds of some wild and cultivated fruits. Nutrition and authenticity aspects. J. Sci. Food Agric. 2019, 99, 546–554. [Google Scholar] [CrossRef]
- Galloway, R. Community Health and Nutrition Programs; A custom publication of the Disease Control Priorities Project; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006; Volume 37. [Google Scholar]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Grzelak-Błaszczyk, K.; Karlińska, E.; Grzęda, K.; Rój, E.; Kołodziejczyk, K. Defatted strawberry seeds as a source of phenolics, dietary fiber and minerals. LWT 2017, 84, 18–22. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Choe, U.; Li, Y.; Yu, L.; Gao, B.; Wang, T.T.; Sun, J.; Chen, P.; Yu, L. Chemical composition of cold-pressed blackberry seed flour extract and its potential health-beneficial properties. Food Sci. Nutr. 2020, 8, 1215–1225. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blackberry products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Grilli, M.; Pittaluga, A.M.; Boggia, R. Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review. Eur. J. Med. Chem. 2019, 183, 111724. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Ayoub, M. Phenolic Compounds and Antioxidant Activity of Blackberry, Black Raspberry and Blueberry Seed Meals. Ph.D. Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2015. [Google Scholar]
- Hassanien, M.F.R. (Ed.) Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Park, M.; Cho, H.; Jung, H.; Lee, H.; Hwang, K.T. Antioxidant and anti- inflammatory activities of tannin fraction of the extract from black raspberry seeds compared to grape seeds. J. Food Biochem. 2014, 38, 259–270. [Google Scholar] [CrossRef]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.I.G.M.; Diniz, C.; Marques, M.P.M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Gupta, B.S.; Jelle, B.P.; Gao, T. Wood facade materials ageing analysis by FTIR spectroscopy. Proc. Inst. Civ. Eng.-Constr. Mater. 2015, 168, 219–231. [Google Scholar] [CrossRef]
- Kragović, M.M.; Daković, A.S.; Milićević, S.Z.; Sekulić, Ž.T.; Milonjić, S.K. Influence of organic cations sorption on the point of zero charge of natural zeolite. Hem. Ind. 2009, 63, 325–330. [Google Scholar] [CrossRef]
- Ramales-Valderrama, R.A.; Vázquez-Durán, A.; Méndez-Albores, A. Biosorption of B-aflatoxins using biomasses obtained from formosa firethorn [Pyracantha koidzumii (Hayata) Rehder]. Toxins 2016, 8, 218. [Google Scholar] [CrossRef]
- Vázquez-Durán, A.; Nava-Ramírez, M.D.J.; Hernández-Patlán, D.; Solís-Cruz, B.; Hernández-Gómez, V.; Téllez-Isaías, G.; Méndez-Albores, A. Potential of kale and lettuce residues as natural adsorbents of the carcinogen aflatoxin B1 in a dynamic gastrointestinal tract-simulated model. Toxins 2021, 13, 771. [Google Scholar] [CrossRef] [PubMed]
- Munagapati, V.S.; Yarramuthi, V.; Nadavala, S.K.; Alla, S.R.; Abburi, K. Biosorption of Cu (II), Cd (II) and Pb (II) by Acacia leucocephala bark powder: Kinetics, equilibrium and thermodynamics. J. Chem. Eng. 2010, 157, 357–365. [Google Scholar] [CrossRef]
- Aguilar-Zuniga, K.; Laurie, V.F.; Moore-Carrasco, R.; Ortiz-Villeda, B.; Carrasco-Sánchez, V. Agro-industrial Waste Products as Mycotoxin Biosorbents: A Review of In Vitro and In Vivo Studies. Food Rev. Int. 2021, 39, 1–17. [Google Scholar] [CrossRef]
| Nutrients (g/100g) | Content | **** Daily Reference Intake, g |
| Moisture | 3.98 ± 0.01 | - |
| Ash | 1.85 ± 0.08 | - |
| Proteins | 13.20 ± 1.02 | 50 |
| Fats | 6.15 ± 0.08 | 70 |
| Carbohydrates | 7.36 ± 0.09 | 260 |
| Dietary fiber | 62.09 ± 0.95 | 30 |
| ADF * | 50.89 ± 0.25 | |
| ADL ** | 24.75 ± 0.28 | |
| NDF *** | 55.86 ± 0.27 | |
| Cellulose | 31.11 ± 0.01 | |
| Hemicellulose | 4.97 ± 0.01 | |
| Minerals (mg/kg) | Content | **** Daily Reference Intake, mg |
| Potassium (K) | 3087.61 ± 15.99 | 2000 |
| Calcium (Ca) | 1568.41 ± 54.52 | 800 |
| Magnesium (Mg) | 1281.40 ± 118.02 | 375 |
| Iron (Fe) | 123.48 ± 8.12 | 14 |
| Zinc (Zn) | 14.51 ± 2.11 | 10 |
| Phenolic Compound | µg/g |
|---|---|
| Gallic acid | 37.2 ± 4.3 |
| Protocatechuic acid | 49.8 ± 5.2 |
| p-coumaric acid | 4.04 ± 0.7 |
| Ellagic acid | 827.2 ± 21.2 |
| Quercetin | 20.2 ± 4.8 |
| Qu-3 rhamnosid | 26.2 ± 5.1 |
| Biosorbent | Functional Groups | ||||
|---|---|---|---|---|---|
| Acidic Groups (mmol/g) | Base Groups (mmol/g) | ||||
| Phenolic | Lactone | Carboxylic | Total | ||
| BBSOC | 0.10 ± 0.02 | 0.85 ± 0.1 | 0.63 ± 0.07 | 1.58 ± 0.12 | 1.18 ± 0.11 |
| AFB1 | Adsorption Index % | |
|---|---|---|
| mg/mL | pH 3 | pH 7 |
| 2 | 3.54 ± 0.34 a | 4.23 ± 0,.21 a |
| 6 | 7.50 ± 0.82 b | 11.62 ± 0.91 b |
| 12 | 24.36 ± 2.67 c | 29.35 ± 2.31 c |
| 18 | 48.35 ± 3.85 d | 50.56 ± 4.69 d |
| 25 | 74.42 ± 5.36 e | 79.24 ± 5.28 e |
| 30 | 85.36 ± 7.11 f | 87.01 ± 8.15 f |
| 35 | 84.91 ± 8.02 f | 86.87 ± 8.94 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljanić, J.; Krstović, S.; Perović, L.; Kojić, J.; Travičić, V.; Bajac, B. Assessment of the Nutritional Benefits and Aflatoxin B1 Adsorption Properties of Blackberry Seed Cold-Pressed Oil By-Product. Foods 2024, 13, 3140. https://doi.org/10.3390/foods13193140
Miljanić J, Krstović S, Perović L, Kojić J, Travičić V, Bajac B. Assessment of the Nutritional Benefits and Aflatoxin B1 Adsorption Properties of Blackberry Seed Cold-Pressed Oil By-Product. Foods. 2024; 13(19):3140. https://doi.org/10.3390/foods13193140
Chicago/Turabian StyleMiljanić, Jelena, Saša Krstović, Lidija Perović, Jovana Kojić, Vanja Travičić, and Branimir Bajac. 2024. "Assessment of the Nutritional Benefits and Aflatoxin B1 Adsorption Properties of Blackberry Seed Cold-Pressed Oil By-Product" Foods 13, no. 19: 3140. https://doi.org/10.3390/foods13193140









