The Role of Lactic Acid Bacteria in Meat Products, Not Just as Starter Cultures
Abstract
1. Introduction
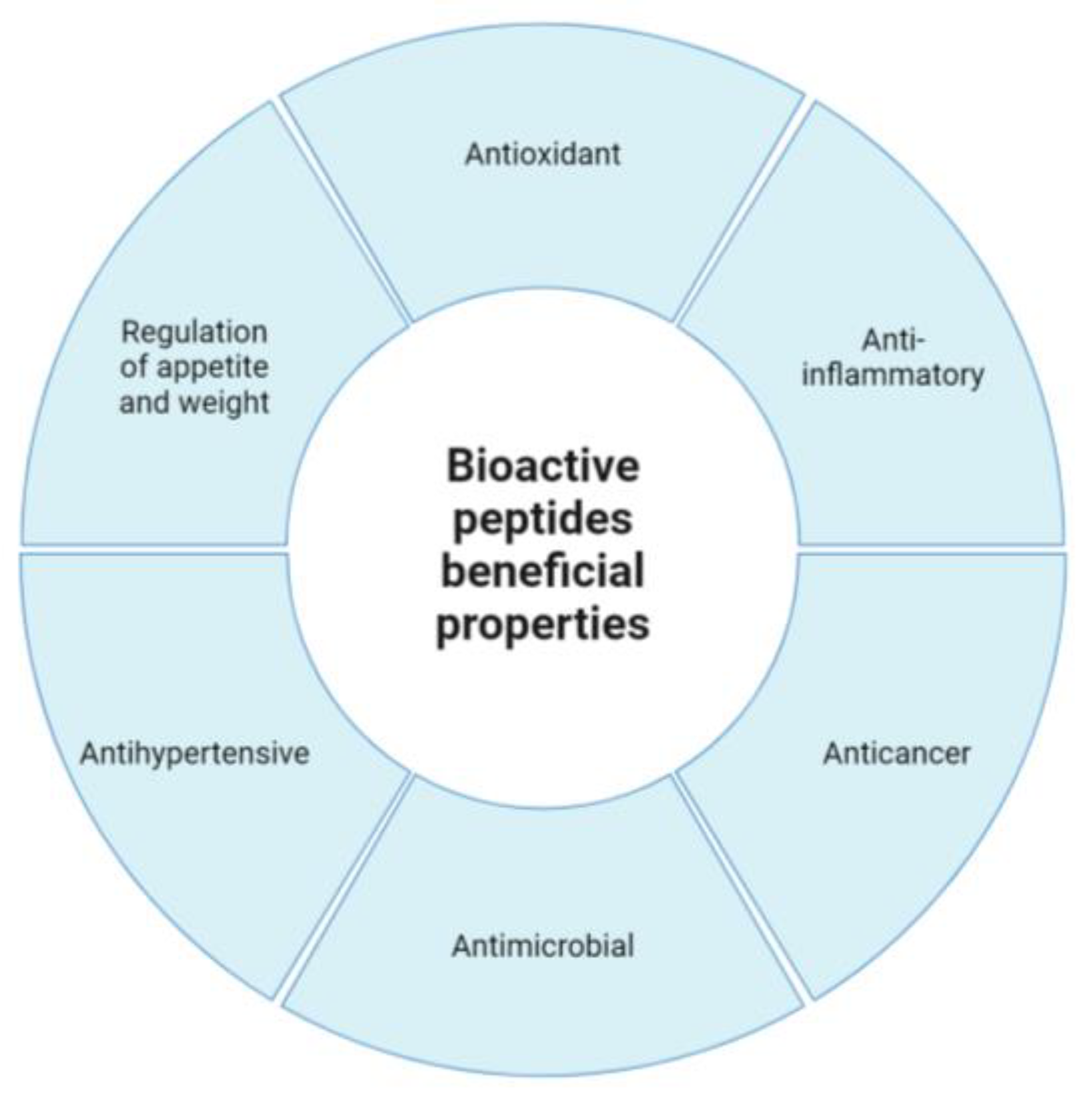
2. Bioactive Peptides
3. Exopolysaccharides
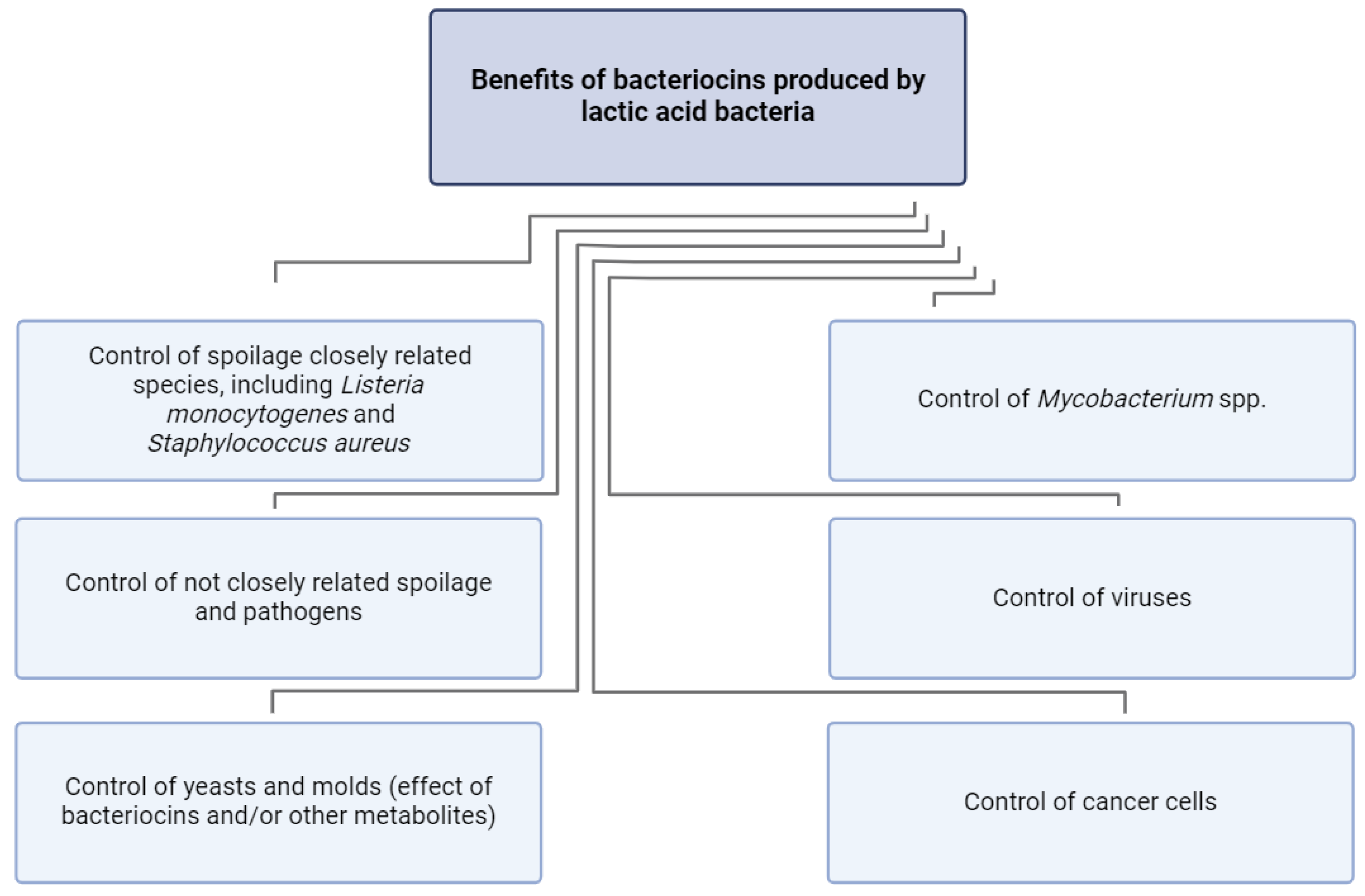
4. Bacteriocins
5. Antioxidant Properties
6. Reduction of Chemical Additives
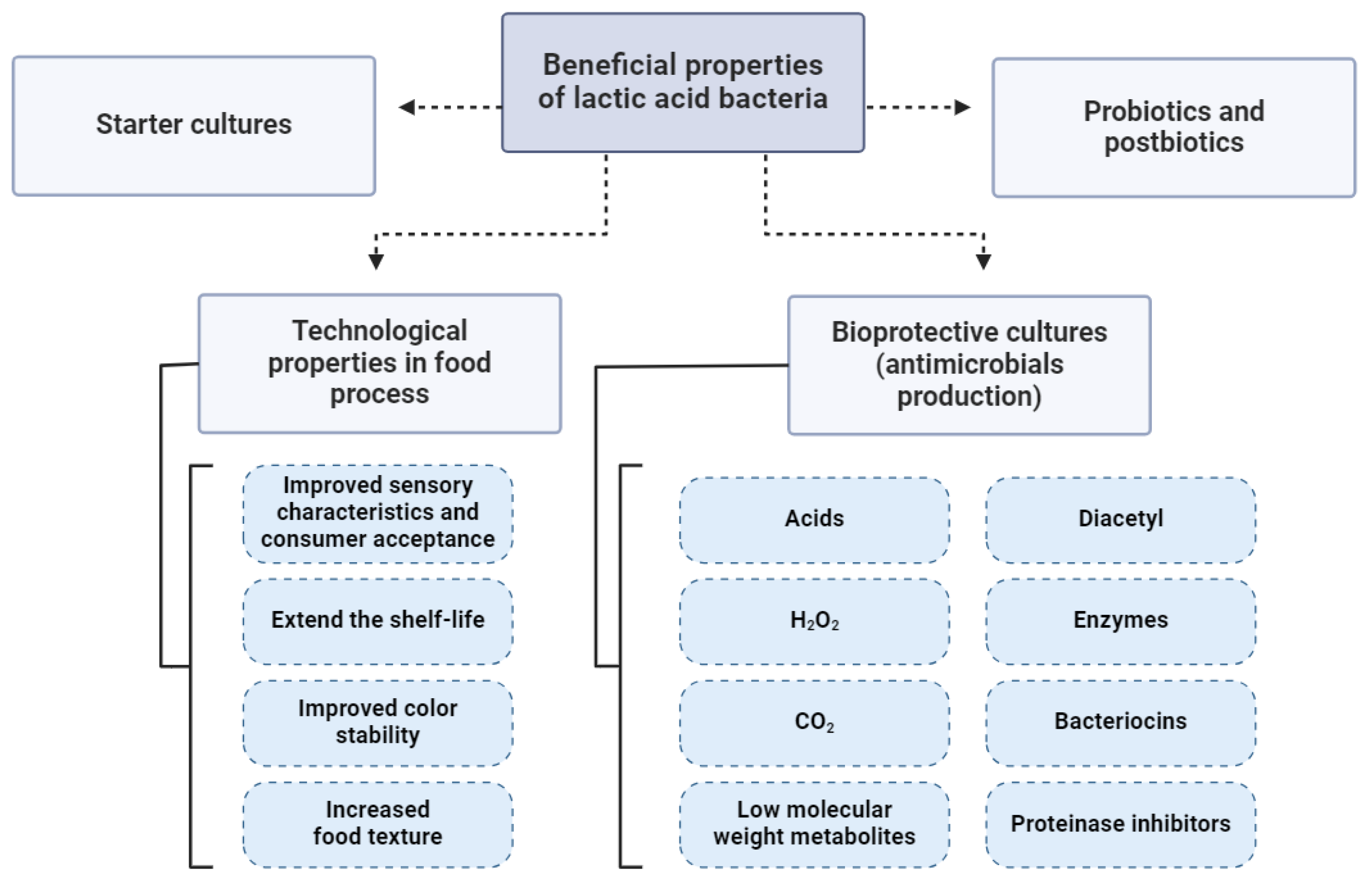
7. Biotechnological and Technological Properties of LABs in Fermented Meat Products
8. Enrichment with GABA
9. Other Beneficial Metabolites
10. Essential Steps in the Selection of LABs for Application in Meat Products
11. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Baretto Penna, A.L.; Venema, K.; Holzapfel, W.H.; Chikindas, M.L. Recommendations for the use of standardized abbreviations for the former Lactobacillus genera, reclassified in the year 2020. Benef. Microbes 2023, 15, 1–4. [Google Scholar] [CrossRef]
- Obayomi, O.V.; Olaniran, A.F.; Owa, S.O. Unveiling the role of functional foods with emphasis on prebiotics and probiotics in human health: A review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Povolotsky, T.L.; Barazany, H.L.; Shacham, Y.; Kolodkin-Gal, I. Bacterial epigenetics and its implication for agriculture, probiotics development, and biotechnology design. Biotechnol. Adv. 2024, 75, 108414. [Google Scholar] [CrossRef]
- Vu, J.; Carvalho, J. Enterococcus: Review of its physiology, pathogenesis, diseases and the challenges it poses for clinical microbiology. Front. Biol. 2011, 6, 357–366. [Google Scholar] [CrossRef]
- Lannes-Costa, P.S.; de Oliveira, J.S.S.; da Silva Santos, G.; Nagao, P.E. A current review of pathogenicity determinants of Streptococcus sp. J. Appl. Microbiol. 2021, 131, 1600–1620. [Google Scholar] [CrossRef] [PubMed]
- Abouloifa, A.; Gaamouche, S.; Ghabbour, N.; Guerrouj, B.E.; Karboune, S.; Saalaoui, E.; Asehraou, A. Lactic acid bacteria from Moroccan traditional foods: Techno-functional, health-promoting, nutraceutical value and application as a starter and bio-preservative agent in the food products. Biores. Tech. Rep. 2024, 27, 101941. [Google Scholar] [CrossRef]
- Guo, H.; Chen, J.; Qiu, H.; Yang, W.; Li, G.; Ma, X.; Liu, J.; Yin, Q.; Zhu, Q. Compound probiotics starter: A solution for aflatoxin B1 reduction and meat quality improvement in fermented chicken jerky. Food Cont. 2024, 165, 110601. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, Y.; Zhu, J.; Wen, R.; Chen, Q.; Kong, B. Technological characterization and flavor-producing potential of lactic acid bacteria isolated from traditional dry fermented sausages in northeast China. Food Microbiol. 2022, 106, 104059. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wang, H.; Song, X.; Xu, N.; Sun, J.; Xu, X. Effects of different mixed starter cultures on microbial communities, taste and aroma compounds of traditional Chinese fermented sausages. Food Chem. X 2024, 21, 101225. [Google Scholar] [CrossRef]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive review of bioactive compounds from lactic acid bacteria: Potential functions as functional food in dietetics and the food industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Al-Dhaheri, A.S.; Mahadin, S.A.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. J. Dairy. Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef]
- Rangel, A.H.d.N.; Bezerra, D.A.F.V.d.A.; Sales, D.C.; Araújo, E.d.O.M.; Lucena, L.M.d.; Porto, A.L.F.; Véras, Í.V.U.M.; Lacerda, A.F.; Ribeiro, C.V.D.M.; Anaya, K. An overview of the occurrence of bioactive peptides in different types of cheeses. Foods 2023, 12, 4261. [Google Scholar] [CrossRef]
