Microbial Food Safety of Sous Vide Cooking Processes of Chicken and Eggs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains
2.2. Foods Preparation
2.3. Sous Vide Cooking
2.4. Microbial Counts and Investigations
2.5. Statistical Analysis
3. Results and Discussion
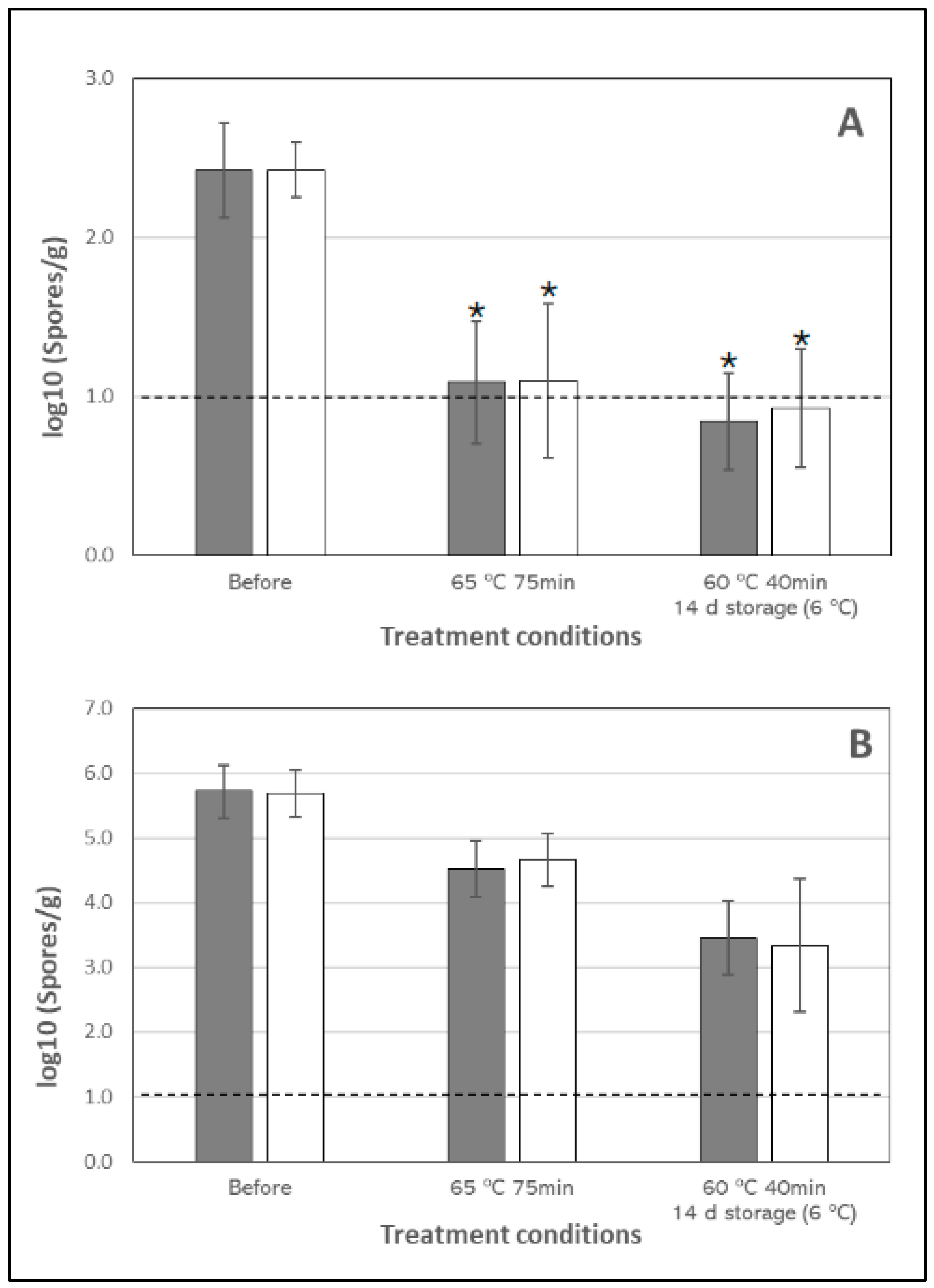
3.1. Inactivation of Clostridum Spores in Chicken Breast
3.2. Inactivation of Campylobacter in Chicken Breast
3.3. Effect on Natural Flora of Chicken Breast
3.4. Inactivation of Salmonella in Egg (Omelet)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Zhang, X.; Mason, S.L.; Bekhit, A.E.A. Sous-vide cooking improves the quality and in-vitro digestibility of Semitendinosus from culled dairy cows. Food Res. Int. 2020, 127, 108708. [Google Scholar] [CrossRef]
- Suleman, R.; Wang, Z.; Aadil, R.M.; Hui, T.; Hopkins, D.L.; Zhang, D. Effect of cooking on the nutritive quality, sensory properties and safety of lamb meat: Current challenges and future prospects. Meat Sci. 2020, 167, 108172. [Google Scholar] [CrossRef]
- Farber, J.M.; Dodds, K. Principles of Modified-Atmosphere and Sous Vide Product Packaging, 1st ed.; Routledge: Lancaster, PA, USA, 2018. [Google Scholar]
- EFSA; ECDC. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Armstrong, G.A.; McIlveen, H. Effects of prolonged storage on the sensory quality and consumer acceptance of sous vide meat-based recipe dishes. Food Qual. Prefer. 2000, 11, 377–385. [Google Scholar] [CrossRef]
- Ramirez, N.; Abel-Santos, E. Requirements for Germination of Clostridium sordellii Spores In Vitro. J. Bacteriol. 2010, 192, 418–425. [Google Scholar] [CrossRef]
- Food Safety Authority of Ireland. Sous Vide and Food Safety. Catering Factsheet Series 2014. Available online: https://www.fsai.ie/publications/sous-vide-and-food-safety (accessed on 11 July 2024).
- Onyeaka, H.; Nwabor, O.; Jang, S.; Obileke, K.; Hart, A.; Anumudu, C.; Miri, T. Sous vide processing: A viable approach for the assurance of microbial food safety. J. Sci. Food Agric. 2022, 102, 3503–3512. [Google Scholar] [CrossRef]
- James, S.J.; James, C. Minimal Processing of Ready Meals (Chapter 32). In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 599–612. [Google Scholar] [CrossRef]
- Stringer, S.C.; Metris, A. Predicting bacterial behaviour in sous vide food. Int. J. Gastron. Food Sci. 2018, 13, 117–128. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Juneja, V.K.; Jones, D.R.; Purohit, A. Thermal Inactivation Kinetics of Three Heat-Resistant Salmonella Strains in Whole Liquid Egg. J. Food Prot. 2019, 82, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, K.A.; Tiwari, U.; Cagney, C.; Walsh, D.; McDowell, D.A.; Duffy, G. Modelling the thermal inactivation of five Campylobacteraceae species. Food Control 2015, 47, 135–140. [Google Scholar] [CrossRef]
