Metabolomics Revealed the Effects of Momordica charantia L. Saponins on Diabetic Hyperglycemia and Wound Healing in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animal Experiments
2.3. Analysis of FBG, Oral Glucose Tolerance Test, Insulin, and LPS
2.4. Determination of Biochemical Indices
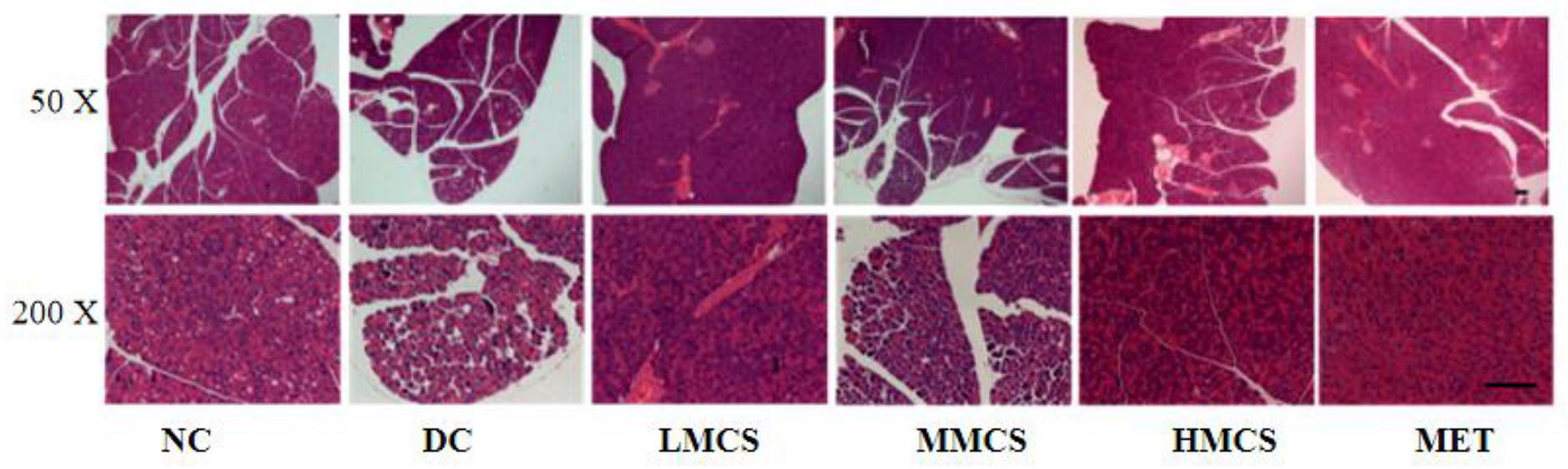
2.5. Pathological Analysis
2.6. Metabolomics Analysis
2.7. Statistical Analysis
3. Results
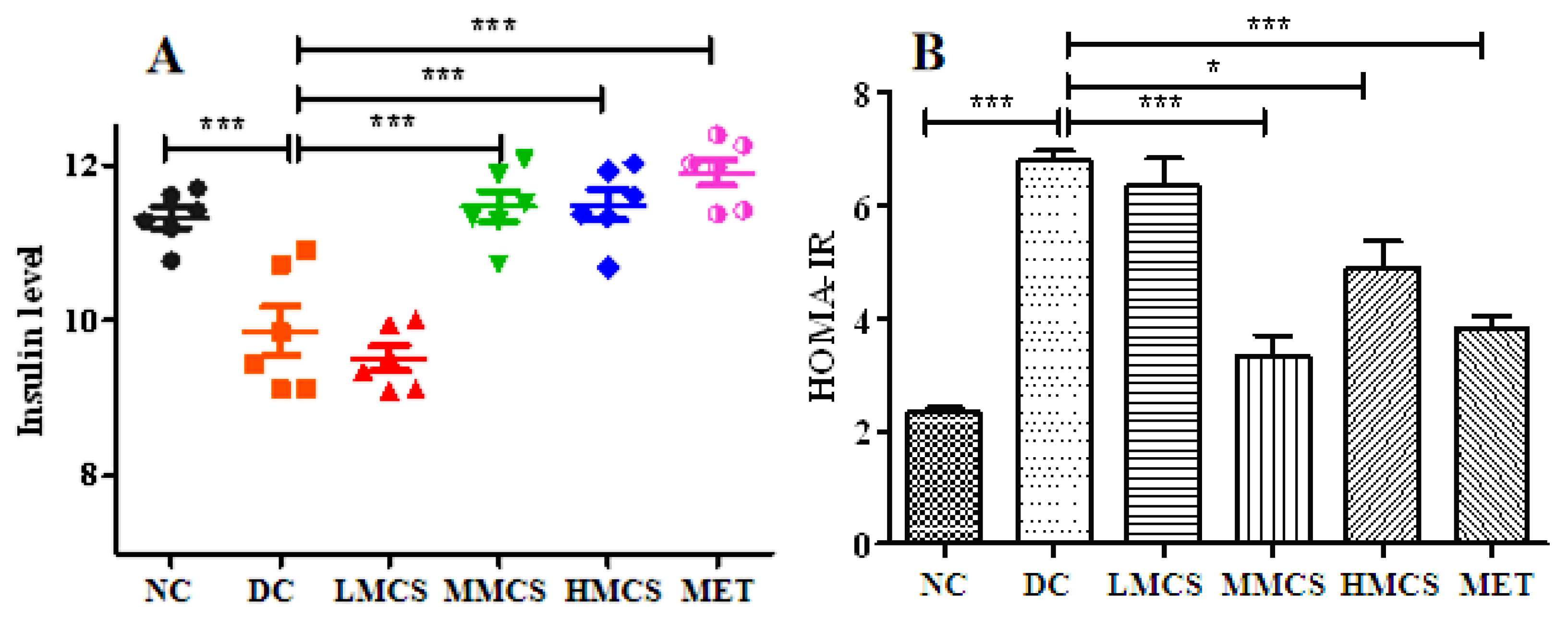
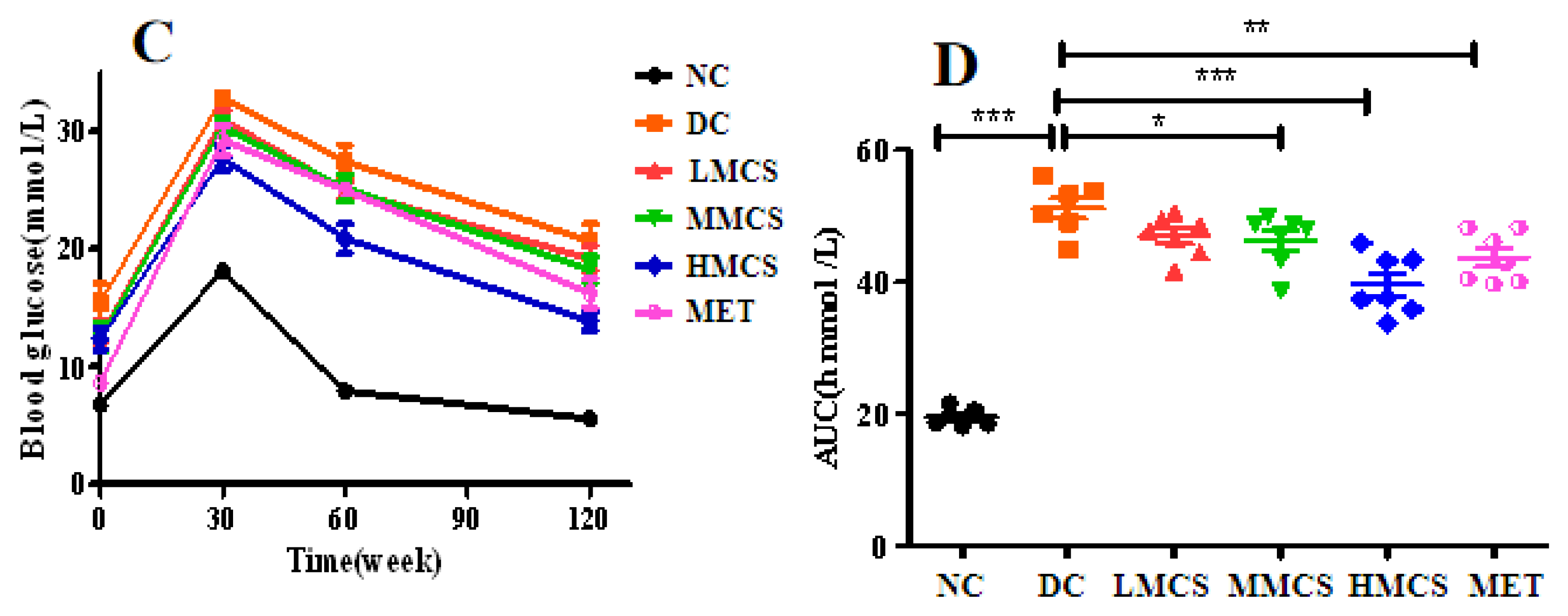
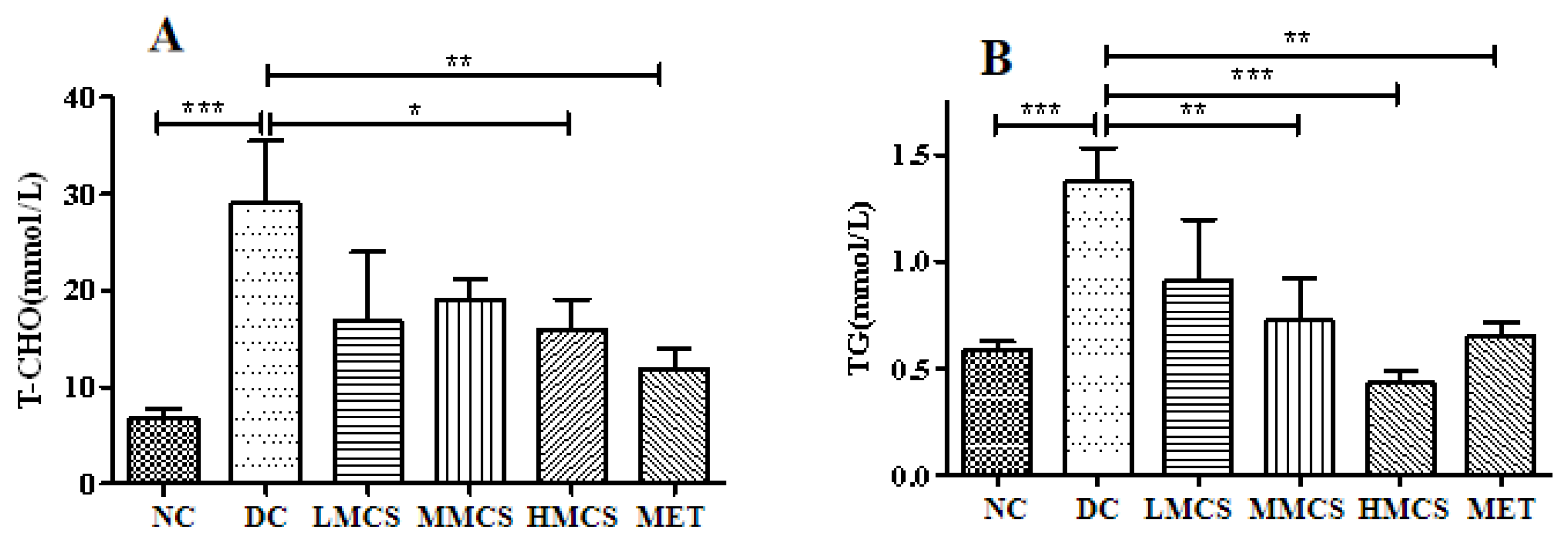
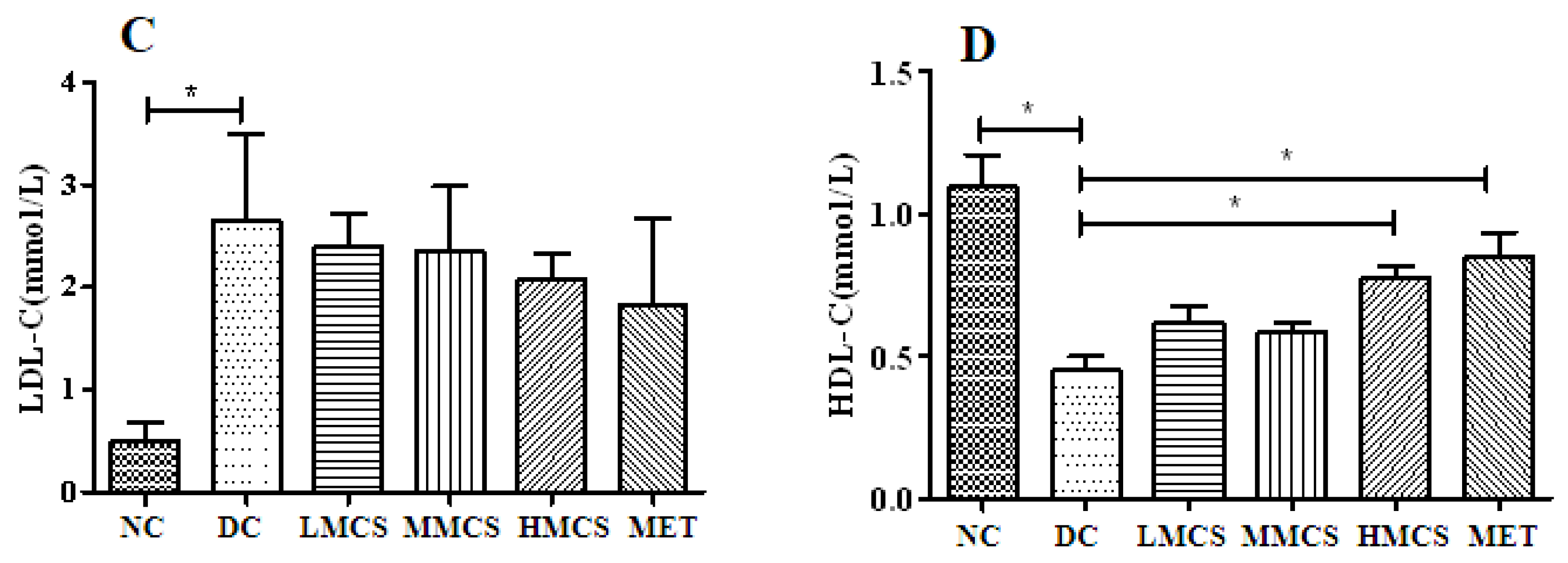
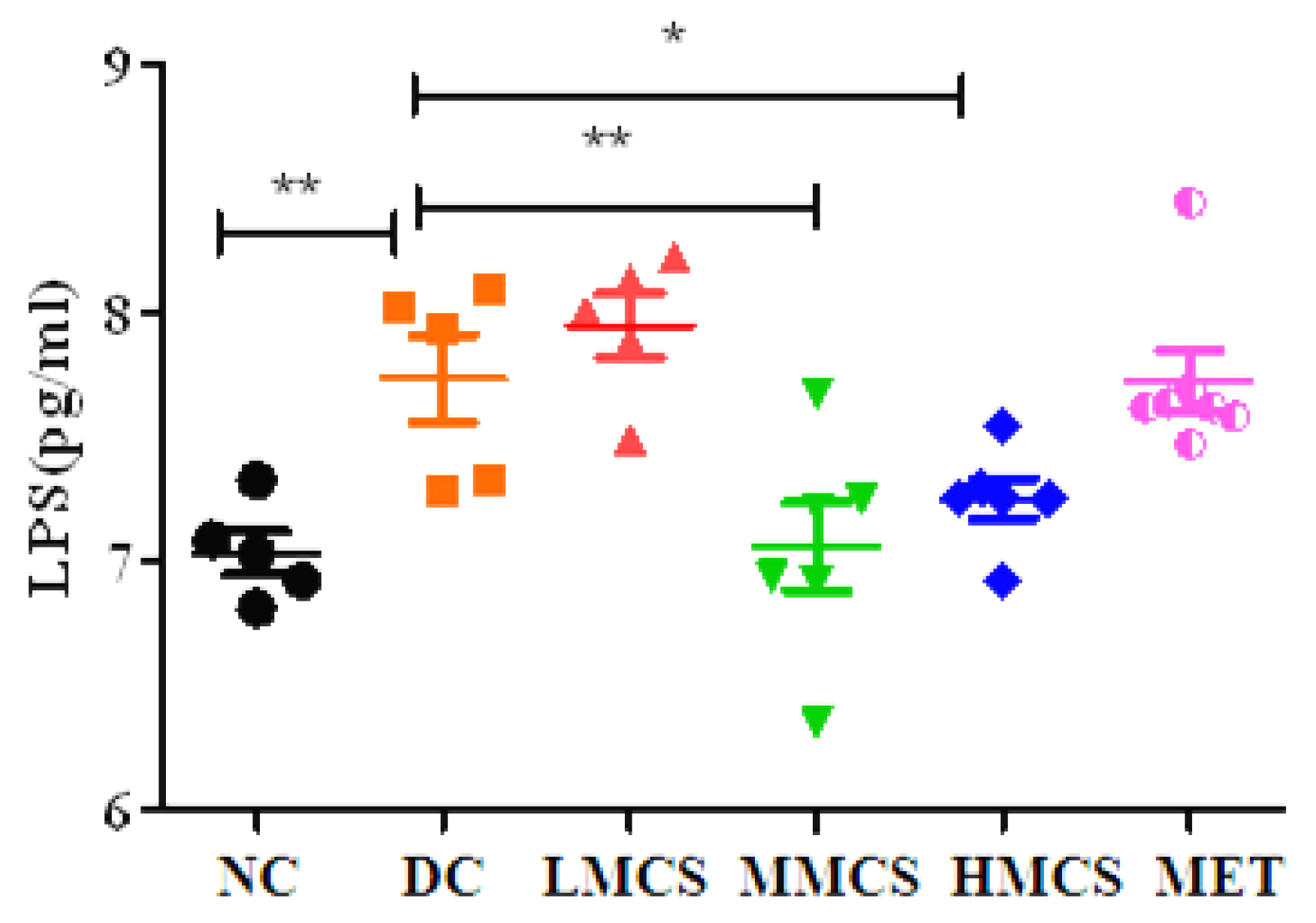
3.1. Effects of MCS on Glucose Homeostasis, Lipid Levels, Chronic Systemic Inflammation, and Pancreatic Histology
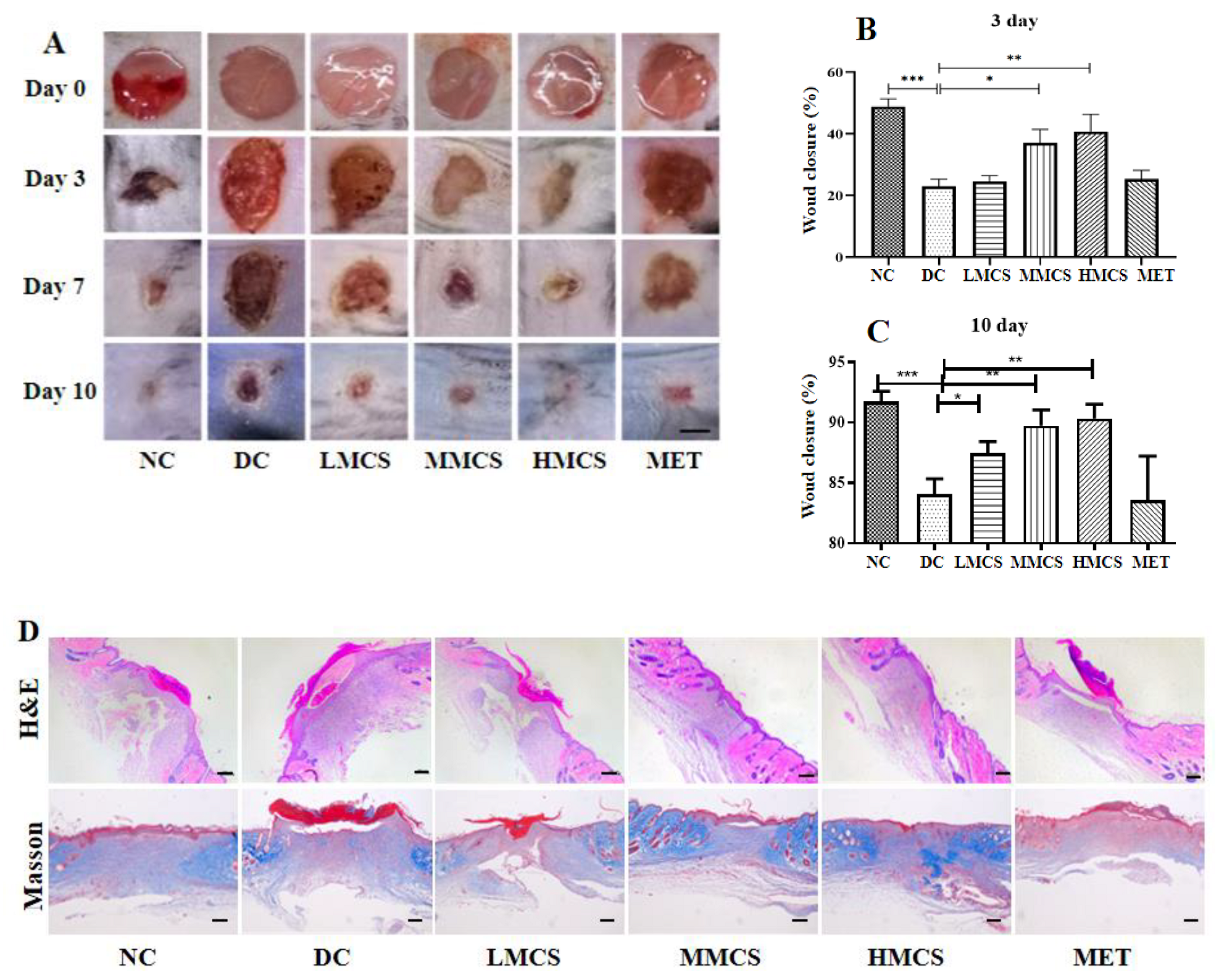
3.2. MCS Promotes Wound Healing in T2DM Mice
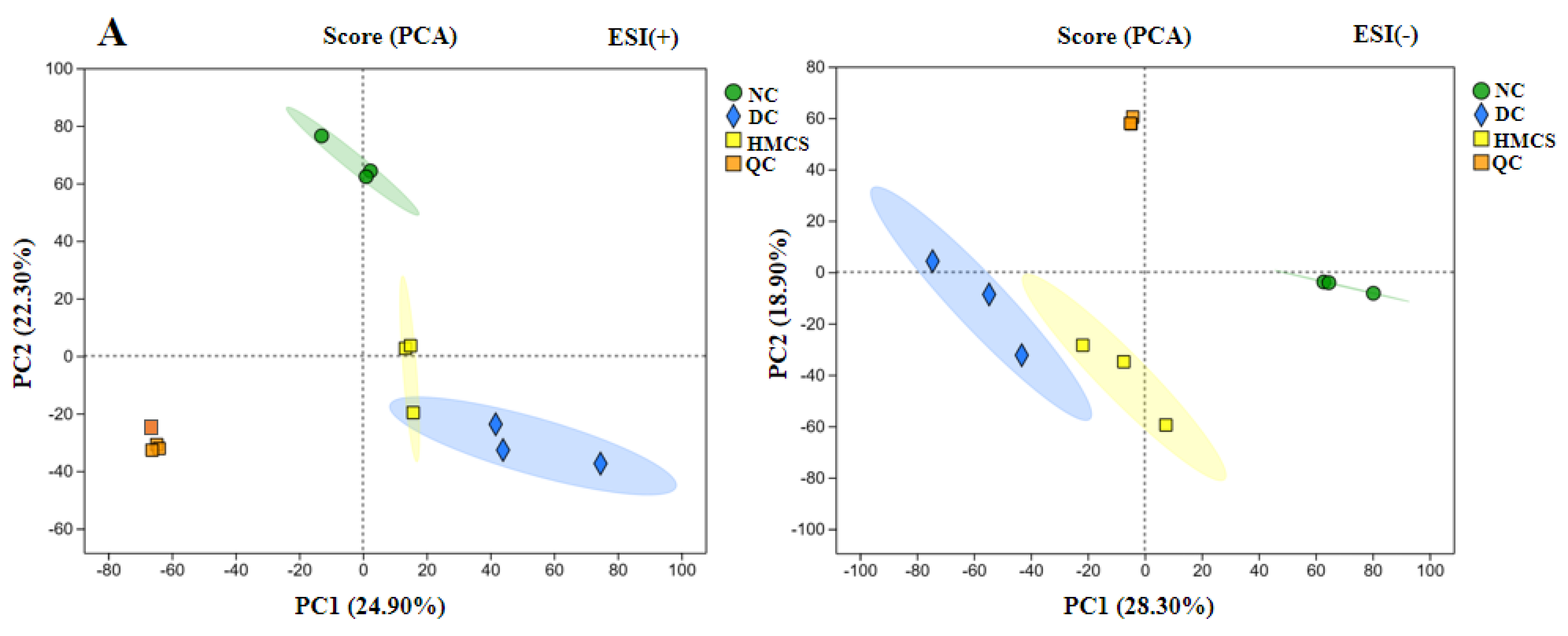
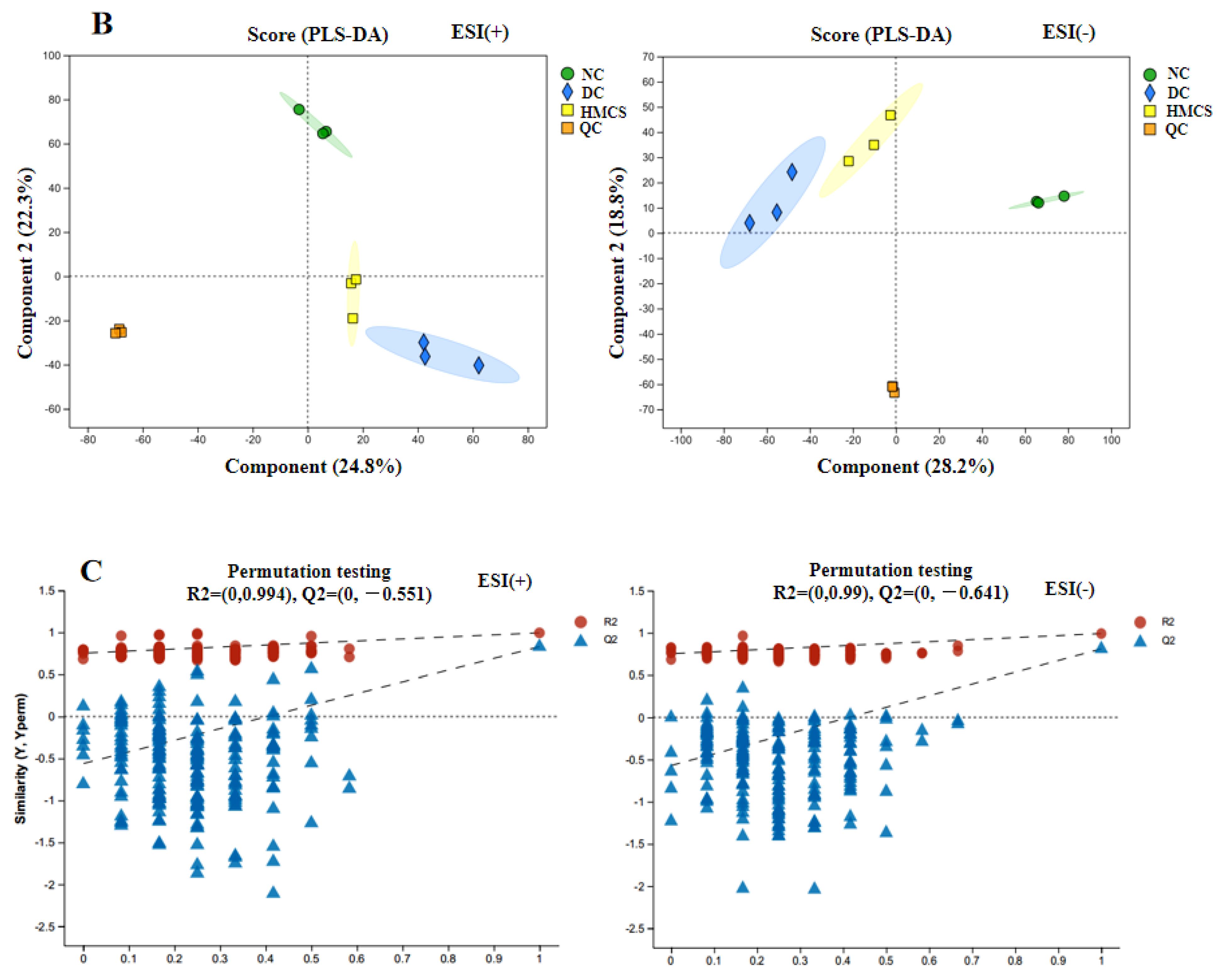
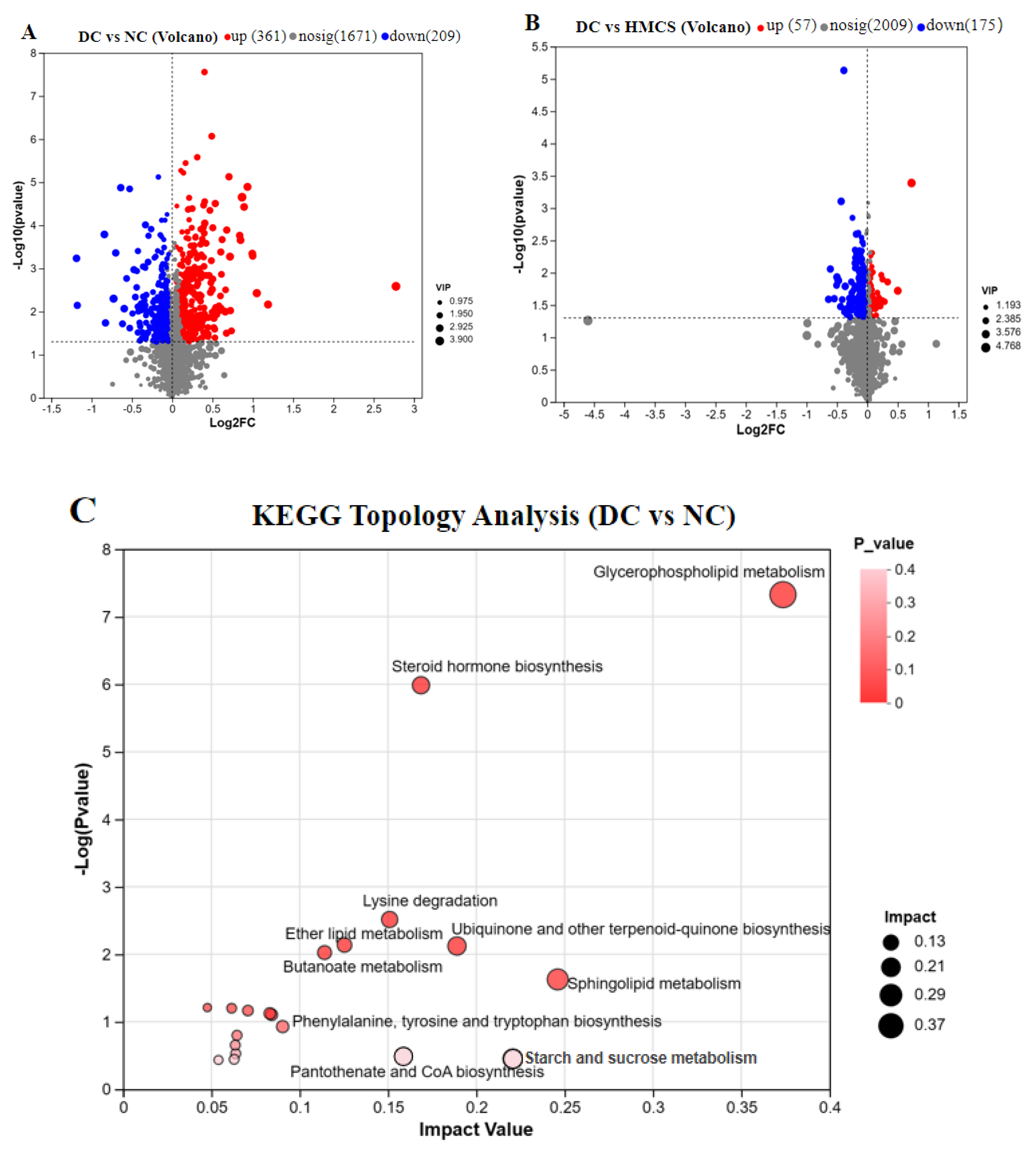
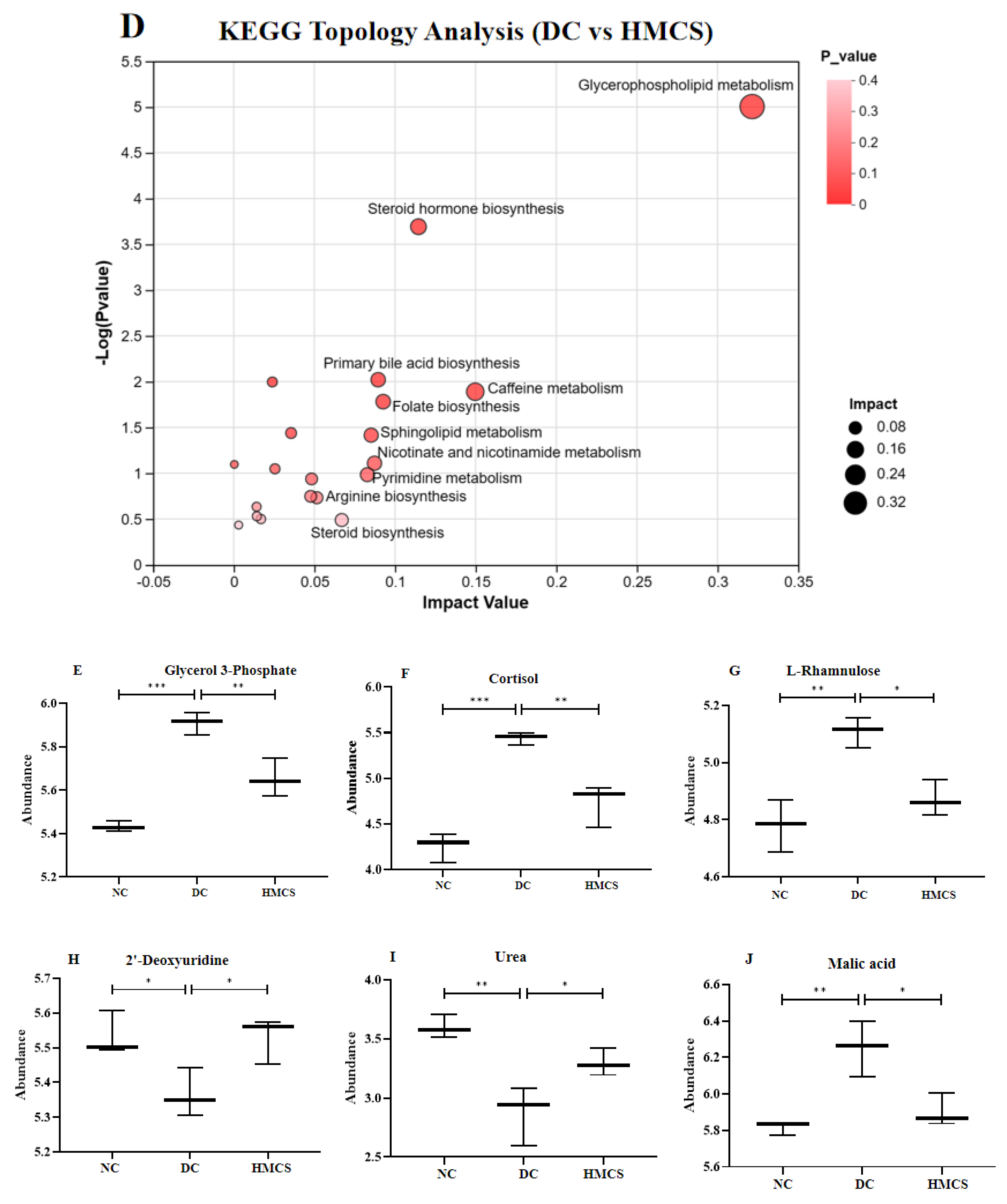
3.3. Effect of MCS on Serum Metabolomics in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | the areas under the glucose-time curve |
| DC | diabetic control |
| FBG | fasting blood glucose |
| FINS | Fasting insulin |
| Gro3P | glycerol-3-phosphate |
| H&E | hematoxylin and eosin |