- Mada, S.B.; Ugwu, C.P.; Abarshi, M.M. Health promoting effects of food-derived bioactive peptides: A review. Int. J. Pept. Res. Ther. 2020, 26, 831–848. [Google Scholar] [CrossRef]
- Atanasova, J.; Dalgalarrondo, M.; Iliev, I.; Moncheva, P.; Todorov, S.D.; Ivanova, I.V. Formation of free amino acids and bioactive peptides during the ripening of Bulgarian white brined cheeses. Prob. Antim. Prot. 2021, 13, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Jafar, S.; Mushtaq, M.; Mudgil, P. Bioactive peptides derived from different sources. In Food Biopolymers: Structural, Functional and Nutraceutical Properties; Gani, A., Ashwar, B.A., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Majura, J.J.; Cao, W.; Chen, Z.; Htwe, K.K.; Li, W.; Du, R.; Zhang, P.; Zheng, H.; Gao, J. The current research status and strategies employed to modify food-derived bioactive peptides. Front. Nutr. 2022, 9, 950823. [Google Scholar] [CrossRef]
- Yılmaz, T.; Şimşek, Ö. Potential health benefits of ropy exopolysaccharides produced by Lactobacillus plantarum. Molecules 2020, 25, 3293. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Lopes Neto, J.H.P.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar] [CrossRef]
- Bhandary, T.; Kurian, C.; Muthu, M.; Anand, A.; Anand, T.; Paari, K.A. Exopolysaccharides derived from probiotic bacteria and their health benefits. J. Pure Appl. Microbiol. 2023, 17, 35–50. [Google Scholar] [CrossRef]
- Salimi, F.; Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef] [PubMed]
- Niyigaba, T.; Liu, D.; Habimana, J.d.D. The Extraction, Functionalities and applications of plant polysaccharides in fermented foods: A review. Foods 2021, 10, 3004. [Google Scholar] [CrossRef] [PubMed]
- Monro, J.A. Digestible and non-digestible polysaccharide roles in reformulating foods for health. In Reformulation as a Strategy for Developing Healthier Food Products; Raikos, V., Ranawana, V., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, N.; Hao, M.; Zhou, J.; Xie, Y.; He, Z. Plant-derived polysaccharides regulated immune status, gut health and microbiota of broilers: A review. Front. Vet. Sci. 2022, 8, 791371. [Google Scholar] [CrossRef]
- Shin, S.Y.; Han, N.S. Leuconostoc spp. as starters and their beneficial roles in fermented foods. In Beneficial Microorganisms in Food and Nutraceuticals; Liong, M.T., Ed.; Microbiology Monographs; Springer: Cham, Switzerland, 2015; Volume 27. [Google Scholar] [CrossRef]
- Choi, G.H.; Holzapfel, W.H.; Todorov, S.D. Diversity of the bacteriocins, their classification and potential applications in combat of antibiotic resistant and clinically relevant pathogens. Crit. Rev. Microbiol. 2023, 49, 578–597. [Google Scholar] [CrossRef]
- Todorov, S.D.; de Melo Franco, B.D.; Tagg, J.R. Bacteriocins of Gram-positive bacteria having activity spectra extending beyond closely-related species. Benef. Microb. 2019, 10, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, Y.; Li, P.; Shang, N. Lactobacillus plantarum derived nanoparticles deliver class IIa bacteriocin as a potential natural food preservatives. LWT-Food Sci. Technol. 2024, 207, 116675. [Google Scholar] [CrossRef]
- Maity, C.; Gupta, A.K. Therapeutic efficacy of probiotic Alkalihalobacillus clausii 088AE in antibiotic-associated diarrhea: A randomized controlled trial. Heliyon 2021, 7, e07993. [Google Scholar] [CrossRef] [PubMed]
- Begenda, D.K.; Yamazaki, K. Application of bacteriocins in food preservation and safety. Food 2007, 1, 137–148. Available online: https://www.academia.edu/24866379/Application_of_Bacteriocins_in_Food_Preservation_and_Safety (accessed on 18 September 2024).