- Abe, H.; Kawasaki, S. Modeling strain variability in Campylobacter jejuni thermal inactivation by quantifying the number of strains required. Int. J. Food Microbiol. 2024, 414, 110618. [Google Scholar] [CrossRef]
- McIntyre, L.; Jorgenson, V.; Ritson, M. Sous vide style cooking practices linked to Salmonella Enteritidis illnesses. Environ. Health Rev. 2017, 60, 42–49. [Google Scholar] [CrossRef]
- Pandita, G.; Bhosale, Y.K.; Choudhary, P. Sous Vide: A Proposition to Nutritious and Superior Quality Cooked Food. ACS Food Sci. Technol. 2023, 3, 592–599. [Google Scholar] [CrossRef]
- Kathuria, D.; Dhiman, A.K.; Attri, S. Sous vide, a culinary technique for improving quality of food products: A review. Trends Food Sci. Technol. 2022, 119, 57–68. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Kim, C.-H.; Joo, S.-T. Control of sous-vide physicochemical, sensory, and microbial properties through the manipulation of cooking temperatures and times. Meat Sci. 2022, 188, 108787. [Google Scholar] [CrossRef]
- Kilibarda, N.; Brdar, I.; Baltic, B.; Markovic, V.; Mahmutovic, H.; Karabasil, N.; Stanisic, S. The safety and quality of sous vide food. Meat Technol. 2018, 59, 38–45. [Google Scholar] [CrossRef]
- Vila-Brugalla, M. Microbiological Safety of Sous-Vide Treatments at Mild Temperatures Applied to Pork Loin. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- González-Fandos, E.; García-Linares, M.C.; Villarino-Rodríguez, A.; García-Arias, M.T.; García-Fernández, M.C. Evaluation of the microbiological safety and sensory quality of rainbow trout (Oncorhynchus mykiss) processed by the sous vide method. Food Microbiol. 2004, 21, 193–201. [Google Scholar] [CrossRef]
- Jeong, K.; Hyeonbin, O.; Shin, S.Y.; Kim, Y.S. Effects of sous-vide method at different temperatures, times and vacuum degrees on the quality, structural, and microbiological properties of pork ham. Meat Sci. 2018, 143, 1–7. [Google Scholar] [CrossRef]
- Bolton, D.J.; McMahon, C.M.; Doherty, A.M.; Sheridan, J.J.; McDowell, D.A.; Blair, I.S.; Harrington, D. Thermal Inactivation of Listeria monocytogenes and Yersinia enterocolitica in Minced Beef under Laboratory Conditions and in Sous-Vide Prepared Minced and Solid Beef Cooked in a Commercial Retort. J. Appl. Microbiol. 2000, 88, 626–632. [Google Scholar] [CrossRef]
- Zavadlav, S.; Blažić, M.; Van de Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Kurp, L.; Danowska-Oziewicz, M.; Kłębukowska, L. Sous Vide Cooking Effects on Physicochemical, Microbiological and Sensory Characteristics of Pork Loin. Appl. Sci. 2022, 12, 2365. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Buonocunto, G.; Puleo, S.; Di Monaco, R.; Anastasio, A.; Vuoso, V.; Smaldone, G.; Baselice, M.; Capuano, F.; et al. The Sous Vide Cooking of Mediterranean Mussel (Mytilus galloprovincialis): Safety and Quality Assessment. Foods 2023, 12, 2900. