Molecular Interaction and Solubilization Efficiency of Neohesperidin in Ternary Systems with Hydroxypropyl-β-cyclodextrin and Meglumine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Co-Solvent Screening
2.3. Determination of Co-Solvent Concentration
2.4. Phase Solubility Study
2.5. Preparation of NH Inclusion Complex
2.6. Saturation Solubility Study
2.7. Characterization of Ternary Inclusion Complex
2.7.1. Fourier Transform Infrared Spectrometer (FTIR)
2.7.2. Powder X-ray Diffraction (PXRD)
2.7.3. Thermal Analyses
2.7.4. SEM
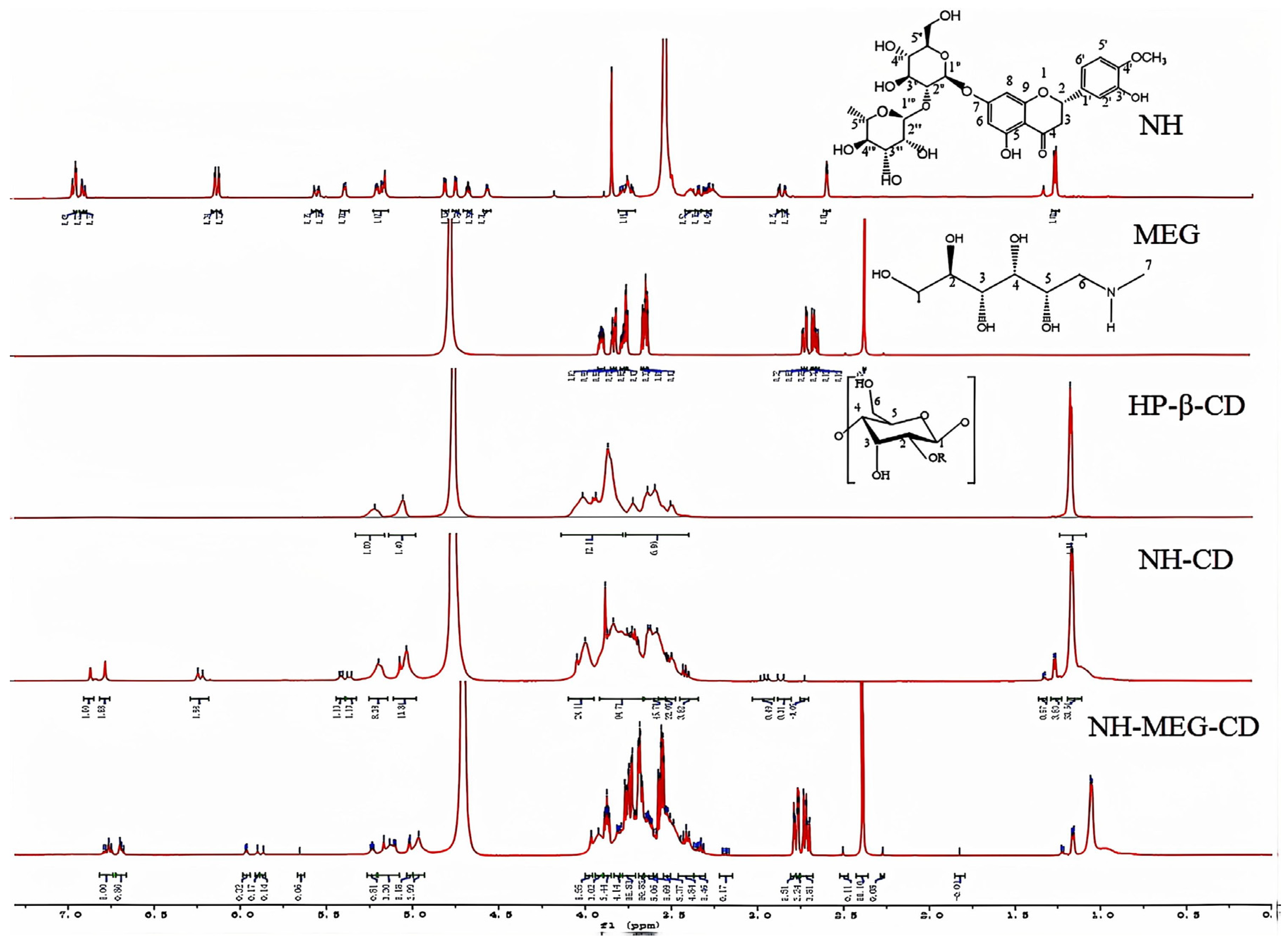
2.7.5. 1H NMR
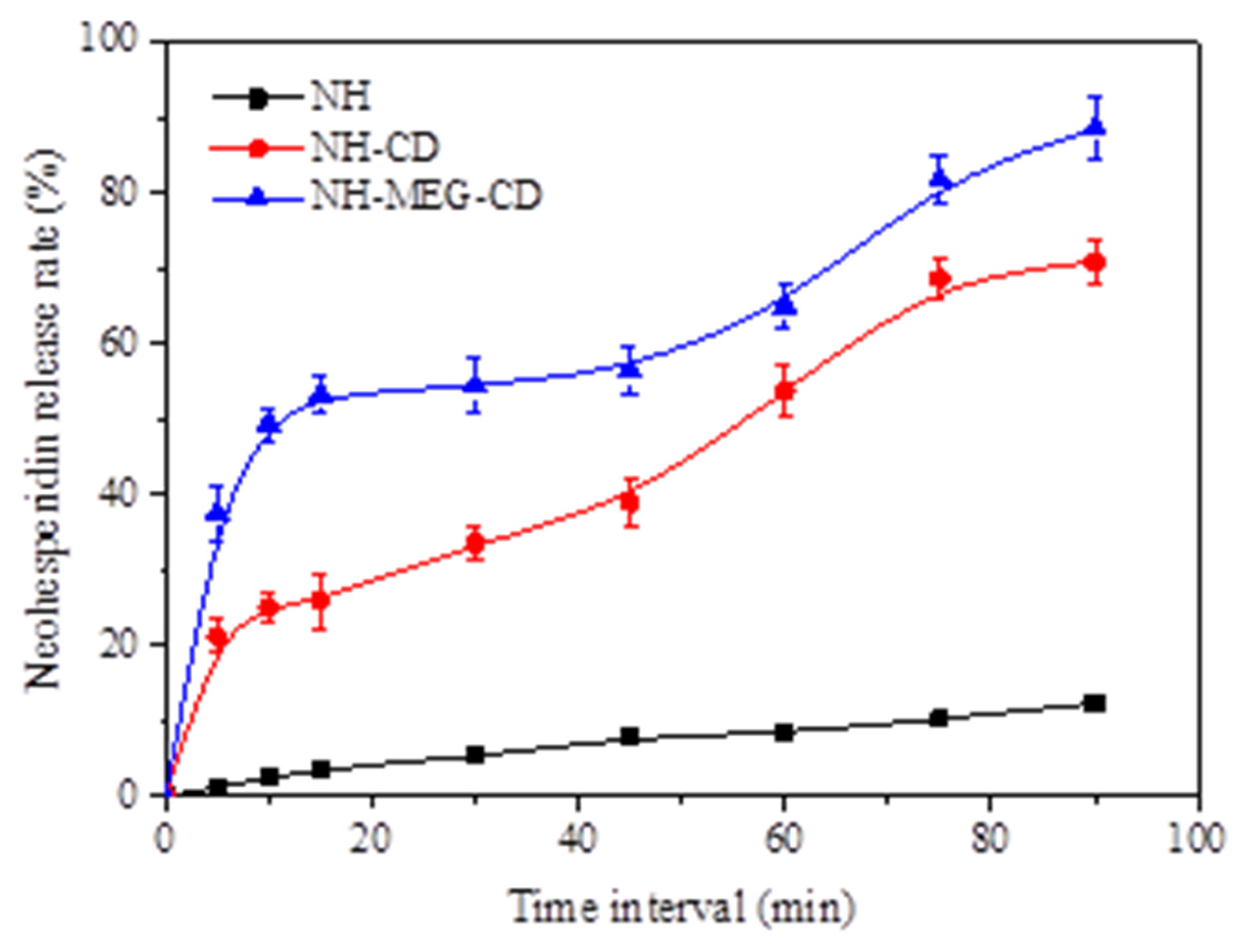
2.7.6. In Vitro Dissolution Test
2.7.7. Molecular Model Construction
2.8. Statistical Analysis
3. Results
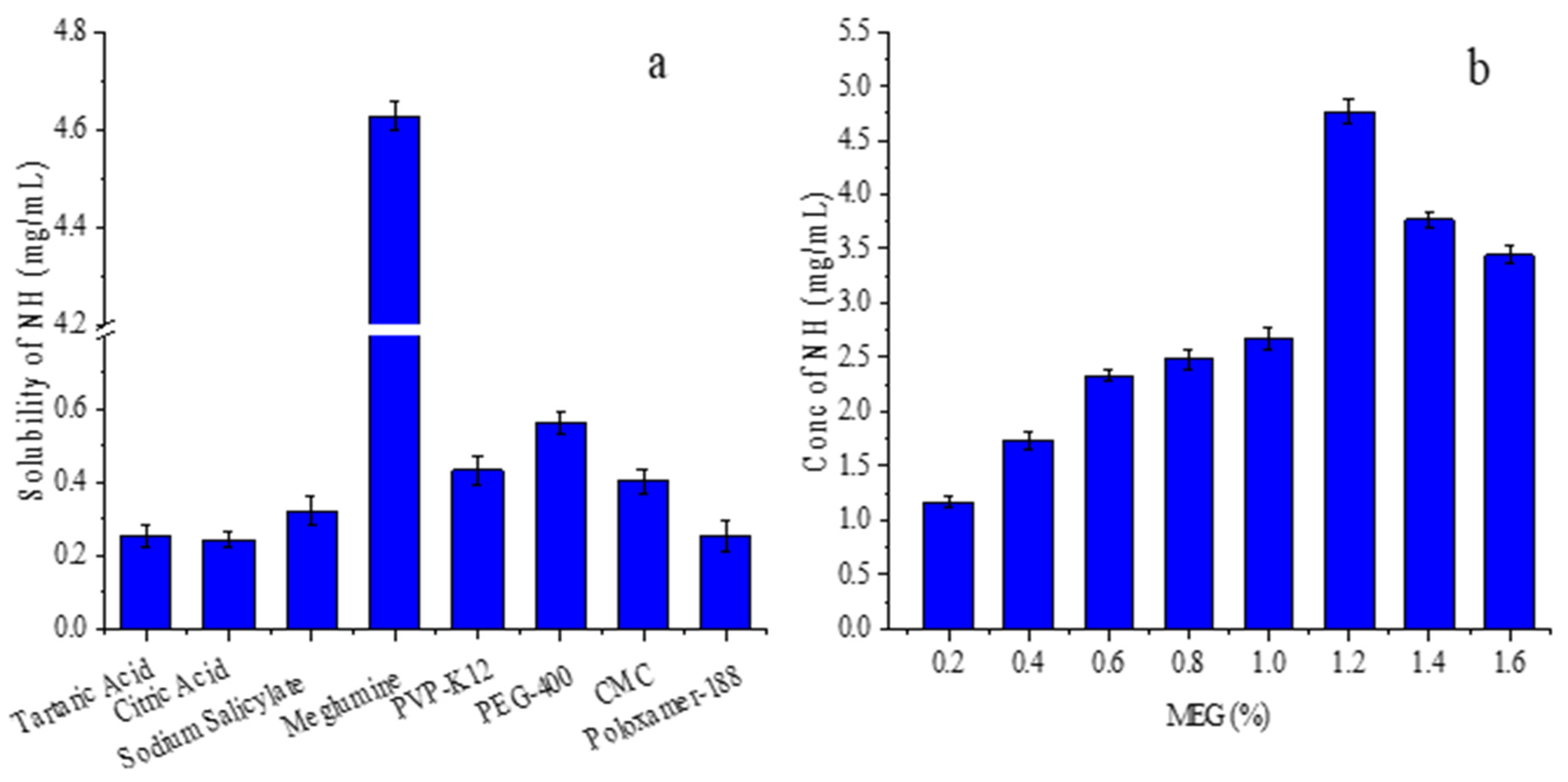
3.1. Selection of Co-Solvent
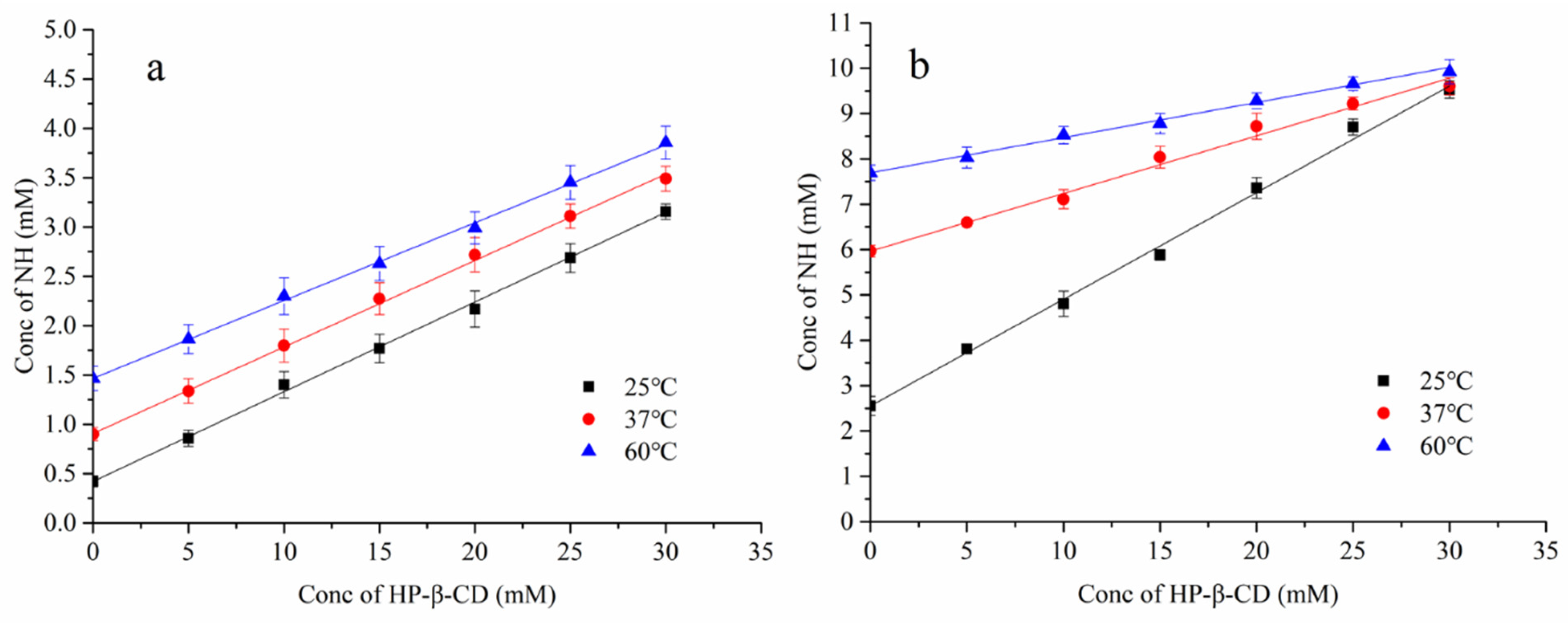
3.2. Study on Phase Solubility
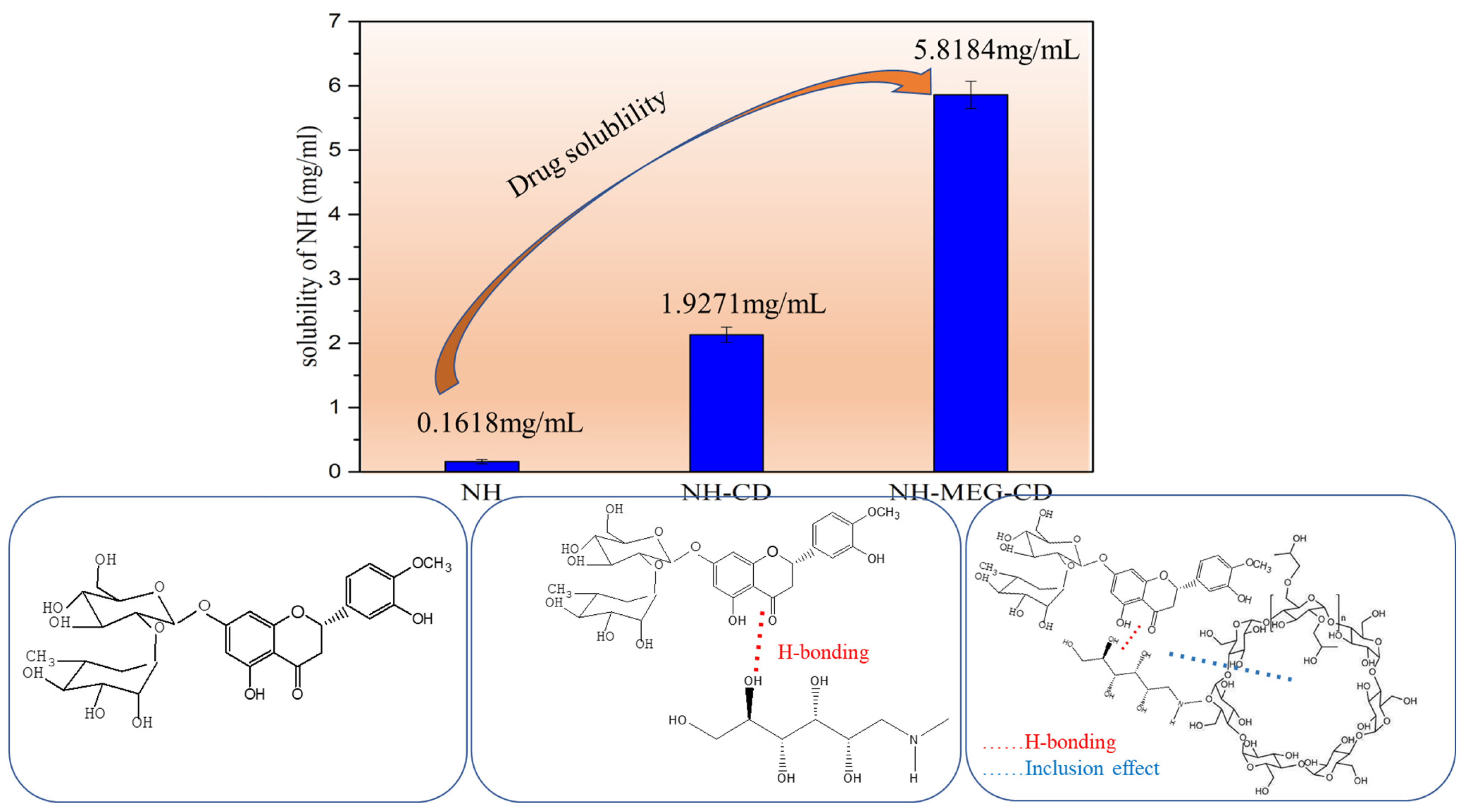
3.3. Saturation Solubility
3.4. Physicochemical Characterization of Inclusion Complex
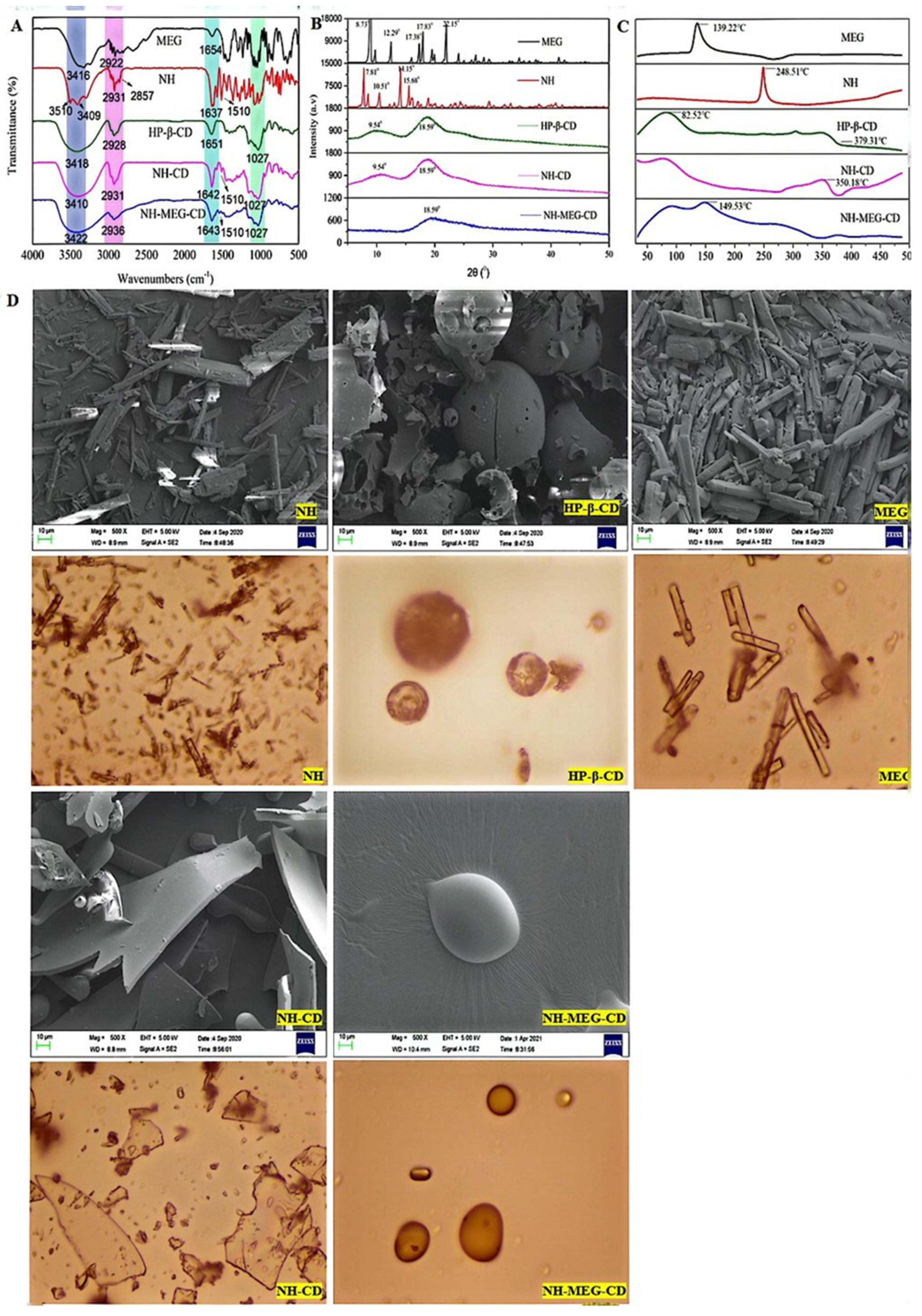
3.4.1. FTIR Analysis
3.4.2. Powder XRD
3.4.3. DSC Analysis
3.4.4. SEM Analysis
3.4.5. 1H NMR Measurements
3.4.6. In Vitro Dissolution Analysis
3.4.7. Computational Simulation of NH Inclusion Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, N.; Wang, C.; Zhu, S. Interaction between pH-shifted ovalbumin and insoluble neohesperidin: Experimental and binding mechanism studies. Food Chem. 2022, 390, 133104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, M.; Li, Y.; Gong, W.; Wang, Y.; Shan, L.; Shao, S.; Gao, C. Formulation