- Correia, L.F.; Pinho, G.S.; Neves, T.J.C.; Vieira, K.C.O.; Maddela, N.R.; Prasad, R.; Winkelstroter, L.K. Nanotechnology innovation combined with bacteriocins as emerging strategy for the development of active and intelligent food packaging. Sust. Chem. Pharm. 2024, 39, 101551. [Google Scholar] [CrossRef]
- Thapar, P.; Kumar Salooja, M. Bacteriocins: Applications in Food Preservation and Therapeutics; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Silva, S.P.M.; Teixeira, J.A.; Silva, C.C.G. Application of enterocin-whey films to reduce Listeria monocytogenes contamination on ripened cheese. Food Microbiol. 2023, 109, 104134. [Google Scholar] [CrossRef]
- Haider, T.; Pandey, V.; Behera, C.; Kumar, P.; Gupta, P.N.; Soni, V. Nisin and nisin-loaded nanoparticles: A cytotoxicity investigation. Drug Devel. Ind. Pharm. 2022, 48, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial activities of bacteriocins: Application in food and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Manivel, B.; Rajkumar, S. Bacteriocin: A Potential Biopreservative in Foods. In Recent Developments in Microbial Technologies; Prasad, R., Kumar, V., Singh, J., Upadhyaya, C.P., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Banerji, R.; Karkee, A.; Saroj, S.D. Bacteriocins against foodborne pathogens (Review). Appl. Biochem. Microbiol. 2022, 58, 518–539. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Casquete, R.; Fonseca, S.C.; Pinto, R.; Castro, S.M.; Todorov, S.; Teixeira, P.; Vaz-Velho, M. Evaluation of the microbiological safety and sensory quality of a sliced cured-smoked pork product with protective cultures addition and modified atmosphere packaging. Food Sci. Technol. Int. 2019, 25, 327–336. [Google Scholar] [CrossRef]
- De Azevedo, P.O.S.; Mendonça, C.M.N.; Seibert, L.; Domínguez, J.M.; Converti, A.; Gierus, M.; Oliveira, R.P.S. Bacteriocin-like inhibitory substance of Pediococcus pentosaceus as a biopreservative for Listeria sp. control in ready-to-eat pork ham. Braz. J. Microbiol. 2020, 51, 949–956. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Nanda, P.K.; Pateiro, M.; Lorenzo, J.M.; Dhar, P.; Das, A.K. Lactic acid bacteria and bacteriocins: Novel biotechnological approach for biopreservation of meat and meat products. Microorganisms 2022, 10, 2058. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Fact. 2014, 13, S3. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Balay, D.R.; Dangeti, R.V.; Kaur, K.; McMullen, L.M. Purification of Leucocin A for use on wieners to inhibit Listeria monocytogenes in the presence of spoilage organisms. Int. J. Food Microbiol. 2017, 255, 25–31. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Antimicrobial antagonists against food pathogens: A bacteriocin perspective. Curr. Opin. Food Sci. 2015, 2, 51–57. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Todorov, S.D. Bacteriocinogenic LAB strains for fermented meat preservation: Perspectives, challenges, and limitations. Prob. Antim. Prot. 2017, 9, 444–458. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Ananou, S.; Garriga, M.; Jofre, A.; Aymerich, T.; Galvez, A.; Maqueda, M.; Martínez-Bueno, M.; Valdivia, E. Combined effect of enterocin AS-48 and high hydrostatic pressure to control food-borne pathogens inoculated in low acid fermented sausages. Meat Sci. 2010, 84, 594–600. [Google Scholar] [CrossRef]