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Nieto, G.; Garrido, M.D.; Bañón, S. Microbial, physical–chemical and sensory spoilage during the refrigerated storage of cooked pork loin processed by the sous vide method. Meat Sci. 2008, 80, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.; Antequera, T.; Martín, A.; Mayoral, A.I.; Ruiz, J. Effect of different temperature–time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Sci. 2013, 9, 572–578. [Google Scholar] [CrossRef]
- Abel, T.; Boulaaba, A.; Lis, K.; Abdulmawjood, A.; Plötz, M.; Becker, A. Inactivation of Listeria monocytogenes in game meat applying sous vide cooking conditions. Meat Sci. 2020, 167, 108164. [Google Scholar] [CrossRef]
- Khalid, T.; Hdaifeh, A.; Federighi, M.; Cummins, E.; Boué, G.; Guillou, S.; Tesson, V. Review of Quantitative Microbial Risk Assessment in Poultry Meat: The Central Position of Consumer Behavior. Foods 2020, 9, 1661. [Google Scholar] [CrossRef]
- Hasani, E.; Kiskó, G.; Dalmadi, I.; Hitka, G.; Friedrich, L.F.; Kenesei, G. Effect of Two-Step Sous Vide Cooking and Storage on Microbiological and Oxidative Stability of Chicken Breast. Foods 2023, 12, 1213. [Google Scholar] [CrossRef]
- Haghighi, H.; Belmonte, A.M.; Masino, F.; Minelli, G.; Lo Fiego, D.P.; Pulvirenti, A. Effect of Time and Temperature on Physicochemical and Microbiological Properties of Sous Vide Chicken Breast Fillets. Appl. Sci. 2021, 11, 3189. [Google Scholar] [CrossRef]
- Song, D.H.; Yang, N.E.; Seomoon, K.M.; Jang, I.S.; Chin, K.B.; Kim, H.W. Sous-vide cooking as a practical strategy to improve quality attributes and shelf stability of reduced-salt chicken breast ham. Poult. Sci. 2023, 102, 102444. [Google Scholar] [CrossRef]
- Kowalska, J.; Miarka, D.; Marzec, A.; Ciurzy’nska, A.; Janowicz, M.; Galus, S.; Kowalska, H. Sous-Vide as an Innovative and Alternative Method of Culinary Treatment of Chicken Breast in Terms of Product Quality and Safety. Appl. Sci. 2023, 13, 3906. [Google Scholar] [CrossRef]
- Shimojima, T.; Shimojima, H.; Morita, Y. Survival of Campylobacter jejuni, Salmonella, and Listeria monocytogenes and Temperature Change in Low-Temperature–Longtime-Cooked Chicken Meat. J. Food Prot. 2022, 85, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Koukou, I.; Stergioti, T.; la Cour, R.; Gkogka, E.; Dalgaard, P. Clostridium sporogenes as surrogate for proteolytic C. botulinum—Development and validation of extensive growth and growth-boundary model. Food Microbiol. 2022, 107, 104060. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.D.; Grecz, N.; Anellis, A. Sporulation of Clostridium botulinum types A, B, and E, Clostridium perfringens, and putrefactive anaerobe 3679 in dialysis sacs. J. Bacteriol. 1963, 85, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, M.; De Luis, R.; Pérez, M.D.; Calvo, M.; Sánchez, L. Selection of high affine peptide ligands for detection of Clostridium tyrobutyricum spores. J. Microbiol. Methods 2009, 79, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.E. Sous Vide for the Home Cook; Paradox Press: New York, NY, USA, 2010. [Google Scholar]