| HDL-C | high-density lipoprotein cholesterol |
| HMCS | high-dose MCS |
| HOMA-IR | the homeostasis model assessment of insulin resistance index |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LDL-C | low-density lipoprotein cholesterol |
| LMCS | low-dose MCS |
| LPS | lipopolysaccharides |
| NC | normal control |
| MC | Momordica charantia L. |
| MCS | Momordica charantia L. saponins |
| MMCS | medium-dose MCS |
| MET | metformin |
| OPLS-DA | orthogonal partial least squares discrimination analysis |
| PCA | principal component analysis |
| PLS-DA | partial least squares discriminant analysis |
| QC | quality control |
| STZ | Streptozotocin |
| TC | total cholesterol |
| TG | triglycerides |
| T2DM | type 2 diabetes mellitus |
References
- Abusalah, M.A.H.; Albaker, W.; Al-Bsheish, M.; Alsyouf, A.; Al-Mugheed, K.; Issa, M.R.; Alumran, A. Prevalence of type 2 diabetes mellitus in the general population of Saudi Arabia, 2000–2020: A systematic review and meta-analysis of observational studies. Saudi J. Med. Med. Sci. 2023, 11, 1–10. [Google Scholar]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers: A review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech. 2019, 9, 4. [Google Scholar]
