The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review
Abstract
:1. Introduction
2. Chemical Composition and Biological Functions of Extracts
2.1. Ethanol Extraction
2.1.1. Phenolic Compounds in Ethanol Extract
Flavonoid in Phenolic Compound Group
Phenolic Acid in Phenolic Compound Group
2.1.2. Lipids and Fatty Acids in Ethanol Extract
Steroids in Lipids and Fatty Acids Group
2.1.3. Terpenoids in Ethanol Extract
2.1.4. Minerals in Ethanol Extract
2.2. Methanol Extraction
2.2.1. Phenolic Compounds in Methanol Extract
Flavonoids in Phenolic Compound Group
Phenolic Acids in Phenolic Compound Group
2.2.2. Anthocyanins in Methanol Extract
2.2.3. Lipids and Fatty Acids in Methanol Extract
Steroid in Lipids and Fatty Acids
2.2.4. Terpenoids in Methanol Extract
2.2.5. Lignans in Methanol Extract
2.2.6. Polysaccharides in Methanol Extract
2.2.7. Carotene in Methanol Extract
2.3. Acetone Extraction
2.3.1. Polyacetylenes in Acetone Extraction
2.3.2. Terpenoids in Acetone Extraction
2.3.3. Carboxylic Acids in Acetone Extraction
2.4. Water and Steam Extraction
2.4.1. Phenolic Compounds in Water and Steam Extraction
Flavonoids in Phenolic Compounds
2.4.2. Anthocyanin in Water and Steam Extract
2.4.3. Essential Oil in Water and Steam Extract
2.4.4. Minerals in Water and Steam Extract
2.4.5. Vitamins in Water and Steam Extract
2.4.6. Polysaccharide in Water and Steam Extract
2.5. Alternative Solvents Extraction and Multi-Solvent Extraction
3. Clinical Trials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shahidi, F. Functional foods: Their role in health promotion and disease prevention. J. Food Sci. 2004, 69, R146–R149. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Cordell, G.A. Natural products in drug discovery–creating a new vision. Phytochem. Rev. 2002, 1, 261–273. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- García-Herrera, P.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Díez-Marqués, C.; Molina, M.; Tardío, J. Nutrient composition of six wild edible Mediterranean Asteraceae plants of dietary interest. J. Food Compos. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef]
- Arora, D.; Rani, A.; Sharma, A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn. Rev. 2013, 7, 179. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Azwanida, N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 1000196. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S. Major phytochemicals: Recent advances in health benefits and extraction method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Avellar, B.K.; Glasser, W.G. Steam-assisted biomass fractionation. I. Process considerations and economic evaluation. Biomass Bioenergy 1998, 14, 205–218. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, D.; Chen, Q.; Shi, C.; Ye, J.; Dai, Z.; Lu, Y. Water-based green and sustainable extraction protocols for value-added compounds from natural resources. Curr. Opin. Green Sustain. Chem. 2023, 40, 100757. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B. Conventional extraction techniques: Solvent extraction. In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 171–189. [Google Scholar]
- Koc, S.; Isgor, B.S.; Isgor, Y.G.; Shomali Moghaddam, N.; Yildirim, O. The potential medicinal value of plants from Asteraceae family with antioxidant defense enzymes as biological targets. Pharm. Biol. 2015, 53, 746–751. [Google Scholar] [CrossRef]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. Bmj 2007, 334, 197. [Google Scholar] [CrossRef]