- Roca, J.; Brugués, S. Cocina con Joan Roca a Baja Temperatura: Descubre una Forma de Cocinar Más Sabrosa, Más Saludable; Editorial Planeta: Barcelona, Spain, 2016. (In Spanish) [Google Scholar]
- Van Asselt, E.D.; Zwietering, M.H. A systematic approach to determine global thermal inactivation parameters for various food pathogens. Int. J. Food Microbiol. 2006, 107, 73–82. [Google Scholar] [CrossRef]
- Komitopoulou, E. 16—Microbiological challenge testing of foods. In Series in Food Science, Technology and Nutrition, Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2011; pp. 507–523. [Google Scholar] [CrossRef]
- Byrne, B.; Dunne, G.; Bolton, D.J. Thermal inactivation of Bacillus cereus and Clostridium perfringens vegetative cells and spores in pork luncheon roll. Food Microbiol. 2006, 23, 803–808. [Google Scholar] [CrossRef]
- El Kadri, H.; Alaizoki, A.; Celen, T.; Smith, M.; Onyeaka, H. The effect of low-temperature long-time (LTLT) cooking on survival of potentially pathogenic Clostridium perfringens in beef. Int. J. Food Microbiol. 2020, 320, 108540. [Google Scholar] [CrossRef]
- Rinaldi, M.; Dall’Asta, C.; Paciulli, M.; Cirlini, M.; Manzi, C.; Chiavaro, E. A novel time/temperature approach to sous vide cooking of beef muscle. Food Bioprocess Technol. 2014, 7, 2969–2977. [Google Scholar] [CrossRef]
- Akoğlu, I.T.; Bıyıklı, M.; Akoğlu, A.; Kurhan, S. Determination of the Quality and Shelf Life of Sous Vide Cooked Turkey Cutlet Stored at 4 and 12 °C. Braz. J. Poult. Sci. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Singh, P.; Sultan, Z.; Pandey, V.K.; Singh, R. Sous vide processing for food quality enhancement: A review. Food Humanit. 2023, 1, 543–552. [Google Scholar] [CrossRef]
- Duan, Z.; Hansen, T.H.; Hansen, R.B.; Salgaard, P.; Knochel, S. Predicting outgrowth and inactivation of Clostridium perfringens in meat products during low temperature long time heat treatment. Int. J. Food Microbiol. 2016, 230, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Nwaizu, C.C.; Ekaette, I. Mathematical modeling for thermally treated vacuum-packaged foods: A review on sous vide processing. Trends Food Sci. Technol. 2022, 126, 73–85. [Google Scholar] [CrossRef]
- Shen, A.; Edwards, A.N.; Sarker, M.R.; Paredes-Sabja, D. Sporulation and Germination in Clostridial Pathogens. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Meng, J.; Genigeorgis, C. Delaying toxigenesis of Clostridium botulinum by sodium lactate in “sous-vide” products. Lett. Appl. Microbiol. 1994, 19, 20–23. [Google Scholar] [CrossRef]