- Kasole, R.; Martin, H.D.; Kimiywe, J. Traditional medicine and its role in the management of diabetes mellitus: “Patients’ and herbalists’ perspectives”. Evid. Based Complement. Altern. Med. 2019, 2019, 2835691. [Google Scholar] [CrossRef]
- Ahmed, R.; Khan, N.A.; Waseem, M.; Khan, J. Phytochemical, medicinal properties and pharmacological studies on bitter gourd (Momordica charantia): A Review. Int. J. Adv. Pharm. Med. Bioal. Sci. 2017, 5, 173–179. [Google Scholar]
- Dandawate, P.R.; Subramaniam, D.; Padhye, S.B.; Anant, S. Bitter melon: A panacea for inflammation and cancer. Chin. J. Nat. Med. 2016, 14, 81–100. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, L.; Li, J.; Wu, D.; Li, C.; Zheng, X.; Zhang, H.; Miao, L.; Ge, X. Momordica charantia saponins administration in low-protein-high-carbohydrate diet improves growth, blood biochemical, intestinal health and microflora composition of juvenile common carp (Cyprinus carpio). Fish Shellfish Immunol. 2023, 140, 108980. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Liu, G.; Zhou, P.; Li, P.; Zhao, Z.; Zhang, R.; Tang, X.; Wang, Z.; Wei, Z.; et al. Saponins from Momordica charantia exert hypoglycemic effect in diabetic mice by multiple pathways. Food Sci. Nutr. 2023, 11, 7626–7637. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, L.; Xu, Y.; Guo, Y.; Wei, L.; Li, X.; Song, W. Antidiabetic effect of Momordica charantia saponins in rats induced by high-fat diet combined with STZ. Electron. J Biotechnol. 2020, 43, 41–47. [Google Scholar] [CrossRef]