- Jang, S.; Kim, M.-S.; Park, T.; Sim, J.H.; Kim, S.-Y. Screening and Identification of an Anti-inflammatory and Anti-adipogenic Constituent from Ligularia taquetii Nakai. Nat. Prod. Commun. 2020, 15, 1934578X19899503. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, M.; Shin, S.; Woo, J.; Son, D.; Ryu, D.; Yoo, J.; Park, D.; Jung, E. Effect of Cirsium japonicum flower extract on skin aging induced by glycation. Molecules 2022, 27, 2093. [Google Scholar] [CrossRef] [PubMed]
- Piątkowska, E.; Biel, W.; Witkowicz, R.; Kępińska-Pacelik, J. Chemical Composition and Antioxidant Activity of Asteraceae Family Plants. Appl. Sci. 2022, 12, 12293. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Georgiev, M.I.; Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Ortan, A.; Georgescu, M.I.; Sutan, A.N.; Zanfirescu, A.; Dinu-Pirvu, C.E.; et al. Mitodepressive, antioxidant, antifungal and anti-inflammatory effects of wild-growing Romanian native Arctium lappa L. (Asteraceae) and Veronica persica Poiret (Plantaginaceae). Food Chem. Toxicol. 2018, 111, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bădărau, A.S.; Swamy, M.K.; Shaw, S.; Maggi, F.; Da Silva, L.E.; López, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium species secondary metabolites chemodiversity and bioactivities. Front. Plant Sci. 2019, 10, 439246. [Google Scholar] [CrossRef] [PubMed]
- Tavassolifar, M.j.; Vodjgani, M.; Salehi, Z.; Izad, M. The influence of reactive oxygen species in the immune system and pathogenesis of multiple sclerosis. Autoimmune Dis. 2020, 2020, 5793817. [Google Scholar] [CrossRef]
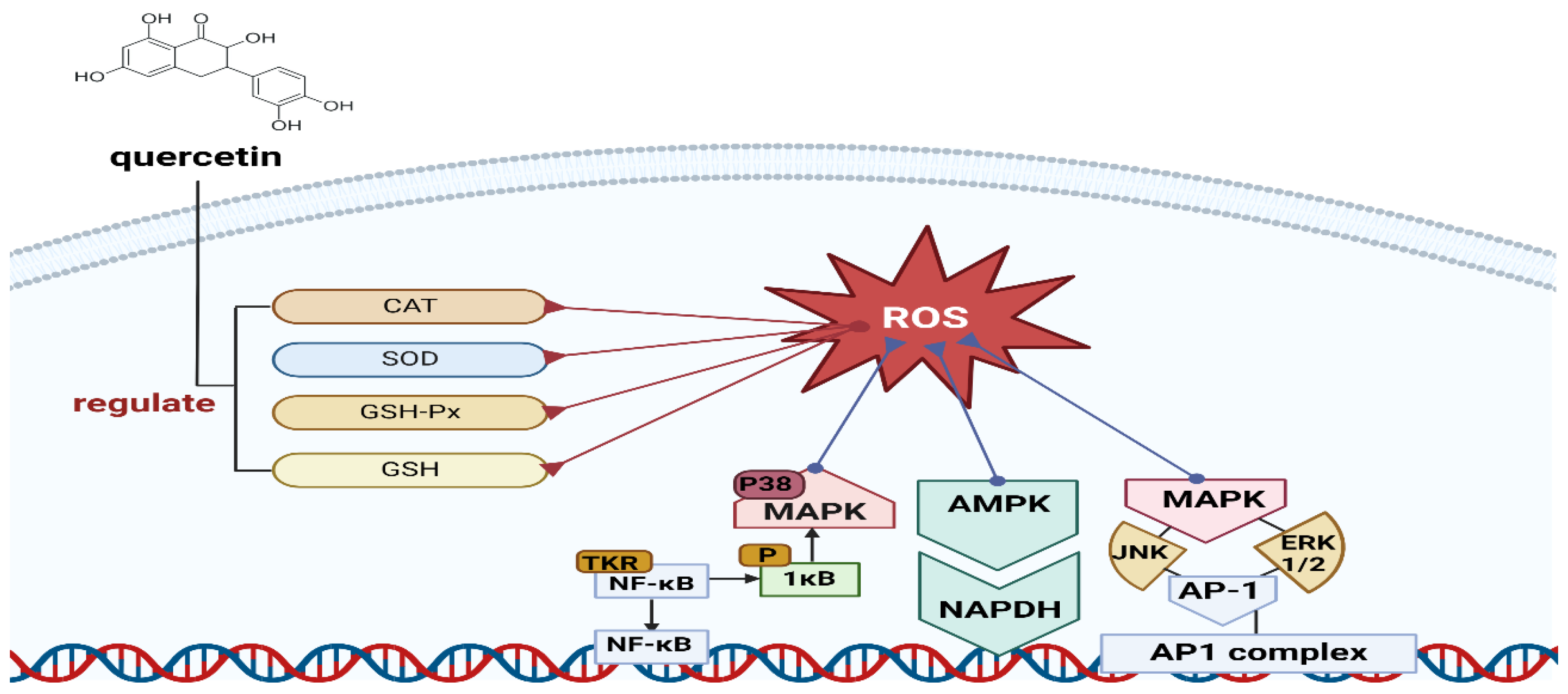
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Perović, J.; Šaponjac, V.T.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient–Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Yuan, X.; Gao, M.; Xiao, H.; Tan, C.; Du, Y. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chem. 2012, 133, 10–14. [Google Scholar] [CrossRef]
- Weber, H. Fatty acid-derived signals in plants. Trends Plant Sci. 2002, 7, 217–224. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Li-Beisson, Y. Plant unusual fatty acids: Learning from the less common. Curr. Opin. Plant Biol. 2020, 55, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Boonen, J.; Baert, B.; Roche, N.; Burvenich, C.; De Spiegeleer, B. Transdermal behaviour of the N-alkylamide spilanthol (affinin) from Spilanthes acmella (Compositae) extracts. J. Ethnopharmacol. 2010, 127, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.; Husna, F.; Gani, B.A.; Garrido, G. The chemical composition of the ethanolic extract from Chromolaena odorata leaves correlates with the cytotoxicity exhibited against colorectal and breast cancer cell lines. J. Pharm. Pharmacogn. Res. 2021, 9, 344–356. [Google Scholar] [CrossRef]
- Gunaherath, G.M.K.B.; Gunatilaka, A.A.L. Plant Steroids: Occurrence, Biological Significance and their Analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Miyazawa, M.; Yagi, N.; Taguchi, K. Inhibitory compounds of α-glucosidase activity from Arctium lappa L. J. Oleo Sci. 2005, 54, 589–594. [Google Scholar] [CrossRef]
- Eshbakova, K.; Aisa, H. Components of Helichrysum arenarium. Chem. Nat. Compd. 2009, 45, 929–930. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Bigović, D.; Janković, T.; Jelačić, S.; Šavikin, K. Sandy Everlasting (Helichrysum arenarium (L.) Moench): Botanical, Chemical and Biological Properties. Front. Plant Sci. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Baskar, A.A.; Al Numair, K.S.; Gabriel Paulraj, M.; Alsaif, M.A.; Muamar, M.A.; Ignacimuthu, S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1, 2-dimethylhydrazine-induced colon cancer. J. Med. Food 2012, 15, 335–343. [Google Scholar] [CrossRef]