and characterization of a ternary inclusion complex containing hydroxypropyl-β-cyclodextrin and meglumine for solubility enhancement of poorly water-soluble ST-246, an anti-smallpox drug. Curr. Drug Deliv. 2017, 14, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Jamil, S.; Ansari, M.J.; Al-Shdefat, R.; Ali, B.E.; Ganaie, M.A.; Abdel-Kader, M.S.; Shakeel, F. Water soluble binary and ternary complexes of diosmin with β-cyclodextrin: Spectroscopic characterization, release studies and anti-oxidant activity. J. Mol. Liq. 2014, 199, 35–41. [Google Scholar] [CrossRef]
- Taupitz, T.; Dressman, J.B.; Klein, S. New formulation approaches to improve solubility and drug release from fixed dose combinations: Case examples pioglitazone/glimepiride and ezetimibe/simvastatin. Eur. J. Pharm. Biopharm. 2013, 84, 208–218. [Google Scholar] [CrossRef]
- Taupitz, T.; Dressman, J.B.; Buchanan, C.M.; Klein, S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example: Itraconazole. Eur. J. Pharm. Biopharm. 2013, 83, 378–387. [Google Scholar] [CrossRef]
- Garnero, C.; Longhi, M. Study of ascorbic acid interaction with hydroxypropyl-β-cyclodextrin and triethanolamine, separately and in combination. J. Pharmaceut. Biomed. Anal. 2007, 45, 536–545. [Google Scholar] [CrossRef]
- Pahuja, P.; Kashyap, H.; Pawar, P. Design and evaluation of HP-β-CD based voriconazole formulations for ocular drug delivery. Curr. Drug Deliv. 2014, 11, 223–232. [Google Scholar] [CrossRef]