- Teoh, S.L.; Latiff, A.A.; Das, S. The effect of topical extract of Momordica charantia (bitter gourd) on wound healing in nondiabetic rats and in rats with diabetes induced by streptozotocin. Clin. Exp. Dermatol. 2009, 34, 815–822. [Google Scholar] [CrossRef]
- Singh, R.; Garcia-Gomez, I.; Gudehithlu, K.P.; Singh, A.K. Bitter melon extract promotes granulation tissue growth and angiogenesis in the diabetic wound. Adv. Skin Wound Care 2017, 30, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Aljohi, A.; Matou-Nasri, S.; Liu, D.; Al-Khafaji, N.; Slevin, M.; Ahmed, N. Momordica charantia extracts protect against inhibition of endothelial angiogenesis by advanced glycation endproducts in vitro. Food Funct. 2018, 9, 5728–5739. [Google Scholar] [CrossRef] [PubMed]
- Monni, G.; Atzori, L.; Corda, V.; Dessolis, F.; Iuculano, A.; Hurt, K.J.; Murgia, F. Metabolomics in prenatal medicine: A review. Front. Med. 2021, 8, 645118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, H.Y.; Park, C.S.; Pyo, M.K.; Yune, T.Y.; Kim, G.W.; Chung, S.H. GS-KG9 ameliorates diabetic neuropathic pain induced by streptozotocin in rats. J. Ginseng Res. 2019, 43, 58–67. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, H.; Han, J.; Dai, X.; Lv, Y.; Sun, T.; Liu, H. Microbiota-derived imidazole propionate inhibits type 2 diabetic skin wound healing by targeting SPNS2-mediated S1P transport. iScience 2023, 26, 108092. [Google Scholar] [CrossRef]
- Vladu, I.M.; Forțofoiu, M.; Clenciu, D.; Forțofoiu, M.C.; Pădureanu, R.; Radu, L.; Cojan, Ș.; Rădulescu, P.M.; Pădureanu, V. Insulin resistance quantified by the value of HOMA-IR and cardiovascular risk in patients with type 2 diabetes. Exp. Ther. Med. 2022, 23, 73. [Google Scholar] [CrossRef]
- Liu, T.; Wang, D.; Zhou, X.; Song, J.; Yang, Z.; Shi, C.; Li, R.; Zhang, Y.; Zhang, J.; Yan, J.; et al. Study on the mechanism of American ginseng extract for treating type 2 diabetes mellitus based on metabolomics. Front. Pharmacol. 2022, 13, 960050. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, A.; Ma, W.; Liu, X.; Luo, P.; Zhan, M.; Zhou, X.; Chen, L.; Zhang, J. Lactobacillus gasseri CKCC1913 mediated modulation of the gut–liver axis alleviated insulin resistance and liver damage induced by type 2 diabetes. Food Funct. 2023, 14, 8504–8520. [Google Scholar] [CrossRef]