- Chakchouk-Mtibaa, A.; Smaoui, S.; Ktari, N.; Sellem, I.; Najah, S.; Karray-Rebai, I.; Mellouli, L. Biopreservative efficacy of bacteriocin BacFL31 in raw ground Turkey meat in terms of microbiological, physicochemical, and sensory qualities. Biocontrol Sci. 2017, 22, 67–77. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Hammou, B.F.; Skali, N.; Idaomar, M.; Abrini, J. The antimicrobial effect of origanum compactum essential oil, nisin and their combination against Escherichia coli in tryptic soy broth (TSB) and in sheep natural sausage casings during storage at 25 and 7 °C. Afr. J. Biotechnol. 2011, 10, 15998–16005. [Google Scholar] [CrossRef]
- Kumar, Y.; Kaur, K.; Shahi, A.K.; Kairam, N.; Tyagi, S.K. Antilisterial, antimicrobial and antioxidant effects of pediocin and Murraya koenigii berry extract in refrigerated goat meat emulsion. LWT-Food Sci. Technol. 2017, 79, 135–144. [Google Scholar] [CrossRef]
- Melero, B.; Diez, A.M.; Rajkovic, A.; Jaime, I.; Rovira, J. Behavior of non-stressed and stressed Listeria monocytogenes and Campylobacter jejuni cells on fresh chicken burger meat packaged under modified atmosphere and inoculated with protective culture. Int. J. Food Microbiol. 2012, 158, 107–112. [Google Scholar] [CrossRef] [PubMed]
- De Martinez, Y.B.; Ferrer, K.; Salas, E.M. Combined effects of lactic acid and nisin solution in reducing levels of microbiological contamination in red meat carcasses. J. Food Prot. 2002, 65, 1780–1783. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Belfiore, C.; Vignolo, G. Combination of bioprotective cultures with EDTA to reduce Escherichia coli O157:H7 in frozen ground-beef patties. Food Control 2011, 22, 1461–1465. [Google Scholar] [CrossRef]
- Gumienna, M.; Górna, B. Antimicrobial food packaging with biodegradable polymers and bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Di Martino, V.; Ferranti, P.; Picariello, L.; Villani, F. Technological properties and bacteriocins production by Lactobacillus curvatus 54M16 and its use as starter culture for fermented sausage manufacture. Food Control 2016, 59, 31–45. [Google Scholar] [CrossRef]
- De Castilho, N.P.A.; Todorov, S.D.; Oliveira, L.L.; Bersot, L.d.S.; Nero, L.A. Inhibition of Listeria monocytogenes in fresh sausage by bacteriocinogenic Lactobacillus curvatus UFV-NPAC1 and its semi-purified bacteriocin. LWT-Food Sci. Technol. 2020, 118, 108757. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Todorov, S.D.; Ivanova, I.; Chobert, J.-M.; Haertlé, T.; Franco, B.D.G.M. Improving safety of salami by application of bacteriocins produced by an autochthonous Lactobacillus curvatus isolate. Food Microbiol. 2015, 46, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.; Silva, J.; Casquete, R.; Queirós, R.; Saraiva, J.A.; Teixeira, P. Combined effect of pediocin bacHA-6111-2 and high hydrostatic pressure to control Listeria innocua in fermented meat sausage. Int. Food Res. J. 2018, 25, 553–560. [Google Scholar]
- Giello, M.; La Storia, A.; De Filippis, F.; Ercolini, D.; Villani, F. Impact of Lactobacillus curvatus 54M16 on microbiota composition and growth of Listeria monocytogenes in fermented sausages. Food Microbiol. 2018, 72, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.C.; Esteves, C.T.; Palazzo, I.C.V.; Darini, A.L.C.; Felis, G.E.; Sechi, L.A.; Franco, B.D.G.M.; De Martinis, E.C.P. Prevalence and characterization of Enterococcus spp. isolated from Brazilian foods. Food Microbiol. 2008, 25, 668–675. [Google Scholar] [CrossRef]
- Dal Bello, B.; Rantsiou, K.; Bellio, A.; Zeppa, G.; Ambrosoli, R.; Civera, T.; Cocolin, L. Microbial ecology of artisanal products from north west of Italy and antimicrobial activity of the autochthonous populations. LWT-Food Sci. Technol. 2010, 43, 1151–1159. [Google Scholar] [CrossRef]