- Bıyıklı, M.; Akoğlu, A.; Kurhan, S.; Akoğlu, I.T. Effect of different Sous Vide cooking temperature-time combinations on the physicochemical, microbiological, and sensory properties of turkey cutlet. Int. J. Gastron. Food Sci. 2020, 20, 100204. [Google Scholar] [CrossRef]
- Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Salek, P.; Pakula, K. Effect of Heat Treatment by the Sous-Vide Method on the Quality of Poultry Meat. Foods 2021, 10, 1610. [Google Scholar] [CrossRef]
- Hong, G.E.; Kim, J.H.; Ahn, S.J.; Lee, C.H. Changes in Meat Quality Characteristics of the Sous-vide Cooked Chicken Breast during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 757–764. [Google Scholar] [CrossRef]
- Patil, K.; Adhikari, M.; Rubinelli, P.; Desiree, K.; Vierck, K.R.; Acuff, J.C. Evaluating the Safety of Sous-Vide Cooking for Beef Products Inoculated with Single Strains of Salmonella enterica and Escherichia coli O157. J. Food Prot. 2024, 87, 100252. [Google Scholar] [CrossRef]
- Verheyen, D.; Altin, O.; Skipnes, D.; Erdogdu, F.; Skåra, T.; Van Impe, J.F. Thermal inactivation of Listeria monocytogenes in the Shaka agitated reciprocal retort: Influence of food matrix rheology and fat content. Food Bioprod. Process. 2021, 125, 22–36. [Google Scholar] [CrossRef]
- Verheyen, D.; Govaert, M.; Seow, T.K.; Ruvina, J.; Mukherjee, V.; Baka, M.; Skåra, T.; Van Impe, J.F. The Complex Effect of Food Matrix Fat Content on Thermal Inactivation of Listeria monocytogenes: Case Study in Emulsion and Gelled Emulsion Model Systems. Front. Microbiol. 2020, 10, 3149. [Google Scholar] [CrossRef]
- Mañas, P.; Pagan, R.; Alvarez, I.; Condón-Usón, S. Survival of Salmonella senftenberg 775 W to current liquid whole egg pasterization treatments. Food Microbiol. 2003, 20, 593–600. [Google Scholar] [CrossRef]
| Species | Strain Designation | Source (Code) |
|---|---|---|
| Salmonella enterica Subsp. enterica | Serotype: 4,5,12:i:1,2; Typhimurium | CECT 4156 |
| Campylobacter coli | serovar 4 | CECT 8205 |
| Campylobacter jejuni | serovar 2 | CECT 8170 |
| Clostridium sporogenes | McClung 2004 | CECT 892 |
| Food | Recipe | Initial Counts (CFU/mL) | Microorganism | Treatment | Storage (6 °C) |
|---|---|---|---|---|---|
| Chicken breast | Natural chicken breast | Non-inoculated | Natural flora | 65 °C, 75 min | - |
| 60 °C, 40 min | 14 d | ||||
| ~102 | Campylobacter Clostridium | 65 °C, 75 min | - | ||
| 60 °C, 40 min | 14 d | ||||
| ~106 | Campylobacter Clostridium | 65 °C, 75 min | - | ||
| 60 °C, 40 min | 14 d | ||||
| Spiced chicken breast | Non-inoculated | Natural flora | 65 °C, 75 min | - | |
| 60 °C, 40 min | 14 d | ||||
| ~102 | Campylobacter Clostridium | 65 °C, 75 min | - | ||
| 60 °C, 40 min | 14 d | ||||