- Okdahl, T.; Wegeberg, A.M.; Pociot, F.; Brock, B.; Størling, J.; Brock, C. Low-grade inflammation in type 2 diabetes: A cross-sectional study from a Danish diabetes outpatient clinic. BMJ Open 2022, 12, e062188. [Google Scholar] [CrossRef]
- Li, G.; Huang, P.; Cui, S.S.; Tan, Y.Y.; He, Y.C.; Shen, X.; Jiang, Q.Y.; Huang, P.; He, G.Y.; Li, B.Y.; et al. Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl. Neurodegener. 2022, 11, 6. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, Y.; Liu, H.; Zhang, Y.; Wei, Z.; Liu, G.; Tang, X.; Jia, X. Structure determination, bitterness evaluation and hepatic gluconeogenesis inhibitory activity of triterpenoids from the Momordica charantia fruit. Food Chem. 2022, 372, 131224. [Google Scholar] [CrossRef] [PubMed]
- Possik, E.; Madiraju, S.M.; Prentki, M. Glycerol-3-phosphate phosphatase/PGP: Role in intermediary metabolism and target for cardiometabolic diseases. Biochimie 2017, 143, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lieu, E.L.; Kelekar, N.; Bhalla, P.; Kim, J. Fructose and mannose in inborn errors of metabolism and cancer. Metabolites 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Pektas, M.B.; Koca, H.B.; Sadi, G.; Akar, F. Dietary fructose activates insulin signaling and inflammation in adipose tissue: Modulatory role of resveratrol. BioMed Res. Int. 2016, 2016, 8014252. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Maurya, N.K. The cortisol connection: Weight gain and stress hormones. Arch. Pharm. Pharm. Sci. 2024, 8, 9–13. [Google Scholar]