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. β-Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2010, 54, 551–558. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Cheng, A.-X.; Lou, Y.-G.; Mao, Y.-B.; Lu, S.; Wang, L.-J.; Chen, X.-Y. Plant Terpenoids: Biosynthesis and Ecological Functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Han, Z.; Wang, M.; Wang, L.; Qu, H.; Li, P.; Wang, C. Chemical analysis of burdock root constituents. Asian J. Chem. 2013, 25, 2573. [Google Scholar] [CrossRef]
- Liao, Z.; Chen, X.; Wu, M. Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic rats. Arch. Pharmacal Res. 2010, 33, 353–362. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Li, D.; Liu, W.; Luo, X.; Zhang, R.; Li, L.; Zhao, J. Anticancer activity and quantitative analysis of flavone of Cirsium japonicum DC. Nat. Prod. Res. 2007, 21, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-S.; Park, J.Y.; Lee, J.; Yoo, H.H.; Hahm, D.-H.; Lee, S.C.; Lee, S.; Hwang, G.S.; Jung, K.; Kang, K.S. Anti-inflammatory effects and corresponding mechanisms of cirsimaritin extracted from Cirsium japonicum var. maackii Maxim. Bioorganic Med. Chem. Lett. 2017, 27, 3076–3080. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value and Bioactive Compounds Characterization of Plant Parts From Cynara cardunculus L. (Asteraceae) Cultivated in Central Greece. Front. Plant Sci. 2018, 9, 459. [Google Scholar] [CrossRef]
- Babich, O.; Larina, V.; Krol, O.; Ulrikh, E.; Sukhikh, S.; Gureev, M.A.; Prosekov, A.; Ivanova, S. In Vitro Study of Biological Activity of Tanacetum vulgare Extracts. Pharmaceutics 2023, 15, 616. [Google Scholar] [CrossRef]
- Jędrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem.-Biol. Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Thiruvengadam, M.; Chung, I.-M.; Nagella, P. Polyphenol composition and antioxidant activity from the vegetable plant Artemisia absinthium L. Aust. J. Crop Sci. 2013, 7, 1921–1926. Available online: https://search.informit.org/doi/10.3316/informit.669038022011805 (accessed on 22 August 2024).
- Mandade, R.; Sreenivas, S.; Choudhury, A. Radical scavenging and antioxidant activity of Carthamus tinctorius extracts. Free Radic. Antioxid. 2011, 1, 87–93. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Konate, K.; Brestic, M. Phenolic Acid Patterns in Different Plant Species of Families Asteraceae and Lamiaceae: Possible Phylogenetic Relationships and Potential Molecular Markers. J. Chem. 2022, 2022, 9632979. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic fractions from dandelion leaves and petals as modulators of the antioxidant status and lipid profile in an in vivo study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Trinh, P.-C.; Thao, L.-T.-T.; Ha, H.-T.-V.; Nguyen, T. DPPH-scavenging and antimicrobial activities of Asteraceae medicinal plants on uropathogenic bacteria. Evid.-Based Complement. Altern. Med. 2020, 2020, 7807026. [Google Scholar] [CrossRef]
- Burlec, A.F.; Pecio, Ł.; Kozachok, S.; Mircea, C.; Corciovă, A.; Vereștiuc, L.; Cioancă, O.; Oleszek, W.; Hăncianu, M. Phytochemical profile, antioxidant activity, and cytotoxicity assessment of Tagetes erecta L. flowers. Molecules 2021, 26, 1201. [Google Scholar] [CrossRef]