- Barillaro, V.; Dive, G.; Bertholet, P.; Evrard, B.; Delattre, L.; Frederich, M.; Ziémons, E.; Piel, G. Theoretical and experimental investigations of organic acids/cyclodextrin complexes and their consequences upon the formation of miconazole/cyclodextrin/acid ternary inclusion complexes. Int. J. Pharm. 2008, 347, 62–70. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Zhao, J.; Liu, Y.; Zhu, X.; Liang, G. Physicochemical characterisation of the supramolecular structure of luteolin/cyclodextrin inclusion complex. Food chem. 2013, 141, 900–906. [Google Scholar] [CrossRef]
- Aloisio, C.; de Oliveira, A.G.; Longhi, M. Solubility and release modulation effect of sulfamerazine ternary complexes with cyclodextrins and meglumine. J. Pharmaceut. Biomed. Anal. 2014, 100, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Méndez, S.G.; Otero Espinar, F.J.; Alvarez, A.L.; Longhi, M.R.; Quevedo, M.A.; Zoppi, A. Ternary complexation of benzoic acid with β-cyclodextrin and aminoacids. Experimental and theoretical studies. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 33–48. [Google Scholar] [CrossRef]
- Dittert, L.W.; Higuchi, T.; Reese, D.R. Phase solubility technique in studying the formation of complex salts of triamterene. J. Pharm. Sci. 1964, 53, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M.; Hoffman, A.; Amidon, G.E.; Amidon, G.L. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: Mechanistic modeling and application to progesterone. J. Pharm. Sci. 2010, 99, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Magnusdottir, A.; Másson, M.; Loftsson, T. Self association and cyclodextrin solubilization of Drugs. J. Pharm. Sci. 2002, 44, 213–218. [Google Scholar] [CrossRef]