- Castro, M.P.; Palavecino, N.Z.; Herman, C.; Garro, O.A.; Campos, C.A. Lactic acid bacteria isolated from artisanal dry sausages: Characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat Sci. 2011, 87, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Cocconcelli, P.S.; Vignolo, G.; Saavedra, L. Occurrence of antilisterial structural bacteriocins genes in meat borne lactic acid bacteria. Food Control 2015, 47, 53–59. [Google Scholar] [CrossRef]
- Yu, W.; Guo, J.; Liu, Y.; Xue, X.; Wang, X.; Wei, L.; Ma, J. Potential impact of combined inhibition by bacteriocins and chemical substances of foodborne pathogenic and spoilage bacteria: A review. Foods 2023, 12, 3128. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Ben Said, L.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024, 22, 556–571. [Google Scholar] [CrossRef]
- Gu, Q. Bacteriocinogenic lactic acid bacteria and antibacterial mechanisms. In Bacteriocins; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Pilevar, Z.; Hosseini, H.; Beikzadeh, S.; Khanniri, E.; Alizadeh, A.M. Application of bacteriocins in meat and meat products: An update. Curr. Nutr. Food Sci. 2020, 16, 120–133. [Google Scholar] [CrossRef]
- Smaoui, S.; Echegaray, N.; Kumar, M.; Chaari, M.; D’Amore, T.; Ali Shariati, M.; Rebezov, M.; Lorenzo, J.M. Beyond conventional meat preservation: Saddling the control of bacteriocin and lactic acid bacteria for clean label and functional meat products. Appl. Biochem. Biotechnol. 2024, 196, 3604–3635. [Google Scholar] [CrossRef]
- Todorov, S.D.; Koep, K.S.; Van Reenen, C.A.; Hoffman, L.C.; Slinde, E.; Dicks, L.M. Production of salami from beef, horse, mutton, blesbok (Damaliscus dorcas phillipsi) and springbok (Antidorcas marsupialis) with bacteriocinogenic strains of Lactobacillus plantarum and Lactobacillus curvatus. Meat Sci. 2007, 77, 405–412. [Google Scholar] [CrossRef]
- Ng, Z.J.; Zarin, M.A.; Lee, C.K.; Tan, J.S. Application of bacteriocins in food preservation and infectious disease treatment for humans and livestock: A review. RSC Adv. 2020, 10, 38937–38964. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, M.; Szymański, P.; Zielińska, D. Synergistic effect of combination of various microbial hurdles in the biopreservation of meat and meat products—Systematic review. Foods 2023, 12, 1430. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Chandrakasan, G.; Rodríguez-Hernández, A.I.; del Rocío López-Cuellar, M.; Palma-Rodriguez, H.-M.; Chavarria-Hernandez, N. Bacteriocin encapsulation for food and pharmaceutical applications: Advances in the past 20 years. Biotechnol. Lett. 2019, 41, 453–469. [Google Scholar] [CrossRef]
- Huang, L.; Teng, W.; Cao, J.; Wang, J. Liposomes as delivery system for applications in meat products. Foods 2022, 11, 3017. [Google Scholar] [CrossRef] [PubMed]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as carriers of bioactive compounds in human nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, A.; Ferrari, G.; Donsì, F. The use of nanocellulose in edible coatings for the preservation of perishable fruits and vegetables. Coatings 2021, 11, 990. [Google Scholar] [CrossRef]