- Park, J.C.; Hur, J.M.; Park, J.G.; Kim, S.C.; Park, J.R.; Choi, S.H.; Choi, J.W. Effects of methanol extract of Cirsium japonicum var. ussuriense and its principle, hispidulin-7-O-neohesperidoside on hepatic alcohol-metabolizing enzymes and lipid peroxidation in ethanol-treated rats. Phytother. Res. 2004, 18, 19–24. [Google Scholar] [CrossRef]
- Babaee, N.; Moslemi, D.; Khalilpour, M.; Vejdani, F.; Moghadamnia, Y.; Bijani, A.; Baradaran, M.; Kazemi, M.T.; Khalilpour, A.; Pouramir, M. Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: A randomized controlled clinical study. DARU J. Pharm. Sci. 2013, 21, 18. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.G.; Cho, E.J.; Choi, K.; Ku, J.; Park, K.-W.; Lee, S. Analysis of phenolic compounds in chwinamul by HPLC/UV. Hortic. Environ. Biotechnol. 2013, 54, 183–189. [Google Scholar] [CrossRef]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem.-Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef]
- Mao, Z.; Gan, C.; Zhu, J.; Ma, N.; Wu, L.; Wang, L.; Wang, X. Anti-atherosclerotic activities of flavonoids from the flowers of Helichrysum arenarium L. MOENCH through the pathway of anti-inflammation. Bioorganic Med. Chem. Lett. 2017, 27, 2812–2817. [Google Scholar] [CrossRef]
- Iqbal, J.; Khan, A.A.; Aziz, T.; Ali, W.; Ahmad, S.; Rahman, S.U.; Iqbal, Z.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A. Phytochemical investigation, antioxidant properties and in vivo evaluation of the toxic effects of Parthenium hysterophorus. Molecules 2022, 27, 4189. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Ross, E.K.; Khatter, S.; Miller, K.; Linseman, D.A. Chemical basis for the disparate neuroprotective effects of the anthocyanins, callistephin and kuromanin, against nitrosative stress. Free Radic. Biol. Med. 2017, 103, 23–34. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yang, B.; Li, J.; Jiang, Y. Effects of various temperatures and pH values on the extraction yield of phenolics from litchi fruit pericarp tissue and the antioxidant activity of the extracted anthocyanins. Int. J. Mol. Sci. 2008, 9, 1333–1341. [Google Scholar] [CrossRef]
- Mishio, T.; Takeda, K.; Iwashina, T. Anthocyanins and other flavonoids as flower pigments from eleven Centaurea species. Nat. Prod. Commun. 2015, 10, 1934578X1501000318. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.-J.; Choung, M.-G. Anthocyanin compositions and biological activities from the red petals of Korean edible rose (Rosa hybrida cv. Noblered). Food Chem. 2011, 129, 272–278. [Google Scholar] [CrossRef]
- Beharav, A.; Ben-David, R.; Malarz, J.; Stojakowska, A.; Michalska, K.; Doležalová, I.; Lebeda, A.; Kisiel, W. Variation of sesquiterpene lactones in Lactuca aculeata natural populations from Israel, Jordan and Turkey. Biochem. Syst. Ecol. 2010, 38, 602–611. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xie, Y.; Hu, H. Antitumor activity and mechanism of costunolide and dehydrocostus lactone: Two natural sesquiterpene lactones from the Asteraceae family. Biomed. Pharmacother. 2020, 125, 109955. [Google Scholar] [CrossRef]
- Morimoto, H.; Sanno, Y.; Oshio, H. Chemical studies on Heliangine: A new sesquiterpene lactone isolated from the leaves of Helianthus tuberosus L. Tetrahedron 1966, 22, 3173–3179. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and Their Derivatives from Plants as Antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef]
- Jafarinia, M.; Jafarinia, M. A review of medicinal properties of some Asteraceae family plants on immune system. Rep. Health Care 2019, 5, 1–7. Available online: https://www.researchgate.net/publication/333557155 (accessed on 22 August 2024).