- Londhe, V.; Shirsat, R. Formulation and characterization of fast-dissolving sublingual film of iloperidone using Box–Behnken design for enhancement of oral bioavailability. AAPS PharmSciTech 2018, 19, 1392–1400. [Google Scholar] [CrossRef]
- Wang, C.; Xia, N.; Yu, M.; Zhu, S. Physicochemical properties and mechanism of solubilised neohesperidin system based on inclusion complex of hydroxypropyl-β-cyclodextrin. Int. J. Food Sci. Technol. 2023, 58, 107–115. [Google Scholar] [CrossRef]
- Xia, N.; Wan, W.; Zhu, S.; Wang, H.; Ally, K. Synthesis and characterization of a novel soluble neohesperidin-copper (II) complex using Ion-exchange resin column. Polyhedron. 2020, 188, 114694. [Google Scholar] [CrossRef]
- Sherje, A.P.; Londhe, V. Ternary inclusion complex of paliperidone with β-cyclodextrin and hydrophilic polymer for solubility and dissolution enhancement. J. Pharm. Innov. 2015, 10, 324–334. [Google Scholar] [CrossRef]
- Suvarna, V.; Thorat, S.; Nayak, U.; Sherje, A.; Murahari, M. Host-guest interaction study of Efavirenz with hydroxypropyl-β-cyclodextrin and l-arginine by computational simulation studies: Preparation and characterization of supramolecular complexes. J. Mol. Liq. 2018, 259, 55–64. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; Rodriguez-Bonilla, P.; Garcia-Carmona, F. Complexation of pinosylvin, an analogue of resveratrol with high antifungal and antimicrobial activity, by different types of cyclodextrins. J. Agric. Food Chem. 2009, 57, 10175–10180. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bonilla, P.; López-Nicolás, J.M.; García-Carmona, F. Use of reversed phase high pressure liquid cromatography for the physicochemical and thermodynamic characterization of oxyresveratrol/β-cyclodextrin complexes. J. Chromatogr. B. 2010, 878, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Munnangi, S.R.; Youssef, A.A.; Narala, N.; Lakkala, P.; Narala, S.; Vemula, S.K.; Repka, M. Drug complexes: Perspective from academic research and pharmaceutical market. Pharm. Res. 2023, 40, 1519–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Q.; Deng, P.; Chen, Q.; Yu, M.; Shang, J.; Li, W. The formation of a host-guest inclusion complex system between β-cyclodextrin and baicalin and its dissolution characteristics. J. Pharm. Pharmacol. 2017, 69, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraja, K.; Khanam, J. Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acid. J. Pharmaceut. Biomed. Anal. 2014, 96, 10–20. [Google Scholar] [CrossRef]
- Patel, A.R.; Vavia, P.R. Preparation and evaluation of taste masked famotidine formulation using drug/β-cyclodextrin/polymer ternary complexation approach. AAPS Pharmscitech 2008, 9, 544–550. [Google Scholar] [CrossRef]