- Hegde, S.M.; Soans, S.H.; Mandapaka, R.T.; Siddesha, J.M.; Archer, A.C.; Agbuna, C.; Achar, R.R. Nanomaterials in Food System Application: Biochemical, Preservation, and Food Safety Perspectives. In Application of Nanotechnology in Food Science, Processing and Packaging; Egbuna, C., Jeevanandam, J.C., Patrick-Iwuanyanwu, K., N. Onyeike, E., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Nanda, A.; Pandey, P.; Rajinikanth, P.S.; Singh, N. Revolution of nanotechnology in food packaging: Harnessing electrospun zein nanofibers for improved preservation—A review. Int. J. Biol. Macromol. 2024, 260 Pt 1, 129416. [Google Scholar] [CrossRef]
- Bano, S. The use of nanoparticles in food preservation and processing. Int. J. Health Sci. 2022, 6, 5254–5265. [Google Scholar] [CrossRef]
- Prakash, J.; Vignesh, K.; Anusuya, T.; Kalaivani, T.; Ramachandran, C.; Sudha Rani, R.; Rubab, M.; Elahi, I.K.F.; Oh, D.-H.; Davanand Venkatasubbu, G. Application of nanoparticles in food preservation and food processing. J. Food Hyg. Saf. 2019, 34, 317–324. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Bielicka, M.; Klekotka, U.; Kalska-Szostko, B. Nanoparticle applications in food—A review. Food Funct. 2023, 14, 2544–2567. [Google Scholar] [CrossRef] [PubMed]
- Naseer, B.; Srivastava, G.; Qadri, O.S.; Faridi, S.A.; Islam, R.U.; Younis, K. Importance and health hazards of nanoparticles used in the food industry. Nanotechnol. Rev. 2018, 7, 623–641. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant activity and probiotic properties of lactic acid bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Bryukhanov, A.L.; Klimko, A.I.; Netrusov, A.I. Antioxidant properties of lactic acid bacteria. Microbiology 2022, 91, 463–478. [Google Scholar] [CrossRef]
- Li, W.; Gao, L.; Huang, W.; Ma, Y.; Muhammad, I.; Hanif, A.; Ding, Z.; Guo, X. Antioxidant properties of lactic acid bacteria isolated from traditional fermented yak milk and their probiotic effects on the oxidative senescence of Caenorhabditis elegans. Food Funct. 2022, 13, 3690–3703. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, S.; Hashemi, S.M.B.; Abedi, E.; Amiri, M.J.; Conte, F.L. Bio-preservation of meat and fermented meat products by lactic acid bacteria strains and their antibacterial metabolites. Sustainability 2023, 15, 10154. [Google Scholar] [CrossRef]
- Soyucok, A.; Basyigit, K.; Kilic, B. The potential use of lactic acid bacteria as antioxidant agent in meat products. In Proceedings of the IX International Agricultural Symposium “Agrosym”, Jahorina, Bosnia and Hercegovina, 4–7 October 2018; pp. 1066–1070. [Google Scholar]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research update on the impact of lactic acid bacteria on the substance metabolism, flavor, and quality characteristics of fermented meat products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Lu, Q.; Sun, L.; Du, S.; Liu, T.; Hou, M.; Ge, G.; Wang, Z.; Jia, Y. Effects of lactic acid bacteria additives on the quality, volatile chemicals and microbial community of Leymus chinensis silage during aerobic exposure. Front. Microbiol. 2022, 13, 938153. [Google Scholar] [CrossRef]
- Rachwał, K.; Gustaw, K. Lactic acid bacteria in sustainable food production. Sustainability 2024, 16, 3362. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canini, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The current and future perspectives of postbiotics. Prob. Antim. Prot. 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.R.; Herrero, A.M.; Jumenez-Colmenero, F.; Menezes, C.R.; Fries, L.L.M. Application of probiotic delivery systems in meat products. Trends Food Sci. Technol. 2015, 43, 120–131. [Google Scholar] [CrossRef]
- Martin, J.G.P.; de Dea Lindner, J. Microbiologia de Alimentos Fermentados, Editora Blucher: Sao Paulo, SP, Brazil, 2022; 552p.