- Atkin, O.; Millar, A.; Gardeström, P.; Day, D. Photosynthesis, carbohydrate metabolism and respiration in leaves of higher plants. In Photosynthesis: Physiology and Metabolism; Springer: Berlin/Heidelberg, Germany, 2000; pp. 153–175. [Google Scholar] [CrossRef]
- Dilworth, L.L.; Riley, C.K.; Stennett, D.K. Chapter 4—Plant constituents: Carbohydrates, oils, resins, balsams, and plant hormones. In Pharmacognosy, 2nd ed.; McCreath, S.B., Clement, Y.N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 49–74. [Google Scholar]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) root components exhibit anti-oxidative and antiplatelet action in an in vitro study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Panfili, G.; Niro, S.; Bufano, A.; D’Agostino, A.; Fratianni, A.; Paura, B.; Falasca, L.; Cinquanta, L. Bioactive compounds in wild Asteraceae edible plants consumed in the Mediterranean diet. Plant Foods Hum. Nutr. 2020, 75, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, M.L.; Liu, J.; Wang, Y.; Hu, J.H.; Wang, M.-H. Sonchus asper extract inhibits LPS-induced oxidative stress and pro-inflammatory cytokine production in RAW264.7 macrophages. Nutr. Res. Pract. 2015, 9, 579–585. [Google Scholar] [CrossRef]
- Konovalov, D. Polyacetylene compounds of plants of the Asteraceae family. Pharm. Chem. J. 2014, 48, 613–631. [Google Scholar] [CrossRef]
- Washino, T.; Yoshikura, M.; Obata, S. New sulfur-containing acetylenic compounds from Arctium lappa. Agric. Biol. Chem. 1986, 50, 263–269. [Google Scholar] [CrossRef]
- Heptinstall, S.; Awang, D.; Dawson, B.; Kindack, D.; Knight, D.; May, J. Parthenolide content and bioactivity of feverfew (Tanacetum parthenium (L.) Schultz-Bip.). Estimation of commercial and authenticated feverfew products. J. Pharm. Pharmacol. 1992, 44, 391–395. [Google Scholar] [CrossRef]
- Godlewska-Żyłkiewicz, B.; Świsłocka, R.; Kalinowska, M.; Golonko, A.; Świderski, G.; Arciszewska, Ż.; Nalewajko-Sieliwoniuk, E.; Naumowicz, M.; Lewandowski, W. Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids. Materials 2020, 13, 4454. [Google Scholar] [CrossRef]
- Llorach, R.; Espin, J.C.; Tomas-Barberan, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef]
- Yun, U.-T.; Cho, J.-Y.; Jeong, E.-Y.; Jo, J.-B.; Lee, E.-H.; Kim, B.-O.; Cho, Y.-J. Biological activities of isolated phenolic compounds from Trachelospermum asiaticum var. intermedium nakai. Food Sci. Preserv. 2017, 24, 282–288. [Google Scholar] [CrossRef]
- Mulabagal, V.; Wang, H.; Ngouajio, M.; Nair, M.G. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. Eur. Food Res. Technol. 2009, 230, 47–53. [Google Scholar] [CrossRef]
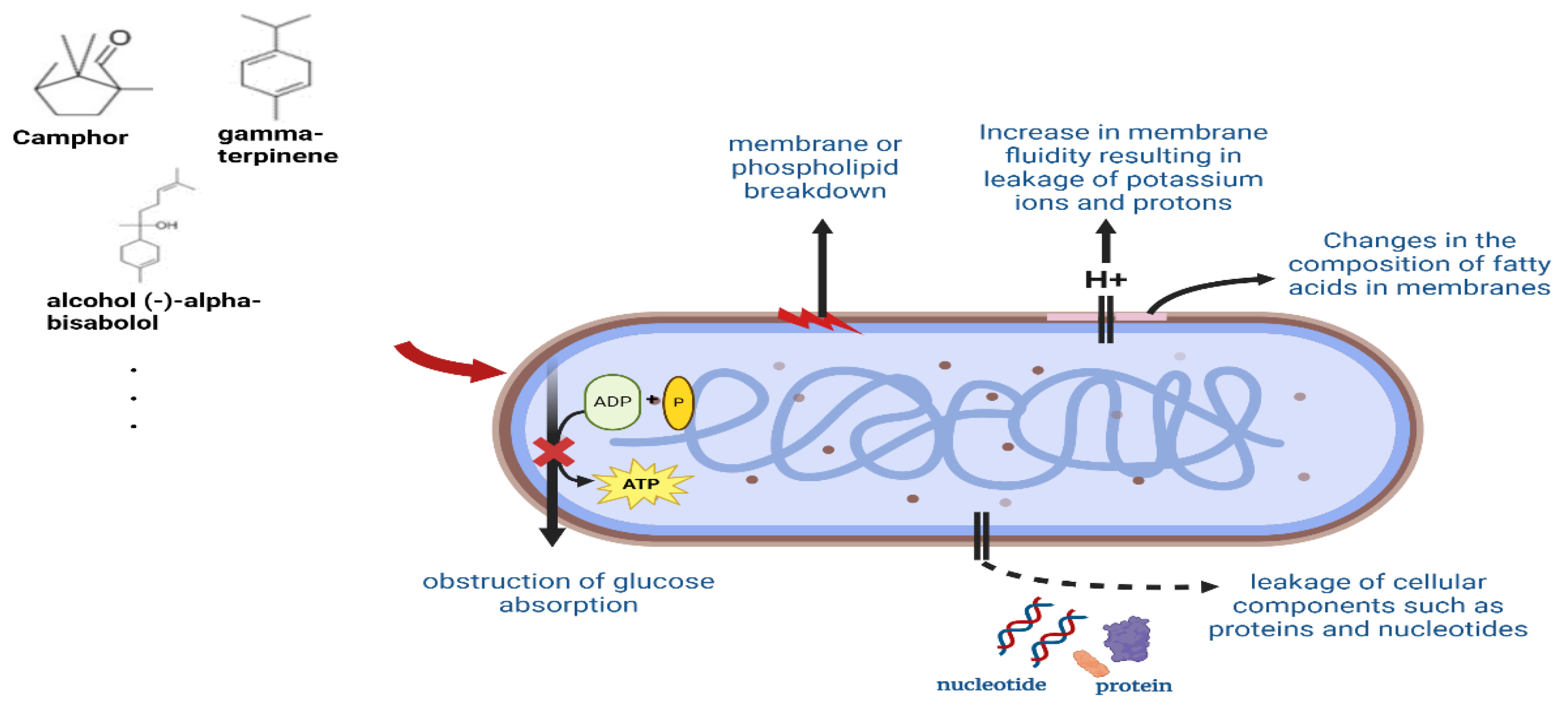