- Patel, P.; Agrawal, Y.; Sarvaiya, J. Cyclodextrin based ternary system of modafinil: Effect of trimethyl chitosan and polyvinylpyrrolidone as complexing agents. Int. J. Biol. Macromol. 2016, 84, 182–188. [Google Scholar] [CrossRef]
- Rakkaew, P.; Suksiriworapong, J.; Chantasart, D. β-Cyclodextrin-based ternary complexes of haloperidol and organic acids: The effect of organic acids on the drug solubility enhancement. Pharm. Dev. Technol. 2018, 23, 715–722. [Google Scholar] [CrossRef]
- Branham, M.L.; Moyo, T.; Govender, T. Preparation and solid-state characterization of ball milled saquinavir mesylate for solubility enhancement. Eur. J. Pharm. Biopharm. 2012, 80, 194–202. [Google Scholar] [CrossRef]
- Londhe, V.Y.; Pawar, A.; Kundaikar, H. Studies on spectral characterization and solubility of hydroxypropyl β-cyclodextrin/iloperidone binary and ternary complexes using different auxiliary agents. J. Mol. Struct. 2020, 1220, 128615. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hou, W.; Tang, M.; Luo, H.; Chen, L.J.; Guan, Y.H.; Sutherland, I.A. Separation of Flavonoids from the Leaves of Oroxylum indicum by HSCCC. Chromatographia 2008, 68, 885–892. [Google Scholar] [CrossRef]
- Schneider, H.J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of Cyclodextrins and Cyclodextrin Complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.S.; Ochoa, R.; Pimenta, A.M.C.; Ferreira, L.A.; Melo, A.L.; Da Silva, J.B.; Sinisterra, R.D.; Demicheli, C.; Frézard, F. Mode of action of β-cyclodextrin as an absorption enhancer of the water-soluble drug meglumine antimoniate. Int. J. Pharm. 2006, 325, 39–47. [Google Scholar] [CrossRef]