- Zdolec, N.; Mikuš, T.; Kiš, M. Chapter 8—Lactic acid bacteria in meat fermentation: Dry sausage safety and quality. In Lactic Acid Bacteria in Food Biotechnology; Ray, R.C., Paramithiotis, S., de Carvalho Azevedo, V.A., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–159. Available online: https://www.sciencedirect.com/science/article/pii/B9780323898751000079 (accessed on 18 September 2024).
- Kröckel, L. The Role of Lactic Acid Bacteria in Safety and Flavor Development of Meat and Meat Products; IntechOpen: London, UK, 2013; Available online: https://www.intechopen.com/chapters/42316 (accessed on 18 September 2024).
- Buckenhüskes, H.J. Selection criteria for lactic acid bacteria to be used as starter cultures for various food commodities. FEMS Microbiol. Rev. 1993, 12, 253–271. [Google Scholar] [CrossRef]
- EFSA. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2022.7666 (accessed on 18 September 2024).
- Barbut, S. Effects of chemical acidification and microbial fermentation on the rheological properties of meat products. Meat Sci. 2005, 71, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Spaziani, M.; Torre, M.D.; Stecchini, M.L. Changes of physicochemical, microbiological, and textural properties during ripening of Italian low-acid sausages. Proteolysis, sensory and volatile profiles. Meat Sci. 2009, 81, 77–85. [Google Scholar] [CrossRef]
- Toldrá, F.; Aristoy, M.C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.S.; Santos, M.R.R.M.; Schwan, R.F. Enumeration, identification and safety proprieties of lactic acid bacteria isolated from pork sausage. Arq. Bras. Med. Vet. Zootec. 2015, 67, 918–926. [Google Scholar] [CrossRef][Green Version]
- Landeta, G.; Curiel, J.A.; Carrascosa, A.V.; Muñoz, R.; de las Rivas, B. Technological and safety properties of lactic acid bacteria isolated from Spanish dry-cured sausages. Meat Sci. 2013, 95, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Sampayo, M.; González, P.; Lombó, F.; Díaz, J. Physicochemical, sensory and microbiological characterization of Asturian Chorizo, a traditional fermented sausage manufactured in Northern Spain. Meat Sci. 2019, 156, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Borovic, B.; Velebit, B.; Moracanin, S.V.; Lakicevic, B.; Baltic, T. Molecular characterization of lactic acid bacteria in Levačka sausage. Procedia Food Sci. 2015, 5, 14–17. [Google Scholar] [CrossRef]
- Diez-Gutierrez, L.; Vicente, L.S.; Barron, L.J.R.; Villaran, M.C.; Chavarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Nejati, F.; Rizzello, C.G.; Di Cagno, R.; Sheikh-Zeinoddin, M.; Diviccaro, A.; Minervini, F.; Gobbetti, M. Manufacture of a functional fermented milk enriched of Angiotensin-I Converting Enzyme (ACE)-inhibitory peptides and γ-amino butyric acid (GABA). LWT Food Sci. Technol. 2013, 51, 183–189. [Google Scholar] [CrossRef]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psych. 2011, 16, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Turkheimer, F.E.; Leech, R.; Expert, P.; Lord, L.-D.; Vernon, A.C. The brain’s code and its canonical computational motifs. From sensory cortex to the default mode network, a multi-scale model of brain function in health and disease. Neurosci. Biobehav. Rev. 2015, 55, 211–222. [Google Scholar] [CrossRef]
- Abdelazez, A.; Abdelmotaal, H.; Evivie, S.E.; Melak, S.; Jia, F.-F.; Khoso, M.H.; Meng, X.-C. Screening potential probiotic characteristics of Lactobacillus brevis strains in vitro and intervention effect on Type I diabetes in vivo. BioMed Res. Int. 2018, 2018, 7356173. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, C.; Liu, C.; Xie, F.; Zhu, K.; Hu, S.Y. Gamma-Aminobutyric Acid Binds to GABAB Receptor to inhibit cholangiocarcinoma cells growth via the JAK/STAT3 pathway. Dig. Dis. Sci. 2013, 58, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Tanibe, R.; Mori, M.; Take, H.; Michihata, T.; Yano, T.; Takahashi, H.; Kimura, B. Microbial and chemical properties of aji-no-susu, a traditional fermented fish with rice product in the Noto Peninsula, Japan. Fish. Sci. 2009, 75, 1499–1506. [Google Scholar] [CrossRef]
- Ratanaburee, A.; Kantachote, D.; Charernjiratrakul, W.; Sukhoom, A. Selection of γ-aminobutyric acid-producing lactic acid bacteria and their potential as probiotics for use as starter cultures in Thai fermented sausages (Nham). Int. J. Food Sci. Technol. 2013, 48, 1371–1382. [Google Scholar] [CrossRef]