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Romeo, F.V.; De Luca, S.; Piscopo, A.; Poiana, M. Antimicrobial effect of some essential oils. J. Essent. Oil Res. 2008, 20, 373–379. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Coté, H.; Boucher, M.-A.; Pichette, A.; Legault, J. Anti-inflammatory, antioxidant, antibiotic, and cytotoxic activities of Tanacetum vulgare L. essential oil and its constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef]
- Gol, N.R.; Noghani, R.Z.; Chamsaz, M. A comparative study of the chemical composition and antioxidant activities of roots, seeds and aerial parts of chicory (Cichorium intybus L.). Int. J. Biosci. 2014, 5, 250–257. [Google Scholar] [CrossRef]
- Alves Gomes Albertti, L.; Delatte, T.L.; Souza de Farias, K.; Galdi Boaretto, A.; Verstappen, F.; van Houwelingen, A.; Cankar, K.; Carollo, C.A.; Bouwmeester, H.J.; Beekwilder, J. Identification of the Bisabolol Synthase in the Endangered Candeia Tree (Eremanthus erythropappus (DC) McLeisch). Front. Plant Sci. 2018, 9, 1340. [Google Scholar] [CrossRef]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial Activity of Essential Oil of Baccharis dracunculifolia DC (Asteraceae) Aerial Parts at Flowering Period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, J.; Jbara, M. Vitamins for enhancing plant resistance. Planta 2016, 244, 529–543. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Skinner, W.; Parkhurst, R. Antioxidant properties of α-tocopherol derivatives and relationship of antioxidant activity to biological activity. Lipids 1970, 5, 184–186. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Gu, G.; Guo, Z. Extraction, degree of polymerization determination and prebiotic effect evaluation of inulin from Jerusalem artichoke. Carbohydr. Polym. 2015, 121, 315–319. [Google Scholar] [CrossRef] [PubMed]
- El Mehy, M. Antioxidant and antimicrobial activity of gamma irradiated chicory (Cichorium intybus L.) leaves and roots. Ann. Agric. Sci. Moshtohor 2018, 56, 51–60. [Google Scholar] [CrossRef]
- Borgo, J.; Wagner, M.S.; Laurella, L.C.; Elso, O.G.; Selener, M.G.; Clavin, M.; Bach, H.; Catalán, C.A.; Bivona, A.E.; Sepúlveda, C.S. Plant Extracts and Phytochemicals from the Asteraceae Family with Antiviral Properties. Molecules 2024, 29, 814. [Google Scholar] [CrossRef]
- Ezzat, M.I.; Ezzat, S.M.; El Deeb, K.S.; El Fishawy, A.M.; El-Toumy, S.A. A new acylated flavonol from the aerial parts of Asteriscus maritimus (L.) Less (Asteraceae). Nat. Prod. Res. 2016, 30, 1753–1761. [Google Scholar] [CrossRef]
- Stojanović, G.; Radulović, N.; Hashimoto, T.; Palić, R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J. Ethnopharmacol. 2005, 101, 185–190. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Garcia-Oliveira, P.; Nuñez-Estevez, B.; Silva, A.; Finimundy, T.C.; Calhelha, R.; Nenadic, M.; Sokovic, M.; Barroso, F.; Simal-Gandara, J. Plants of the Family Asteraceae: Evaluation of Biological Properties and Identification of Phenolic Compounds. Chem. Proc. 2021, 5, 51. [Google Scholar] [CrossRef]