- Luqman, A.; Götz, F. The ambivalent role of skin microbiota and adrenaline in wound healing and the interplay between them. Int. J. Mol. Sci. 2021, 22, 4996. [Google Scholar] [CrossRef]
- Wu, Z.G.; Yin, W.X.; Luo, H.L.; Si, Y.K.; Sun, M.Q.; Liao, L.C. Metabolic abnormalities associated with ketamine-associated bladder toxicity based on metabolomics. Acta Pharm. Sin. 2020, 1849–1854. [Google Scholar]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol. 2015, 10, 1444–1458. [Google Scholar] [CrossRef]
- Li, X.; Ban, Z.; Yu, F.; Hao, W.; Hu, X. Untargeted metabolic pathway analysis as an effective strategy to connect various nanoparticle properties to nanoparticle-induced ecotoxicity. Environ. Sci. Technol. 2020, 54, 3395–3406. [Google Scholar] [CrossRef]
- Trichez, D.; Auriol, C.; Baylac, A.; Irague, R.; Dressaire, C.; Carnicer-Heras, M.; Heux, S.; François, J.M.; Walther, T. Engineering of Escherichia coli for Krebs cycle-dependent production of malic acid. Microb. Cell Fact. 2018, 17, 113. [Google Scholar] [CrossRef]
| Group | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| NC | 4.71 ± 0.83 a | 4.27 ± 0.41 a | 4.16 ± 0.85 a | 4.78 ± 0.32 a | 4.88 ± 0.65 a |