- Thuy, B.D.T.; Nguyen, T.A.; Vandamme, P. Isolation, screening, identification and optimization of culture parameters to produce γ-aminobutyric acid by Lactiplantibacillus pentosus R13, an isolate from ruoc (fermented shrimp paste). Appl. Food Biotechnol. 2022, 9, 1–8. [Google Scholar] [CrossRef]
- López, M.S.; Sciarini, L.S.; Salvucci, E.J.; Pérez, G.T. Autochthonous lactic acid bacteria in gluten-free sourdoughs produce nutritional and technological improvements in quinoa and buckwheat breads. Int. J. Gastron. Food Sci. 2024, 37, 100970. [Google Scholar] [CrossRef]
- Joshi, T.J.; Salini, S.V.; Mohan, L.; Nandagopal, P.; Arakal, J.J. Functional metabolites of probiotic lactic acid bacteria in fermented dairy products. Food Humanit. 2024, 3, 100341. [Google Scholar] [CrossRef]
- Aouadhi, C.; Turki, M.; Maaroufi, A. Screening and characterization of lactic acid bacteria with antibacterial effect against heat-resistant spore outgrowth of Bacillus sporothermodurans. Int. Dairy. J. 2024, 158, 106047. [Google Scholar] [CrossRef]
- Figueroa, R.H.H.; López-Malo, A.; Mani-López, E. Antimicrobial activity and applications of fermentation from lactic acid bacteria—A review. Sust. Food Technol. 2024, 2, 292–306. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Mozuriene, E.; Krungleviciute, V.; Novoslavskij, A.; Santini, A.; Rozentale, I.; Juodeikiene, G.; Cizeikiene, D. The impact of lactic acid bacteria with antimicrobial properties on biodegradation of polycyclic aromatic hydrocarbons and biogenic amines in cold smoked pork sausages. Food Control 2017, 71, 285–292. [Google Scholar] [CrossRef]
- Coban, H.B. Organic acids as antimicrobial food agents: Applications and microbial productions. Bioproc. Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Nychas, G.J.E.; Luo, X.; Zhu, L.; Mao, Y.; Dong, P.; Zhang, Y. Utilizing lactic acid bacteria and their metabolites for controlling Listeria monocytogenes in meat products: Applications, limitations, and future perspectives. Trends Food Sci. Technol. 2024, 152, 104699. [Google Scholar] [CrossRef]
- Molina, G.E.S.; Shetty, R.; Xiao, H.; Watjen, A.P.; Tovar, M.; Bang-Berthelsen, C.H. Development of a novel lactic acid bacteria starter culture approach: From insect microbiome to plant-based fermentations. LWT Food Sci. Technol. 2022, 167, 113797. [Google Scholar] [CrossRef]
- Kang, D.; Fung, D.Y.C. Effect of diacetyl on controlling Escherichia coli O157:H7 and Salmonella Typhimurium in the presence of starter culture in a laboratory medium and during meat fermentation. J. Food Protect. 1999, 62, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L. Microbial bioprotection: An opportunity to improve safety and quality of meat products in a sustainable way. Meat Sci. 2024, 2024, 109576. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Laiño, J.E.; Juarez del Valle, M.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; Font de Valdez, G.; Savoy de Giori, G.; Sesma, F. B-Group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; Lopes, P.; Spano, G. Lactic acid bacteria producing B-group vitamins: A great potential for functional cereals products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Recent update on lactic acid bacteria producing riboflavin and folates: Application for food fortification and treatment of intestinal inflammation. J. Appl. Microbiol. 2021, 130, 1412–1424. [Google Scholar] [CrossRef]
- Peng, X.; Ed-Dra, A.; Yue, M. Whole genome sequencing for the risk assessment of probiotic lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2023, 63, 11244–11262. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, K.O.; Campos, G.Z.; Scafuro Lima, J.M.; Rocha, R.d.S.; Vaz-Velho, M.; Todorov, S.D. The Role of Lactic Acid Bacteria in Meat Products, Not Just as Starter Cultures. Foods 2024, 13, 3170. https://doi.org/10.3390/foods13193170
Carneiro KO, Campos GZ, Scafuro Lima JM, Rocha RdS, Vaz-Velho M, Todorov SD. The Role of Lactic Acid Bacteria in Meat Products, Not Just as Starter Cultures. Foods. 2024; 13(19):3170. https://doi.org/10.3390/foods13193170
Chicago/Turabian StyleCarneiro, Kayque Ordonho, Gabriela Zampieri Campos, João Marcos Scafuro Lima, Ramon da Silva Rocha, Manuela Vaz-Velho, and Svetoslav Dimitrov Todorov. 2024. "The Role of Lactic Acid Bacteria in Meat Products, Not Just as Starter Cultures" Foods 13, no. 19: 3170. https://doi.org/10.3390/foods13193170
APA StyleCarneiro, K. O., Campos, G. Z., Scafuro Lima, J. M., Rocha, R. d. S., Vaz-Velho, M., & Todorov, S. D. (2024). The Role of Lactic Acid Bacteria in Meat Products, Not Just as Starter Cultures. Foods, 13(19), 3170. https://doi.org/10.3390/foods13193170