- Berge, S.M.; Bighley, L.D.; Monkhouse, D.C. Pharmaceutical salts. J. Pharm. Sci. 1977, 66, 1–19. [Google Scholar] [CrossRef]
| Time (min) | Flow Rate (mL/min) | Percent of Mobile Phase Substances (%) | |
|---|---|---|---|
| A | B | ||
| 0 | 1.000 | 80 | 20 |
| 20 | 1.000 | 0 | 100 |
| 25 | 1.000 | 0 | 100 |
| 26 | 1.000 | 80 | 20 |
| 30 | 1.000 | 80 | 20 |
| Tem (°C) | Linear Regression Function | R2 | K/(L·moL−1) | |
|---|---|---|---|---|
| NH-CD | 25 | A = 0.0904c + 0.4231 | 0.9973 | 826.75 |
| 37 | A = 0.0886c + 0.9003 | 0.9983 | 600.82 | |
| 60 | A = 0.0788c + 1.4654 | 0.9981 | 331.17 | |
| NH-MEG-CD | 25 | A = 0.2352c + 2.5518 | 0.9926 | 2558.28 |
| 37 | A = 0.1272c + 5.9644 | 0.9926 | 900.07 | |
| 60 | A = 0.07752c + 7.6943 | 0.9897 | 325.36 |
| Assignment | δfree (ppm) | Δδ (δComplex − ΔFree Drug) (ppm) | ||
|---|---|---|---|---|
| NH-CD | NH-MEG-CD | |||
| NH | Rha-CH3 | 1.154 | 0.003 | −0.008 |
| H-3 | 2.793 | −0.008 | −0.002 | |
| OCH3 | 3.772 | −0.011 | −0.051 | |
| Rha-H-1 | 5.011 | −0.029 | −0.049 | |
| Glu-H-1 | 5.125 | −0.012 | −0.018 | |
| OH | 5.181 | −0.038 | −0.019 | |
| H-2 | 5.392 | −0.018 | - | |
| H-8 | 6.122 | 0.064 | 0.259 | |
| H-6 | 6.141 | 0.072 | 0.243 | |
| H-6′ | 6.901 | −0.087 | −0.007 | |
| H-2′ | 6.933 | −0.012 | −0.256 | |
| H-5′ | 6.941 | −0.009 | −0.177 | |
| OH-3′ | 9.092 | − | − | |
| OH-5 | 12.025 | − | − | |
| MEG | H-1 | 3.8137 | − | −0.003 |
| H-2 | 3.6981 | − | −0.015 | |
| H-3 | 3.6620 | − | 0.004 | |
| H-4 | 3.6584 | − | 0.003 | |