| DC | 14.54 ± 1.99 b | 19.28 ± 5.40 b | 15.54 ± 0.84 b | 17.64 ± 2.15 b | 15.58 ± 1.02 b |
| LMCS | 14.65 ± 1.58 b | 17.90 ± 2.00 b | 12.84 ± 2.86 b | 14.14 ± 2.80 c | 13.66 ± 1.36 c |
| MMCS | 14.30 ± 1.12 b | 14.82 ± 1.21 b | 12.64 ± 1.76 b | 11.36 ± 0.96 c | 7.20 ± 0.48 d |
| HMCS | 14.60 ± 1.92 b | 17.67 ± 1.93 b | 13.56 ± 2.59 b | 12.48 ± 1.72 c | 9.52 ± 1.54 e |
| MET | 14.58 ± 2.84 b | 19.16 ± 1.78 b | 12.70 ± 2.48 b | 12.40 ± 2.67 c | 8.56 ± 0.23 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Luo, Z.; Li, S.; Liu, S.; Wang, C. Metabolomics Revealed the Effects of Momordica charantia L. Saponins on Diabetic Hyperglycemia and Wound Healing in Mice. Foods 2024, 13, 3163. https://doi.org/10.3390/foods13193163
Zhao D, Luo Z, Li S, Liu S, Wang C. Metabolomics Revealed the Effects of Momordica charantia L. Saponins on Diabetic Hyperglycemia and Wound Healing in Mice. Foods. 2024; 13(19):3163. https://doi.org/10.3390/foods13193163
Chicago/Turabian StyleZhao, Dengdeng, Zixuan Luo, Shasha Li, Shuwen Liu, and Chan Wang. 2024. "Metabolomics Revealed the Effects of Momordica charantia L. Saponins on Diabetic Hyperglycemia and Wound Healing in Mice" Foods 13, no. 19: 3163. https://doi.org/10.3390/foods13193163