- da Silva, A.G.; Machado, E.R.; de Almeida, L.M.; Menezes Nunes, R.M.; Giesbrecht, P.C.P.; Costa, R.M.; Costa, H.B.; Romão, W.; Kuster, R.M. A clinical trial with Brazilian arnica (Solidago chilensis Meyen) glycolic extract in the treatment of tendonitis of flexor and extensor tendons of wrist and hand. Phytother. Res. 2015, 29, 864–869. [Google Scholar] [CrossRef]
- Cho, I.-J.; Choung, S.Y.; Hwang, Y.-C.; Ahn, K.J.; Chung, H.Y.; Jeong, I.-K. Aster spathulifolius Maxim extract reduces body weight and fat mass in obese humans. Nutr. Res. 2016, 36, 671–678. [Google Scholar] [CrossRef]
- Kuan, C.-M.; Liang, C.-H.; Chuang, W.-H.; Lin, T.-Y.; Hsu, P.-K. Ameliorating Effect of Crassocephalum rabens (Asteraceae) Extract on Skin Aging: A Randomized, Parallel, Double-Blind, and Placebo-Controlled Study. Nutrients 2022, 14, 2655. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Ogbalu, O.; Williams, J. The use of Ageratum conyzoides L. [Asteraceae] as a therapeutic measure in the treatment of breast myiasis sores in rural women and associated bacteria. IOSR J. Pharm. Biol. Sci. 2014, 9, 44–50. [Google Scholar] [CrossRef]
- Ikeda, M.; Sato, A.; Mochizuki, N.; Toyosaki, K.; Miyoshi, C.; Fujioka, R.; Mitsunaga, S.; Ohno, I.; Hashimoto, Y.; Takahashi, H. Phase I trial of GBS-01 for advanced pancreatic cancer refractory to gemcitabine. Cancer Sci. 2016, 107, 1818–1824. [Google Scholar] [CrossRef]
- Moghadam, M.H.; Ghasemi, Z.; Sepahi, S.; Rahbarian, R.; Mozaffari, H.M.; Mohajeri, S.A. Hypolipidemic effect of Lactuca sativa seed extract, an adjunctive treatment, in patients with hyperlipidemia: A randomized double-blind placebo-controlled pilot trial. J. Herb. Med. 2020, 23, 100373. [Google Scholar] [CrossRef]
- Keshavarzi, A.; Akrami, R.; Zarshenas, M.M.; Zareie, S.; Ghadimi, T.; Najafi, A.; Rostami Chijan, M.; Dehghan, A.; Zarenezhad, E. Evaluation of the Effect of Cichorium intybus L. on the Liver Enzymes in Burn Patients: A Randomized Double-Blind Clinical Trial. Int. J. Clin. Pract. 2024, 2024, 1016247. [Google Scholar] [CrossRef]
- Amiri, M.; Navabi, J.; Shokoohinia, Y.; Heydarpour, F.; Bahrami, G.; Behbood, L.; Derakhshandeh, P.; Momtaz, S.; Farzaei, M.H. Efficacy and safety of a standardized extract from Achillea wilhelmsii C. Koch in patients with ulcerative colitis: A randomized double blind placebo-controlled clinical trial. Complement. Ther. Med. 2019, 45, 262–268. [Google Scholar] [CrossRef]
- Jenabi, E.; Fereidoony, B. Effect of Achillea millefolium on relief of primary dysmenorrhea: A double-blind randomized clinical trial. J. Pediatr. Adolesc. Gynecol. 2015, 28, 402–404. [Google Scholar] [CrossRef]
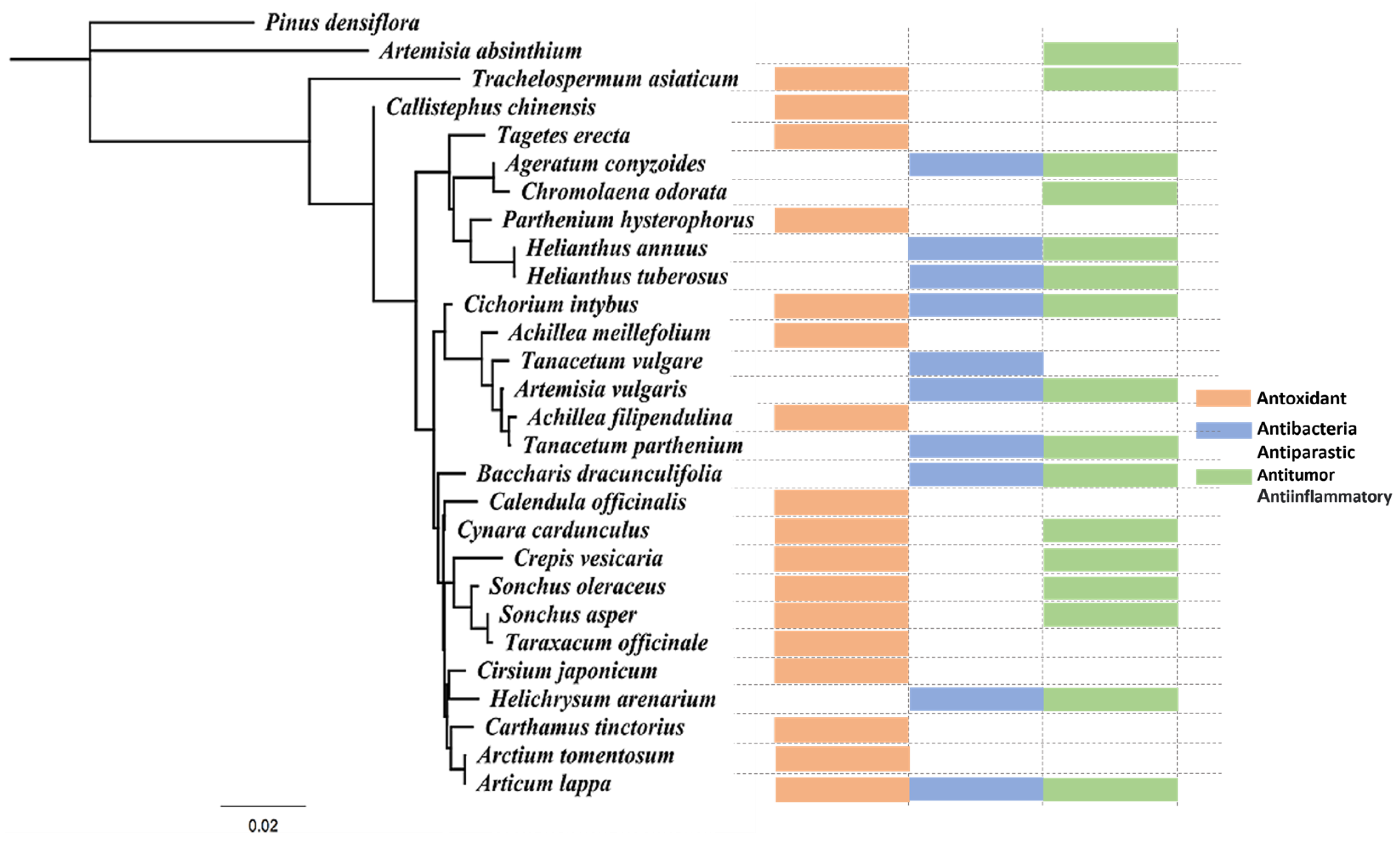
| Extract | Extract Portion | Extract Method | Target Organ | Experimental Effect | Reference |