| H-5 | 3.5532 | − | 0.009 | |
| H-6a | 2.6108 | − | 0.156 | |
| H-6b | 2.5601 | − | 0.157 | |
| H-7 | 2.2677 | − | 0.121 | |
| HP-β-CD | H-1 | 5.004 | −0.043 | −0.023 |
| H-2 | 3.644 | 0.033 | −0.025 | |
| H-3 | 3.938 | −0.019 | −0.066 | |
| H-4 | 3.422 | −0.012 | −0.005 | |
| H-5 | 3.793 | −0.089 | −0.074 | |
| H-6 | 3.862 | −0.051 | −0.054 | |
| CH3 | 1.063 | −0.004 | −0.006 | |
| Complex | Energy Difference Mode kcal/moL | Interaction Energy Mode kcal/moL | ||||
|---|---|---|---|---|---|---|
| Del Total Energy | Del vdW | Del Electro | Total Energy | Vdw | Electrostat | |
| binary | −23.6324 | −48.8362 | −16.5153 | −149.2773 | −112.369 | −36.9083 |
| ternary | −29.1803 | −55.4772 | −19.2427 | −165.3823 | −126.669 | −38.7133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, N.; Liu, Y.; Gao, D.; Zhu, S. Molecular Interaction and Solubilization Efficiency of Neohesperidin in Ternary Systems with Hydroxypropyl-β-cyclodextrin and Meglumine. Foods 2024, 13, 3143. https://doi.org/10.3390/foods13193143
Xia N, Liu Y, Gao D, Zhu S. Molecular Interaction and Solubilization Efficiency of Neohesperidin in Ternary Systems with Hydroxypropyl-β-cyclodextrin and Meglumine. Foods. 2024; 13(19):3143. https://doi.org/10.3390/foods13193143
Chicago/Turabian StyleXia, Na, Yanquan Liu, Dan Gao, and Siming Zhu. 2024. "Molecular Interaction and Solubilization Efficiency of Neohesperidin in Ternary Systems with Hydroxypropyl-β-cyclodextrin and Meglumine" Foods 13, no. 19: 3143. https://doi.org/10.3390/foods13193143