| ~106 | Campylobacter Clostridium | 65 °C, 75 min | - | ||
| 60 °C, 40 min | 14 d | ||||
| Liquid egg | Natural omelet | ~102 | Salmonella | 75 °C, 10 min | - |
| 70 °C, 5 min | 7 d | ||||
| ~106 | Salmonella | 75 °C, 10 min | - | ||
| 70 °C, 5 min | 7 d | ||||
| Spiced omelet | ~102 | Salmonella | 75 °C, 10 min | - | |
| 70 °C, 5 min | 7 d | ||||
| ~106 | Salmonella | 75 °C, 10 min | - | ||
| 70 °C, 5 min | 7 d |
| Parameter | Additives (Spices) | Inoculated 102 | Inoculated 106 | ||
|---|---|---|---|---|---|
| 65 °C 75 min | 60 °C 40 min 14 D | 65 °C 75 min | 60 °C 40 min 14 D | ||
| Clostridium spores | Natural | >1.23 ± 0.5 *,** | >1.36 ± 0.38 *,** | 1.19 a ± 0.17 | 2.26 b ± 0.23 |
| Spiced | >1.18 ± 0.37 *,** | >1.32 ± 0.29 *,** | 1.02 a ± 0.35 | 2.34 ab ± 1.05 | |
| Campylobacter spp. | Natural | >2.19 ± 0.18 *** | >2.19 ± 0.18 *** | >5.87 ± 0.21 *** | >5.87 ± 0.21 *** |
| Spiced | >2.07 ± 0.44 *** | >2.07 ± 0.44 *** | >5.77 ± 0.16 *** | >5.77 ± 0.16 *** | |
| Parameter | Additives (Spices) | Initial Counts (log10 CFU/g) | Inactivation Rates | |
|---|---|---|---|---|
| 65 °C 75 min INST | 60 °C 40 min 14 D | |||
| Total aerobic counts | Natural | 5.06 ± 0.09 | 4.06 a ± 0.09 | 3.46 b ± 0.09 |
| Spiced | 4.73 ± 0.52 | 3.08 bc ± 0.51 | 2.94 c ± 0.33 | |
| Enterobacteriaceae | Natural | 2.84 a ± 0.03 | >1.84 ±0.03 * | >1.84 ± 0.03 * |
| Spiced | 2.00 b ± 0.00 * | >1.00 ± 0.00 * | >1.00 ± 0.00 * | |
| Pseudomonas | Natural | 4.06 ± 0.29 | >2.06 ± 0.29 * | >2.06 ± 0.29 * |
| Spiced | 3.40 ± 0.35 | >1.40 ± 0.35 * | >1.40 ± 0.35 * | |
| LAB | Natural | 3.28 ± 0.31 | >2.28 ± 0.31 * | >2.28 ± 0.31 * |
| Spiced | 2.95 ± 0.60 | >1.95 ± 0.60 * | >1.95 ± 0.60 * | |
| Yeast and molds | Natural | 2.00 ±0.00 * | >1.00 ± 0.00 * | >1.00 ± 0.00 * |
| Spiced | 2.40 ± 0.35 | >1.40 ± 0.35 * | >1.40 ± 0.35 * | |
| Inoculated 102 | Inoculated 106 | ||||
|---|---|---|---|---|---|
| Product | Initial Concentration | 75 °C 10 min | 70 °C 5 min 7 D | 75 °C 10 min | 70 °C 5 min 7 D |
| Natural omelet | 5.33 ± 0.3 | >1.76 ± 0.19 *,** | >1.76 ± 0.19 *,** | 4.33 ± 0.3 *,*** | 4.33 ± 0.3 *,*** |
| Spiced omelet | 5.35 ± 0.18 | >1.84 ± 0.15 *,** | >1.84 ± 0.15 *,** | 4.35 ± 0.18 *,*** | 4.35 ± 0.18 *,*** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, M.; Lavilla, M.; Amárita, F. Microbial Food Safety of Sous Vide Cooking Processes of Chicken and Eggs. Foods 2024, 13, 3187. https://doi.org/10.3390/foods13193187
Romeo M, Lavilla M, Amárita F. Microbial Food Safety of Sous Vide Cooking Processes of Chicken and Eggs. Foods. 2024; 13(19):3187. https://doi.org/10.3390/foods13193187
Chicago/Turabian StyleRomeo, Miguel, Maria Lavilla, and Félix Amárita. 2024. "Microbial Food Safety of Sous Vide Cooking Processes of Chicken and Eggs" Foods 13, no. 19: 3187. https://doi.org/10.3390/foods13193187