|---|---|---|---|---|---|
| Solidago chilensis Meyen | Stems, leaves, petioles, and flowers | 92.8% ethanol for 24 h in a percolator. | Tendonitis of flexor and extensor tendons of wrist and hand | Muscle strength improved; pain reduction effect improved | [103] |
| Aster spathulifolius Maxim | Leaves | 50% ethanol at 60 °C for 4 h | Lipid | Body weight, BMI, and fat mass were significantly, decreased | [104] |
| Crassocephalum rabens | Stems, leaves, petioles, and flowers | 95% ethanol (1:10 w/v) at 40 °C for 3 h | Skin | Skin brightness improved | [105] |
| Achillea millefolium | Flowers | [extraction condition unknown] | Kidney | Mean plasma concentrations of basal nitrite and nitrate decreased. | [106] |
| Ageratum conyzoides L. | Leaves, stems and flowers | 70% ethanol for 4 h | Breast skin | Pain relief provided | [107] |
| Arctium lappa L. | Fruit | [extraction condition unknown] | Pancreatic cancer | Anti-tumor effect increased | [108] |
| Lactuca sativa | Seeds | 80% ethanol [extraction time unknown] | Liver | LDL levels reduced, but HDL level is no significantly changed | [109] |
| Cichorium intybus L. | Seeds | Distilled water for 10 min | Liver | Liver enzymes were reduced in burn patients | [110] |
| Achillea wilhelmsii | Leaves, stems and flowers | 70% ethanol at room temperature for 24 h | Colon | No significant difference between the number of bowel movements and degree of improvement in rectal bleeding | [111] |
| Calendula officinalis | Flowers | 70% ethanol for 72 h | Mucous membrane of the mouth | Strength of oropharyngeal mucositis has improved | [58] |
| Cirsium japonicum | Flowers | 70% ethanol at room temperature | Skin | Skin elasticity was improved, wrinkles were relieved, and aging was prevented. | [22] |
| Achillea Millefolium | Flowers | Hot water for 10 min | Lower abdomen | Alleviating menstrual pain | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Jayakody, J.T.M.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. https://doi.org/10.3390/foods13193151
Lee J-E, Jayakody JTM, Kim J-I, Jeong J-W, Choi K-M, Kim T-S, Seo C, Azimi I, Hyun J, Ryu B. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods. 2024; 13(19):3151. https://doi.org/10.3390/foods13193151
Chicago/Turabian StyleLee, Ji-Eun, Jayakodyge Thilini Madushani Jayakody, Jae-Il Kim, Jin-Woo Jeong, Kyung-Min Choi, Tae-Su Kim, Chan Seo, Iman Azimi, Jimin Hyun, and Bomi Ryu. 2024. "The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review" Foods 13, no. 19: 3151. https://doi.org/10.3390/foods13193151
APA StyleLee, J.-E., Jayakody, J. T. M., Kim, J.-I., Jeong, J.-W., Choi, K.-M., Kim, T.-S., Seo, C., Azimi, I., Hyun, J., & Ryu, B. (2024). The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods, 13(19), 3151. https://doi.org/10.3390/foods13193151








